Introduction
Pancreatic cancer is one of the leading causes of
cancer-related mortality in the US, since the majority of affected
patients present with advanced stages of surgically inoperable
disease (1,2). The identification of effective
therapeutic and prevention strategies (3–5)
against this metastatic disease is of high priority and has public
health significance.
Previous studies revealed the link between microRNAs
(miRNAs) and human cancer, thus yielding interest in using them as
targets for cancer therapies. miRNAs are a class of highly
conserved, non-coding RNAs that are approximately 19–25 nucleotides
long (6–8). Biogenesis of miRNA results from
primary miRNAs (pri-miRNAs), endogenously expressed transcripts of
hairpin RNAs. These pri-miRNAs are cleaved by the ribonuclease
Drosha in the nucleus in order to generate 72–100 nucleotide
precursor miRNAs (pre-miRNAs). The nuclear protein Exportin 5
translocates pre-miRNAs, in the fold-back structure, to the
cytoplasm. Cytoplasmic ribonuclease Dicer then digests the
pre-miRNAs to form short double-stranded miRNA duplexes. The miRNA
duplexes separate into mature, single-stranded miRNAs of 19–25
nucleotides. These mature miRNAs are able to bind the RNA-induced
silencing complex to align with target miRNAs at their 3′
untranslated region (UTR). Such interaction leads to translational
repression or cleavage of miRNAs and the subsequent down-modulation
of the respective genes.
Several miRNAs are involved in the regulation of
gene expression, a critical aspect of many biological processes,
including cell development, differentiation, apoptosis and
proliferation (7–12). Recent studies have implicated miRNAs
in the development of human malignancies (9–12) and
have documented the oncogenic (overexpressed) and tumor-suppressor
(underexpressed) roles of miRNAs in several neoplasms. Notably,
previous findings have established the role of miRNA-16 (miR-16) as
a tumor suppressor in a variety of human cancers (13–15).
Moreover, Lu et al (16)
demonstrated that the expression of miR-16 was globally higher in
normal cells compared to cancer cells. miR-16 is involved in cell
growth and apoptosis pathways in human malignancies, including
chronic lymphocytic leukemia (13)
and gastric carcinoma (14), by
negatively regulating Bcl-2. The anti-apoptotic protein, Bcl-2, is
also overexpressed in human pancreatic cancer (17,18).
Previously, Bold et al (18)
suggested that an increased BCL2 expression correlates with
apoptotic resistance and metastatic potential, since the
deregulation of BCL2 expression may be involved in the metastatic
progression of pancreatic carcinoma. Findings of this study showed
that the enforced overexpression of miR-16 mimic in pancreatic
adenocarcinoma cells attenuates cell growth with the simultaneous
repression of the BCL2 gene.
Materials and methods
Cell culture and treatment
Human pancreatic adenocarcinoma BxPC-3 cells were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). Cells were grown in RPMI with supplements of 10% fetal
bovine serum and 50 μg/ml of gentamicin at 37°C in a 5%
CO2 humidified atmosphere.
Transfection of miRNA mimic and
inhibitor
Cells were plated at a density of 1.8×105
cells/ml in T75 flasks and incubated overnight at 37°C. The
following day, cells were either mock-transfected or transfected
with 27 nM miR-16 miRIDIAN miRNA hairpin inhibitor or miR-16 miRNA
mimic and their appropriate negative controls, using the
Dharmafect2 transfection reagent (Dharmacon, Lafayette, CO, USA) in
accordance with the manufacturer’s protocol. miRIDIAN mimic and
inhibitor oligos were prepared by dissolution in 1X small
interfering RNA (siRNA) buffer. Diluted oligos were placed in Tube
1, and transfection reagent was maintained in Tube 2 in serum-free
medium. Reagents were mixed gently, followed by incubation at room
temperature (RT) for 5 min. The contents in Tubes 1 and 2 were then
combined and incubated at RT for 30 min. Subsequently, complete
medium with 10% serum was added to the mixture followed by addition
to the cells. The transfected cells were allowed to grow overnight.
The transfection medium was replaced after 24 h with fresh
medium.
miRNA Northern blot analysis
Total RNA (5 μg) was loaded and separated on a 15%
denatured urea gel. After electrophoresis and transfer, the blot
was hybridized with corresponding biotin pre-labeled miRNA probe
(Signosis Inc., Sunnyvale, CA, USA). To normalize the
hybridization, a biotin pre-labeled RNU48 probe was mixed with
miRNA probe for co-hybridization. The hybridized probes were then
monitored with streptavidin-HRP and chemiluminescent detection.
Luciferase activity assay
pGL3-BCL2 3′ UTR sense and antisense constructs were
prepared as previously described (13). For luciferase reporter assay, human
pancreatic cancer cells were co-transfected in a 96-well plate with
100 ng of the firefly luciferase reporter vector and 10 ng of the
pEGFPC2 expression vector (transfection control; Clonetech) either
alone or in combination with 50 nM miR-16 mimic oligo or 50 nM
mimic negative control using the Dharmafect Duo transfection
reagent. Luciferase activity was normalized to GFP expression. The
relative expression was determined for the mimic negative control
and miR-16-transfected samples.
Western immunoblotting
Following the designated treatment, total cellular
proteins were extracted. Equal protein from each sample was
fractionated by SDS-PAGE and blotted onto a nitrocellulose membrane
(GE Healthcare, NJ, USA). Membranes were probed with the Bcl-2
antibody (Upstate Biotechnology, Syracuse, NY, USA) followed by
incubation with a HRP-conjugated secondary antibody (3,4).
Finally, immunodetection was carried out by the enhanced
chemiluminescence method (GE Healthcare). Immunodetection with the
β-actin antibody (Sigma, St. Louis, MO, USA) served as a protein
loading control.
Clonogenic cell survival assay
Cells were washed 24 h post-transfection with
phosphate-buffered saline and then seeded in triplicate (20,000
cells/10-cm dish). The cells were allowed to grow for an additional
2 weeks. The medium was changed every 4 days. The cells were then
fixed with 4% buffered formalin (Electron Microscopy Sciences,
Hatfield, PA, USA) and stained with 0.1% crystal violet for
visualization and photography (19,20).
Results
Overexpression of miR-16 impairs
pancreatic cancer cell clonal growth
To understand whether miR-16 regulates pancreatic
adenocarcinoma cell proliferation, we used a commercially available
miR-16 mimic (mature). miR-16 mimics and inhibitors as well as the
respective negative controls were transfected into BxPC-3 cells.
The overexpression or silencing of miR-16 was verified by Northern
blotting of the transfected cells (Fig.
1B). At the 48-h transfection, we achieved a significant
expression of miR-16 over mock-transfected and scrambled
control-containing cells (Fig. 1B).
The clonogenic cell survival assay (19,20)
was employed to determine the long-term survival ability of BxPC-3
cells following transfection with miR-16 and its inhibitor oligos.
Notably, the overexpression of miR-16 significantly decreased the
clonogenic cell survival of pancreatic tumor cells when compared to
the mimic negative control (Fig. 1,
panel II vs. panel I), whereas silencing of the same miR-16 had no
effect on cell survival (Fig. 1A,
panel III vs. panel IV). Due to the low endogenous level, silencing
of miR-16 did not enhance cell survival when compared to the
control.
 | Figure 1miR-16 impairs the colony formation
ability of pancreatic tumor cells and down-regulates Bcl-2/FGF-2.
The transfection of miR-16 mimic, inhibitor and the respective
negative controls was carried out using Dharmafect2 transfection
reagent as described in Materials and methods. (A) Clonogenic cell
survival assay. Cells were washed 2 times with phosphate-buffered
saline and seeded in triplicate (20,000 cells/10-cm dish) 24 h
post-transfection. Cells were allowed to grow for an additional 2
weeks, followed by staining with 0.1% crystal violet for
visualization and photography. Panel I, mimic negative control;
panel II, miR-16 mimic oligonucleotide; panel III, inhibitor
negative control; panel IV, miR-16 inhibitor. (B) Northern blot
analysis. RNA was isolated from the negative control, miR-16 mimic-
and inhibitor-transfected BxPC-3 cells and subjected to Northern
blotting. Lane 1, mock; lane 2, mimic negative control; lane 3,
miR-16 mimic; lane 4, inhibitor negative control; lane 5, miR-16
inhibitor; lane M, molecular weight markers. To normalize the
hybridization, RNU48 probe was used as an internal control. |
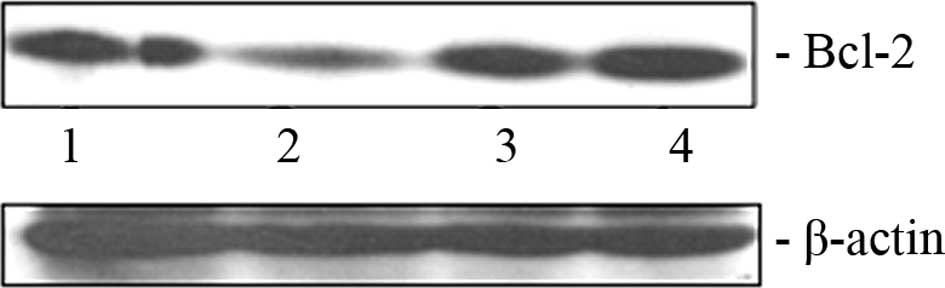
Exogenous expression of miR-16
reciprocally regulates Bcl-2 expression in BxPC-3 cells
As mentioned previously, miR-16 has the ability to
down-modulate anti-apoptotic protein Bcl-2 in diverse cancer types.
We attempted to ascertain whether the transfection of miR-16 mimic
oligonucleotide exerts any effect on the Bcl-2 level in pancreatic
cancer cells. A decrease in the Bcl-2 protein level in the BxPC-3
cells (Fig. 2, lane 2) was clearly
evident due to the ectopic overexpression of miR-16. However, the
level of Bcl-2 did not decrease when the hairpin inhibitor
oligonucleotide of miR-16 was transfected into these cells
(Fig. 2, lane 4).
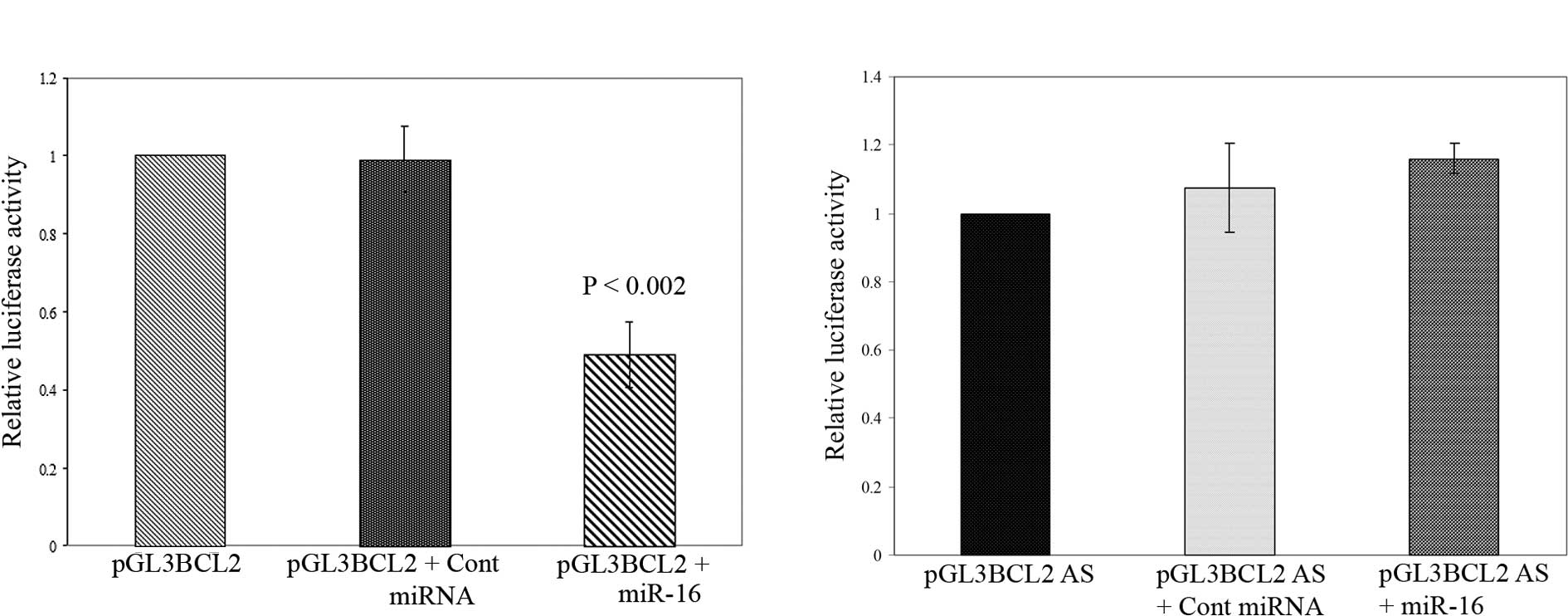
Post-transcriptional repression of BCL2
by miR-16 in pancreatic cancer cells
The decrease in Bcl-2 protein in
miR-16-overexpressed cells may be the direct effect of miRNA::mRNA
complementarity or by an indirect interaction with other unknown
targets. To address this issue, we used luciferase reporter
constructs where the 3′ UTR sequence of the human BCL2 gene
contained a presumed miR-16 complementary site that was fused into
a luciferase reporter plasmid in both sense and antisense
orientations (immediately downstream of the stop codon of
luciferase). The resulting plasmids and control plasmid (pEGFPC2)
were introduced into BxPC-3 pancreatic cancer cells together.
Notably, as shown in Fig. 3A, the co-transfection of the sense
orientation constructs (pGL3 BCL2) with miR-16 mimic oligo resulted
in a significant reduction in firefly luciferase reporter activity
when normalized against GFP (P<0.002). In contrast, the
transfection of miR-16 oligo in the presence of the luciferase
vector containing BCL2 3′ UTR in the antisense orientation
terminated this suppression (Fig.
3B). Moreover, the co-transfection of mimic negative control
miRNA had no effect on the luciferase activity of pGL3 BCL2 and
pGL3 BCL2 AS. Thus, our observation suggests that miR-16 directly
regulates Bcl-2 expression in pancreatic cancer cells through the
target site of 3′ UTR of BCL2 mRNA at the post-transcriptional
level.
Discussion
Pancreatic cells often become malignant during
tumorigenesis when cells acquire traits such as the ability to
evade apoptosis, replicate indefinitely and engage in persistent
angiogenesis. These malignant cells acquire resistance to therapy
due to multiple genetic alterations at each stage of tumorigenesis,
thereby imparting a selective advantage of growth over normal
cells. In this respect, the ability of miR-16 to attenuate the
growth of pancreatic adenocarcinoma cells by down-modulation of the
anti-apoptotic gene BCL2 is significant. Our finding is potentially
significant for utilizing miRNA-16 as a therapeutic tool for
pancreatic cancer treatment. The role of miR-16 as a tumor
suppressor was primarily documented (13) in B-cell chronic lymphocytic
leukemia, which is a common form of adult leukemia. Among other
studied potential tumor suppressor miRNAs, let-7 was found to be
consistently under-expressed in lung cancers compared to normal
adjacent tissues (21). Similar to
our finding, the overexpression of tumor suppressor let-7g
miRNA was shown to impair lung cancer cell proliferation and
promote cell death in vitro and in vivo (22,23).
Notably, the effect of anticancer drugs and
chemopreventive agents that modulate cell proliferation and
apoptotic signaling on miRNA expression profiles has also been
explored. In this context, Blower et al (24) suggested the potential role of
miRNAs, such as let-7i, miR-16 and miR-21, in the anticancer drug
response when tested in NCI-60 human cancer cell lines. Recently,
the pro-apoptotic action of EGCG, a significant component of green
tea, has been shown to be mediated by the up-regulation of miR-16
and down-regulation of Bcl-2 in hepatocellular carcinoma cells
(25). Previously, Scott et
al (26) demonstrated a
functional link between histone deacetylase inhibition and the
presence of miR-27a/27b miRNA. In addition, all-trans-retinoic acid
treatment of leukemic cells resulted in the differential expression
of a number of miRNAs, including let-7 and the miR-16 family
(27). More importantly, Weidhaas
et al (22) suggested that
miRNAs may be potential targets for altering resistance to
cytotoxic anticancer therapy.
Since miRNA-16 overexpression diminishes the
proliferation of human pancreatic cancer cells, it can be used in
conjunction with chemotherapeutic agents such as gemcitabine to
combat pancreatic cancer. miRNAs have multiple target genes and may
decrease the expression levels of genes with various biological
functions. As opposed to the silencing of individual genes, this
multi-targeted approach can revolutionize therapeutic strategies
against pancreatic cancer by exerting a stronger inhibitory effect
on tumor growth. Therefore, this novel observation implicating the
antiproliferative effect of miR-16 on pancreatic adenocarcinoma
cells, may provide new insights into treating pancreatic
cancer.
Acknowledgements
This study was supported, in part, by NIH Grants
CA137476 (A. Basu) and CA 109181 (S. Haldar). We also thank D.
Haldar and K. Haas for the experimental assistance.
References
|
1
|
Jamal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer Statistics. CA Cancer J Clin. 57:43–66.
2007.
|
|
2
|
Goggins M: Identifying molecular markers
for the early detection of pancreatic neoplasia. Semin Oncol.
34:303–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Basu A and Haldar S: 2-Methoxyestradiol
mediated signaling network in pancreatic cancer. Front Biosci.
14:2170–2178. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basu A, Castle VP, Bouziane M, Bhalla K
and Haldar S: Crosstalk between extrinsic and intrinsic cell death
pathways in pancreatic cancer: synergistic action of estrogen
metabolite and ligands of death receptor family. Cancer Res.
66:4309–4318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qanungo S, Das M, Haldar S and Basu A:
Epigallocatechin-3-gallate induces mitochondrial membrane
depolarization and caspase-dependent apoptosis in pancreatic cancer
cells. Carcinogenesis. 26:958–967. 2005. View Article : Google Scholar
|
|
6
|
Zeng Y: Principles of micro-RNA production
and maturation. Oncogene. 25:6156–6162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dalmay T and Edwards DR: MicroRNAs and the
hallmarks of cancer. Oncogene. 25:6170–6175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kent OA and Mendell JT: A small piece in
the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
Oncogene. 25:6188–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Negrini M, Nicoloso MS and Calin GA:
MicroRNAs and cancer – new paradigms in molecular oncology. Curr
Opin Cell Biol. 21:470–479. 2009.
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M,
Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M and
Croce CM: miR-15 and miR-16 induce apoptosis by targeting BCL2.
Proc Natl Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia L, Zhang D, Du R, et al: miR15-b and
miR-16 modulate multidrug resistance by targeting BCL2 in human
gastric cancer cells. Int J Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D’Urso L, Pagliuca A, Biffoni M, Labbaye C,
Bartucci M, Muto G, Peschle C and De Maria R: The miR-15a-miR-16-1
cluster controls prostate cancer by targeting multiple oncogenic
activities. Nat Med. 14:1271–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
Downing JR, Jacks T, Horvitz HR and Golub TR: MicroRNA expression
profiles classify human cancers. Nature. 435:834–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galante JM, Mortenson MM, Bowles TL,
Virudachalam S and Bold RJ: ERK/BCL-2 pathway in the resistance of
pancreatic cancer to anoikis. J Surg Res. 152:18–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bold RJ, Virudachalam S and McConkey DJ:
BCL2 expression correlates with metastatic potential in pancreatic
cancer cell lines. Cancer. 92:1122–1129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Munshi A, Hobbs M and Meyn RE: Clonogenic
cell survival assay. Methods Mol Med. 110:21–28. 2005.
|
|
20
|
Basu A and Haldar S: Antiproliferative and
proapoptotic effects of benzyl isothiocyanate on human pancreatic
cancer cells is linked to death receptor activation and RasGAP/Rac1
down-modulation. Int J Oncol. 35:593–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roush S and Slack FJ: The let-7 family of
microRNAs. Trends Cell Biol. 18:505–516. 2008. View Article : Google Scholar
|
|
22
|
Weidhaas JB, Babar I, Nallur SM, Trang P,
Roush S, Boehm M, Gillespie E and Slack FJ: MicroRNAs as potential
agents to alter resistance to cytotoxic anticancer therapy. Cancer
Res. 67:11111–11116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar MS, Erkeland SJ, Pester RE, Chen CY,
Ebert MS, Sharp PA and Jacks T: Suppression of non-small cell lung
tumor development by the let-7 microRNA family. Proc Natl Acad Sci
USA. 105:3903–3908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blower PE, Chung JH, Verducci JS, Lin S,
Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM,
Weinstein JN and Sadee W: MicroRNAs modulate the chemosensitivity
of tumor cells. Mol Cancer Ther. 7:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsang WP and Kwok TT: Epigallocatechin
gallate up-regulation of miR-16 and induction of apoptosis in human
cancer cells. J Nutr Biochem. 21:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scott GK, Mattie MD, Berger CE, Benz SC
and Benz CC: Rapid alteration of microRNA levels by histone
deacetylase inhibition. Cancer Res. 66:1277–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garzon R, Pichiorri F, Palumbo T,
Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder
H, Calin GA, Liu CG, Andreeff M and Croce CM: MicroRNA gene
expression during retinoic acid-induced differentiation of human
acute promyelocytic leukemia. Oncogene. 26:4148–4157. 2007.
View Article : Google Scholar : PubMed/NCBI
|