Introduction
The incidence of malignant pleural mesothelioma is
on the increase and is anticipated to increase further in future
decades as a result of widespread exposure to asbestos particularly
in unprotected countries (1,2).
Epidemiologically, analyses using an age-cohort model in Japan
showed that there may be approximately 100,000 deaths due to
pleural mesothelioma in the next 40 years (3). Based on US mesothelioma incidence data
from 1973 to 2000, it was estimated that the total number of male
mesothelioma cases in 2003–2054 will reach approximately 71,000
(4). Thus, it is crucial to develop
treatment modalities for the therapy of malignant mesothelioma, and
an appropriate animal model is necessary for this purpose. Reports
exist on peritoneal mesothelioma induction by chemicals or fibers
in conventional rats (5–8) or pleural mesotheliomas in genetically
modified animals such as the p53 knockout mouse (9). Lardinois et al also reported
the efficacy of the intrapleural application of cisplatin in an
immune-competent rat model inoculated with mesothelioma cells
(10). Since pleural malignant
mesothelioma is the most common form of mesothelioma in humans
(11), a bioassay model featuring
similar lesions in otherwise normal animals would be optimal.
However, to our knowledge no report of pleural mesothelioma in
experimental wild-type animals using direct infusion of particles
currently exists. In our previous study, 0.2 ml of polymer gel was
infused directly into the left cavity of the thorax by thoracotomy
to occupy a certain thoracic cavity volume and to examine the
influence of physical pulmonary collapse. We demonstrated that a
pronounced mesothelial cell reaction to the infused polymer was
evident on the left lung surfaces and parietal pleura (12).
In the present study, thoracotomy was performed to
allow for the infusion of particles directly into the thoracic
cavity of A/J mice. In order to assess differences in the reaction
depending on the shape of the infused particles, fiber-shaped
potassium octatitanate (TISMO) and granular-shaped micro- and
nano-size order titanium dioxide (TiO2) were employed as
test materials. To contribute to the mechanistic analysis, iron
staining with Berlin blue and immunostaining for calretinin were
also performed.
Materials and methods
Chemicals
Potassium octatitanate fibers, trade name TISMO, and
the chemical formula K2O·nTiO2, were supplied
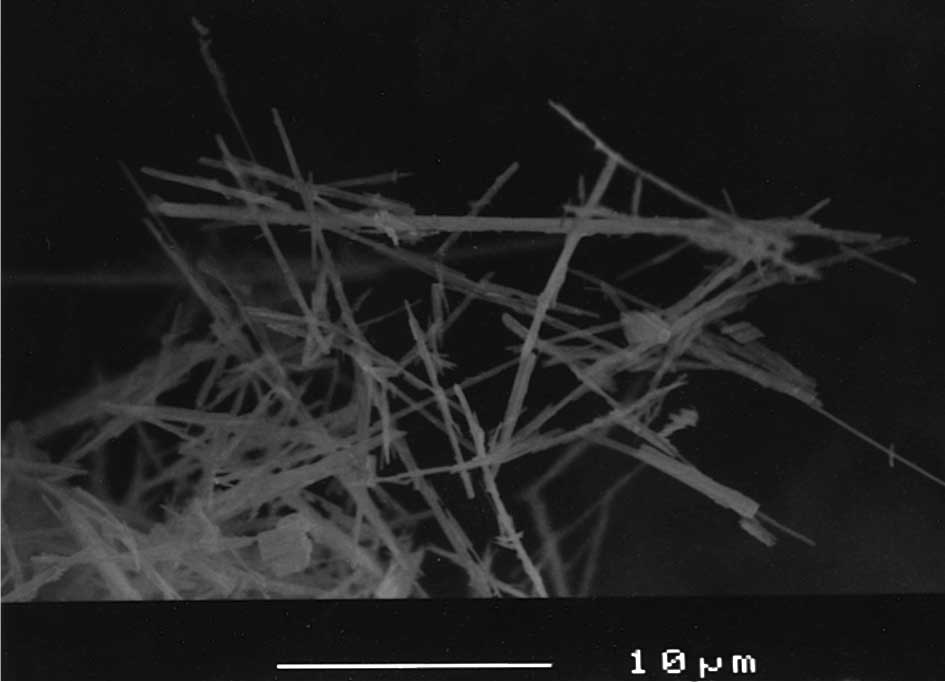
by Otsuka Chemical Co., Ltd. (Osaka, Japan) with dimensions mostly
<50 μm in length and <2 μm in width [Fig. 2; scanning electron microscope (SEM)
image, JSM-6400 (Joel Ltd., Tokyo, Japan), at a magnification
×3,000]. Granular-shaped TiO2 with particle diameters of
<5 μm (Rutile form, lot. TCG4139) (TiO2 micro) and
~80 nm (lot. DPN0960) (TiO2 nano) were purchased from
Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The particles
were all suspended in saline (Otsuka isotonic sodium chloride
solution; Otsuka Pharmaceutical Factory, Inc., Tokushima,
Japan).
Animals
Female A/J mice (5 weeks old), purchased from
Shizuoka Laboratory Animal Center (Shizuoka, Japan), were
maintained in the Division of Animal Experimentation, Life Science
Research Center, Kagawa University, according to the Institutional
Regulations for Animal Experiments. The protocols of the
experiments were approved by the Animal Care and Use Committee of
Kagawa University. The animals were housed in polycarbonate cages
with white wood chips for bedding and given free access to drinking
water and a basal diet, Oriental MF (Oriental Yeast Co., Ltd.,
Tokyo, Japan), under controlled conditions of humidity (60±10%),
lighting (12-h light/dark cycle) and temperature (24±2°C). The
experiments were started after a 2-week acclimatization period.
Experimental design and tissue
preparation
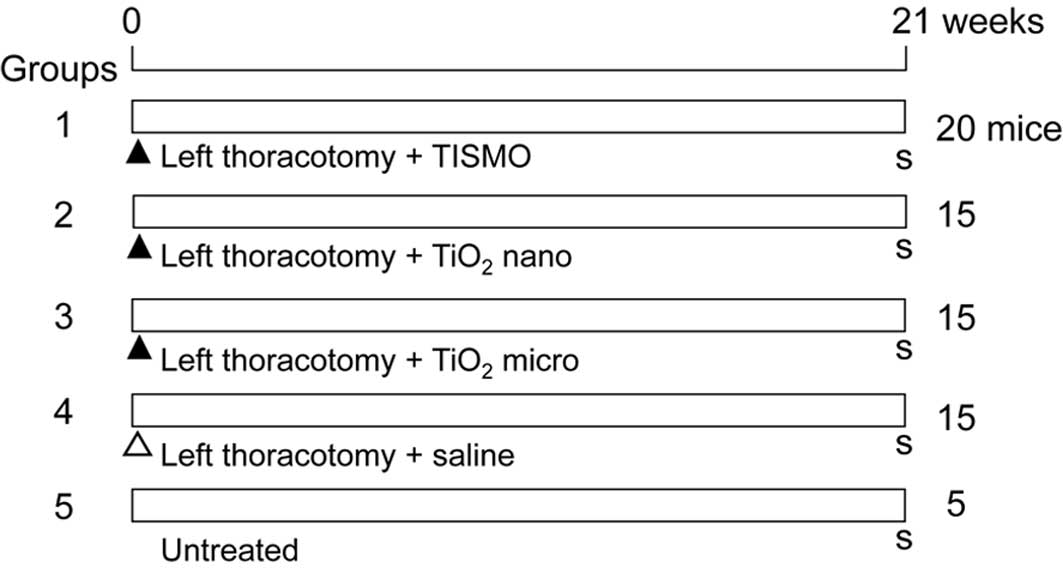
Fig. 1 shows the
design for the experiment. A total of 70 mice at 7 weeks of age
were divided into 5 groups of 20 (Group 1), 15 (Groups 2, 3 and 4)
and 5 (Group 5) mice. At the beginning of the experiment, the 65
mice of Groups 1–4 underwent a left thoracotomy and were
administered 1.5 mg of TISMO particles (Group 1), TiO2
nano (Group 2), TiO2 micro (Group 3) (all suspended in
0.2 ml saline) or the vehicle (Group 4, saline control group). Each
mouse was administered intraperitoneally 0.2 ml pentobarbital
sodium (Nembutal, Dainippon Sumitomo Pharma Co., Ltd., Osaka,
Japan) with a 10 times dilution (0.06–0.1 ml/10 g body weight).
Under deep anesthesia, a skin incision (~7 mm) was performed on the
left axilla. After confirmation of the location of the thoracic
wall, a thoracotomy was completed with an incision (~5 mm) between
the ribs. The left lung was observed directly through this open
hole, and atelectasis was confirmed. Following test particle
infusion into the left thoracic cavity, the skin was clipped
together to close the thorax. The experiment was terminated after
21 weeks, and all of the groups were sacrificed under deep
anesthesia.
At autopsy, the lungs, liver and kidneys were
removed. The lungs were initially weighed, followed by the trachea
and heart. The organs were then rinsed and infused with 10%
neutral-buffered formalin. The lung weight was calculated by
subtraction of the trachea and heart weight. The organs were
immersed in 10% neutral-buffered formalin for 1 week, and sections
were routinely processed for embedding in paraffin for
histopathological examination of hematoxylin and eosin-stained
sections. Sections were also prepared for special staining with
Berlin blue to detect iron accumulation and immunostaining of
calretinin to identify mesothelial cells (13).
Immunostaining analysis
The lungs were immunostained by the avidin-biotin
complex (ABC) method, and all staining processes from
deparaffinization to counterstaining with hematoxylin were
performed automatically using the Ventana Discovery™ staining
system (Ventana Medical Systems, AZ, USA). Anti-mouse calretinin
monoclonal antibody, clone 5A5, purchased from Novocastra
Laboratories Ltd. (Newcastle upon Tyne, UK) was employed at a 1:100
dilution.
Statistical analysis
The body and organ weight data were analyzed by the
Tukey-Kramer test (multi-comparison test) and the incidences of
lung lesions by the Chi-square test.
Results
Two mice died (1 each in Groups 2 and 3) following
thoracotomy. The general condition in all of the groups consisted
of no marked changes during the experimental period. Final body and
organ weights are shown in Table I.
The body weights of Group 1 were significantly greater than those
of Group 4. The absolute weights of the lungs of Group 1 and the
livers of Group 5 demonstrated significant differences in
comparison to Group 4. However, the relative weights were similar,
without significant variation.
 | Table IBody and organs weights of the
mice. |
Table I
Body and organs weights of the
mice.
| Groups | 1 (n=20) | 2 (n=14) | 3 (n=14) | 4 (n=15) | 5 (n=5) |
|---|
| Left thoracotomy | + | + | + | + | − |
|---|
| Treatment | TISMO | TiO2
nano | TiO2
micro | Saline | Untreated |
|---|
| Body weight (g) | 32.3±7.7a | 25.0±2.1 | 26.1±2.2 | 27.4±3.6 | 32.5±4.2 |
| Lungs |
| Absolute (g) | 0.28±0.04a | 0.19±0.03 | 0.19±0.02 | 0.21±0.03 | 0.23±0.02 |
| Relative (%) | 0.91±0.26 | 0.76±0.13 | 0.73±0.08 | 0.76±0.11 | 0.70±0.10 |
| Liver |
| Absolute (g) | 1.20±0.16 | 1.10±0.22 | 1.09±0.09 | 1.18±0.14 | 1.44±0.15a |
| Relative (%) | 3.85±0.76 | 4.43±1.01 | 4.17±0.34 | 4.33±0.38 | 4.44±0.32 |
| Kidneys |
| Absolute (g) | 0.29±0.04 | 0.26±0.04 | 0.25±0.02 | 0.28±0.02 | 0.31±0.03 |
| Relative (%) | 0.93±0.18 | 1.03±0.18 | 0.98±0.10 | 1.02±0.10 | 0.97±0.10 |
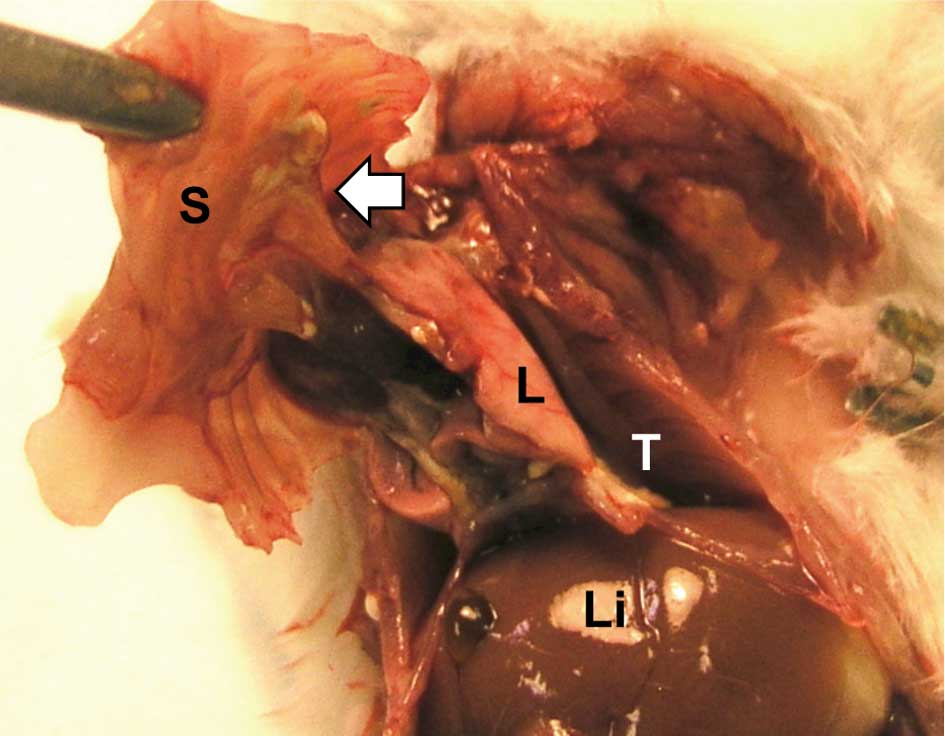
Grossly, the infused TISMO, TiO2 micro-
or TiO2 nano-particles formed discrete masses in the
cavity of the chest. Severe adhesions around the lung to the thorax
were observed in the TISMO-treated group (Group 1) (Fig. 3), while less or no adhesion was
evident in the TiO2 nano- or TiO2
micro-treated groups (Groups 2 and 3, respectively). The control
groups (Groups 4 and 5) were without inflammatory lesions.
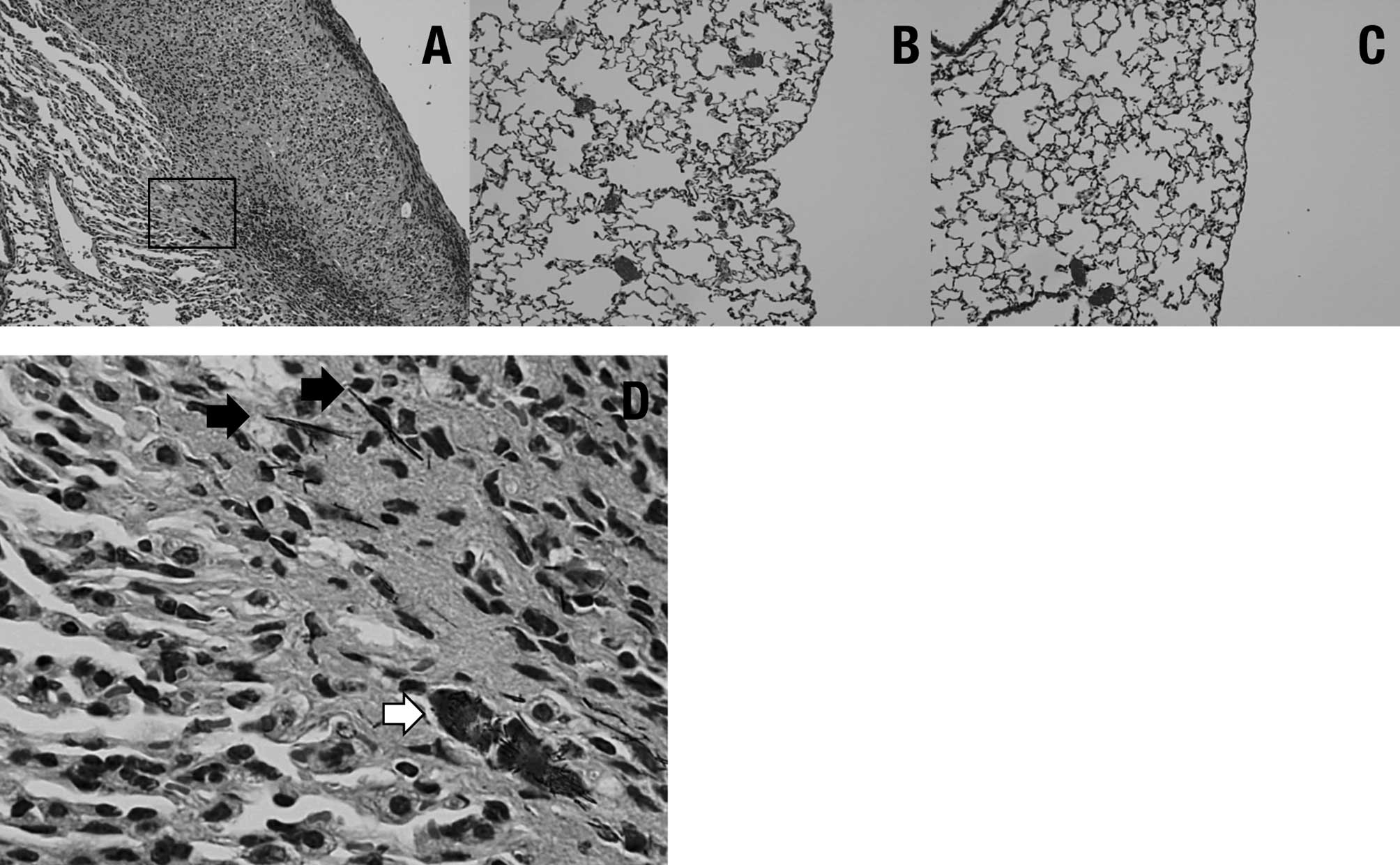
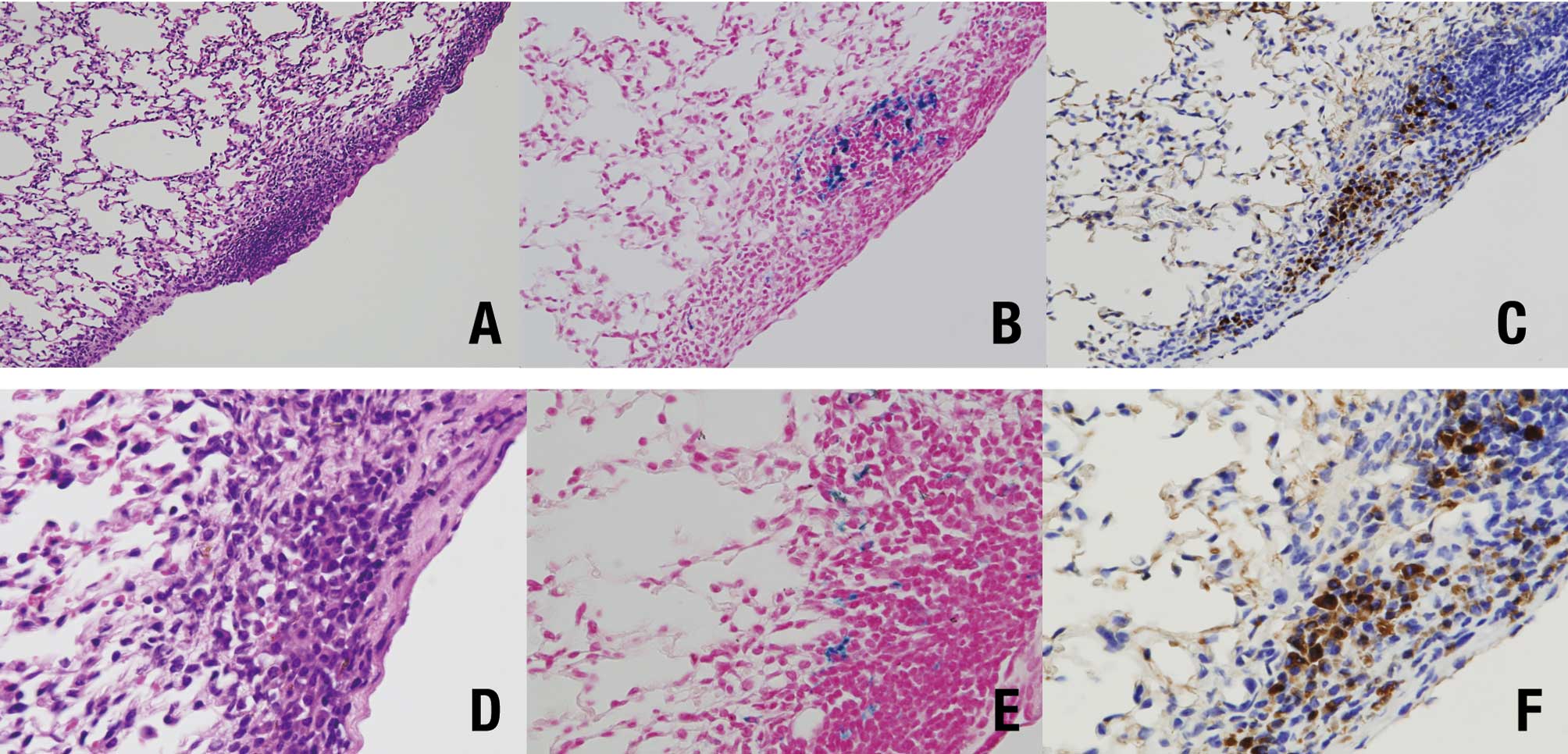
Histopathologically, pleural thickening was observed
after TISMO (Group 1) (Fig. 4A),
but not TiO2 nano or TiO2 micro treatment
(Groups 2 and 3, respectively) (Fig. 4B
and C). In the TISMO-treated group (Group 1), fibers were
detected, not only in areas of pleural thickening, but also within
alveoli (Fig. 4D). However,
inflammatory reactions were limited to the pleura. Table II shows the incidences of lung
lesions. In the TISMO-treated group (Group 1), pleural thickening
and particles in the alveoli were detected at significantly greater
incidences than in the other groups. In the particle-treated groups
(Groups 1, 2 and 3), deposits were observed in the lymph nodes
around the lungs. In the lungs, adenomas were noted in all of the
groups, but without significant intergroup differences. Positive
spots of Berlin blue staining (Fig. 5B
and E) were detected in the area of pleural thickening with
TISMO fibers (Fig. 5A and D), but
not in alveoli or in the other groups. Calretinin-positive cells
were also observed in essentially the same lesions (Fig. 5C and F). In the liver, fatty
degeneration was observed in 1 mouse in Groups 1 and 4,
respectively. In the kidneys, tubular necrosis was noted in 1 mouse
of Group 2. No other marked lesions were detected.
 | Table IIHistopathological findings of the
lung. |
Table II
Histopathological findings of the
lung.
| Groups | 1 (n=20) | 2 (n=14) | 3 (n=14) | 4 (n=15) | 5 (n=5) |
|---|
| Left thoracotomy | + | + | + | + | − |
|---|
| Treatment | TISMO | TiO2
nano | TiO2
micro | Saline | Untreated |
|---|
| Incidence % |
| Pleural
thickening | 100.0a (20/20) | 7.1 (1/14) | 14.3 (2/14) | 0.0 (0/15) | 0.0 (0/5) |
| Particles in the
alveoli | 100.0a (20/20) | 0.0 (0/14) | 0.0 (0/14) | 0.0 (0/15) | 0.0 (0/5) |
| Particles in the
lymph nodes | 75.0b (15/20) | 92.9b (13/14) | 100.0b (14/14) | 0.0 (0/15) | 0.0 (0/5) |
| Adenoma
(lung) | 5.1 (3/20) | 35.7 (5/14) | 7.1 (1/14) | 13.3 (2/15) | 20.0 (1/5) |
Discussion
In the present study, the particles, TISMO,
TiO2 nano and TiO2 micro, essentially
consisted of the same material, i.e., titanium, but the shape
(fiber or granular) was different. Only the fiber-shaped TISMO
induced mesothelial cell reactions. To our knowledge, this is the
first such study. Previously, fibrous particles greater than 1 μm
in length, with a length-to-width ratio >10:1, were believed to
have particular biological relevance for mesothelial risk (14). The TISMO used in our experiments,
with dimensions mostly less than 50 μm in length and less than 2 μm
in width, meets these criteria. Results of the present study show
that it is not necessarily the chemical composition, but rather the
physical characteristics of the material used that determined the
reactions, including the severe adhesion and mesothelial cell
accumulation.
The chemical formula of TISMO is
K2O·nTiO2, although TISMO containing
K2O is chemically different from TiO2. It is
likely that the fomula containing K2O induced the
mesothelial cell reactions. Therefore, the difference in the
influence between the TISMO and TiO2 particles by
focusing attention only on their shape may be relevant. Fewer
mesothelial cell reactions induced both by TiO2 micro-
and TiO2 nano-particles, which have the same chemical
formula, were noted. It was thus proven that the mesothelial cell
reactions are not influenced by particle size. Additionally, the
TISMO results provide important reference and additional data
concerning particle shape. To obtain detailed findings regarding
the shape, future experiments should be performed with fiber-shaped
and fine particles of identical chemical composition, such as
milled fiber-shaped TISMO.
The Berlin blue staining used in this study showed
iron accumulation around TISMO fibers in the thickened pleura,
co-locating with immunostaining for calretinin, indicative of
mesothelioma cells, although this was not noted in the pleura
treated with the TiO2 nano- or micro-particles. DNA
damage and apoptosis from iron-derived reactive oxygen species are
regarded as important for malignant mesothelioma development
(15–17). Furthermore, iron as a component of
asbestos or as a consequence of asbestos-induced pathology is a
significant candidate for underlying carcinogenic mechanisms, since
iron overload is identified as being carcinogenic (18). Recently, intraperitoneal or
intrascrotal application of multi-wall carbon nanotube (MWCNT) was
reported to induce mesotheliomas (19,20).
Notably, TISMO contains no iron component, therefore the iron
accumulation is likely to have derived from an endogenous source in
the body. In our previous studies in which TISMO fibers
administered by intratracheal instillation to F344 rats (4 mg per
rat) were used, only mild inflammatory reactions were observed
compared to quartz particles in the lungs after 28 days (21,22).
Results of other studies of TISMO administration by chronic
inhalation also found limited inflammation in the lung (23,24).
However, inhaled fibers of TISMO may be able to penetrate the
alveoli to the pleura, as demonstrated here in reverse, and this
would elucidate the induction of pleural mesothelioma by inhalation
of asbestos in humans.
The mesothelial cells in our reactive lesions showed
only slight atypia, and it was difficult to make a diagnosis of
malignant pleural mesothelioma. The experimental period of 21 weeks
was determined based on a previous study (20) whose results indicated that the
mortality rate due to mesothelioma increased approximately 130 days
after intraperitoneal injection of MWCNT or crocidolite. However,
the animals used in this study were not wild-type, but rather
p53-heterozygous. Future studies in which the experiment is
repeated using a longer time period need to be conducted.
No intergroup differences were observed in the
incidence of lung adenoma. A/J female mice, a mouse strain which
frequently develops lung tumors and is used commonly as a lung
carcinogenesis model (25–27), were employed in the present study to
assess the influence of particles or fibers on lung proliferative
lesions. We assessd the influence on lung tumors without any
chemicals, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
and tobacco-specific N-nitrosamine (28), to strongly initiate the effects.
Subsequently, the present study may have had a short experimental
time period. Exposure to asbestos which causes mesothelioma has a
primarily occupational etiology, and the majority of cases are male
(29,30). When considering the strain used for
malignant pleural mesothelioma, female mice were shown to be in
conflict with the etiological evidence. Thus, male mice should be
used for future experiments on mesothelioma.
In conclusion, the present study demonstrated that
only fiber-shaped TISMO induced severe reactions of the mesothelium
in the pleura, and these reactions involved iron accumulation
derived from endogenous sources. The results indicate that the risk
of mesothelial cell reaction does not depend on particle size, but
may depend on the shape.
Acknowledgements
The authors thank Dr Malcolm A. Moore for the
critical reading of the manuscript. This study was supported, in
part, by Grants-in-Aid for Cancer Research from the Ministry of
Health, Labour and Welfare of Japan.
References
|
1
|
Metintas S, Metintas M, Ucgun I and Oner
U: Malignant mesothelioma due to environmental exposure to
asbestos: follow-up of a turkish cohort living in a rural area.
Chest. 122:2224–2229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murayama T, Takahashi K, Natori Y and
Kurumatani N: Estimation of future mortality from pleural malignant
mesothelioma in Japan based on an age-cohort model. Am J Ind Med.
49:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Price B and Ware A: Mesothelioma trends in
the united states: An update based on surveillance, epidemiology,
and end results program data for 1973 through 2003. Am J Epidemiol.
159:107–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim Y, Ton TV, DeAngelo AB, et al: Major
carcinogenic pathways identified by gene expression analysis of
peritoneal mesotheliomas following chemical treatment in f344 rats.
Toxicol Appl Pharmacol. 214:144–151. 2006. View Article : Google Scholar
|
|
6
|
Kamstrup O, Ellehauge A, Collier CG and
Davis JM: Carcinogenicity studies after intraperitoneal injection
of two types of stone wool fibres in rats. Ann Occup Hyg.
46:135–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krajnow A and Lao I: Assessment of
carcinogenic effect of aluminosilicate ceramic fibers produced in
Poland. Animal experiments Med Pr. 51:19–27. 2000.PubMed/NCBI
|
|
8
|
Crosby LM, Morgan KT, Gaskill B, Wolf DC
and DeAngelo AB: Origin and distribution of potassium
bromate-induced testicular and peritoneal mesotheliomas in rats.
Toxicol Pathol. 28:253–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jongsma J, van Montfort E, Vooijs M, et
al: A conditional mouse model for malignant mesothelioma. Cancer
Cell. 13:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lardinois D, Jung FJ, Opitz I, et al:
Intrapleural topical application of cisplatin with the surgical
carrier vivostat increases the local drug concentration in an
immune-competent rat model with malignant pleuromesothelioma. J
Thorac Cardiovasc Surg. 131:697–703. 2006. View Article : Google Scholar
|
|
11
|
Moore AJ, Parker RJ and Wiggins J:
Malignant mesothelioma. Orphanet J Rare Dis. 3:342008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yokohira M, Hashimoto N, Yamakawa K, Saoo
K, Kuno T and Imaida K: Lack of promoting effects from physical
pulmonary collapse in a female a/j mouse lung tumor initiated with
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nnk) with
remarkable mesothelial cell reactions in the thoracic cavity by the
polymer. Exp Toxicol Pathol. (In press).
|
|
13
|
Shield PW and Koivurinne K: The value of
calretinin and cytokeratin 5/6 as markers for mesothelioma in cell
block preparations of serous effusions. Cytopathology. 19:218–223.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedrichs KH and Molik B: Microscopic
observations on some fibrous dust samples. Zentralbl Bakteriol
Mikrobiol Hyg. 181B:216–225. 1985.PubMed/NCBI
|
|
15
|
Kamp DW: Asbestos-induced lung diseases:
An update. Transl Res. 153:143–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang L, Nagai H, Ohara H, et al:
Characteristics and modifying factors of asbestos-induced oxidative
DNA damage. Cancer Sci. 99:2142–2151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aung W, Hasegawa S, Furukawa T and Saga T:
Potential role of ferritin heavy chain in oxidative stress and
apoptosis in human mesothelial and mesothelioma cells: implications
for asbestos-induced oncogenesis. Carcinogenesis. 28:2047–2052.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toyokuni S: Iron-induced carcinogenesis:
the role of redox regulation. Free Radic Biol Med. 20:553–566.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakamoto Y, Nakae D, Fukumori N, et al:
Induction of mesothelioma by a single intrascrotal administration
of multi-wall carbon nanotube in intact male fischer 344 rats. J
Toxicol Sci. 34:65–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takagi A, Hirose A, Nishimura T, et al:
Induction of mesothelioma in p53+/− mouse by
intraperitoneal application of multi-wall carbon nanotube. J
Toxicol Sci. 33:105–116. 2008.
|
|
21
|
Yokohira M, Takeuchi H, Yamakawa K, et al:
Bioassay by intratracheal instillation for detection of lung
toxicity due to fine particles in f344 male rats. Exp Toxicol
Pathol. 58:211–221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokohira M, Kuno T, Yamakawa K, et al: An
intratracheal instillation bioassay system for detection of lung
toxicity due to fine particles in f344 rats. J Toxicol Pathol.
22:1–10. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikegami T, Tanaka A, Taniguchi M, et al:
Chronic inhalation toxicity and carcinogenicity study on potassium
octatitanate fibers (tismo) in rats. Inhal Toxicol. 16:291–310.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ikegami T, Taniguchi M, Singer AW, et al:
Inhalation toxicity of potassium octatitanate fibers (tismo) in
rats following 13 weeks of aerosol exposure. Inhal Toxicol.
12:415–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gordon T and Bosland M: Strain-dependent
differences in susceptibility to lung cancer in inbred mice exposed
to mainstream cigarette smoke. Cancer Lett. 275:213–220. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharma S, Gao P and Steele VE: The
chemopreventive efficacy of inhaled oltipraz particulates in the
b[a]p-induced a/j mouse lung adenoma model. Carcinogenesis.
27:1721–1727. 2006.PubMed/NCBI
|
|
27
|
Yokohira M, Takeuchi H, Saoo K, et al:
Establishment of a bioassay model for lung cancer chemoprevention
initiated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nnk)
in female a/j mice. Exp Toxicol Pathol. 60:469–473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeuchi H, Saoo K, Yokohira M, et al:
Pretreatment with 8-methoxypsoralen, a potent human cyp2a6
inhibitor, strongly inhibits lung tumorigenesis induced by
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female a/j mice.
Cancer Res. 63:7581–7583. 2003.
|
|
29
|
Pukkala E, Martinsen JI, Lynge E, et al:
Occupation and cancer – follow-up of 15 million people in five
nordic countries. Acta Oncol. 48:646–790. 2009.
|
|
30
|
Madkour MT, El Bokhary MS, Awad Allah HI,
Awad AA and Mahmoud HF: Environmental exposure to asbestos and the
exposure-response relationship with mesothelioma. East Mediterr
Health J. 15:25–38. 2009.PubMed/NCBI
|