Introduction
Intracranial non-germinomatous malignant germ cell
tumors (NGMGCTs) include embryonal carcinoma, endodermal sinus
tumor (also called yolk sac tumor), choriocarcinoma, teratoma
(including immature teratoma and teratoma with malignant
transformation) and mixed germ cell tumor. Historically, these
tumors were rarely identified and were usually mistaken as a single
category. However, different patterns of recurrence and survival
were reported among different subtypes (1–3).
Therefore, the relative roles of surgical resection, radiotherapy,
chemotherapy and gamma knife surgery in the management of patients
with such lesions have remained controversial (4). Between 1995 and 2007, 223 cases of
germ cell tumors (GCTs) were treated in Shanghai Huashan Hospital,
of which 39 cases (17.5%) were NGMGCTs. All the afore-mentioned
cases were pathologically inspected and verified. The clinical
features of these 39 cases of intracranial NGMGCTs were analyzed
for the diagnosis and treatment of this disease.
Materials and methods
Patient population
A retrospective review of medical records from 1995
to 2007 identified 39 patients with intracranial NGMGCTs. In this
group of medical cases, the male to female ratio was 33:6 and the
age 2–40 years (median 14.2±3.5) (Table
I). In 19 cases, the tumor was located in the pineal region,
while in another 11 it was located in the sellar region. Two tumors
were located in the lateral ventricle, 3 in the third ventricle, 1
in the thalamus, 1 in the basal ganglia, 1 in the septum pellucidum
and 1 tumor in the temporal lobe.
 | Table ICharacteristics of intracranial
non-germinomatous germ cell tumors in 39 patients. |
Table I
Characteristics of intracranial
non-germinomatous germ cell tumors in 39 patients.
| | Age (years) | Tumor location |
|---|
| |
|
|
|---|
| Histology | No. of patients | Median | Range | Pineal region | Sellar region | Thalamus or basal
ganglia | Lateral
ventricle | Third ventricle | Temporal lobe | Septum
pellucidum |
|---|
| Teratoma | | | | | | | | | - | - |
| Immature | 15 | 14.6 | 8–28 | 11 | 4 | - | - | - | - | - |
| Malignant
transformation | 0 | - | - | - | - | - | - | - | - | - |
| Embryonal
carcinoma | 7 | 13.9 | 3–26 | 4 | 1 | - | - | 1 | 1 | - |
| Yolk sac tumor | 2 | 7.0 | 2–12 | - | - | - | 2 | - | - | - |
| Choriocarcinoma | 0 | - | - | - | - | - | - | - | - | - |
| Mixed germ cell
tumor | | | | - | - | - | - | - | - | - |
| Mainly germinoma or
teratoma | 13 | 12.8 | 8–22 | 4 | 6 | 2 | - | - | - | 1 |
| Mainly malignant
element | 2 | 29.5 | 19–40 | - | - | - | - | 2 | - | - |
| Total | 39 | 14.2 | 2–40 | 19 | 11 | 2 | 2 | 3 | 1 | 1 |
Clinical manifestations
There were 19 cases of pineal region tumor, 14 cases
(77.78%) with obstructed hydrocephalus, resulting in intracranial
pressure, 6 (33.33%) with diplopia, 6 (33.33%) with hypopsia and 1
case (5.56%) with precocious puberty.
A total of 11 cases were located at the sellar
region, including 9 (81.82%) with hypopsia, 5 (45.45%) with
polydipsia and polyuria and 1 case (9.09%) with menstrual
disorder.
There were 3 cases of third ventricle tumor that
suffered from headache, impaired vision and parinaud syndrome.
Other clinical manifestations, including alalia and weakness of
limbs occurred on account of the location of the tumor.
Imaging analysis
Of the 38 patients that underwent CT scans, 35
manifested heterodense lesion, 3 lesions were isodense, 14 had
hydrocephalus and 7 had calcification. Of the 36 patients that
underwent magnetic resonance imaging (MRI), the tumors were
hypointense and hyperintense in T1-weighted and T2-weighted images,
respectively, with one exception that showed heterointensity on the
T1-weighted image. The tumors were enhanced in the
contrast-enhanced MRI.
Serum levels of tumor markers
There were 21 patients who underwent a plasma AFP
examination prior to surgery. AFP was elevated in 13 patients
(68.42%). Nineteen patients underwent plasma β-HCG examination
prior to surgery and 8 showed an elevated plasma β-HCG
(42.11%).
Treatment
Patients were treated surgically first. Then, they
received radiotherapy and/or chemotherapy. Some patients also
received gamma knife surgery following surgery.
Statistical analysis
The median follow-up for the 34 patients was 42
months (range 6–108), with 5 patients being lost to follow-up.
According to the classification of Matsutani et al patients
were grouped into intermediate prognosis and poor prognosis groups
based on the histology of the tumor (14). Overall survival and intracranial
control rates were calculated actuarially according to the
Kaplan-Meier method and COX regression and were measured from the
day of surgery. Differences between groups were estimated using the
log-rank test. A probability level of 0.05 was chosen for
statistical significance. Statistical analysis was performed with
the state software package (version 10.0).
Results
Surgical outcomes
The tumor was totally removed in 28 cases,
sub-totally in 5, partially in 4 and biopsy was performed in 2
cases. One patient succumbed to pleura empyema after surgery.
Surgical complications included central nervous system infection (2
cases, 5.12%), high fever (2 cases, 5.12%), diabetes insipidus (3
cases, 7.69%) and hydrocephalus (8 cases, 20.51%). The patients
improved after treatment.
Histology
All 39 cases were pathologically inspected and
verified. There were 15 cases of mixed germ cell tumor, 15 of
immature teratoma, 7 of embryonal carcinoma and 2 cases of yolk sac
tumor (Table I).
Follow-up
Thirty-four patients were followed up. Following
surgery, 25 patients underwent radiotherapy and 15 underwent
chemotherapy. Following tumor resection, 4 patients received gamma
knife surgery on account of the recurrent tumor in 3 cases and
residual tumor in 1 case. The common 5-year survival rate was
36.8%. In our study, the survival curve of embryonal carcinoma
patients is similar to those of immature teratoma and mixed germ
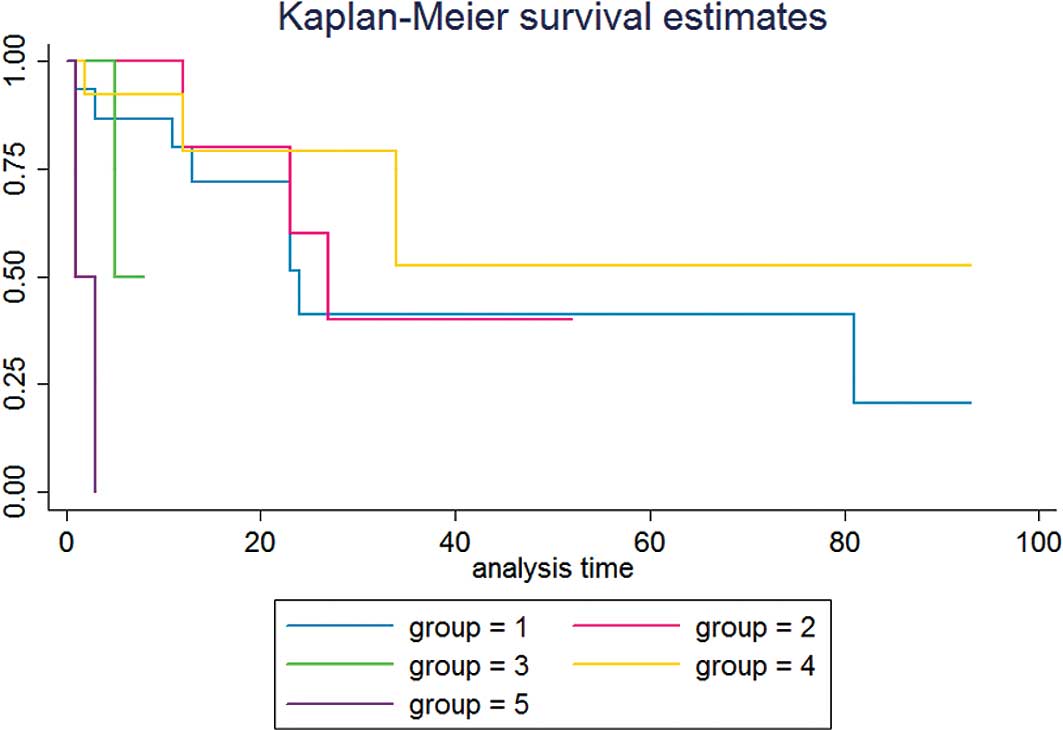
cell tumor mainly composed of germinoma or teratoma (Fig. 1). In particular, for immature
teratoma and embryonal carcinoma patients, the median survival time
was very close at 24 vs. 27 months, respectively.
The intermediate prognosis group comprised 35 cases,
including immature teratoma, embryonal carcinoma and mixed germ
cell tumor mainly composed of germinoma and teratoma. The common
5-year survival rate of the intermediate prognosis group was 42.6%.
The COX regression analysis showed a statistically insignificant
correlation between gender, age, total resection and survival
(P<0.05). However, chemotherapy combined with radiotherapy
significantly affected survival time (P=0.039). The 5-year survival
rates of patients who did and did not receive chemotherapy combined
with radiotherapy post-operation were 62.4 and 25%,
respectively.
In the case of immature teratoma, patients who
received gamma knife surgery after surgery had a 5-year survival
rate of 100%. A significant difference (P=0.0049) was noted in
comparison to the 5-year survival rate of patients who did not
receive gamma knife surgery. Post-operative radiotherapy and
chemotherapy had no significant impact on the 5-year survival rate
(P>0.05).
The poor prognosis group comprised 4 patients who
exhibited yolk sac tumor, choriocarcinoma and mixed germ cell tumor
mainly composed of yolk sac tumor or choriocarcinoma. The 1-year
survival rate was 25% and the 5-year survival rate was 0%.
Discussion
According to the WHO classification of intracranial
tumors, germ cell tumors (GCTs) are categorized into germinoma and
non-germinomatous germ cell tumors (NGGCTs). The latter include
teratoma (classified into mature, immature and teratoma with
malignant transformation), embryonal carcinoma, yolk sac tumor,
choriocarcinoma and mixed germ cell tumors (5). The NGMGCTs include teratoma
(classified into immature and teratoma with malignant
transformation), embryonal carcinoma, yolk sac tumor,
choriocarcinoma and mixed germ cell tumors (6).
Records from our hospital showed that in cases
concerning the primary central nervous system among children under
the age of 18, GCTs consists of 8.9% (NGMGCTs 39.7%; male-female
ratio 4.67:1; median age 13.18 years) (7). NGMGCTs commonly exist either at the
pineal or sellar region. The incidence of pineal region is higher
than that of sellar, especially for yolk sac tumor (8). In our study, 19 cases of this group
were observed at the pineal and 11 at the sellar region.
The clinical manifestations of NGMGCTs are often
related to the location and size of tumor. The special feature of
NGMGCTs in computed tomography (CT) or magnetic resonance imaging
(MRI) was rare (9–11). In our group, 38 patients underwent
CT scans and 36 underwent MRI examination. However, it is difficult
to clarify the subtype of NGMGCTs only by imaging analysis.
Monitoring the α-fetoprotein (AFP) and β-HCG of
serum or cerebrospinal fluid carries a measure of significance to
the diagnosis and treatment of NGMGCTs. Sano (12) supported that if AFP is positive, the
tumor is yolk sac or mixed germ cell tumor that contains components
of yolk sac tumor. An increase in β-HCG suggests that the
choriocarcinoma or mixed germ cell tumor contains components of
choriocarcinoma. Matsutani (13)
pointed out that the β-HCG of the serum shows a 100% increase in
choriocarcinoma and a 50% increase in embryonic carcinoma.
In this group, 21 patients underwent a plasma AFP
examination prior to surgery. AFP was elevated in 13 patients
(68.42%). Plasma β-HCG examination was performed in 19 patients
prior to surgery and 8 showed elevated plasma β-HCG (42.11%). We
found AFP was sensitive for mixed germ cell tumor mainly composed
of yolk sac tumor, embryonal carcinoma and mixed germ cell tumor
mainly composed of germinoma and teratoma. The elevated rates of
AFP were 100% in mixed germ cell tumor mainly composed of yolk sac
tumor, 80% in embryonal carcinoma and 75% in mixed germ cell tumor
mainly composed of germinoma and teratoma. β-HCG was sensitive for
mixed germ cell tumor mainly composed of yolk sac tumor and mixed
germ cell tumor mainly composed of germinoma and teratoma, and the
elevated rates were 100 and 83.3%, respectively. However, in
immature teratoma the expression of AFP and β-HCG was not
sensitive. The results demonstrated 4 cases (57.1%) of AFP increase
and 1 case (16.7%) of β-HCG increase.
Grouping strategy
Intracranial NGMGCTs should be treated with
different modalities based on their diverse pathological
categories. Matsutani et al (14) analyzed 153 cases of germ cell
tumors, classified them into three groups with different prognosis
and proposed a treatment guideline appropriate for the categories.
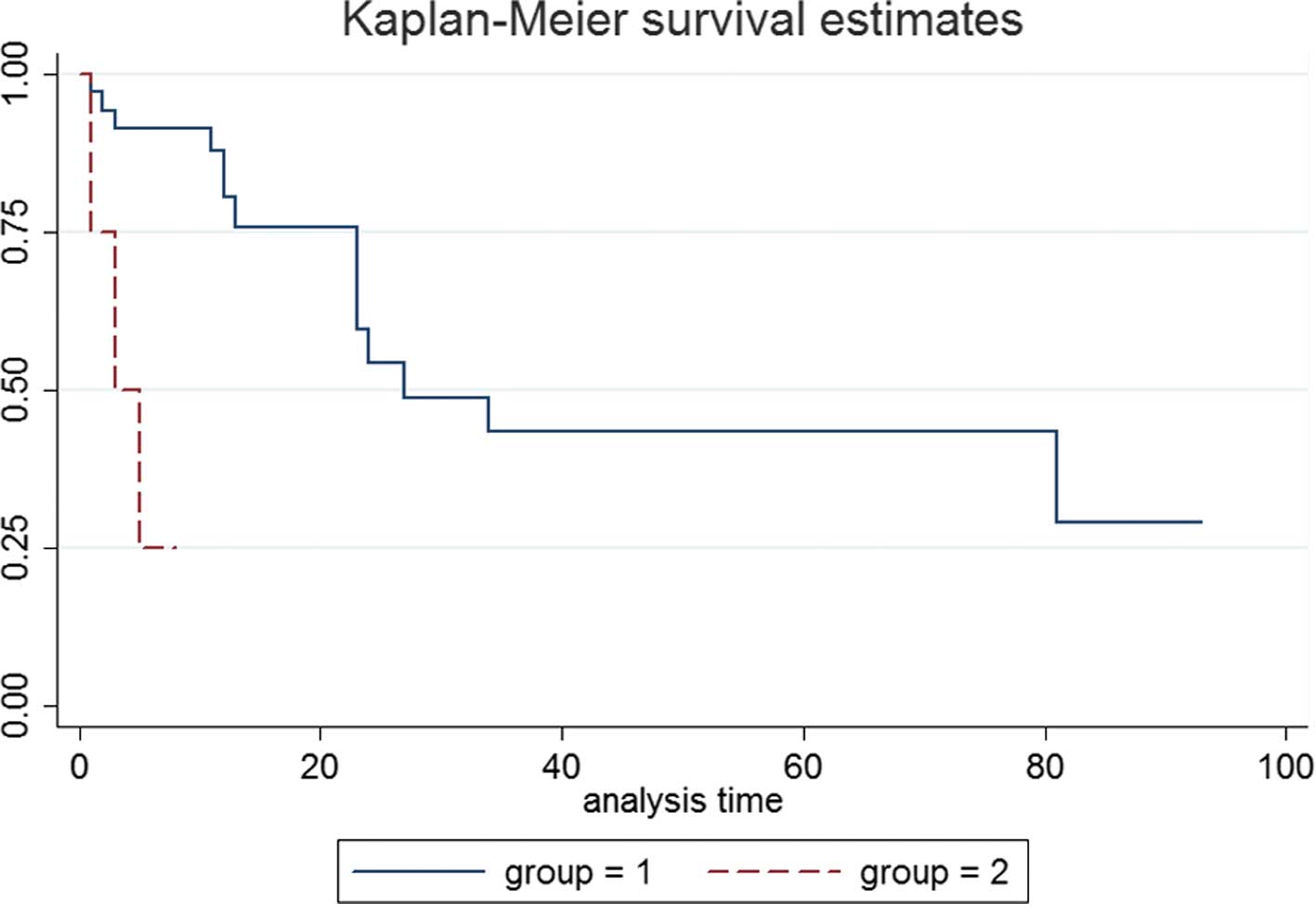
Our study showed that the survival curve of embryonal carcinoma
patients is similar to that of immature teratoma and mixed germ
cell tumor mainly composed of germinoma or teratoma. Therefore, it
was determined that patients with embryonal carcinoma, immature
teratoma or mixed germ cell tumor mainly composed of germinoma or
teratoma were classified into the intermediate prognosis group. On
the other hand, patients with yolk sac and mixed germ cell tumor
with mostly yolk sac elements were grouped into the poor prognosis
group. The survival curves between the two groups showed a
significant difference (P=0.0003) (Fig.
2). Sano (15) showed that
NGMGCTs originate from different cells in different phases of the
embryo. This author further noted that the earlier the phase the
more aggressive the tumor. Since embryonal carcinoma originates
from a trilaminar embryo, the malignant degree may lie between the
yolk sac tumor and germ cell tumor or teratoma. By administering
appropriate treatment, a similar outcome to germ cell tumors and
teratoma may be achieved. This is in agreement with our theory in
that the embryonal carcinoma can be grouped into the intermediate
prognosis group.
Intermediate prognosis group
For the intermediate prognosis group of NGMGCTs, the
treatment effects are negative. The analysis performed by Aoyama
et al (16) regarding the
clinical treatment effects of 24 cases of intracranial teratoma
indicated that the survival rate of 5 immature teratoma cases was a
mere 44%. As to the analysis of 111 GCTs performed by Sawamura
et al (8), the 5-year
survival rate of 9 immature teratomas proved to be 67%. Moreover,
Ogawa et al (17) reported
24 cases of intermediate prognosis of NGMGCTs. The 5-year survival
rate was 68%. However, the analysis performed by Ge Jia et
al (18) showed a 0% 5-year
survival rate in 18 patients who underwent intracranial immature
teratoma surgery plus radiotherapy out of 37 mature and immature
intracranial teratoma cases.
The relationship of total resection and clinical
outcome is a topic of contention (15,19,20).
Our COX regression analysis on the intermediate prognosis group of
intracranial NGMGCTs showed a statistically insignificant
correlation between total resection and survival (P=0.139).
However, chemotherapy combined with radiotherapy significantly
affected survival time (P=0.039). Kretschmar et al (21) also supported that pre-radiation
chemotherapy has a response rate of 55% on NGMGCTs and is thus
effective. Robertson et al (22) reported 18 patients receiving
multi-modality ‘sandwich’ therapy
(chemotherapy/radiation-chemotherapy): 3 or 4 cycles of neoadjuvant
chemotherapy with cisplatin and VP-16 + radiation therapy + 4
cycles post-radiation chemotherapy with vinblastine, bleomycin,
VP-16 and carboplatin. Findings of the study by these authors
showed 4-year actuarial event-free and total survival rates to be
67 and 74%, respectively. Thus, it is likely that high-dose
chemotherapy and radiotherapy are useful in the improvement of
prognosis.
For immature teratoma, radiochemotherapy exerts a
positive effect on eliminating residual tumor cells, but the
sensitivity of such treatment modalities has yet to be elucidated.
However, an analysis of the survival curve of after-treatment gamma
knife surgery demonstrated a significant difference, with a P-value
of 0.0049. In the immature teratoma group, patients who had gamma
knife surgery are alive (100% 5-year survival rate). This suggests
that gamma knife is highly sensitive to residual tumors, although
further data are required to confirm our conclusion. Cho et
al (23) analyzed 7 pineal
region tumors (including 1 case of immature teratoma), treated with
gamma knife surgery, 6 of which have been regulated (the size of
immature teratoma was reduced by 40%). These authors concluded that
gamma knife surgery is effective on the pineal region tumor,
regardless of the tissue pathology. Numerous clinical analyses
concerning the treatment effects of gamma knife on GCT were also
conducted, all of which revealed a controlling rate of GCT greater
than 50% (24–26).
Poor prognosis group
The treatment for the poor prognosis group of NGMGCT
patients was not ideally effective. Ogawa et al (17) analyzed the treatment of 41 NGMGCT
patients and found the 5-year survival rate to be 8% in the poor
prognosis group. In our study, only 1 out of 4 patients grouped in
the poor prognosis group are currently alive. Despite surgical
resection combined with post-operative radiotherapy and
chemotherapy, the 5-year survival rate was under 25% (22). Baranzelli et al (27) treated 13 patients with chemotherapy
alone post-operatively and had 12 recurrences. Matsutani et
al (14) treated 11 patients
with surgery, chemotherapy and radiotherapy. These patients reached
a 3-year survival rate of 27.3% with choriocarcinoma (0%), yolk sac
tumor (33.3%) and mixed germ cell tumor that included both of the
above-mentioned elements (9.3%).
It is crucial to improve the treatment effectiveness
for the poor prognosis patients after gross total resections.
Schild et al (3) indicated
that patients who underwent subtotal resections or biopsies had
significantly poorer survival rates compared to patients who
underwent complete resection. The 3-year survival rate was 0% for
patients that underwent biopsy alone and 32% for patients who
underwent subtotal resection, compared to 73% for patients who
underwent macroscopic total resection (P=0.0001). The 4 patients in
our poor prognosis group underwent total resections.
Neoadjuvent therapy may be useful in the treatment
of NGMGCTs of the poor prognosis group (28), and more encouraging results are
anticipated.
Therefore, we suggest that intracranial NGMGCTs be
strictly classified according to their pathological categories
before administering pathology-specific standard treatment. Surgery
remains the first choice of treatment wherever possible. This type
of treatment not only aids in the decrease of intracranial pressure
and reduction of hydrocephalus, but, more importantly, also
achieves pathology results that lead to appropriate treatment
modalities.
NGMGCTs are divided into intermediate and poor group
based on the prognosis (P=0.0003). Embryonal carcinoma is
classified as intermediate prognosis group due to its similar
prognosis with immature teratoma and mixed tumors mainly composed
of germinoma or teratoma.
In the intermediate prognosis group, patients with
immature teratoma elements in the tumor do not require chemotherapy
and radiotherapy if the lesion is completely resected. These
patients should be closely followed up with CT and MRI
post-operatively. If residual tumor is found or recurrences occur
during follow-up of patients undergoing gross total resections,
gamma knife surgery may be employed as a complement. However, for
tumors other than immature teratoma, total resection combined with
post-operative chemo-therapy, radiotherapy and/or gamma knife
surgery is ideal On the other hand, in the poor prognosis group,
the treatment results were found to be unsatisfactory.
Subsequently, total resection combined with radiotherapy and
chemotherapy is currently the only treatment option available.
References
|
1
|
Balmaceda C, Heller G, Rosenblum M, et al:
Chemotherapy without irradiation – a novel approach for newly
diagnosed cns germ cell tumors: results of an international
cooperative trial. The first international central nervous system
germ cell tumor study. J Clin Oncol. 14:2908–2915. 1996.
|
|
2
|
Edwards MS, Hudgins RJ, Wilson CB, Levin
VA and Wara WM: Pineal region tumors in children. J Neurosurg.
68:689–697. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schild SE, Haddock MG, Scheithauer BW, et
al: Non-germinomatous germ cell tumors of the brain. Int J Radiat
Oncol Biol Phys. 36:557–563. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Packer RJ, Cohen BH and Cooney K:
Intracranial germ cell tumors. Oncologist. 5:312–320. 2000.
|
|
5
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SK, Cho BK, Paek SH, et al: The
detection of p53 gene mutation using a microdissection technique in
primary intracranial germ cell tumors. Int J Oncol. 18:111–116.
2001.PubMed/NCBI
|
|
7
|
Zhang R, Shen WQ and Zhou LF: Primary
pediatric central nervous system tumors statistic: study of 763
cases in a single institution. Zhonghua Yi Xue Za Zhi. 87:442–447.
2007.PubMed/NCBI
|
|
8
|
Sawamura Y, Ikeda J, Shirato H, Tada M and
Abe H: Germ cell tumours of the central nervous system: treatment
consideration based on 111 cases and their long-term clinical
outcomes. Eur J Cancer. 34:104–110. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou LF, Chen XC and Shi YQ: Modern
Neurosurgery. Zhou LF: Fudan Press Shanghai; Shanghai: pp. 468–476.
2001
|
|
10
|
Fujimaki T, Matsutani M, Funada N, et al:
CT and MRI features of intracranial germ cell tumors. J Neurooncol.
19:217–226. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sumida M, Uozumi T and Kiya K: MRI of
intracranial germ cell tumors. Neuroradiology. 37:32–37. 1995.
View Article : Google Scholar
|
|
12
|
Sano K: Pathogenesis of intracranial germ
cell tumors reconsidered. J Neurosurg. 90:258–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsutani M, Sano K, Takakura K, Fujimaki
T and Nakamura O: Combined treatment with chemotherapy and
radiation therapy for intracranial germ cell tumors. Childs Nerv
Syst. 14:59–62. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsutani M, Sano K, Takakura K, et al:
Primary intracranial germ cell tumors: a clinical analysis of 153
histologically verified cases. J Neurosurg. 86:446–455. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sano K: So-called intracranial germ cell
tumors: are they really of germ cell origin? Br J Neurosurg.
9:391–401. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoyama H, Shirato H, Yoshida H, et al:
Retrospective multi-institutional study of radiotherapy for
intracranial non-germinomatous germ cell tumors. Radiother Oncol.
49:55–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogawa K, Toita T, Nakamura K, et al:
Treatment and prognosis of patients with intracranial
non-germinomatous malignant germ cell tumors: a multiinstitutional
retrospective analysis of 41 patients. Cancer. 98:369–376. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia G, Zhang YQ, Ma ZY, Luo SQ and Dai K:
The clinical study on the treatment of intracranial mature teratoma
and immature teratoma: 37 case reports. Chin J Neurosurg.
19:334–336. 2003.
|
|
19
|
Calaminus G, Bamberg M, Baranzelli MC, et
al: Intracranial germ cell tumors: a comprehensive update of the
European data. Neuropediatrics. 25:26–32. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itoyama Y, Kochi M, Kuratsu J, et al:
Treatment of intracranial non-germinomatous malignant germ cell
tumors producing alpha-fetoprotein. Neurosurgery. 36:459–466. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kretschmar C, Kleinberg L, Greenberg M,
Burger P, Holmes E and Wharam M: Pre-radiation chemotherapy with
response-based radiation therapy in children with central nervous
system germ cell tumors: a report from the children’s oncology
group. Pediatr Blood Cancer. 48:285–291. 2007.PubMed/NCBI
|
|
22
|
Robertson PL, DaRosso RC and Allen JC:
Improved prognosis of intracranial non-germinoma germ cell tumors
with multimodality therapy. J Neurooncol. 32:71–80. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho SY, Park CK, Chung HT, Peak SH and Kim
DG: Gamma knife surgery for the pineal region tumors. J Korean
Neurosurg Soc. 40:342–345. 2006.
|
|
24
|
Kobayashi T, Kida Y and Mori Y:
Stereotactic gamma radiosurgery for pineal and related tumors. J
Neurooncol. 54:301–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasegawa T, Kondziolka D, Hadjipanayis CG,
Flickinger JC and Lunsford LD: Stereotactic radiosurgery for CNS
non-germinomatous germ cell tumors. Report of four cases. Pediatr
Neurosurg. 38:329–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Endo H, Kumabe T, Jokura H and Tominaga T:
Stereotactic radiosurgery followed by whole ventricular irradiation
for primary intracranial germinoma of the pineal region. Minim
Invasive Neurosurg. 48:186–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baranzelli MC, Patte C, Bouffet E, et al:
Nonmetastatic intracranial germinoma: the experience of the French
society of pediatric oncology. Cancer. 80:1792–1797. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kochi M, Itoyama Y, Shiraishi S, Kitamura
I, Marubayashi T and Ushio Y: Successful treatment of intracranial
non-germinomatous malignant germ cell tumors by administering
neoadjuvant chemotherapy and radiotherapy before excision of
residual tumors. J Neurosurg. 99:106–114. 2003. View Article : Google Scholar
|