Introduction
Hypoxic regions within tumors frequently appear
during the processes of growth and progression, resulting in the
activation of hypoxia-inducible factors (HIFs). Three HIFs (HIF-1,
−2 and −3) that regulate transcriptional programs in response to
low oxygen level have been identified. HIF-1 was the first family
member to be characterized and is composed of a α and β subunit.
The HIF-1β subunit is a constitutively expressed nuclear protein,
while the HIF-1α subunit is regulated by hypoxia, a variety of
growth factors and cytokines, as well as tumor-modifying genes.
Almost 100 target proteins were identified as being transactivated
by HIF-1 and these are involved in various cellular processes
including glucose uptake and metabolism, angiogenesis,
erythropoiesis, cell proliferation, apoptosis and invasion
(1,2). Overexpression of HIF-1α protein has
not only been demonstrated in tumor cell lines (3) and a variety of human tumors, including
bladder, breast, colon, ovarian, pancreas, prostate and kidney
(4), but also correlates with poor
patient outcome in a wide range of tumor types (5–7). HIF-2
was the second member of the family to be characterized and as
HIF-2α is structurally similar to HIF-1α, it heterodimerizes with
the β unit before inducing target gene expression. In contrast to
HIF-1α, the expression of HIF-2α is more restricted (8). HIF-3 is the third member of the family
and its exact role has yet to be clearly defined. HIF-1 is
considered to be the most important family member as it is known to
be crucial for tumorigenesis (9).
However, there are certain controversial, even conflicting,
findings concerning its role in the modulation of tumor growth and
apoptosis. Therefore, this study aimed to determine the effect of
HIF-1 on tumor growth, using the human metastatic breast cancer
cell line (MDA-MB-231), and to probe the underlying control
mechanisms using small interfering RNA (siRNA).
Materials and methods
Cell cultures and reagents
Human breast cancer cell lines MDA-MB-231, MCF-7,
MDA-MB-468, SK-BR-3 and MDA-MB-453 were obtained from the
Department of Pathology, Peking University Medical Science Center,
and were cultured in DMED medium or RPMI-1640 with 10% fetal bovine
serum at 37°C in a humidified environment of 5% CO2.
Cells were subcultured in 6- or 24-well plates until 70–80%
confluent. After extensive washing with phosphate-buffered saline
(PBS), cells were serum-starved for various time points according
to each experimental protocol. A HIF-1α antibody was purchased from
Sigma while antibodies against caspase 3, B cell lymphoma (Bcl-2),
Bax and α-tubulin, as well as specific secondary antibodies were
purchased from the Beyotime Institute of Biotechnology. siRNA
duplexes targeting HIF-1α mRNA and transfection reagents were
purchased from Santa Cruz Biotechnology.
Transfection of MDA-MB-231 cell line with
hypoxia-inducible factor-1α small interference RNA or control small
interference RNA
Cells were transfected with either siRNA duplexes
targeting HIF-1α mRNA (HIF-1α: sense, 5-UCAAGUUGCUGGUCAUCAGdTdT-3
and antisense, 5-CUGAUGACCAGCAACUUGAdTdT-3) or with control siRNA
not targeting any known genes, as previously described (10). MDA-MB-231 cells were transfected at
a final siRNA duplex concentration of 80 pmol in 6-well plates
according to the manufacturer’s protocol. After 6 h of
transfection, 1 ml of normal growth medium containing twice the
normal serum and antibiotic concentration was added to the culture
medium. The cells were then incubated for a further 18–24 h.
Western blotting
Following serum starvation, cells were washed with
ice-cold PBS and lysed in 2% SDS, 100 mM DTT, 60 mM Tris, pH 6.8.
Total cell protein was quantified using the Bradford assay.
Proteins were separated on 10 or 12% SDS-PAGE gels and transferred
to PVDF membranes. The membranes were incubated with monoclonal
antibodies against HIF-1α, caspase 3, Bcl-2 and Bax and specific
secondary antibodies, and visualization was achieved via enhanced
chemiluminescence and exposure to photographic film as previously
described (11). The blot was
stripped and re-probed with an antibody for α-tubulin as an
internal control.
Cell proliferation assay
MDA-231 cells (1×105/ml) expressing
HIF-1α or control siRNA were placed in 24-well plates and incubated
in RPMI-1640 + 10% FCS for 9 days. Live cell numbers were regularly
determined on alternate days by trypan blue staining and cell
counting. Each condition was performed in quadruplicate with three
counts per replicate and the average number of cells was
calculated.
Flow cytometry
After appropriate treatment, cells transfected with
HIF-α or control siRNA were stained with either propidium iodide
(PI) or Annexin V-fluorescent isothiocyanate (FITC) in combination
with PI according to the manufacturer’s protocol. Cell cycle status
and apoptosis were then analysed by flow cytometry.
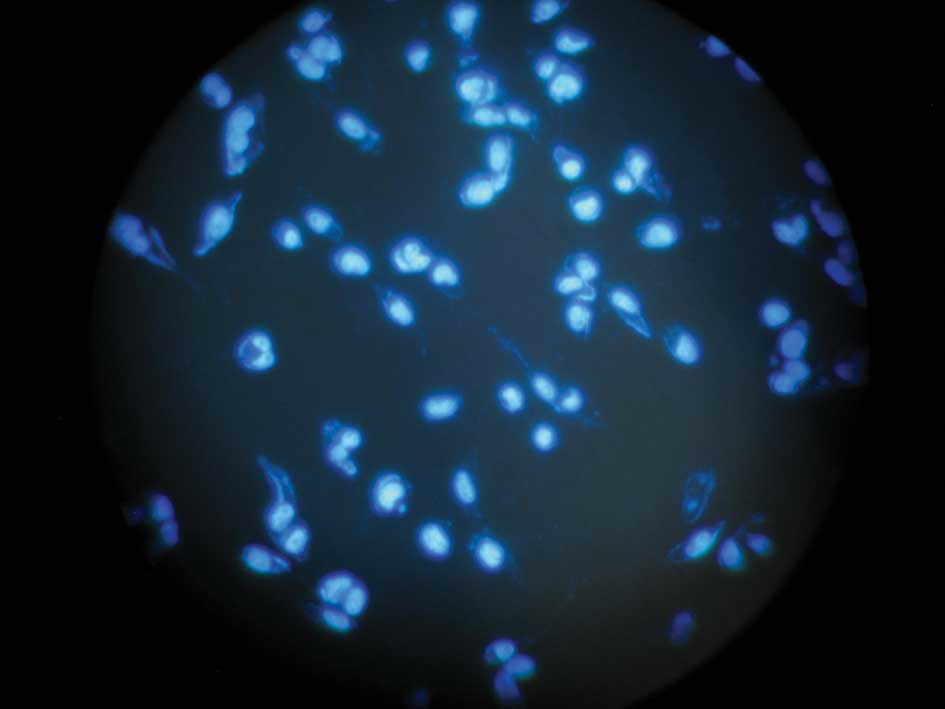
Hoechst 33258 stain
After a time period of serum starving, cells were
washed twice with PBS, fixed in 4% paraformaldehyde in PBS, treated
with 2 μM Hoechst 33258 dye and examined under fluorescence
microscopy. Cells with condensed and fragmented DNA were considered
to be apoptotic.
Caspase 3 activity assay
Caspase 3 activity was determined using an assay kit
(purchased from BIB) according to the manufacturer’s protocol. In
brief, MDA-MB-231 cells (1×105/ml) were incubated in
serum-free medium, harvested in PBS and centrifuged at 500 g for 5
min. The cells were lysed on ice for 10 min and then centrifuged at
13,000 rpm for 1 min at 4°C. The supernatant was harvested and 80
μg of total protein was incubated with buffer containing 10 mM
dithiothreitol and 5 μl of Ac-DEVD-pNA (final concentration 200 μM)
at 37°C. The chromophore P-nitroanilide was determined at 405 nm
with a fluorescence microplate reader.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD) and statistical analysis was performed using a Student’s
t-test. Results were considered significant if p<0.05
Results
Basic expression of hypoxia-inducible
factor-1α protein in breast cancer cell lines
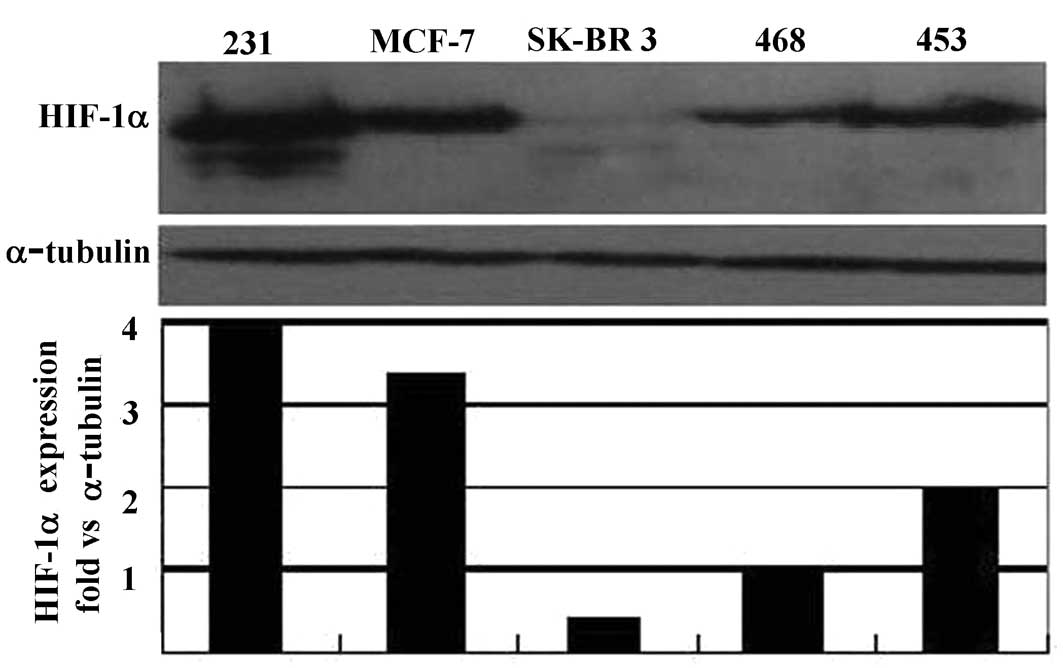
The basic expression of HIF-1α protein in a number
of breast cancer cell lines, with different backgrounds, including
MDA-MB-231, MCF-7, MDA-MB-468, SK-BR-3 and MDA-MB-453, was assessed
after 24 h of serum starvation. Whole cell proteins were extracted
and probed by Western blotting using an anti-HIF-1α monoclonal
antibody. Our results showed that the MDA-MB-231 cell line
expressed a higher level of HIF-1α protein than any of the other
cell lines tested (Fig. 1A). We
also found that this cell line grew faster than other cell lines.
To determine whether there was a change in HIF-1α protein levels
following long-term serum starvation, we cultured the cells in
serum-free medium for 12–60 h. Results showed no change in protein
levels when compared to those treated with serum (Fig. 1B). This suggests that the persistent
expression of HIF-1α plays a key role in protecting cell growth
under long-term serum deprivation.
Down-regulation of hypoxia-inducible
factor-1α protein by hypoxia-inducible factor-1α small interference
RNA
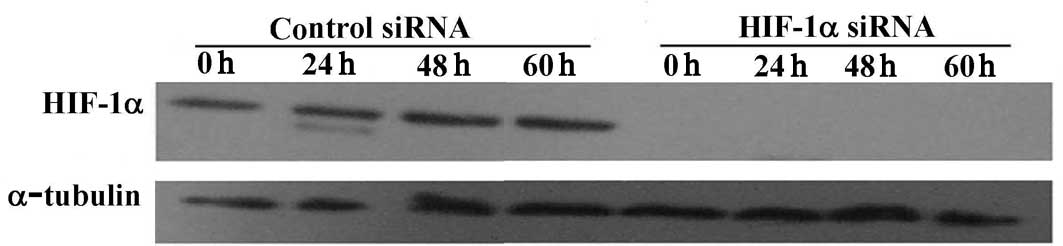
MDA-MB-231 cells were transfected with either HIF-1α
siRNA duplex (a HIF-1α target-specific 20–25 nt siRNA designed to
knock down gene expression) or a control duplex (a non-targeting
20–25 nt siRNA designed as a negative control). To examine the
efficiency of HIF-1α siRNA, cells were cultured in a serum-free
medium for various times under normoxic conditions or for 6 h under
hypoxic conditions. Whole cell proteins were extracted and probed
by Western blotting. HIF-1α protein expression was completely
blocked when the transfected cells were cultured in serum or were
serum-starved for 24–60 h (Fig.
2A). HIF-1α was also significantly reduced under hypoxic
conditions (Fig. 2B).
Role of hypoxia-inducible factor-1α in
cell growth under serum deprivation
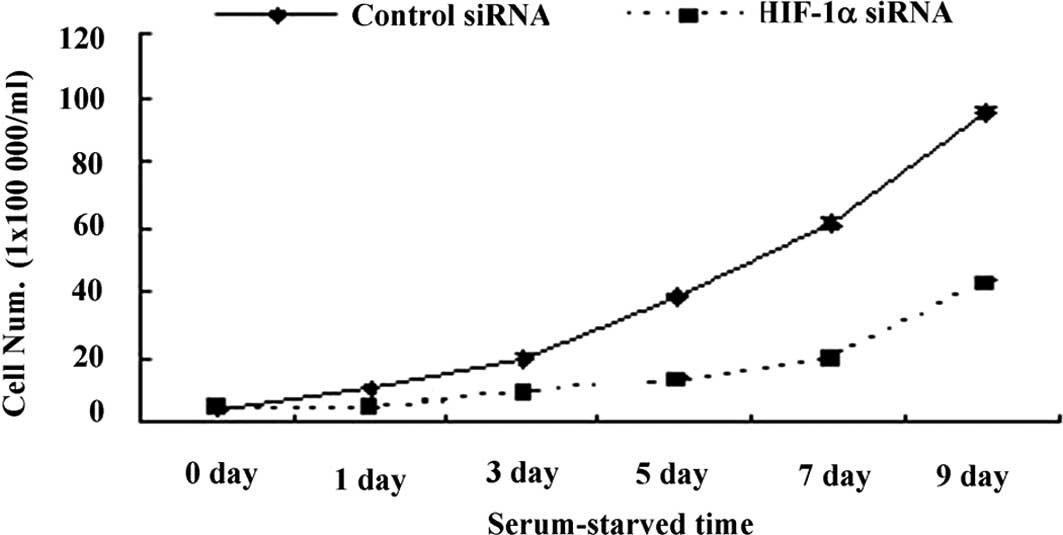
To investigate the role of HIF-1 in cell growth,
1×105/ml cells transfected with either HIF-1α or control
siRNA were grown in 24-well plates for 9 days. Cell numbers were
counted every other day and growth curves established. The results
revealed that cell growth was markedly inhibited in the group with
HIF-1α siRNA compared to that with control siRNA (Fig. 3), suggesting a potential role for
HIF-1 in protecting cell growth under serum starvation.
Effect of hypoxia-inducible factor-1 on
cell cycle and apoptosis
Tumor growth depends on the number of cells
proliferating (growth fraction) and the number of cells dying (cell
loss). The growth fraction is calculated from the percentage of
cells in the S, G2 and M phases of the cell cycle. Cell loss
involves cells undergoing apoptosis and necrosis. Using flow
cytometry, the cell cycle was assessed following serum starvation
for varying time periods and no significant difference was found
between cells treated with HIF-1α siRNA and those in the control
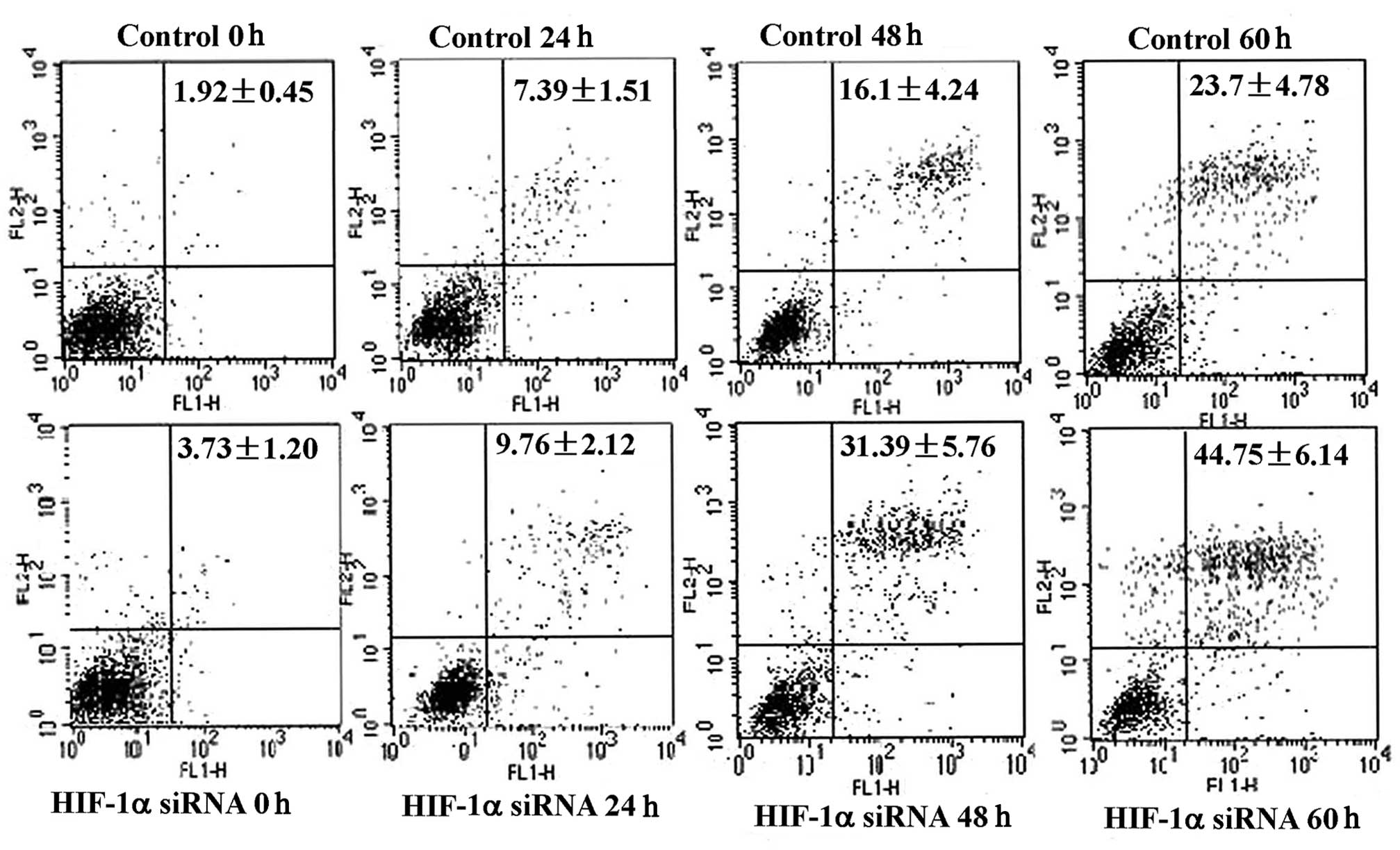
group (data not shown). Levels of apoptosis were then assessed by
flow cytometry using Annexin-V FITC and PI staining. More apoptotic
cells were found in the HIF-1α siRNA-treated group compared with
the controls and this difference was significant after 48 h
(Fig. 4). Furthermore, when the
HIF-1α siRNA-treated cells were stained with Hoechst 33258
(Fig. 5A and B), more condensation
or fragmentation of DNA was noted. This result morphologically
confirmed the flow cytometry results. The results therefore suggest
that HIF-1 promotes cell growth under serum deprivation by
protecting cells from apoptosis rather than by shifting the cell
cycle from G0–G1 to G2-S.
Anti-apoptosis of hypoxia-inducible
factor-1: Involvement of caspase cascade and B cell lymphoma
The caspase cascade is a typical pathway for the
initiation of apoptosis and Bcl-2 is a key modulating factor in
this cascade. In order to determine the anti-apoptotic role of
HIF-1 cells, with either HIF-1α or control siRNA, were
serum-starved for various times and protein extracts were probed
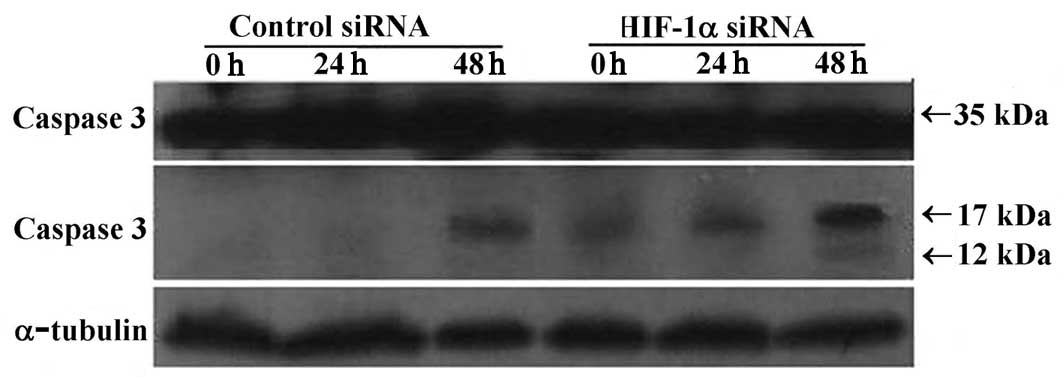
with anti-caspase 3, Bcl-2 and Bax antibodies. The anti-caspase 3
antibody can detect procaspase 3 (35 kDa) and its activated
fragments (molecular weight 17 and 12 kDa). Our results showed that
levels of procaspase 3 did not change in either of the treatment
groups. However, in the HIF-1α siRNA-treated group, the activated
fragment (17 kDa) was present in all samples from 0–48 h of serum
starvation and a weak expression of the 12 kDa fragment was noted
at 48 h. In contrast, the activated fragment (17 kDa) was only
detected after 48 h of serum starvation in the control siRNA group
(Fig. 6A). Increased caspase 3
activity was observed at all time points (0–60 h of serum
starvation) following transfection with HIF-1α siRNA when compared
to the control group (Fig. 6B).
This observation was in-line with the detection of activated
caspase 3 fragments by Western blotting and suggests a role for
HIF-1 in the inactivation of the caspase cascade. Bcl-2 and Bax are
two members of the Bcl-2 family that play contradictory roles in
the regulation of apoptosis. Our results showed no change in the
Bax protein expression (data not shown). However, the expression of
Bcl-2 (28 kDa molecular weight) was detected in cells transfected
with either HIF-1α or control siRNA and, notably, a larger band was
also detected in cells transfected with HIF-1α siRNA (Fig. 6C). This larger band may be due to
multiple site phosphorylation of Bcl-2, which would result in an
increase in molecular weight and may be related to the loss of
HIF-1 followed by apoptosis.
Discussion
HIF-1 is crucial for tumor progression involving
growth, invasion and metastasis (12).
Tumor cells adapt to a lack of oxygen and nutrients,
continue to grow and escape necrosis and apoptosis. Previous
studies suggested that HIF-1 promotes tumor growth or suppresses
apoptosis (13–15). However, other studies found that
HIF-1 inhibited tumor growth and promoted hypoxia-induced apoptosis
(16,17). Bafilomycin A1, a potential
anticancer agent, was also found to restrict cell proliferation and
tumor growth by inhibiting the degradation of the HIF-1α protein
(18). A further study using a
three-dimensional model found that HIF-1 promoted hepatoma cell
growth, but that a HIF-1 deficient counterpart showed increased
proliferation and a higher rate of apoptosis than a wild-type
hepatoma (19). Since tumor growth
depends on the balance between the growth fraction and cell loss
(necrosis and apoptosis, respectively), our study aimed to
determine the role of HIF-1 in tumor growth and the possible
mechanisms involved.
Detection of the basic expression of HIF-1α protein
in a number of breast cancer cell lines provided further evidence
for the universal expression of HIF-1α protein in tumor cells
(3). MDA-MB-231, a metastatic
breast cancer cell line showed a relatively higher level of HIF-1α
protein compared to other cell lines analyzed, implying a possible
correlation between HIF-1 expression and a malignant phenotype.
Sustained growth with the expression of HIF-1α protein under
long-term serum starvation suggested a potential relationship
between HIF-1 and cell growth. Blocking the expression of HIF-1α
using siRNA down-regulated cell growth confirmed previous studies
indicating that suppression of HIF-1 decreases cell proliferation
in vitro (13–15,20–22).
Our study not only confirmed the role of HIF-1 in promoting growth,
but also showed that apoptotic inhibition, as opposed to a shift in
the cell cycle from G0 to G1, contributed to the role of HIF-1 in
cell growth.
Apoptosis is triggered by a variety of factors such
as death receptors, stress (including serum deprivation and growth
factor depletion), free radicals, ionizing radiation, and factors
released from cytotoxic T cells. Under stress, cytochrome C
released an apoptotic inhibitor from the mitochondrial interaction
with Apaf-1 resulting in the recruitment of procaspase 9, which
activates caspase cascades. The effector caspases 3, 6 and 7, are
downstream of the activator caspases and cleave various targets
leading to apoptosis. Precursor caspase 3, a 35-kDa protein, is
cleaved into 17 and 12 kDa fragments following activation (23). When HIF-1α protein was blocked we
detected an activated fragment of caspase 3 (17 kDa) from 0–48 h
serum starvation. A 12 kDa fragment was also faintly detected after
48 h of starvation. These findings demonstrate that the
interruption of HIF-1 promotes cell apoptosis by activating the
caspase cascade.
The Bcl-2 family comprises a number of members of
which Bad, Bid, Bax, Bim and Bik are pro-apoptotic proteins while
Bcl-2 and Bcl-xL are anti-apoptotic proteins (24). Pro-apoptotic members promote the
release of cytochrome C from mitochondria in response to stress
factors, while anti-apoptotic members inhibit this process
(25). A number of studies showed
that Bcl-2 undergoes multiple phosphorylations (26,27)
resulting in a molecular mobility shift of the Bcl-2 protein and
loss of anti-apoptotic function (27,28).
Consistent with these studies, we also noticed a molecular mobility
shift of the Bcl-2 protein in the HIF-1α siRNA group, but not in
the control group, suggesting that loss of HIF-1 results in the
phosphorylation of Bcl-2 followed by loss of its anti-apoptotic
function, resulting in the release of cytochrome C from
mitochondria and further activation of the caspase cascade. Our
study has therefore shown that HIF-1 protects cell growth under
serum-deprived conditions via the inhibition of apoptosis.
Acknowledgements
We would like to thank Dr Lynne Bingle for editorial
assistance with the manuscript. This study was supported by the
National Natural Science Foundation of China (30560057 and
30860331), The Inner Mongolia Natural Science Foundation
(200508010911) and the Key Project of the Science and Technology
Inner Mongolia Medical College (NY2004ZD001).
Abbreviations:
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
siRNA
|
small interference RNA
|
|
Bcl-2
|
B cell lymphoma
|
References
|
1
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Volm M and Koomagi R: Hypoxia-inducible
factor (HIF-1) and its relationship to apoptosis and proliferation
in lung cancer. Anticancer Res. 20:1527–1533. 2000.PubMed/NCBI
|
|
3
|
Zhong H, Mabjeesh N, Willard M and Simons
J: Nuclear expression of hypoxia-inducible factor 1alpha protein is
heterogeneous in human malignant cells under normoxic conditions.
Cancer Lett. 181:233–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jubb AM, Pham TQ, Hanby AM, et al:
Expression of vascular endothelial growth factor, hypoxia inducible
factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin
Pathol. 57:504–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osada R, Horiuchi A, Kikuchi N, Yoshida J,
Hayashi A and Ota M: Expression of hypoxia-inducible factor 1alpha,
hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in
epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau
gene: nuclear expression of hypoxia-inducible factor 1alpha is an
independent prognostic factor in ovarian carcinoma. Hum Pathol.
38:1310–1320. 2007.
|
|
6
|
Yoshimura H, Dhar DK, Kohno H, et al:
Prognostic impact of hypoxia-inducible factors 1{alpha} and
2{alpha} in colorectal cancer patients: correlation with tumor
angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res.
10:8554–8560. 2004.
|
|
7
|
Kubo T, Sugita T, Shimose S, Matsuo T,
Arihiro K and Ochi M: Expression of hypoxia-inducible
factor-1{alpha} and its relationship to tumour angiogenesis and
cell proliferation in cartilage tumours. J Bone Joint Surg Br.
90B:364–370. 2008.
|
|
8
|
Wiesener MS, Jurgensen JS, Rosenberger C,
et al: Widespread, hypoxia-inducible expression of HIF-2alpha in
distinct cell populations of different organs. FASEB J. 17:271–273.
2003.PubMed/NCBI
|
|
9
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ameri K, Lewis CE, Raida M, Sowter H, Hai
T and Harris AL: Anoxic induction of ATF-4 through
HIF-1-independent pathways of protein stabilization in human cancer
cells. Blood. 103:1876–1882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi YH, Wang YX, Bingle L, et al: In vitro
study of HIF-1 activation and VEGF release by bFGF in the T47D
breast cancer cell line under normoxic conditions: involvement of
PI-3K/Akt and MEK1/ERK pathways. J Pathol. 205:530–536. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1 alpha in brain tumors – association with angiogenesis, invasion,
and progression. Cancer. 88:2606–2618. 2000.
|
|
13
|
Gillespie DL, Zhong H, Scalzitti JM,
Laughner E, Simons JW and Semenza GL: Silencing of hypoxia
inducible factor-1alpha by RNA interference attenuates human glioma
cell growth in vivo. Clin Cancer Res. 15:2441–2448. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kilic M, Kasperczyk H, Fulda S and Debatin
KM: Role of hypoxia inducible factor-1 alpha in modulation of
apoptosis resistance. Oncogene. 26:2027–2038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Kon T, Wang H, et al: Enhancement
of hypoxia-induced tumor cell death in vitro and radiation therapy
in vivo by use of small interfering RNA targeted to
hypoxia-inducible factor-1{alpha}. Cancer Res. 64:8139–8142.
2004.PubMed/NCBI
|
|
16
|
Krick S, Eul BG, Hanze J, et al: Role of
hypoxia-inducible factor-1{alpha} in hypoxia-induced apoptosis of
primary alveolar epithelial type II cells. Cell Mol Biol.
32:395–403. 2005.
|
|
17
|
Mack FA, Rathmell WK, Arsham AM, Gnarra J,
Keith B and Simon MC: Loss of pVHL is sufficient to cause HIF
dysregulation in primary cells but does not promote tumor growth.
Cancer Cell. 3:75–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim JH, Park JW, Kim MS, Park SK, Johnson
RS and Chun YS: Bafilomycin induces the p21-mediated growth
inhibition of cancer cells under hypoxic conditions by expressing
hypoxia-inducible factor-1{alpha}. Mol Pharmacol. 70:1856–1865.
2006.PubMed/NCBI
|
|
19
|
Emerling BM, Platanias LC, Black E, et al:
Mitochondrial reactive oxygen species activation of p38
mitogen-activated protein kinase is required for hypoxia signaling.
Mol Cell Biol. 25:4853–4862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida D, Kim K, Noha M and Teramoto A:
Anti-apoptotic action by hypoxia inducible factor 1-alpha in human
pituitary adenoma cell line, HP-75 in hypoxic condition. J
Neurooncol. 78:217–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takahashi Y, Nishikawa M and Takakura Y:
Inhibition of tumor cell growth in the liver by RNA
interference-mediated suppression of HIF-1alpha expression in tumor
cells and hepatocytes. Gene Ther. 15:572–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Y, Wang W, Qu X and Sun S: Effects of
hypoxia inducible factor-1alpha (HIF-1alpha) on the growth and
adhesion in tongue squamous cell carcinoma cells. Indian J Med Res.
129:154–163. 2009.PubMed/NCBI
|
|
23
|
Yang JY, Walicki J, Michod D, Dubuis G and
Widmann C: Impaired akt activity down-modulation, caspase-3
activation, and apoptosis in cells expressing a caspase-resistant
mutant of RasGAP at position 157. Mol Biol Cell. 16:3511–3520.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei MC, Zong WX, Cheng EH, et al:
Proapoptotic BAX and BAK: a requisite gateway to mitochondrial
dysfunction and death. Science. 292:727–730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amamoto K, Ichijo H and Korsmeyer SJ:
BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-Terminal
protein kinase pathway normally activated at G2/M. Mol Cell Biol.
19:8469–8478. 1999.PubMed/NCBI
|
|
27
|
Kondo E, Ichijo H and Korsmeyer SJ:
Expression of phosphorylated Ser70 of Bcl-2 correlates with
malignancy in human colorectal neoplasms. Clin Cancer Res.
11:7255–7263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blagosklonny MV, Schulte T, Nguyen P,
Trepel J and Neckers LM: Taxol-induced apoptosis and
phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a
novel c-Raf-1 signal transduction pathway. Cancer Res.
56:1851–1854. 1996.PubMed/NCBI
|