Introduction
Soft tissue sarcomas (STSs) are heterogeneous
malignant mesenchymal tumors, histologically classified based on
morphological characteristics (1).
STSs often display highly aggressive behavior with a tendency
towards early metastasis. Although the same histological categories
of STSs exist, various grades of malignancy with different
prognosis are included (2). A
variety of clinicopathological factors, such as tumor size, depth
and histological grade, have been studied to predict the malignant
potential of STSs (1–4). It is necessary to select the
appropriate therapy for patients with STSs due to their different
pathobiological behavior and prognostic significance. A number of
prognostic or biological markers were studied in STSs and reported
to be significant prognostic markers (5–19).
Minichromosome maintenance complex (MCM2-7) and
Geminin are important in the prevention of DNA re-replication in
the cell cycle. MCM proteins are expressed throughout the whole
cell cycle, including cells that exit the G0 and enter the G1 phase
(20). Geminin is present from G1-S
transition to early M phases (21).
Thus, MCM is the G0/G1/S/G2/M-phase marker and Geminin the
S/G2/M-phase marker. MCM proteins are known to contribute to the
regulation of transcription, chromatin remodeling and checkpoint
responses. The activated MCM complex appears to play a key role in
the DNA unwinding step, acting as a DNA helicase (22,23).
Following the initiation of DNA replication during the cell cycle,
Geminin inhibits the re-uploading of the MCM complex onto chromatin
and prevents DNA re-replication in the same cell cycle (24–26).
MCM proteins were suggested as potential prognostic
markers in a variety of human malignancies, including prostate and
breast cancer and bronchial adenocarcinoma (19,27–34).
Overexpression of Minichromosome maintenance protein 7 (MCM7) was
reported to actively contribute to tumor formation, progression and
malignant conversion. Moreover, MCM7 was considered a useful
proliferation marker in colonic, prostate, endometrial and cervical
cancers (30,31). In addition, Geminin is overexpressed
in breast, colorectal and prostate cancers (33–38).
However, the clinicopathological importance of MCM7 and Geminin
expression in STSs has yet to be thoroughly investigated.
The present study examined MCM7 and Geminin
expression levels in human STSs to clarify the correlation with the
clinicopathological factors, in comparison with the proliferation
marker, Ki-67 expression.
Materials and methods
Surgical specimens
A total of 109 patients with localized STSs were
selected from the files of the Division of Organ Pathology, Faculty
of Medicine, Tottori University and the affiliated teaching
hospitals. Of the 109 patients, 106 were recruited following
surgery and 3 following biopsy, during the period between 1981 and
2007. The clinical information was collected from the medical
records regarding clinical presentation, age, gender, tumor
location, tumor size, distant metastasis and pre-operative
treatment.
Radiotherapy (30–50 Gy) was performed in 25
patients, chemotherapy in 27 and both in 13 patients, as a
pre-operative treatment. Mean follow-up periods were 51.4 months
(range 1–228).
All of the specimens were fixed in 10% buffered
formalin and embedded in paraffin wax. Serial sections (4-μm) were
stained using hematoxylin and eosin, and periodic acid-Schiff
reaction. Immunohistochemistry was performed to detect
immunoreactivity for α-smooth muscle actin, desmin, caldesmon,
S-100 protein, vimentin, CD34 and epithelial membrane antigen. A
histologic diagnosis was established according to the World Health
Organization classification (39).
Histological grades were assigned according to the French
Federation of the Cancer Center Sarcoma Group (FNCLCC) system,
based on necrosis, number of mitoses and the degree of tumor
differentiation (40).
Immunohistochemistry
Immunohistochemistry was performed using a standard
streptavidin-biotin-peroxidase complex technique (SAB method).
Briefly, paraffin-embedded tumor tissue specimens were cut into
4-μm sections, dewaxed in xylene, rehydrated through a graded
series of ethanol solution and rinsed in distilled water for 5 min.
Tissue sections were retrieved using a microwave (MI-77; Azumaya,
Tokyo, Japan) in citrate buffer (0.01 M, pH 6.0) at 95°C for 20
min. After cooling to room temperature, endogenous peroxidase
activity was blocked by incubation with 0.6% hydrogen peroxide in
methanol for 30 min. After rinsing with phosphate-buffered saline,
the sections were incubated with blocking serum (2% fetal bovine
serum) at room temperature for 20 min and incubated at 4°C
overnight, with the primary antibody as follows: mouse anti-MCM7
antibody (diluted 1:100; Santa Cruz Biotechnology, Santa Cruz, CA,
USA); rabbit anti-Geminin antibody (diluted 1:100; Santa Cruz
Biotechnology); and mouse anti-Ki-67 antibody (MIB-1, diluted 1:50;
Dako, Glostrup, Denmark). Incubation with biotinylated anti-mouse
IgG (Nichirei, Tokyo, Japan) for MCM7 and Ki-67, and the secondary
antibody, anti-rabbit IgG (Nichirei) for Geminin, was carried out
for 30 min, followed by incubation with a streptavidin
biotinylated-HRP complex for 30 min. The immunoreaction was
visualized with 3,3′-diaminobenzidine and 100 μl hydrogen peroxide
in 0.05 M Tris-HCl buffer (pH 7.6). Finally, the sections were
counterstained with hematoxylin.
Evaluation of immunohistochemical
findings
To evaluate MCM7, Geminin and Ki-67 expression
levels, positive tumor cell nuclei were captured by CCD camera in
the most distinctly labeled area. Subsequently, counts were
performed in high-magnification fields using the FLOVEL Image
Filing System FlvFs (FLOVEL Inc., Tachikawa, Japan). The percentage
of positive cells was determined for each antibody by two authors
who were unaware of the clinicopathological variables. At least
1,000 tumor cells for MCM7, Geminin and Ki-67 were counted. The
labeling indices (LIs) were determined by counting the number of
positive cells (expressed as a percentage). MCM7, Geminin and Ki-67
LIs in the 109 STSs were classified as high expression if they were
equal to or more than median LIs. To confirm the specificity of the
immunostaining results, sections that immunoreacted without the
primary antibodies were used as negative controls.
Statistical analysis
Statistical analysis was performed using Excel 2003
(Microsoft, USA) with the add-in software StatView version program
5.0 (Abacus Concept, USA). The correlation between MCM7 and Geminin
LIs and Ki-67 LI was calculated using the Spearman rank order
correlation test. The Mann-Whitney U test was used when the
categorical variables of interest were two and the Kruskal-Wallis
test was used when the variables were three or more. Survival
curves were calculated using the Kaplan-Meier method. Univariate
analysis was performed using the log-rank test and multivariate
analysis was performed using a Cox proportional hazards regression
model in a stepwise manner. Hazard ratios were reported with 95%
confidence intervals (CI). P<0.05 was considered to be
statistically significant.
Results
Table I shows the
characteristics of the 109 patients with STSs. There were 67 men
and 42 women with a mean age of 55.8 years (range 0.1–93). The
tumors were located in the extremities in 90 patients and in the
trunk in 19 patients. Tumors were >5 cm in 77 patients and <5
cm in 32 patients. The distant metastases were absent in 68
patients and present in 41 patients. Histological grades 1, 2 and 3
were identified in 27, 51 and 31 cases, respectively. The tumors
consisted of 40 leiomyosarcomas, 34 liposarcomas, 18 synovial
sarcomas, 5 myxofibrosarcomas, 5 epithelioid sarcomas, 4 malignant
fibrous histiocytomas (MFH)/undifferentiated pleomorphic sarcomas
(UPS) and 3 rhabdomyosarcomas.
 | Table ICorrelation between LIs for MCM7,
Geminin and Ki-67, and clinicopathological profiles. |
Table I
Correlation between LIs for MCM7,
Geminin and Ki-67, and clinicopathological profiles.
| Variables | No. | MCM7 LIs | P-value | Geminin LIs | P-value | Ki-67 LIs | P-value |
|---|
| All patients | 109 | 17.4±15.7 | | 7.45±8.51 | | 14.2±14.9 | |
| Age (mean 55.8
years) | | | NS | | NS | | NS |
| ≥55.8 | 56 | 20.4 | | 8.14 | | 16.5 | |
| <55.8 | 53 | 15.0 | | 6.71 | | 11.9 | |
| Gender | | | NS | | NS | | NS |
| Male | 67 | 17.1 | | 7.24 | | 14.6 | |
| Female | 42 | 18.8 | | 8.24 | | 14.2 | |
| Tumor location | | | NS | | NS | | NS |
| Extremity | 90 | 18.6 | | 7.58 | | 14.8 | |
| Trunk | 19 | 13.9 | | 6.81 | | 11.7 | |
| Tumor size | | | NS | | NS | | NS |
| ≥5 cm | 77 | 18.0 | | 7.80 | | 14.4 | |
| <5 cm | 32 | 17.2 | | 6.59 | | 13.8 | |
| Distant
metastasis | | | <0.01 | | <0.01 | | <0.01 |
| Absent | 68 | 11.5 | | 4.71 | | 8.64 | |
| Present | 41 | 28.2 | | 12.00 | | 23.5 | |
| Histological
gradea | | | <0.01 | | <0.01 | | <0.01 |
| 1 | 27 | 5.37 | | 1.18 | | 2.05 | |
| 2 | 51 | 17.1 | | 7.69 | | 14.8 | |
| 3 | 31 | 29.7 | | 12.50 | | 23.9 | |
| Histologic
type |
| Leiomyosarcoma | 40 | 24.6 | | 10.20 | | 21.5 | |
| Liposarcoma | 34 | 6.6 | | 1.74 | | 4.35 | |
|
Well-differentiated | 16 | | | | | | |
| Myxoid | 13 | | | | | | |
| Pleomorphic | 2 | | | | | | |
|
Dedifferentiated | 1 | | | | | | |
| Round cell | 1 | | | | | | |
| Synovial
sarcoma | 18 | 28.2 | | 12.10 | | 16.5 | |
| Monophasic | 8 | | | | | | |
| Biphasic | 10 | | | | | | |
| Epithelioid
sarcoma | 5 | 11.2 | | 8.24 | | 18.3 | |
|
Myxofibrosarcoma | 5 | 12.8 | | 7.90 | | 14.4 | |
| MFH/UPS | 4 | 12.1 | | 3.45 | | 11.5 | |
| Pleomorphic | 3 | | | | | | |
| Inflammatory | 1 | | | | | | |
|
Rhabdomyosarcoma | 3 | 18.1 | | 11.10 | | 13.7 | |
| Alveolar | 2 | | | | | | |
| Embryonal | 1 | | | | | | |
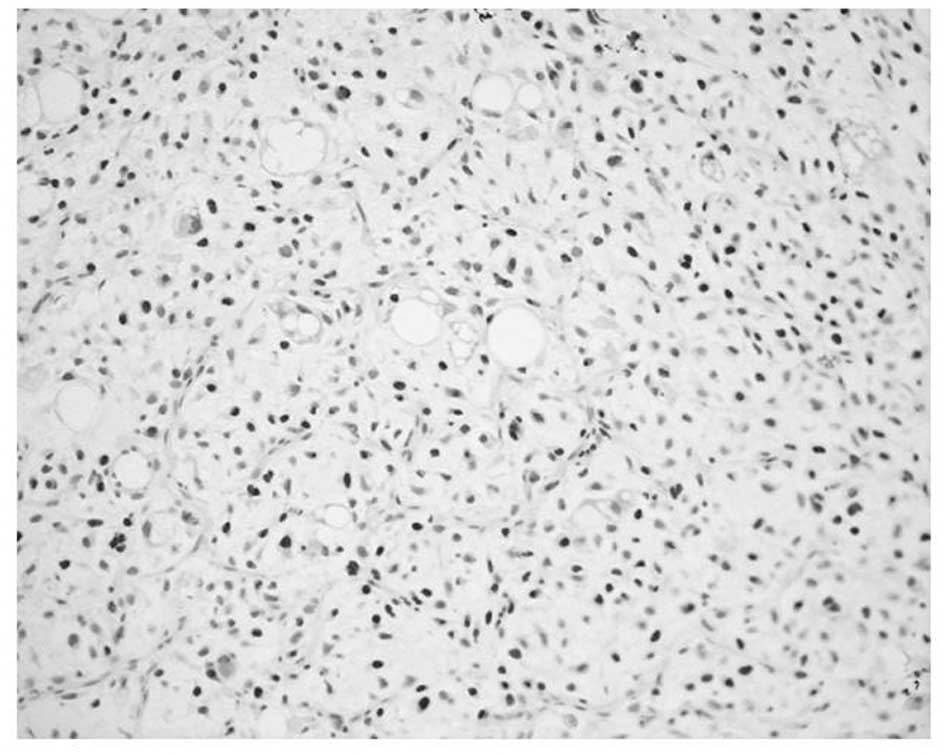
MCM7 and Geminin expression was observed in all STSs
examined. Their immunoreactivity was noted in the nuclei of the
tumor cells, as shown in Fig. 1.
Table I shows the association of
MCM7, Geminin and Ki-67 LIs with the clinicopathological factors of
the 109 patients with STSs. Mean LIs of MCM7, Geminin and Ki-67
were 17.4±15.7, 7.45±8.51 and 14.2±14.9%, respectively. The LIs
were highest for MCM7, followed by Ki-67 and Geminin. Significant
correlations were noted between the LIs of the three molecules and
distant metastasis (P<0.01) or histological grade (P<0.01),
respectively. On the other hand, no correlation was observed
between the LIs and the remaining clinicopathological factors,
including age, gender, tumor location or tumor size. Among the
histologic type, MCM7 and Geminin LIs were highest in synovial
sarcoma, followed by leiomyosarcoma. MCM7 LIs were higher than
Ki-67 LIs in all histologic types of tumors, except for
myxofibrosarcoma and epithelioid sarcoma. On the other hand,
Geminin LIs were lower than Ki-67 LIs in all histologic types of
tumors.
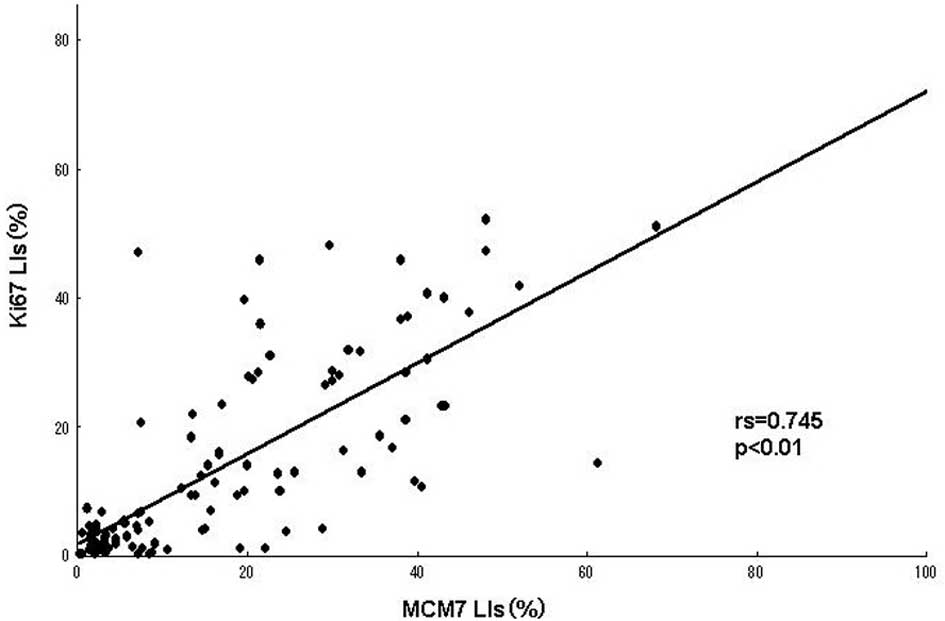
A positive linear correlation was found between MCM7
and Ki-67 in the analyzed samples (Fig.
2A), as well as between Geminin and Ki-67 (Fig. 2B), with Spearman’s correlation
coefficients of rs=0.745 (P<0.01) and rs=0.604 (P<0.01),
respectively.
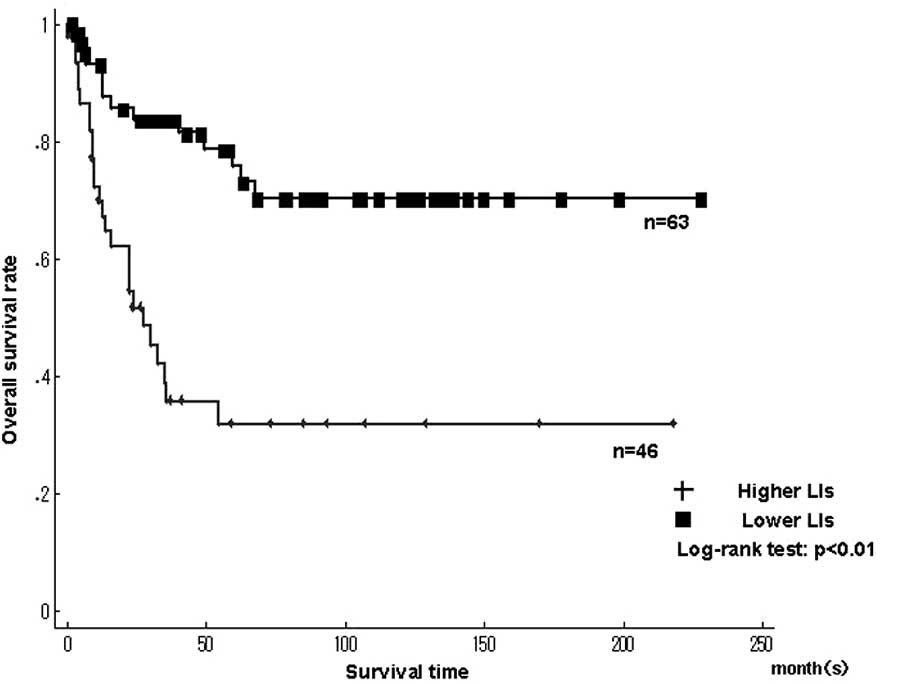
Fig. 3 shows the
Kaplan-Meier survival curves for MCM7 and Geminin LIs in patients
with 109 STSs. The patients were classified into higher and lower
LIs, which were divided by the median LIs. A significantly poorer
prognosis was found in patients with higher LIs for MCM7
(>17.4%), Geminin (>7.45%) and Ki-67 LIs (>14.2%) compared
to patients with lower LIs (P<0.01), respectively (Fig. 3A and B).
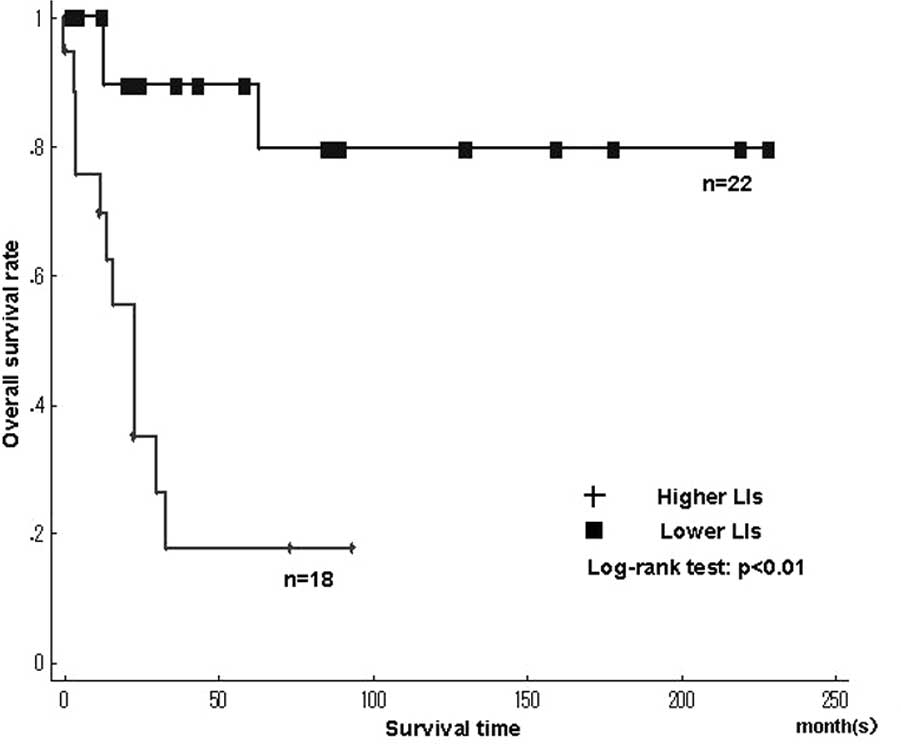
Fig. 4 shows the
Kaplan-Meier survival curves for MCM7 and Geminin LIs in the
leiomyosarcomas. Among the histologic types, the overall survival
rate was significantly worse in leiomyosarcomas with higher LIs of
MCM7 and Geminin than in those with lower LIs (MCM7, P<0.01;
Geminin, P<0.01)(Fig. 4A and B).
The overall survival rate was significantly worse in liposarcomas
with higher LIs of Ki-67 than in those with lower LIs (Ki-67,
P=0.02). On the other hand, the overall survival for Ki-67 LIs in
leiomyosarcomas, MCM7 and Geminin LIs in liposarcomas, as well as
MCM7, Geminin and Ki-67 LIs in synovial sarcomas did not show
significant differences between higher and lower LIs. Survival with
myxofibrosarcoma, epithelioid sarcoma, MFH/UPS and rhabdomyosarcoma
was not determined, since the small number of cases did not enable
the required statistical analysis.
We performed univariate Cox regression analyses to
evaluate the contributions of potential prognostic markers to
overall survival (Table II).
Overall survival was significantly correlated with higher LIs for
MCM7 (relative risk (RR)=3.70; 95% CI 1.92–7.16; P<0.01), higher
LIs for Geminin (RR=5.25; 95% CI 2.75–10.0; P<0.01) and higher
LIs for Ki-67 (RR=3.16; 95% CI 1.68–5.95; P<0.01). Patients with
distant metastasis (RR=14.7; 95% CI 6.10–35.4; P<0.01) and
histological grade (grade 1 vs. 2: RR=3.38, 95% CI 0.99–11.6,
P=0.052 and grade 1 vs. 3: RR=7.75, 95% CI 2.29–26.2, P<0.01)
were also significantly correlated with the overall survival rate,
respectively.
 | Table IIUnivariate and multivariate analysis
of clinicopathological factors for overall survival. |
Table II
Univariate and multivariate analysis
of clinicopathological factors for overall survival.
| Relative risk | 95% CI | P-value |
|---|
| Univariate
analysis |
| MCM7 LIs |
| <17.4% | 1 | - | - |
| >17.4% | 3.70 | 1.92–7.16 | <0.01 |
| Geminin LIs |
| <7.45% | 1 | - | - |
| >7.45% | 5.25 | 2.75–10.0 | <0.01 |
| Ki-67 LIs |
| <14.2% | 1 | - | - |
| >14.2% | 3.16 | 1.68–5.95 | <0.01 |
| Distant
metastasis |
| Absent | 1 | - | - |
| Present | 14.7 | 6.10–35.4 | <0.01 |
| Histological
gradea |
| 1 | 1 | - | - |
| 2 | 3.38 | 0.99–11.6 | 0.05 |
| 3 | 7.75 | 2.29–26.2 | <0.01 |
| Multivariate
analysis |
| Geminin LIs |
| <7.45% | 1 | - | - |
| >7.45% | 2.72 | 1.23–5.99 | 0.01 |
The Cox proportional hazards regression model was
conducted, adjusting for age (≥55.8 or <55.8 years), gender
(male or female), tumor location (extremity or trunk), tumor size
(≥5 or <5 cm), histological grade (1, 2 or 3), MCM7 LIs (higher
or lower), Geminin LIs (higher or lower) and Ki-67 LIs (higher or
lower). Distant metastasis (absent or present) was excluded, since
it was not present at diagnosis, but developed during the
follow-up. In multivariate analyses, higher LIs of Geminin
(RR=2.72, 95% CI 1.23–5.99, P=0.013), but not of MCM7 (RR=1.64, 95%
CI 0.678–3.990, P=0.27), Ki-67 (RR=1.02, 95% CI 0.440–2.347,
P=0.97) and histological grade (RR=2.07, 95% CI 0.555–7.734,
P=0.28), were identified as independent prognostic factors of poor
prognosis in the 109 STSs (Table
II).
Discussion
STSs are relatively rare but aggressive tumors,
representing 1% of adult and 15% of pediatric malignancies.
Although the prognosis of patients with STSs has improved, a subset
of STSs shows an aggressive clinical course and results in
tumor-related mortality (1,2). Therefore, a number of studies have
been designed to investigate the prognostic factors of STSs, such
as Ki-67, p53, cyclin D1, EGFR, cyclin A, CD40, CD44, VEGF, EZH2,
FOXO1, c-Myc, osteopontin, Bcl-2, ERBB2, KIT, IGF-1R and MCM2,
since they may aid in determining the course of adjuvant therapy
and the length of follow-up (5–19). The
present study shows the prognostic significance of MCM7, Geminin
and Ki-67, using immunohistochemical methods in 109 STSs.
MCM proteins were reported to be correlated with the
histologic grade in other types of cancer, including prostate
cancer, lung adenocarcinoma and renal cell carcinoma (27–34).
We previously reported the positive relation between the expression
of MCM2 and the prognosis of patients with human malignant fibrous
histiocytoma (19). The trimeric
complex of MCM4, MCM6 and MCM7 was found to possess DNA helicase
activity in an in vitro study. This complex also interacts
with and is regulated by another trimeric complex, comprising MCM2,
MCM3 and MCM5, to form a hexameric complex. Furthermore, MCM7 is
the only family member whose promoter contains an E-box sequence,
which is the binding site for members of the MYC transcription
factor family. MCM7 is also a direct target of MYCC and MYCN
(31,41,42).
Therefore, we focused on the significance of MCM7 expression in
STSs.
Geminin is a protein that blocks re-replication of
the gene in the same cycle. It is present in S, G2 and M phases of
the cell cycle, but absent in the G1 phase (20,21).
Numerous previous studies examined the effect of Geminin expression
on the overall survival of patients with breast, colorectal and
prostatic cancers and showed that a higher expression of Geminin
was a poor prognostic marker in patients with these types of cancer
(33–35,37,38).
On the other hand, Shrestha et al found that the
relationship between Geminin expression and survival in patients
with high-grade astrocytic tumors contradicted the previous
findings (36), and that the
overexpression of Geminin was associated with a longer survival of
patients with high-grade astrocytic tumors. These contradictory
results may partially explain why the overexpression of Geminin may
increase radiosensitivity, leading to a better prognosis after
radiotherapy, even though the Geminin expression is elevated with
increasing tumor grade. However, direct evidence for this
increasing sensitivity has yet to be presented (36). In the present study, a higher
Geminin LI was a significant poor prognostic marker in patients
with STSs, but the small number of cases with pre-operative
radiotherapy did not enable confirmation of the relationship
between Geminin LIs and radiosensitivity.
Gerdes et al first reported that the Ki-67
antigen was a nuclear protein associated with cellular
proliferation (43). Ki-67 was
present throughout the complete cell cycle, except for the early G1
phase, which is associated with poor prognosis of the disease
(43–45). A Ki-67 LI was correlated with the
poor prognosis of patients with several malignancies (28,34,43).
Ki-67 has been used as a common and valuable prognostic marker in
STSs (8,9,44,45).
We also showed that MCM7 and Geminin LIs are positively correlated
with Ki-67 LIs.
The survival rate in the 109 patients calculated by
Kaplan-Meier analysis showed significant correlations between
patient prognosis and the LIs of MCM7, Geminin and Ki-67. As a
result, higher LIs of these markers were negative prognostic
factors in the 109 STSs. The survival rate demonstrated a
significant correlation between patient prognosis and MCM7 and
Geminin LIs in leiomyosarcomas, and between patient prognosis and
Ki-67 LIs in liposarcomas. On the other hand, the survival rates in
other histologic types were not confirmed in this study due to the
small number of cases. The results suggest that MCM7 and Geminin in
STSs, as well as Ki-67, are useful prognostic markers.
Furthermore, multivariate analyses revealed that
higher Geminin LIs, but not higher those of MCM7 and Ki-67, were
independent prognostic factors for survival in the 109 STSs. Our
data suggest that Geminin LIs are a more useful prognostic marker
than those of MCM7 and Ki-67 in STSs.
Acknowledgements
We thank Mr. N. Itaki, Ms. T. Yamasaki and Ms. M.
Iwatani for the excellent technical assistance.
References
|
1
|
Stefanovski PD, Bidoli E, De Paoli A, et
al: Prognostic factors in soft tissue sarcomas: a study of 395
patients. Eur J Surg Oncol. 28:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomita Y, Aozasa K, Myoui A, et al:
Histologic grading in soft-tissue sarcomas – an analysis of 194
cases including agnor count and mast-cell count. Int J Cancer.
54:194–199. 1993.
|
|
3
|
Levine EA: Prognostic factors in soft
tissue sarcoma. Semin Surg Oncol. 17:23–32. 1999. View Article : Google Scholar
|
|
4
|
Engellau J, Bendahl PO, Persson A, et al:
Improved prognostication in soft tissue sarcoma: independent
information from vascular invasion, necrosis, growth pattern, and
immunostaining using whole-tumor sections and tissue microarrays.
Hum Pathol. 36:994–1002. 2005. View Article : Google Scholar
|
|
5
|
Ahlen J, Wejde J, Brosjo O, et al:
Insulin-like growth factor type 1 receptor expression correlates to
good prognosis in highly malignant soft tissue sarcoma. Clin Cancer
Res. 11:206–216. 2005.PubMed/NCBI
|
|
6
|
Bramwell VHC, Tuck AB, Wilson SM, et al:
Expression of osteopontin and HGF/Met in adult soft tissue tumors.
Cancer Biology and Therapy. 4:1336–1341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huuhtanen RL, Blomqvist CP, Bohling TO, et
al: Expression of cyclin A in soft tissue sarcomas correlates with
tumor aggressiveness. Cancer Res. 59:2885–2890. 1999.PubMed/NCBI
|
|
8
|
Jensen V, Sorensen FB, Bentzen SM, et al:
Proliferative activity (MIB-1 index) is an independent prognostic
parameter in patients with high-grade soft tissue sarcomas of
subtypes other than malignant fibrous histiocytomas: a
retrospective immunohistological study including 216 soft tissue
sarcomas. Histopathology. 32:536–546. 1998. View Article : Google Scholar
|
|
9
|
Lopes JM, Nesland JM, Reis JS and Holm R:
Differential Ki67 and bcl-2 immunoexpression in solid-glandular and
spindle cell components of biphasic synovial sarcoma: a double
immunostaining assessment with cytokeratin and vimentin.
Histopathology. 40:464–471. 2002. View Article : Google Scholar
|
|
10
|
Oda Y, Tateishi N, Matono H, et al:
Chemokine receptor CXCR4 expression is correlated with VEGF
expression and poor survival in soft-tissue sarcoma. Int J Cancer.
124:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ottaiano A, De Chiara A, Perrone F, et al:
Prognostic value of CD40 in adult soft tissue sarcomas. Clin Cancer
Res. 10:2824–2831. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peiper M, Sato T, Zurakowski D, et al:
CD44s expression is associated with improved survival in soft
tissue sarcoma. Anticancer Res. 24:1053–1056. 2004.PubMed/NCBI
|
|
13
|
Sato O, Wada T, Kawai A, et al: Expression
of epidermal growth factor receptor, ERBB2 and KIT in adult soft
tissue sarcomas – a clinicopathologic study of 281 cases. Cancer.
103:1881–1890. 2005.
|
|
14
|
Tsiatis AC, Herceg ME, Keedy VL, et al:
Prognostic significance of c-Myc expression in soft tissue
leiomyosarcoma. Mod Pathol. 22:1432–1438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vesely K, Jurajda M, Nenutil R and Vesela
M: Expression of p53, cyclin D1 and EGFR correlates with
histological grade of adult soft tissue sarcomas: a study on tissue
microarrays. Neoplasma. 56:239–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaga K, Osaki M, Kidani K, Shomori K,
Yoshida H and Ito H: High expression of enhancer of zeste homologue
2 indicates poor prognosis in patients with soft tissue sarcomas.
Mol Med Rep. 1:633–639. 2008.PubMed/NCBI
|
|
17
|
Yudoh K, Kanamori N, Ohmori K, Yasuda T,
Aoki M and Kimura T: Concentration of vascular endothelial growth
factor in the tumour tissue as a prognostic factor of soft tissue
sarcomas. Br J Cancer. 84:1610–1615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Tomita Y, Ch’ng E, et al:
Prognostic significance of phosphorylated FOXO1 expression in soft
tissue sarcoma. Ann Surg Oncol. 16:1925–1937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osaki M, Yamashita H, Shomori K, Yoshida H
and Ito H: Expression of minichromosome maintenance-2 in human
malignant fibrous histiocytomas: Correlations with Ki-67 and P53
expression and apoptosis. Int J Mol Med. 10:161–168.
2002.PubMed/NCBI
|
|
20
|
Lindner K, Gregan J, Montgomery S and
Kearsey SE: Essential role of MCM proteins in premeiotic DNA
replication. Mol Biol Cell. 13:435–444. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishitani H and Lygerou Z: Control of DNA
replication licensing in a cell cycle. Genes to Cells. 7:523–534.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishimi Y: A DNA helicase activity is
associated with an MCM4, -6 and -7 protein complex. J Biol Chem.
272:24508–24513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forsburg SL: Eukaryotic MCM proteins:
beyond replication initiation. Microbiol Mol Biol Rev. 68:109–131.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGarry TJ and Kirschner MW: Geminin, an
inhibitor of DNA replication, is degraded during mitosis. Cell.
93:1043–1053. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lygerou Z and Nurse P: Cell cycle. License
withheld – Geminin blocks DNA replication. Science. 290:2271–2273.
2000.PubMed/NCBI
|
|
26
|
Wohlschlegel JA, Kutok JL, Weng AP and
Dutta A: Expression of geminin as a marker of cell proliferation in
normal tissues and malignancies. Am J Pathol. 161:267–273. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez MA, Pinder SE, Callagy G, et al:
Minichromosome maintenance protein 2 is a strong independent
prognostic marker in breast cancer. J Clin Oncol. 21:4306–4313.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashimoto K, Araki K, Osaki M, et al: MCM2
and Ki-67 expression in human lung adenocarcinoma: prognostic
implications. Pathobiology. 71:193–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nariculam J, Loddo M, Masters J, Williams
G and Feneley M: MCM-2 expression in clinically localised prostate
cancer. Eur Urol Suppl. 7:7382008. View Article : Google Scholar
|
|
30
|
Padmanabhan V, Callas P, Philips G,
Trainer TD and Beatty BG: DNA replication regulation protein MCM7
as a marker of proliferation in prostate cancer. J Clin Pathol.
57:1057–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishihara K, Shomori K, Fujioka S, et al:
Minichromosome maintenance protein 7 in colorectal cancer:
Implication of prognostic significance. Int J Oncol. 33:245–251.
2008.PubMed/NCBI
|
|
32
|
Shimizu M, Nikaido T, Kato K, et al:
Expression of replication-licensing factors MCM2 and MCM3 in normal
endometrium and endometrial carcinomas. In: 9th International
Menopause Society World Congress on the Menopause; pp. 159–162.
1999
|
|
33
|
Torres-Rendon A, Roy S, Craig GT and
Speight PM: Expression of MCM2, geminin and Ki67 in normal oral
mucosa, oral epithelial dysplasias and their corresponding
squamous-cell carcinomas. Br J Cancer. 100:1128–1134. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vargas PA, Cheng Y, Barrett AW, Craig GT
and Speight PM: Expression of Mcm-2, Ki-67 and geminin in benign
and malignant salivary gland tumours. J Oral Pathol Med.
37:309–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salabat MR, Melstrom LG, Strouch MJ, et
al: Geminin is overexpressed in human pancreatic cancer and
downregulated by the bioflavanoid apigenin in pancreatic cancer
cell lines. Mol Carcinogen. 47:835–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shrestha P, Saito T, Hama S, et al:
Geminin: a good prognostic factor in high-grade astrocytic brain
tumors. Cancer. 109:949–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishihara K, Shomori K, Tamura T, Fujioka
S, Ogawa T and Ito H: Immunohistochemical expression of geminin in
colorectal cancer: Implication of prognostic significance. Oncol
Rep. 21:1189–1195. 2009.PubMed/NCBI
|
|
38
|
Gonzalez MA, Tachibana KK, Chin SF, et al:
Geminin predicts adverse clinical outcome in breast cancer by
reflecting cell-cycle progression. J Pathol. 204:121–130. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fletcher CDM, Unni KK and Mertens F: World
Health Organization Classification of Tumours. Pathology and
Genetics of Tumours of Soft tissue and Bone. IARC Press; Lyon:
2002
|
|
40
|
Guillou L, Coindre JM, Bonichon F, et al:
Comparative study of the National Cancer Institute and French
Federation of Cancer Centers Sarcoma Group grading systems in a
population of 410 adult patients with soft tissue sarcoma. J Clin
Oncol. 15:350–362. 1997.
|
|
41
|
Eisenman RN: Deconstructing Myc. Genes
Dev. 15:2023–2030. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shohet JM, Hicks MJ, Plon SE, et al:
Minichromosome maintenance protein MCM7 is a direct target of the
MYCN transcription factor in neuroblastoma. Cancer Res.
62:1123–1128. 2002.PubMed/NCBI
|
|
43
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell-cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal-antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
44
|
Hoos A, Stojadinovic A, Mastorides S, et
al: High Ki-67 proliferative index predicts disease specific
survival in patients with high-risk soft tissue sarcomas. Cancer.
92:869–874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hasegawa T, Yamamoto S, Yokoyama R, Umeda
T, Matsuno Y and Hirohashi S: Prognostic significance of grading
and staging systems using MIB-1 score in adult patients with soft
tissue sarcoma of the extremities and trunk. Cancer. 95:843–851.
2002. View Article : Google Scholar : PubMed/NCBI
|