Introduction
Melanoma is characterized by rapid growth,
mortality, unpredictable behaviour and resistance to chemotherapy,
and surgery is currenlty considered to be the only effective
therapy (1). The American Cancer
Society estimated that melanoma accounted for 8,420 deaths in 2008
(2). Despite numerous years of
continuous research, the histological characteristics and related
mechanisms of melanoma remain unclear. Linearly patterned
programmed cell necrosis (LPPCN), which was previously neglected by
pathologists, was recently reported by Zhang et al (3) as being a special programmed cell death
(PCD) in highly aggressive melanoma. LPPCN is morphologically
characterized by loss of cell-cell adhesion, concentrated cytoplasm
and the cellular nucleus being stained darker than that of the
environmental cells in hematoxylin and eosin (H&E)-stained
sections. More importantly, cells that underwent LPPCN were
distributed in patterns of lines and/or networks among the
environmental tumor cells. LPPCN is similar to neither apoptosis
nor conventional necrosis commonly observed in most tumoral
tissues.
The mechanism involved in apoptosis is genetically
regulated cell death and is a type of physiologically active death.
However, a cell dying by conventional necrosis is regarded as a
victim of extrinsic events beyond its control, meaning that
conventional necrosis is a pathologic death and a passive process.
Apoptosis is morphologically characterized by karyolysis, pyknosis
(deep staining of nuclear mass), karyorrhexis and the formation of
condensed cell bodies (apoptotic bodies) (4). This ordered morphology depends on the
ability of the dying cell to engage in ATP-dependent processes of
self-degradation. By contrast, necrosis can be defined
morphologically by electron-lucent cytoplasm, swelling of cellular
organelles and loss of plasma membrane integrity (5). Yuan et al (6) and Zong et al (7) described programmed necrosis as a novel
cell death phenomenon in ischemic brain injury animal models.
Morphologically, programmed necrosis exhibits characteristics that
are different from the apoptotic bodies and DNA fragmentation
typical of apoptosis, and different from the morphological features
described above in conventional necrosis. Furthermore, apoptotic
cells distribute randomly, and cells dying to conventional necrosis
distribute in clusters and inflammatory cells occur in surroundings
of necrosis in H&E-stained sections, whereas cells that
underwent LPPCN formed a line and/or network pattern. Thus, it
appears that neither the morphological change nor the mechanism
involved in LPPCN is similar to apoptosis or conventional
necrosis.
The mechanism of this special cell death involved in
LPPCN should be elucidated. Zhang et al (3) suggested that LPPCN is similar to
mitochondria-dependent apoptosis-signaling. Additionally, Endo G,
which is an essential member of the mitochondria-dependent
apoptosis signaling pathway, may play a role in the process of
LPPCN. Recent technological advances have helped define the
function and mechanism of programmed necrosis. Accumulating
evidence has confirmed that autophagy plays an important role in
cell survival and disintegration (8). Autophagy has also been suggested as a
possible mechanism for non-apoptotic and/or non-necrosis cell
death, despite evidence from a number of species that it is a
survival strategy in times of stress (9). In autophagy, a double-membrane
structure known as an autophagosome, sequesters a section of
cytoplasm and fuses with the lysosome/vacuole to deliver its
contents into the lysosomal/vacuolar lumen. The encoding
microtubule-associated protein light chain 3 (LC3), is an important
autophagy-related protein with a mammalian ortholog of Atg8 in
yeast. Atg4B is a critical regulator of LC3 (10). LC3 is localized to the
autophagosomal membrane, and is widely used as a key molecule to
monitor autophagosome formation and autophagy activity in mammalian
systems. PCD has captured the attention of various cancer
investigators. Numerous studies on the role of autophagy in PCD
type II or non-apoptotic death are available (12–15).
However, the role of autophagy in oncogenesis and progress remains
unclear, and mechanisms that induce autophagy and regulate its
outcome in human cancers have yet to be elucidated.
In this study, LPPCN was examined via morphological
and experimental evidence. Moreover, the correlations between the
expression of LC3 and Atg4B and LPPCN were investigated in order to
identify the role of the autophagy-related proteins in the process
of LPPCN, as well as to determine the mechanism and the
clinicopathologic significance of LPPCN in melanoma.
Materials and methods
Patients
This study utilized 70 primary tumor specimens of
human melanoma obtained from patients who consecutively underwent
surgical resection in the Tianjin Medical University Cancer
Institute and Hospital in China between January 1999 and October
2005. The patients comprised 27 women and 43 men with ages ranging
from 28–81 (mean 54.96; SD 12.60). During the final follow-up, 12
patients had survived and 58 had succumbed within 113.5 months,
with the median survival time being 29.5 months.
Clinical and histopathological
evaluation
The tissue slides of 70 melanoma patients were
reviewed using the World Health Organization Classification of
Tumours Pathology and Genetics of Skin Tumours (11). Tissue sections of 5 μm were cut from
the formalin-fixed, paraffin-embedded representative tissue blocks
of melanoma and were stained with H&E. The quantities and
distribution of LPPCN, and other characteristics involved in LPPCN,
were evaluated in all 70 sections. The number of positive cells per
×200 field was then assessed. The percentage of cells that
underwent LPPCN was rated as: cases with <1% positive cells were
rated as zero, 1–25% positive cells as 1 point, 26–50% positive
cells as 2 points and >51% positive cells as 3 points.
Clinicopathological parameters were obtained from patient medical
records and files kept at the Department of Pathology, and included
information on gender, age, tumor location, survival status,
survival time, lymph node status, distant metastasis, AJCC stage
(i.e., based on the American Joint Committee On Cancer Staging
System), tumor thickness, coventional necrosis, histological
subtypes, melanin, cellular phenotype, tumor-infiltrating
lymphocytes and nucleolus.
Immunohistochemical staining
Serial 4μm sections cut from formalin-fixed,
paraffin-embedded tumor tissue for immunohistochemical analysis
were deparaffinized and hydrated through a series of xylenes and
alcohols prior to immunohistochemical staining (using the alkaline
phosphatase-streptavidin method) for Ki-67, HIF-1α, LC3 and Atg4B.
The sections were rehydrated in phosphate-buffered saline (PBS),
and antigen retrieval was performed using microwave heating for 5
min in 10 melanoma citrate buffer solution (pH 6.0) for survivin.
The sections were incubated with monoclonal mouse anti-HIF-1α
(clone Sc-53546; Santa Cruz Biotechnology, dilution of 1:50),
anti-Ki67 (ready-to-use; Zhongshan, Beijing, China), rabbit
polyclonal anti-LC3 (Novus Biologicals, USA, dilution of 1:50), and
rabbit polyclonal anti-Atg4B (Abzoom, USA, dilution of 1:50).
Following incubation, the sections were rinsed with PBS and
incubated with biotinylated goat anti-mouse or goat anti-rabbit IgG
for 20 min at 37°C. The sections were then incubated with
3,3′-diaminobenzidine (DAB) chromogen for 5–10 min at room
temperature and washed with distilled water. Finally, the slides
were washed for 5 min in running tap water and counterstained with
Harris hematoxylin.
Immunohistochemical analysis
A positive value was recorded for HIF-1α and Ki67
immunoreactivity when nuclear staining was observed. Using
cytoplasm staining, LC3 and Atg4B were found to be positive.
Semi-quantitative expression levels were determined by assessing
the percentage and intensity of the stained tumor cells. The
percentage of positive cells was rated per HPF using a
magnification of ×200 as follows: cases with <1% positive cells
were rated as zero, 1–25% positive cells as 1 point, 26–50%
positive cells as 2 points and >51% positive cells as 3 points.
The staining intensity was rated as: 1 point for weak, 2 points for
moderate and 3 points for strong intensity. The points for staining
intensity and the percentage of positive cells were added together.
The specimens were then classified into three groups according to
their overall score: negative expression for 0–1 point, weak
expression for 2–4 points and a strong expression for 5–6
points.
Total RNA isolation and real-time
PCR
Primers were designed and synthesized by Takara
Biotechnology Co., Ltd. Real-time PCR analysis was carried out to
determine the mRNA expression of LC3 and Atg4B using the Gene AMP
PCR system 7500 sequence detector. A separate PCR assay for the
mRNA of the β-actin housekeeping gene was performed to verify
general mRNA integrity. Total RNA was extracted from 30 melanoma
tumor samples frozen at −80°C using TRIzol reagent. The optical
density (OD) 260/280 ratio for all 70 samples was 1.9–2.0. RNA was
then converted into cDNA using Taq Man reverse transcriptase
reagents (Applied Biosystems). cDNA was used as the template that
was amplified in a 25-ml reaction mixture using the following
conditions: denaturation at 94°C for 5 min, then 35 cycles of 94°C
for 30 sec, the optimal annealing temperature for 45 sec and 55°C
for 40 sec. The primer sequences used for the matrix
metalloproteinase LC3 (Accession no.: AF087871.1) detection were
5′-GGCGCTTACAGCTCAATGCTAAT-3′ (sense) and 5′-AAT
TTCATCCCGAACGTCTCCTG-3′ (antisense). The primer sequences used for
Atg4B (Accession no.: NM_013325.4) detection were
5′-ATGGAGGAAATCAGAAGGTTGT-3′ (sense) and 5′-TCGTTGATGTCCGTGAGCC-3′
(antisense). The primers used to amplify β-actin were
5′-ATCCGTAAAGAC CTCTATGCCAAC-3′ (sense) and 5′-ATGGAGCCACCGAT
CCACA-3′ (antisense). The resultant products of LC3, Atg4B and
β-actin amplification were 163, 185 and 174 base pairs,
respectively. The CT value (the cycle number at which the
fluorescence crosses the threshold) was determined and the formula
2−ΔΔCt was used to determine the relative quantity of
the amplified fragment. Each sample was tested in triplicate and
the mean value was used.
Statistical analysis
Statistical analysis was performed in this study
using SPSS 13.0. Pearson’s χ2 test. Bivariate
correlations were used to determine the different parameters
associated with the distribution of LPPCN. A t-test was used to
analyze the mRNA expression of LC3 and Atg4B. The Kaplan-Meier
method and the log-rank test were employed to evaluate survival
analysis where p<0.05 was considered to be significant.
Results
Morphological characteristics of linearly
patterned programmed cell necrosis
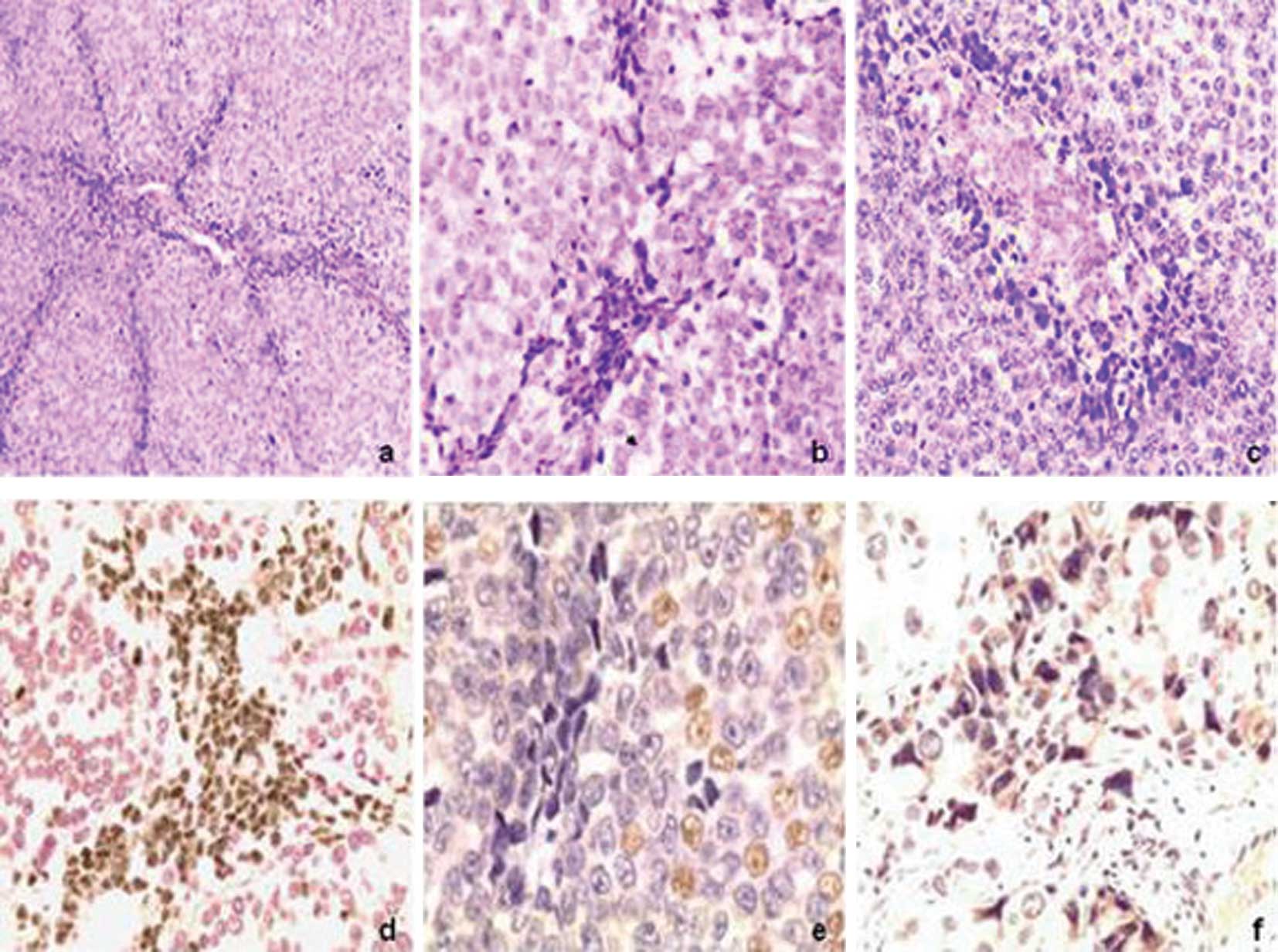
In the H&E-stained sections of tumor tissues of
melanoma, cells that underwent LPPCN were characterized by darker
staining than that of the environmental cells, loss of cell-cell
adhesion, concentrated cytoplasm, cellular nucleus adopting a
different pattern or heteromorphies (e.g., triangulum, polygon,
fusiform and deformed pattern) and bolus-shaped and homogeneous
chromatin. The cells were distributed in patterns of lines and/or
networks among the environmental tumor cells. The LPPCN lines
and/or networks observed were occasionally accompanied with
neovasculogenic networks or conventional necrosis. Inflammation
cells were absent in the environmental district of LPPCN (Fig. 1a–f). The morphological changes of
LPPCN are similar to neither apoptosis, which is typically
characterized by apoptotic bodies, nor conventional necrosis, which
is typically characterized by electron-lucent cytoplasm, swelling
of cellular organelles, loss of plasma membrane integrity and the
inflammatory reaction presents.
Correlations between linearly patterned
programmed cell necrosis and clinical and histopathological
parameters
A total of 54.29% (38/70) of the specimens observed
exhibited the LPPCN phenomenon. The appearance of LPPCN has a close
positive correlation with the following parameters in melanoma:
tumor thickness (p=0.016), histological subtype (p=0.029) and
conventional necrosis (p=0.000), but has a negative correlation
with melanin (p=0.001). The presence of LPPCN did not show any
association with gender, age, lymph node invasion, AJCC stage,
cellular phenotype, tumor-infiltrating lymphocytes or nucleolus
(p>0.05). Table I shows the
clinical and histopathological characteristics involved in
LPPCN.
 | Table ICorrelations between LPPCN and
clinical and histopathological parameters in melanoma. |
Table I
Correlations between LPPCN and
clinical and histopathological parameters in melanoma.
| Parameter | Total no. | LPPCN | p-value |
|---|
| − | + | Positive rate, % |
|---|
| Gender | | | | | 0.099 |
| Male | 43 | 23 | 20 | 46.5 | |
| Female | 27 | 9 | 18 | 66.7 | |
| Age | | | | | 0.253 |
| ≤55 | 38 | 15 | 23 | 60.5 | |
| <55 | 32 | 17 | 15 | 46.9 | |
| Tumor location | | | | | 0.071 |
| Trunk | 43 | 16 | 27 | 62.8 | |
| Extremities | 27 | 16 | 11 | 40.7 | |
| LN metastasis | | | | | 0.150 |
| Absent | 35 | 13 | 22 | 62.9 | |
| Present | 35 | 19 | 16 | 45.7 | |
| DM | | | | | 0.224 |
| Absent | 45 | 23 | 22 | 48.9 | |
| Present | 25 | 9 | 16 | 64.0 | |
| AJCC stage | | | | | 0.866 |
| I+II | 27 | 12 | 15 | 55.6 | |
| III+IV | 43 | 20 | 23 | 53.5 | |
| Tumor thickness | | | | | 0.018 |
| ≤17mm | 17 | 12 | 5 | 29.4 | |
| >17mm | 53 | 20 | 33 | 62.3 | |
| Histology | | | | | 0.025 |
| A+ N+Un | 58 | 23 | 35 | 60.3 | |
| SSM | 12 | 9 | 3 | 25.0 | |
| Melanin | | | | | 0.003 |
| Absent | 33 | 9 | 24 | 72.7 | |
| Present | 37 | 23 | 14 | 37.8 | |
| Nucleolus | | | | | 0.222 |
| Absent | 36 | 19 | 17 | 47.2 | |
| Present | 34 | 13 | 21 | 61.8 | |
| CP | | | | | 0.190 |
| Spindle | 28 | 10 | 18 | 64.3 | |
| Epithelium | 27 | 16 | 11 | 40.7 | |
| Diphase | 15 | 6 | 9 | 60.0 | |
| T-IL | | | | | 0.459 |
| Absent | 34 | 14 | 20 | 58.8 | |
| Present | 36 | 18 | 18 | 50.0 | |
| Conventional
necrosis | | | | | 0.000 |
| Absent | 32 | 25 | 7 | 21.9 | |
| Present | 38 | 7 | 31 | 81.6 | |
Correlation between LPPCN and
immunohistochemical activities
Immunohistochemical staining for Ki67 showed that
cells that underwent LPPCN were negative, while tumor cells that
were distant to LPPCN were positive. For HIF-1α, cells that
underwent LPPCN were found to be positive. The positive rates of
Ki67 and HIF-1α in the group where LPPCN was present were higher
than those in the group without LPPCN (p<0.05), a finding that
is the same as the results of LC3 and Atg4B. Correlation analysis
showed that the expression of both LC3 and Atg4B are closely
correlated (p=0.010). Data further showed that the presence of
LPPCN is related with the Ki67 index (p=0.000), LC3 (p=0.01) and
Atg4B (p=0.01) (Table II).
 | Table IICorrelations between LPPCN and
immunohistochemical parameters in melanoma. |
Table II
Correlations between LPPCN and
immunohistochemical parameters in melanoma.
| Protein | LPPCN (p) | LC3 (p) | Atg4B (p) | Ki67 (p) |
|---|
| LC3 | 0.01a | | | |
| Atg4B | 0.01a | 0.010a | | |
| Ki67 | 0.000a | 0.200 | 0.015a | |
| Hif-1α | 0.000a | 0.004a | 0.000a | 0.000a |
Expression of LC3 and Atg4B mRNA
Real-time PCR was performed to detect LC3 and Atg4B
mRNA expression in the LPPCN-positive and -negative groups in
melanoma. The expression of LC3 and Atg4B mRNA in the
LPPCN-positive group was higher than that of the LPPCN-negative
group. The difference was significant (p=0.000 and p=0.02),
respectively (Table III).
 | Table IIIExpression of LC3 and Atg4B mRNA in
melanoma. |
Table III
Expression of LC3 and Atg4B mRNA in
melanoma.
| Genes | Group | χ̄ ± SD | t-test | p-value |
|---|
| Atg4B | | | 2.63 | 0.020 |
|
LPPCN+ | 3.605±0.881 | | |
|
LPPCN− | 2.372±0.950 | | |
| LC3 | | | 6.204 | 0.000 |
|
LPPCN+ | 5.793±1.467 | | |
|
LPPCN− | 2.124±0.963 | | |
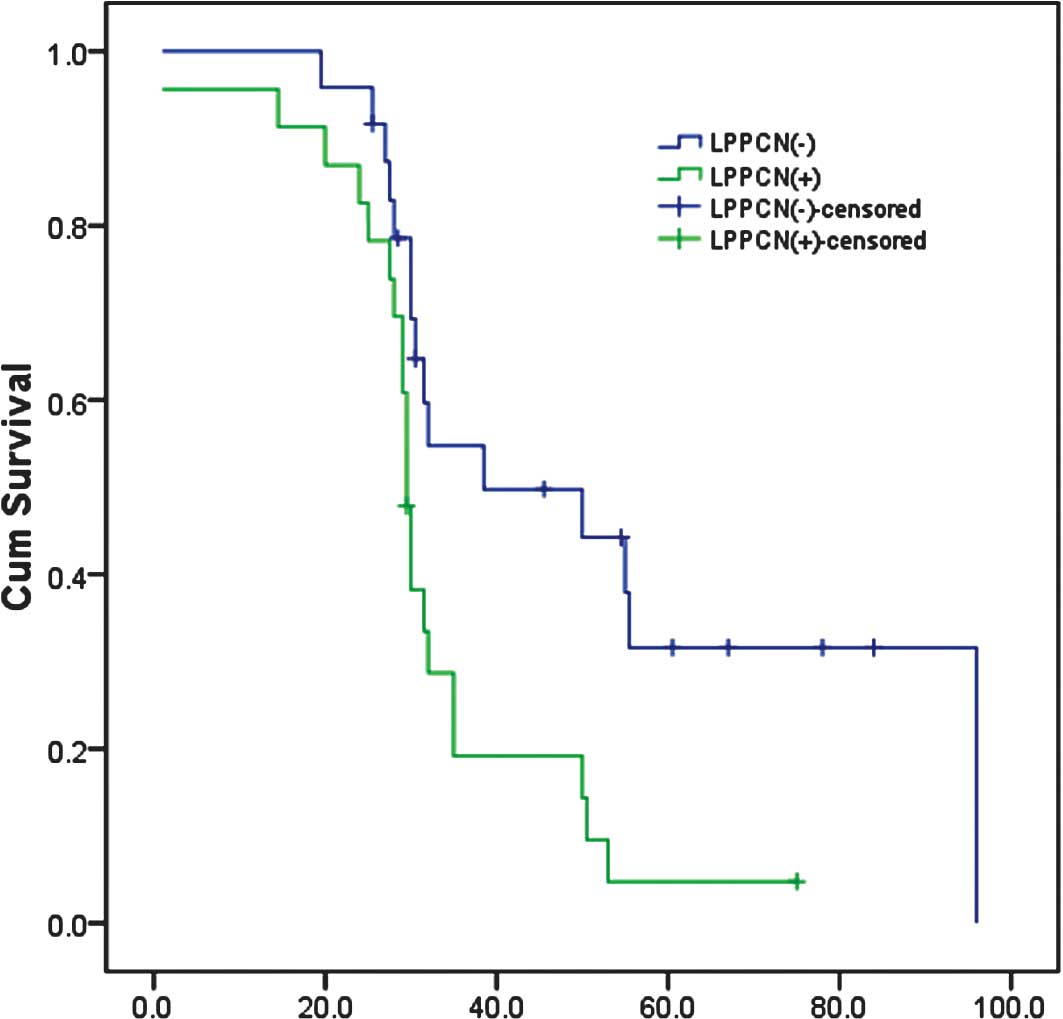
Survival analysis
Kaplan-Meier survival analysis showed that the
survival time for patients with the presence of LPPCN was
significantly shorter than that for patients without LPPCN. The
average survival time of cases with and without the presence of
LPPCN was 28.870±2.567 and 53.255±6.809 months, respectively
(p<0.05). In addition, an analysis of the results showed that
parameters such as tumor thickness, histological subtype, melanin,
conventional necrosis, cellular phenotype, tumor-infiltrating
lymphocytes, lymph node invasion and Ki67 index had a strong
influence on the survival of patients with melanoma (p<0.05)
(Fig. 2).
Discussion
No efficient chemotherapy for melanoma currently
exists (16,17). Even in low-incidence countries such
as China, the status remains a major concern. A novel phenomenon of
cell death known as LPPCN is noted in melanoma (3). This study, for the first time,
carefully observed and described tumor cell subpopulations that
underwent LPPCN. It confirmed that the LPPCN phenomenon is a novel
but common characteristic in more aggressive melanoma.
Morphological characteristics associated with LPPCN have been
sparsely described by pathologists thus far. Subsequently, we
carefully evaluated the distribution and quantification of LPPCN in
human melanoma. Cells that underwent LPPCN were morphologically
characterized by loss of cell-cell adhesion, concentrated cytoplasm
and a cellular nucleus stained darkly in H&E-stained sections.
The cells were distributed in patterns of lines and/or networks
within the environmental tumor cells. The cell clusters and/or
networks were always accompanied with neovasculogenesis networks or
conventional necrosis, but inflammatory cells were absent in the
environmental district of LPPCN.
Based on morphological observations of LPPCN,
immunohistochemical experiments were performed to determine the
formation mechanisms of LPPCN. Findings show that LPPCN is a
special type of PCD that can be activated by many factors, such as
tissue hypoxia, drugs and toxins (18). Additionally, cells underwent
programmed necrosis regulated by the tumor cells themselves under
tumor microenvironmental conditions (19,20).
In this study, cells that underwent LPPCN tested negative for Ki67,
but positive for HIF-1α by immunohistochemical staining. This
phenomenon suggests that the formation of dying cell networks
through LPPCN may be initiated in the local hypoxic
microenvironment under which HIF-1α, which mainly mediates the
hypoxic response, was up-regulated, and that these cells underwent
programmed necrosis (3). Notably,
the distribution and morphological changes of LPPCN are typically
different from apoptosis and conventional necrosis. Cells that
underwent LPPCN formed clusters, lines or networks. By contrast,
apoptosis reflects scattered cells in randomly distributed tumor
tissues. Necrosis usually occurs within the lamellar tracts of
contiguous cells in H&E-stained sections (21). Furthermore, morphologically, LPPCN
cells showed loss of cell-cell adhesion, concentrated cytoplasm,
pyknosis, karyorrhexis and karyolysis; characteristics that are
different from the apoptotic bodies and DNA fragmentation
identified. By contrast, necrosis was characterized by a pyknotic
nucleus, cytoplasmic swelling, progressive disintegration of the
cytoplasmic membranes and inflammation cell reaction. Additionally,
apoptotic cell death required energy in the form of ATP. We were
therefore able to infer that, under hypoxic microenvironments which
were unable to supply sufficient energy to cell death, LPPCN most
likely avoided consuming energy to conform to the hypoxic
microenvironment.
The hypothesis that cells perish by mechanisms other
than apoptosis has been gaining momentum (6). Technological advances have aided in
defining the role and mechanism of programmed necrosis, as well as
the role of autophagy in cell survival and disintegration (8). Autophagy is also considered to be a
potential mechanism for non-apoptotic and/or non-necrosis cell
death despite evidence from a number of species that it is a
survival strategy in times of stress (6). In this study, immunohistochemical
results show that the overexpression of LC3 and Atg4B at the
protein and mRNA levels in the LPPCN-positive group as opposed to
the LPPCN-negative group in melanoma, as well as the expression of
LC3 and Atg4B, are closely correlated. Thus, we proposed that
autophagy plays a crucial role in LPPCN. Endo G was thought to play
a role in the process of LPPCN in a study by Zhang et al
(3). We speculated whether
apoptotic and autophagic mechanisms contribute to LPPCN as certain
findings indicated in curcumin-induced K562 cell death (22). Numerous consecutive experiments are
required in order to verify this novel phenomenon.
According to Zong et al (5), chemicals such as Ca2+
chelators, ROS scavengers, PARP inhibitors, calpain inhibitors and
cathepsin inhibitors have been shown to inhibit programmed necrotic
cell death. Studies using gene knockout models in C. elegans
and mice have also been useful in showing that programmed necrotic
cell death can be prevented (5).
Determining the regulatory mechanisms for LPPCN is crucial. A
better understanding of the regulating factors involved in
programmed necrosis should allow future studies to evaluate the
role of LPPCN in solid tumors more thoroughly.
In 1971, Folkman (23) hypothesized that tumor expansion and
metastasis are dependent upon angiogenesis. Angiogenesis is a
well-orchestrated sequence of events that involves endothelial cell
migration, proliferation, degradation of tissue, new capillary
vessel (sprout) and loop formation (anastomosis), as well as the
blood flow associated with the nascent network (24). However, a crucial point that remains
to be elucidated is how a solid tumor with compact cell mass and a
high IFP (25) interior is able to
provide spacial functions to facilitate endothelial cell migration
and proliferation. The findings regarding LPPCN may clarify this
concern during angiogenesis.
Prior to vasularization, various solid tumors are
found in animals that live as tiny spheroids or ellipsoids of a few
millimeters in diameter, and which are dependent on simple
diffusion for absorption of nutrients and release of catabolites
(23). Tumors become dormant at a
diameter of only a few millimeters due to the absence of blood
vessels. However, once vascularized, these tumors are released from
the dormant phase and begin exponential growth (26). In a microenvironment without
adequate oxygen and blood supply, certain tumor cells are likely to
be controlled by regulators of PCD, such as Endo G, LC3 and Atg4B.
LPPCN is then initiated and results in a channel-shaped,
vessel-like empty space left by the dead cells. Subsequently, blood
vascular networks can be constructed via the proliferation of the
endothelial cells migrating towards the middle of the tumor
following the networks left by LPPCN. Without endothelial cell
ingression into the tumor that would allow vessel formation, the
tumor would undergo conventional necrosis. Therefore, we speculated
that LPPCN was an early stage event in tumoral
neovascularization.
This study provides a comprehensive investigation of
the associations between the occurrence of LPPCN and various
clinicopathological parameters in melanoma. The results indicated
that LPPCN has a close correlation with various clinicopathological
parameters in melanoma, including tumor thickness, histological
subtype, conventional necrosis and melanin. In this study, the
Kaplan-Meier method and log-rank test data showed that the survival
time of patients with LPPCN-positive tumors is shorter than that of
patients without LPPCN. This is similar to the result of Zhang
et al (3). Therefore, we
assume that LPPCN correlates with a poor patient outcome.
In conclusion, the data from our experiment
confirmed that LPPCN extensively exists in melanoma. The cellular
subpopulations that underwent LPPCN were previously ignored by
pathologists. LPPCN may be a special type of PCD, and an autophagic
mechanism may contribute to it. LPPCN has a close correlation with
certain histopothalogical characteristics and signifies a poor
prognosis for patients with melanoma. We speculated that LPPCN was
an early stage event in tumoral neovascularization. Although the
mechanism involved in LPPCN remains unclear and subsequent
investigations are necessary to verify this novel phenomenon, LPPCN
can be considered a novel target in the process of antiangiogenesis
treatment, leading to an evolutionary strategy for melanoma in the
future.
Acknowledgements
This study was supported by a grant from the
National Nature Science Foundation of China (Grant No.
30770828).
References
|
1
|
McKinnon JG, Yu XQ, McCarthy WH, et al:
Prognosis for patients with thin cutaneous melanoma: long-term
survival data from New South Wales Central Cancer Registry and the
Sydney Melanoma Unit. Cancer. 98:1223–1231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society. Cancer Facts And
Figures. GA: American Cancer Society; Atlanta: 2008
|
|
3
|
Zhang SW, Li M, Zhang DF, et al: Hypoxia
influences linearly patterned programed cell necrosis and tumor
blood supply patterns formation in melanoma. Lab Invest.
89:575–586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assuncao Guimaraes C and Linden R:
Programmed cell deaths. Apoptosis and alternative deathstyles. Eur
J Biochem. 271:1638–1650. 2004.PubMed/NCBI
|
|
5
|
Zong WX and Thompson CB: Necrotic death as
a cell fate. Genes Dev. 20:1–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan J, Lipinski M and Degterev A:
Diversity in the mechanisms of neuronal cell death. Neuron.
40:401–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zong WX, Ditsworth D, Bauer DE, et al:
Alkylating DNA damage stimulates a regulated form of necrotic cell
death. Genes Dev. 18:1272–1282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edinger AL and Thompson CB: Death by
design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitanaka C and Kuchino Y:
Caspase-independent programmed cell death with necrotic morphology.
Cell Death Differ. 6:508–515. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satoo K, Noda NN, Kumeta H, et al: The
structure of Atg4B-LC3 complex reveals the mechanism of LC3
processing and delipidation during autophagy. EMBO J. 28:1341–1350.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Philip EL, Burg G, David W and Sarasain A:
World Health Organization Classification of Tumours Pathology and
Genetics of Skin Tumours. IARC Press; Lyon: 2006
|
|
12
|
Bursch W, Ellinger A, Gerner C, Fröhwein U
and Schulte-Hermann R: Programmed cell death (PCD). Apoptosis,
autophagic PCD, or others? Ann NY Acad Sci. 926:1–12. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tolkovsky AM: Mitochondrial disappearance
from cells: a clue to the role of autophagy in programmed cell
death and disease? Biochimie. 84:233–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larsen KE and Sulzer D: Autophagy in
neurons: a review. Histol Histopathol. 17:897–908. 2002.PubMed/NCBI
|
|
15
|
Cuervo AM: Autophagy: in sickness and in
health. Trends Cell Biol. 14:70–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osterlind A: Epidemiology on malignant
melanoma in Europe. Acta Oncol. 31:903–908. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karim-Kos HE, de Vries E, Soerjomataram I,
Lemmens V, Siesling S and Coebergh JW: Recent trends of cancer in
Europe: a combined approach of incidence, survival and mortality
for 17 cancer sites since the 1990s. Eur J Cancer. 44:1345–1389.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yakolev AG and Faden AI: Mechanisms of
neural cell death: implications for development of neuroprotective
treatment strategies. NeuroRx. 1:5–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Proskuryakov SY, Gabai VL and
Konoplyannikov AG: Necrosis is an active and controlled form of
programmed cell death. Biochemistry (Mosc). 67:387–408. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lugovskoy AA, Degterev AI, Fahmy AF, et
al: A novel approach for characterizing protein ligand complexes:
molecular basis for specificity of small-molecule Bcl-2 inhibitors.
J Am Chem Soc. 124:1234–1240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: the significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar
|
|
22
|
Jia YL, Li J, Qin ZH and Liang ZQ:
Autophagic and apoptotic mechanisms of curcumin-induced death in
K562 cells. J Asian Nat Prod Res. 11:918–928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tannock IF and Hayashi S: The
proliferation of capillary endothelial cells. Cancer Res. 32:77–82.
1972.PubMed/NCBI
|
|
26
|
Folkman J and Hochberg M: Self-regulation
of growth in dimensions. J Exp Med. 138:745–753. 1973. View Article : Google Scholar : PubMed/NCBI
|