Introduction
Tumor hypoxia is a significant factor in tumor
physiology and treatment, as it appears to be closely associated
with tumorigenesis, metastasis and chemoresistance (1). Hypoxia is a common characteristic of
locally advanced solid tumors. Under this condition, reduced oxygen
levels (hypoxia) lead to a set of cellular adaptations, including
increased angiogenesis, erythropoiesis and a switch to glycolytic
metabolism. Mounting evidence indicates that the effect of hypoxia
on malignant progression is mediated by a series of hypoxia-induced
proteomic and genomic changes that activate angiogenesis, anaerobic
metabolism and other processes that enable tumor cells to survive
or escape their oxygen-deficient environment. The critical
regulatory gene that functions when the oxygen level in tissues is
low is the transcription factor hypoxia-inducible factor-1 (HIF-1)
(2,3). Clinical studies have suggested that
HIF-1 is a significant regulator of tumor cell adaptation to
hypoxic stress and is crucial in cervical malignant progression and
outcome (4,5). HIF-1 plays an essential role in the
maintenance of oxygen homeostasis in metazoan organisms (3). The DNA binding complex of HIF-1 is a
heterodimer comprising HIF-1α and HIF-1β subunits, both of which
are basic helix-loop-helix transcription factors (6). HIF-1α is constitutively expressed
(7), but under normoxic conditions
is hydroxylated at specific proline residues resulting in
ubiquitination through the interaction with von Hippel-Lindau
factor suppressor protein (pVHL) and proteosomal degradation
(8,9). Under hypoxic conditions, proline
hydroxylation is inhibited, preventing association with pVHL.
Subsequently, HIF-1α accumulates and associates with HIF-1β to form
a heterodimer that accumulates in the nucleus and activates a
specific set of genes by binding to hypoxic response elements in
the promoter region (10). The
HIF-1α protein complex mediates transcriptional responses to
hypoxia by binding to hypoxia response elements on specific target
genes (2), but the role of HIF-1 in
tumor growth and development remains uncertain as a number of in
vivo studies have drawn conflicting results. For example,
certain studies have shown that the loss of HIF-1 function inhibits
both angiogenesis and tumor growth (11–13),
while other studies showed impaired growth ability, but no effect
on angiogenesis (12,14–16).
The main reason for this phenomenon appears to be that HIF is not
expressed in monolayer culture cells under normal culture
circumstances. Therefore, the appropriate in vitro model
simulating the realistic situation in vivo is the most
important factor in the study of HIF functional analysis.
In this study, the three-dimensional spheroid
culture method was employed to study the possible role of HIF-1α in
the biological behavior of the cervical tumor cell line HeLa in
vitro. A vector was constructed to express antisense HIF-1α
(anti-HIF-1α-pEGFP) and transfect the latter into HeLa cells. Cell
proliferation, apoptosis and migration were compared among the
anti-HIF-1α-pEGFP-transfected (HIF-1α-blocked), pEGFP-transfected
(mock, as a plasmid-transfection control) and untransfected cells.
HIF-1α was found to play a potentially pivotal role in the
malignant phenotype of HeLa cells in vitro.
Materials and methods
Cell culture
The human cervical carcinoma HeLa cell line was
obtained from the American Type Culture Collection (ATCC, VA,
Manassas, USA) and was cultured in RPMI growth medium supplemented
with 10% fetal bovine serum (FBS). Cells were maintained at 37˚C in
a humidified atmosphere with 5% CO2. For the hypoxic
culture, cells were maintained in an incubator with 1%
O2.
Vector construction
RNA was isolated from HeLa cells using
TRIzolTM Reagent (Gibco BRL, USA) according to the
manufacturer's instructions. RNA (2 μg) was used for cDNA synthesis
by reverse transcription. The RNA samples were incubated at 70˚C
for 5 min with 0.5 μg oligo(deoxythymidine) primers in a final
volume of 10 μl and then at 37˚C for 60 min in a 25-μl reaction
volume containing 125 mmol/l deoxynucleotide triphosphate, 200
units Muloney murine leukemia virus reverse transcriptase and
Muloney murine leukemia virus RT buffer (Promega, USA). The cDNAs
obtained were amplified by using the cloning primers: 5′ CGG GAT
CCG GTG ATT TGG ATA TTG AAG ATG AC 3′ (upper) and 5′ GAA GAT CTC
ACT CAC AAC GTA ATT CAC ACA TA 3′ (lower). The PCR profile was 95˚C
for 1 min, 94˚C for 40 sec, 58˚C for 40 sec and 72˚C for 1 min for
30 cycles, followed by extension for 7 min at 72˚C. The amplified
products were purified using a PCR kit (New England Biotech, UK),
ligated with pEGFP vector (Promega) by following the instruction
manual. The recombinant plasmid was then screened by digestion and
sequencing to confirm the blocked sequences of HIF-1, and was
termed anti-HIF-1α-pEGFP.
Vector transfection and clone
selection
HeLa cells were transfected with 3 μg pEGFP (as a
blank control) or 3 μg anti-HIF-1α-pEGFP according to the protocol
provided with the Lipofectamine 2000 transfection reagent (Life
Technologies, Inc.). Briefly, 2×105 cells were plated in
6-well plates and incubated with the appropriate plasmid DNA and
Lipofectamine 2000 in serum-free medium for 5 h. Equal volumes of
media containing 20% FBS were then added. After 24 h, the media
were replaced with media containing 1 mg/ml G418. Surviving
colonies were selected after 2 weeks and maintained in 300 μg/ml
G418. Positive cell clones were selected and amplified. Changes in
HIF-1α levels were confirmed by Western blotting in the hypoxic
environment.
Spheroid culture
HeLa cells, the anti-HIF-1α-pEGFP transfected
counterpart and the control blank vector-transfected cells were
cultured to 95% confluence, seeded into agarose-coated 24-well
plates at a density of 2,000 cells/well and cultured. Each well
contained 200 μl of tissue culture medium, and the spheroids were
fed every other day by carefully aspirating 100 μl of spent medium
and replacing it with the same quantity of fresh medium.
Western blot analysis
Cells were lysed in a lysis buffer containing 50 mm
Tris, pH 7.4, 150 mm NaCl, 0.5% NP-40, 50 mm NaF, 1 mm
Na3VO4, 1 mm phenylmethylsulfonyl fluoride,
25 mg/ml leupeptin and 25 mg/ml aprotinin. The lysates were cleared
by centrifugation, and the supernatants were collected. Equal
amounts of lysate protein were used for the Western blot analyses
with the indicated antibodies. Specific signals were visualized
using the ECL chemiluminescence detection kit (Amersham, Arlington
Heights, IL, USA).
Analysis of cell proliferation and
apoptosis by flow cytometry
After trypsinization for cell detachment, the cells
were incubated in 50% FBS for 15 min to restore membrane integrity
and centrifuged for 5 min at 1,200 rpm. Detached cells were stored
via retention of the culture medium and recovered by
centrifugation. Apoptotic cells were detected by assaying the
Annexin V binding by flow cytometry (commercially available test,
provided by Boehringer Mannheim). To exclude necrotic cells, we
double-stained the cells with 5 μg/ml propidium iodide (PI) in PBS.
Cells were fixed with 75% ethanol and digested with DNase-free
RNase in PBS containing 5 μg/ml PI for DNA staining for 45 min at
37˚C. PI and forward light scattering were detected using the flow
cytometer FACSCalibur (Beckton-Dickinson) equipped with Cell Quest
software. The data were analyzed using Cell Fit software. The
experiment was repeated three times.
Spheroid invasion assays
Cell motility was assessed using the HABM-HEC model.
Multicellular spheroids were plated at 100 spheroids/well in the
upper chamber of the model. The outer chambers were filled with 0.5
ml of media containing 10% FBS. After 24 h, cells migrating to the
undersurface of the filters were counted. The same five microscopic
fields were used to count the number of cells passing to the
undersurface of each filter.
Statistical analysis
All experiments were repeated at least three times.
The Student's t-test was used to evaluate the differences between
the experimental and control groups. P<0.05 was considered to be
statistically significant.
Results
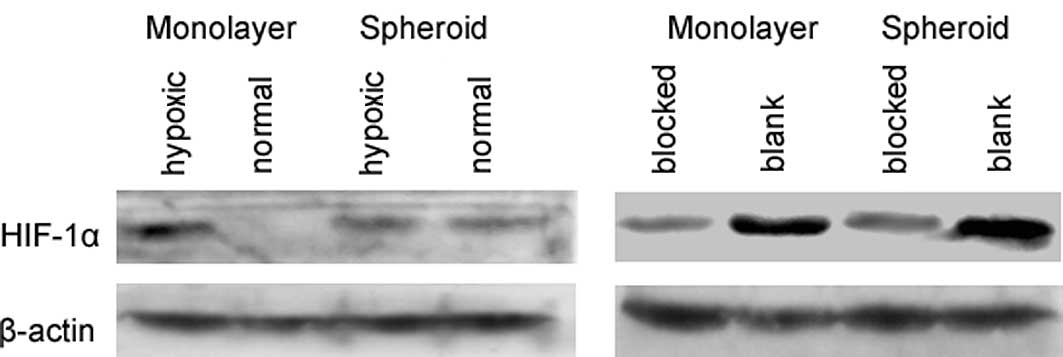
Expression of HIF-1α protein in the
monolayer-cultured HeLa cells and the multicellular spheroids
In the monolayer-cultured HeLa cells, no HIF-1α
protein expression was detected under normal culture conditions.
However, we observed HIF-1α expression under hypoxic conditions.
Nevertheless, in the multicellular tumor spheroids, HIF-1α was
expressed in hypoxic and normal cultures (Fig. 1A). A significantly decreased HIF-1α
expression was noted in the anti-HIF-1α-pEGFP-transfected (blocked)
HeLa cells under hypoxic conditions, compared to the blank pEGFP
vector-transfected cells in the monolayer culture. Similar results
were also obtained in the multicellular spheroids (Fig. 1B). These results confirm that HIF-1α
is expressed under hypoxic conditions and that the multicellular
tumor spheroid was an ideal model of hypoxia in vitro.
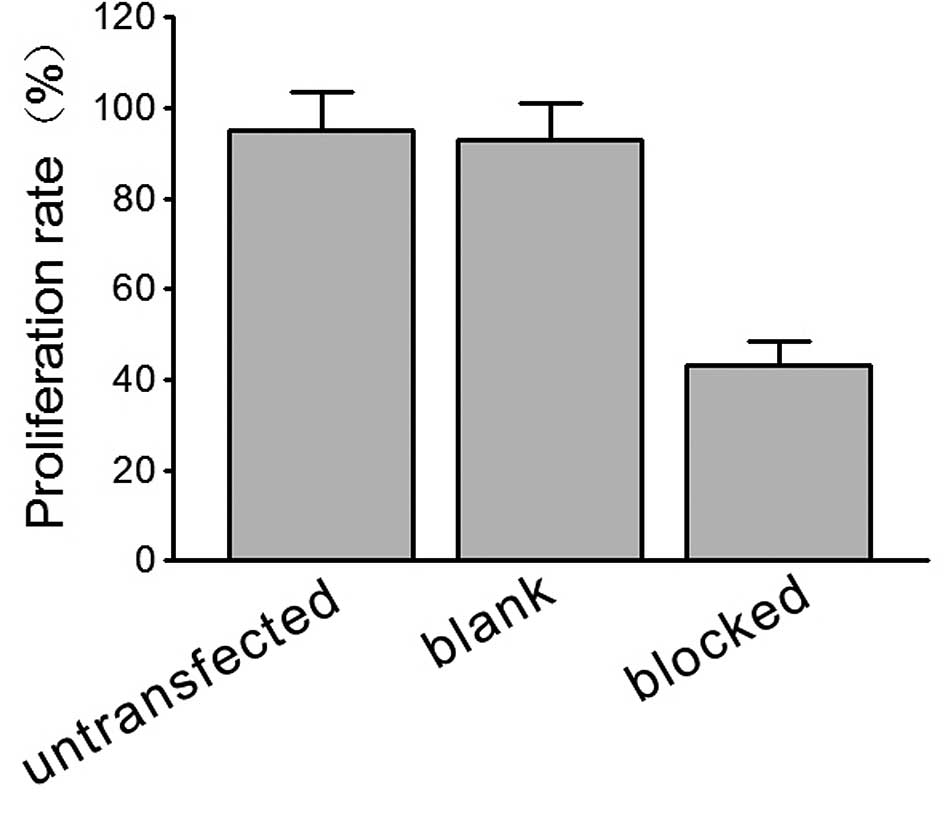
Effect of HIF-1α on multicellular
spheroid growth and apoptosis
In the HIF-1α-blocked HeLa cells, a marked decrease
in proliferation was observed in the HeLa cell spheroids when
compared to the blank pEGFP vector-transfected spheroid cells under
normal culture conditions, as assessed by flow cytometry and the
counting of cell numbers (Fig.
2).
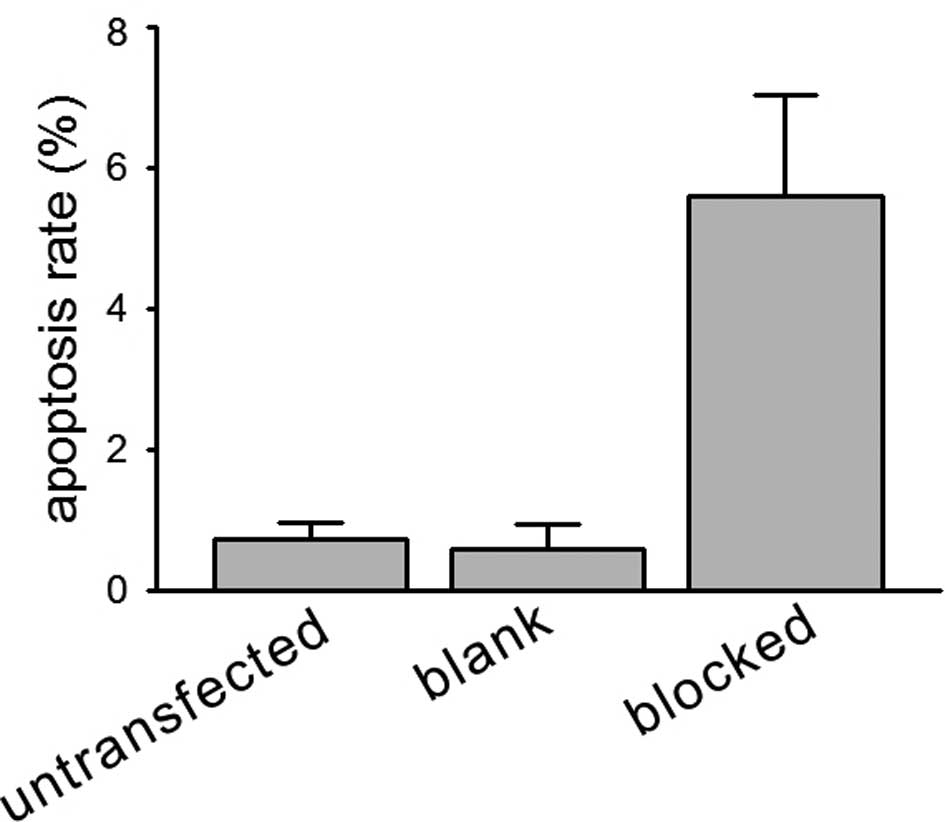
Concomitantly, when the apoptotic indices were
compared, HIF-1α-blocked HeLa cell spheroids had higher fold levels
of apoptosis than those of the blank vector-transfected cell
spheroids (5.6 vs. 0.6%) in the normal culture (Fig. 3).
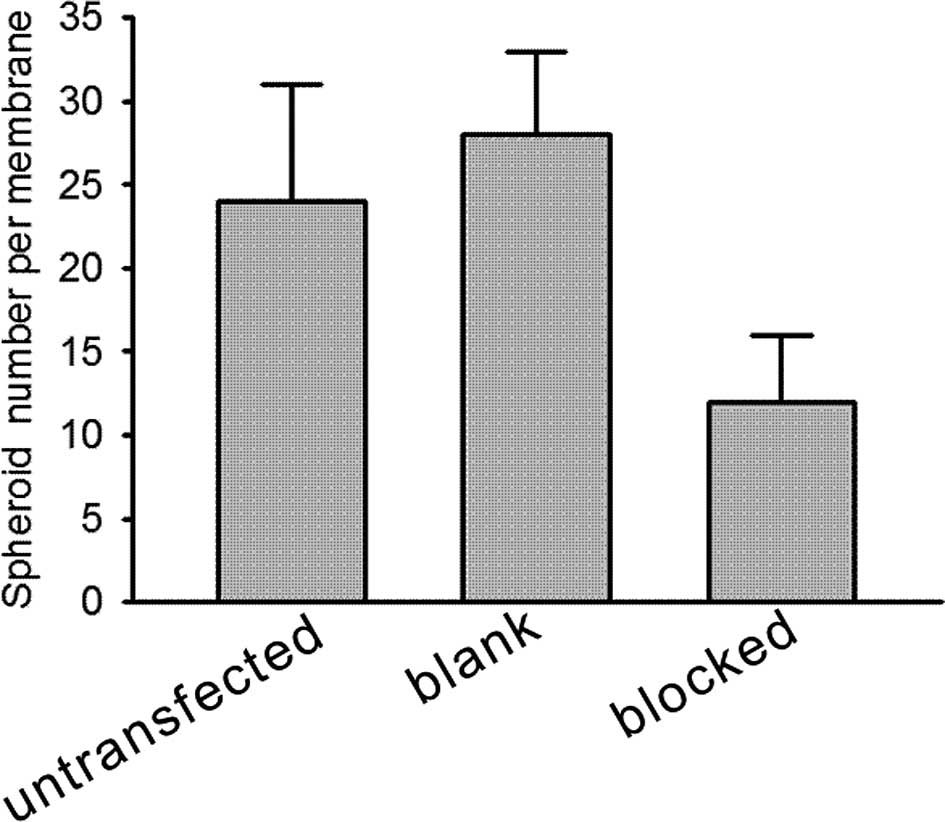
HIF-1α protein promotes the invasive
ability of HeLa cells
To evaluate the effect of HIF-1α on cell invasive
ability, an invasion assay was performed in vitro by testing
the cells invading from the top well to the lower chamber. Our data
showed that the invasion rate of the HIF-1α-blocked HeLa cells was
much lower than that of the blank vector-transfected HeLa cells in
the spheroids under normal conditions (Fig. 4, P<0.01). Findings of this study
indicate that HIF-1α protein promotes the invasive activity of
tumor cells in a three-dimensional spheroid culture in
vitro.
Discussion
Cells under hypoxic conditions express a series of
genes that allow for survival and proliferation. HIF-1α regulates
the expression of more than 30 target genes (11), most of which play roles in tumor
malignant behaviors, such as proliferation, invasion and metastasis
(17,18). HIF-1α expression is a common feature
of solid human tumors and has been reported in many different tumor
types (5,19–25).
Moreover, the overexpression of HIF-1α was found to be a poor
prognostic indicator in a variety of tumors (4,26–28).
This study focused on whether HIF-1α is involved in
the cervical tumor malignant phenotype by affecting proliferation,
apoptosis and tumor invasion of HeLa cells in vitro.
Therefore, we established interference for the inhibition of HIF-1α
in HeLa cells. Since HIF-1α rapidly undergoes ubiquitin-mediated
degradation during normoxia, we detected HIF-1α expression in
monolayer cultured cells and in multicellular spheroids,
respectively. HIF-1α was not detected in HeLa cells in the
monolayer culture in a normal culture condition. However, it
displayed a strong increase at the protein level in multicellular
spheroids under the same condition or in a monolayer under hypoxic
conditions. The main cause of this phenomenon may be that in
three-dimensional spheroid culture, oxygen diffusion was limited by
the depth of the fluid medium and the smaller surface area to the
volume compared to that in monolayer culture cells. Thus, the
spheroid culture is an ideal model for the study of the mechanism
of HIF-1α in vitro.
We transfected the antisense HIF-1α plasmid into the
human cervical cancer cell line HeLa. Western blotting showed that
the HIF-1α expression was markedly down-regulated in the cloned
antisense plasmid-transfected cells in the monolayer under a
hypoxic condition or in multicellular spheroids in a normal culture
condition.
We then compared the cell proliferation between
HIF-1α-blocked and blank plasmid-transfected cells. We found that
spheroid HIF-1α-blocked HeLa cells decreased proliferative ability
when compared to the blank plasmid-transfected cells in the normal
culture condition. The apoptotic rate of HIF-1α-blocked cells was
also significantly reduced in the spheroid cultured cells when
compared to the monolayer cultured cells. Additionally, the
HIF-1α-blocked HeLa cell spheroids had higher fold levels of
apoptosis than the normal HeLa cells (5.6% of cells in the
HIF-1α-blocked HeLa cell spheroids compared to 0.6% in the HeLa
cells). Thus, in the spheroids, HIF-1α has a dual role in the
regulation of cell division and resistance to apoptosis. Studies
have reported that hypoxia causes cell death partly by involving
the pro-apoptotic HIF-regulated factor BNip3 (26,29,30).
Nevertheless, in spheroids, overall HIF-1 has an anti-apoptotic
effect as measured by the inhibition of caspase-3 activation in the
proliferating compartment and by the final growth rate of the
spheroid (31).
When the cell cycle was analyzed, enhanced
transition from the G1 into the S phase was noted under hypoxic
conditions. However, Wang et al (32) showed that the loss of HIF-1α caused
an increased progression into the S phase and abolished
hypoxia-induced growth arrest. Goda et al found that HIF-1α
was required for cell cycle arrest during hypoxia and that BrdUrd
labeling was increased in HIF-1α null B cells in culture (33), which was also observed in HIF-1α
null chondrocytes in vivo (34). Taken together, the findings appear
to be contradictory to our observation that the overall growth rate
was slower in the anti-HIF-1α HeLa spheroids. Other studies have
shown that HIF-1α-defective tumor cell lines grow more quickly than
those with functional HIF-1α in normoxia (12). However, we observed no difference in
the growth rates of the HeLa and HIF-1 dysfunctional HeLa cell
lines in the normoxic monolayer culture.
HIF-1α protein has been found to be overexpressed in
multiple types of human cancer and distant metastatic tissues
(18). This overexpression of
HIF-1α may occur very early in carcinogenesis before histological
evidence of angiogenesis or invasion (18). Regarding cell migration, the data
presented in this study suggest a molecular mechanism by decreasing
the protein level of HIF-1α as an anti-metastatic strategy.
However, the discrepancy between the extent by which anti-HIF-1α
decreases the HIF-1α level and expression levels of its target
genes must be considered. Transactivation of target genes by HIF-1α
is cell-type specific; thus, it should not be expected that the
same battery of genes reported would be transactivated by HIF-1α in
other cell lines. Furthermore, the data presented in this study did
not distinguish between direct and indirect regulation of the
identified target genes by HIF-1α. Nevertheless, our results
indicate that antisense affects multiple steps in the complex
process of invasion by inhibiting HIF-1α.
This study therefore supports the hypothesis that
HIF-1α is a significant regulator of adaptive processes that
promote tumor cell malignant phenotypes, such as proliferation,
anti-apoptosis and invasive ability. The results of previous
pre-clinical and clinical studies have established the theory that
tumor hypoxia may promote malignant progression by a number of
mechanisms, including an increased expression of transcription
factors and gene products involved in tumor propagation and the
induction of genomic instability. Therefore, in developing
treatment strategies for cancer patients, it is reasonable to
consider approaches aimed at ameliorating tumor hypoxia in an
effort to maximize the effects of cancer therapy.
Acknowledgements
This study was supported by the National Science
Foundation of China (nos. 30700895, 30770913, 30571950, 30271358
and 30370657); Major Innovation Medicine program (2009ZX09103- 738)
and the ‘973’ Program of China (no. 2009CB521808).
References
|
1
|
Hockel M and Vaupel P: Tumor hypoxia:
definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semenza GL: Life with oxygen. Science.
318:62–64. 2007. View Article : Google Scholar
|
|
3
|
Brahimi-Horn MC and Pouyssegur J:
Harnessing the hypoxia-inducible factor in cancer and ischemic
disease. Biochem Pharmacol. 73:450–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bachtiary B, Schindl M, Potter R, et al:
Overexpression of hypoxia-inducible factor 1alpha indicates
diminished response to radiotherapy and unfavorable prognosis in
patients receiving radical radiotherapy for cervical cancer. Clin
Cancer Res. 9:2234–2240. 2003.
|
|
5
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|
|
6
|
Giordano FJ and Johnson RS: Angiogenesis:
the role of the microenvironment in flipping the switch. Curr Opin
Genet Dev. 11:35–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang LE, Arany Z, Livingston DM and Bunn
HF: Activation of hypoxia-inducible transcription factor depends
primarily upon redox-sensitive stabilization of its alpha subunit.
J Biol Chem. 271:32253–32259. 1996. View Article : Google Scholar
|
|
8
|
Maxwell PH, Wiesener MS, Chang GW, et al:
The tumour suppressor protein VHL targets hypoxia-inducible factors
for oxygen-dependent proteolysis. Nature. 399:271–275. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cockman ME, Masson N, Mole DR, et al:
Hypoxia inducible factor-alpha binding and ubiquitylation by the
von Hippel-Lindau tumor suppressor protein. J Biol Chem.
275:25733–25741. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang BH, Rue E, Wang GL, Roe R and
Semenza GL: Dimerization, DNA binding, and transactivation
properties of hypoxia-inducible factor 1. J Biol Chem.
271:17771–17778. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maxwell PH, Dachs GU, Gleadle JM, et al:
Hypoxia-inducible factor-1 modulates gene expression in solid
tumors and influences both angiogenesis and tumor growth. Proc Natl
Acad Sci USA. 94:8104–8109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hopfl G, Wenger RH, Ziegler U, et al:
Rescue of hypoxia-inducible factor-1alpha-deficient tumor growth by
wild-type cells is independent of vascular endothelial growth
factor. Cancer Res. 62:2962–2970. 2002.PubMed/NCBI
|
|
13
|
Kung AL, Wang S, Klco JM, Kaelin WG and
Livingston DM: Suppression of tumor growth through disruption of
hypoxia-inducible transcription. Nat Med. 6:1335–1340. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryan HE, Lo J and Johnson RS: HIF-1 alpha
is required for solid tumor formation and embryonic
vascularization. EMBO J. 17:3005–3015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryan HE, Poloni M, McNulty W, et al:
Hypoxia-inducible factor-1alpha is a positive factor in solid tumor
growth. Cancer Res. 60:4010–4015. 2000.PubMed/NCBI
|
|
16
|
Chen J, Zhao S, Nakada K, et al:
Dominant-negative hypoxia-inducible factor-1 alpha reduces
tumorigenicity of pancreatic cancer cells through the suppression
of glucose metabolism. Am J Pathol. 162:1283–1291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erler JT, Bennewith KL, Nicolau M, et al:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao D, Corle C, Seagroves TN and Johnson
RS: Hypoxia-inducible factor-1alpha is a key regulator of
metastasis in a transgenic model of cancer initiation and
progression. Cancer Res. 67:563–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al: Relation of hypoxia inducible factor 1 alpha and 2 alpha
in operable non-small cell lung cancer to angiogenic/molecular
profile of tumours and survival. Br J Cancer. 85:881–890. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Talks KL, Turley H, Gatter KC, et al: The
expression and distribution of the hypoxia-inducible factors
HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and
tumor-associated macrophages. Am J Pathol. 157:411–421. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blancher C, Moore JW, Talks KL, Houlbrook
S and Harris AL: Relationship of hypoxia-inducible factor
(HIF)-1alpha and HIF-2alpha expression to vascular endothelial
growth factor induction and hypoxia survival in human breast cancer
cell lines. Cancer Res. 60:7106–7113. 2000.
|
|
22
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, et al: Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis,
and chemoradiotherapy outcome of squamous cell head-and-neck
cancer. Int J Radiat Oncol Biol Phys. 53:1192–1202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koukourakis MI, Giatromanolaki A,
Skarlatos J, et al: Hypoxia inducible factor (HIF-1α and HIF-2α)
expression in early esophageal cancer and response to photodynamic
therapy and radiotherapy. Cancer Res. 61:1830–1832. 2001.
|
|
24
|
Sermeus A, Cosse JP, Crespin M, et al:
Hypoxia induces protection against etoposide-induced apoptosis:
molecular profiling of changes in gene expression and transcription
factor activity. Mol Cancer. 7:272008. View Article : Google Scholar
|
|
25
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
26
|
Peng XH, Karna P, Cao Z, Jiang BH, Zhou M
and Yang L: Cross-talk between epidermal growth factor receptor and
hypoxia-inducible factor-1alpha signal pathways increases
resistance to apoptosis by up-regulating survivin gene expression.
J Biol Chem. 281:25903–25914. 2006. View Article : Google Scholar
|
|
27
|
Shibaji T, Nagao M, Ikeda N, et al:
Prognostic significance of HIF-1 alpha overexpression in human
pancreatic cancer. Anticancer Res. 23:4721–4727. 2003.PubMed/NCBI
|
|
28
|
Bos R, van der Groep P, Greijer AE, et al:
Levels of hypoxia-inducible factor-1alpha independently predict
prognosis in patients with lymph node negative breast carcinoma.
Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramanathan M, Luo W, Csoka B, et al:
Differential regulation of HIF-1alpha isoforms in murine
macrophages by TLR4 and adenosine A(2A) receptor agonists. J Leukoc
Biol. 86:681–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng X, Ruas JL, Cao R, et al:
Cell-type-specific regulation of degradation of hypoxia-inducible
factor 1 alpha: role of subcellular compartmentalization. Mol Cell
Biol. 26:4628–4641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leek RD, Stratford I and Harris AL: The
role of hypoxia-inducible factor-1 in three-dimensional tumor
growth, apoptosis, and regulation by the insulin-signaling pathway.
Cancer Res. 65:4147–4152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang G, Reisdorph R, Clark RE Jr,
Miskimins R, Lindahl R and Miskimins WK: Cyclin dependent kinase
inhibitor p27(Kip1) is upregulated by hypoxia via an ARNT dependent
pathway. J Cell Biochem. 90:548–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goda N, Ryan HE, Khadivi B, McNulty W,
Rickert RC and Johnson RS: Hypoxia-inducible factor 1alpha is
essential for cell cycle arrest during hypoxia. Mol Cell Biol.
23:359–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schipani E, Ryan HE, Didrickson S,
Kobayashi T, Knight M and Johnson RS: Hypoxia in cartilage:
HIF-1alpha is essential for chondrocyte growth arrest and survival.
Genes Dev. 15:2865–2876. 2001.PubMed/NCBI
|