Introduction
Renal cell carcinoma (RCC) is a common malignancy
(1) and >35% of patients dying
with RCC have skeletal metastases (2). These skeletal metastases cause
devastating complications including intractable bone pain,
pathological fractures, and hypercalcemia (2–4). Thus,
bone metastasis in RCC is one of the major causes of increased
morbidity and eventual mortality and a therapeutic target in RCC
patients. Clinically their care is challenging for the orthopaedic
surgeon because of the highly lytic, vascular nature of the tumors
(5). In order to affect any
improvement in survival from this inherently chemo- and
radio-resistant disease (6–8), a better understanding of the molecular
mechanisms involved in RCC growth in bone is required.
Generally, it has been recognized that cytokine
secretion by RCC cells into the local microenvironment modulates
host immune response, tumor growth and metastasis (9). RCC are highly vascular tumors, which
overproduce angiogenic factors such as vascular endothelial growth
factor (VEGF) (9,10). Most cancer cells produce
transforming growth factor-β (TGF-β) and a high level of TGF-β
secretion is thought to increase the malignant potential of the
tumor. Increased plasma levels of TGF-β were described as a tumor
marker and prognostic factor in RCC (11). The pro-inflammatory cytokines
interleukin-6 (IL-6) and interleukin-8 (IL-8) were also
predominantly produced by RCC (12–15).
IL-6 in particular is an autocrine growth factor for RCC and seems
to be tumor protecting against cytotoxic tumor-infiltrating
lymphocytes (11,16–17).
The importance of the TGF-α/EGF-R signaling pathway in RCC bone
metastasis has been elucidated by Weber et al (9).
We reasoned these phenotypic changes might be
responsible for the mechanism of preferential metastasis of RCC to
bone. To examine this hypothesis, we established bone-seeking
(ACHN-BO) clones of the human RCC cell line ACHN by repeated
sequential passages in nude mice and in vitro of metastatic
cells obtained from bone. These clones were examined for
distinguishing biological characteristics and compared with the
ACHN parental cells (ACHN-P) in vivo and in vitro. We
found that the bone-seeking clone of ACHN cells has several
different biological properties from those of the ACHN parental
cells. Our data suggest that these phenotypic changes may allow RCC
cells to develop and accelerate bone metastases.
Material and methods
Cell cultures
ACHN cells, a human renal cancer line, were from
China Center of Type Culture Collection (CCTCC), Wuhan (China).
ACHN cells were maintained in DMEM/F12 (1:1) medium supplemented
with 10% heat-inactivated fetal bovine serum. All cell lines were
incubated at 37°C in a humidified atmosphere of 5% CO2
in air.
Animals
For selection in vivo, 6- to 8-week-old
female nude mice (BALB/c-nu) were obtained from the Experimental
Animal Center of Huazhong University of Science and Technology
(Wuhan, China). All animals in our study were housed under
pathogen-free conditions and maintained according to the guidelines
of the Committee on Animals of Huazhong University of Science and
Technology.
In vivo selection of ACHN-BO
Female nude mice (BALB/c-nu, 5-week-old) were
anesthetized with 5% Rompun/10% Ketavet in 0.9% NaCl/10 g body
weight before injection. We injected 0.1 ml of tumor cells
(5×105 cells) into the lateral tail vein of anesthetized
BALB/c-nu mice using an insulin syringe as described by Otsuka
et al (18). Mice with
osteolytic lesions in the hind limbs caused by ACHN-P line detected
by radiography (osteolytic lesions in hind limbs were monitored
every 7 days starting from day 28) were sacrificed and the affected
hind limb was separated from the body. Skin and muscles were
removed and the hind limb was mashed with a piston through a sieve
in a petridish containing 10 ml of 0.9% NaCl medium. Tumor cell
suspension was collected from the petridish into a T-75 flask. The
next day, cells were washed twice with PBS to wash off mouse bone
marrow cells that did not attach to the plate. After 3 weeks, a
population of human cancer cells was obtained. These subpopulations
(ACHN-BO1) were again inoculated into the lateral tail
vein of anesthetized female BALB/c-nu. Following four passages of
in vivo selection a highly bone metastatic cell line,
ACHN-BO4, was obtained. The mice were sacrificed when
they lost >10% of their body weight.
Radiographic analysis of osteolytic
lesions
Osteolytic lesions in hind limbs were monitored
every 7 days starting from day 28 using a Digital Faxitron small
animal X-ray cabinet (Faxitron X-Ray, Wheeling, IL, USA) at 35 kV
tube voltage, 0.3 mA current and 4 sec exposure time. Lesion area
in hind limbs was quantified using image analysis software
(Analysis Software Imaging System GmbH, Germany).
Bone histology
For histological examination, hind limbs were
removed from mice after being sacrificed, fixed in 4% buffered
formalin (Merck & Co. Inc., USA), decalcified in 10% EDTA
(Sigma-Aldrich, Munich, Germany), dehydrated and embedded in
paraffin. Tissue sections of 4 μm thick were cut and stained with
hematoxylin and eosin (H&E) using standard protocols.
Cell growth assay
To assess possible impacts of in vivo
selection on ACHN cells malignant biological behaviors. The cells
were trypsinized, counted, plated and assayed for cell
proliferation, migration and invasion in triplicate experiments by
our research group (19). For
proliferation assays, cells in the log-growth phase were harvested,
suspended at a density of ~1×104 cells/μl and seeded
into triplicate wells of 96-well plates at 100 μl/well. After 24 h
of culture, 50 μl of 1X MTT was added to each well. The plates were
then incubated at 37°C for 4 h. After removal of the supernatants,
the precipitates were solubilized in DMSO (150 μl/well) and shaken
for 20 min. The absorbances of the wells were measured at a
wavelength of 450 nm.
Sterile polycarbonate membrane filters (Corning
Inc., New York, NY) with 8-μm pores were coated with 6 μg/ml
Matrigel gelatin (BD Co., Franklin Lakes, NJ). The filters were
hydrated with 200 μl of serum-free medium at 37°C for 60 min before
use. Cells (5×104) were seeded into the top chambers of
6-well plates, and the lower chambers were filled with 500 μl of
DMEM/F12 (1:1) medium containing 10% FBS for 24 h. The filters were
fixed with 95% alcohol and stained with hematoxylin for 15 min. The
cells on the upper surface were gently removed with a cotton swab
and the cells on the lower surface of the filters were quantified
under a microscope at a magnification of ×400.
For invasion assays, Matrigel-coated sterile 8-μm
polyethylene filters were rehydrated as described above. The lower
chambers of 24-well plates were filled with 1 ml of DMEM/F12 (1:1)
medium containing 10 μg of fibronectin as a chemoattractant and 0.5
ml of serum-free DMEM/F12 (1:1) containing 5×104
ACHN-P(BO) cells was added to the upper chambers for 48 h.
Subsequently, the cells were stained with hematoxylin and the
numbers of cells that had invaded the filters were recorded.
Cell apoptosis assay
Cells were harvested using trypsin/EDTA in
phosphate-buffered saline (PBS; Biochrom), counted, and collected
by centrifugation in PBS. Phosphatidylserine (PS) exposure on the
outer leaflet of the plasma membrane was detected using the
fluorescent dye Annexin V-FITC Apoptosis Detection kit according to
the manufacturer’s instructions. In brief, cells were rinsed with
ice-cold PBS and then resuspended in 200 μl of binding buffer.
Annexin V stock solution (10 μl) was added to the cells and
incubated for 30 min at 4°C. The cells were then further incubated
with 5 μl propidium iodide (PI) and were immediately analyzed on a
FACSC-LSR (Becton Dickinson) equipped with CellQuest (Becton
Dickinson) software; ~5×105 cells were collected in each
of the samples and 5×104 cells were analyzed.
ELISAs to measure production of growth
factors and cytokines
The production and secretion of TGF-α, VEGF, bFGF,
and IL-6 (Uscn Life Science Inc., Wuhan, China) by the ACHN-P cell
line and the highly metastatic subpopulation (ACHN-BO4)
were determined in cell culture supernatants after 48 h of culture
using a quantitative sandwich enzyme immunoassay technique (ELISA)
according to manufacturer’s instructions. Triplicates were
measured.
Data analysis
Data are expressed as the means±standard deviations.
Statistical analyses were performed using Student’s t-test.
Differences were considered to be statistically significant when
the P-value was <0.05.
Results
The establishment of ACHN-BO cell
line
In the first cycle of inoculation, 25% (3/12) of the
transplanted mice with ACHN-P cells inoculated through the lateral
tail vein had tumor clones in bone (the time point was 6 weeks),
the remaining 75% (9/12) of the transplanted mice were observed for
4 months and no bone metastasis and no tumor-related death were
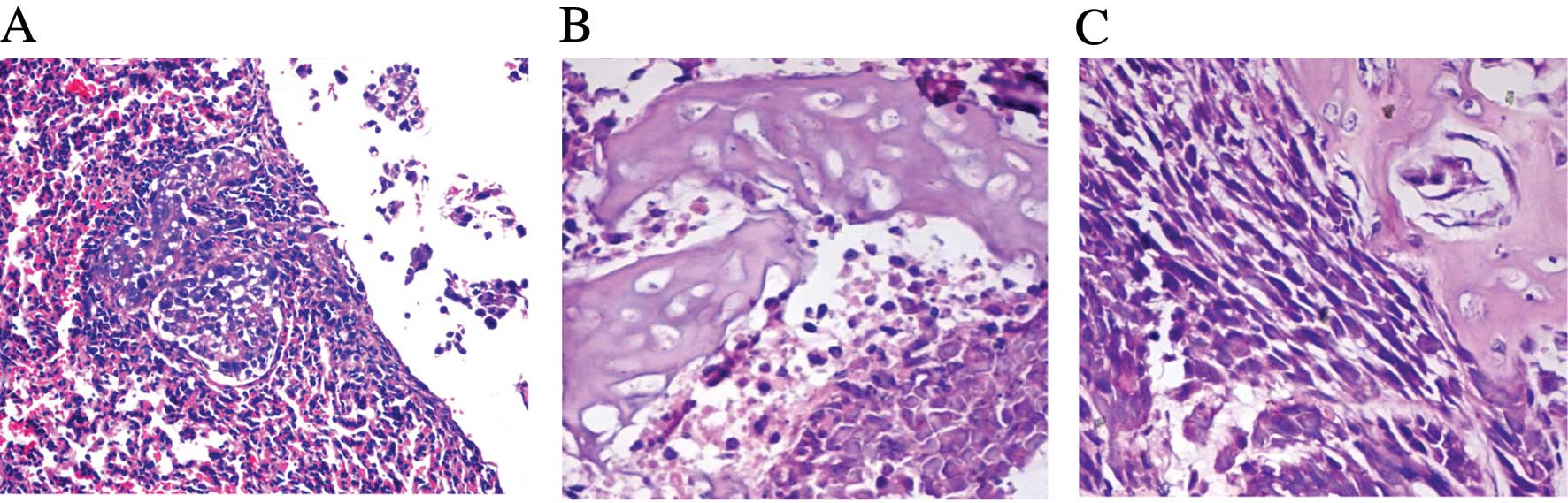
found. The lung metastasis rates were 100% (11/11) (Fig. 1A). The bone metastasis tumor clones
were dissected and used for primary cell culture; these bone
metastasis tumor cells were confirmed as ACHN-BO1 cells with
histological analysis (Fig. 1B) and
chromosomal karyotype analysis (data not shown). In the second
cycle of inoculation, ~42% (5/12) of the transplanted mice had bone
metastasis. Starting with the third cycle, higher percentage of the
bone metastasis in the transplanted mice was observed. All of the
transplanted mice had bone metastasis after the fifth inoculation
(12/12). Most of the bone metastasis sites were spine and the four
limbs. In addition, the tumor cells can also translocate to adrenal
glands, sub-mandibular glands, lung and lymph nodes, etc. We named
the sixth cycled bone metastasis cells as ACHN-BO6 with
histological analysis confirmation (Fig. 1C).
Symptoms of the RCC bone metastasis model
resemble clinical setting
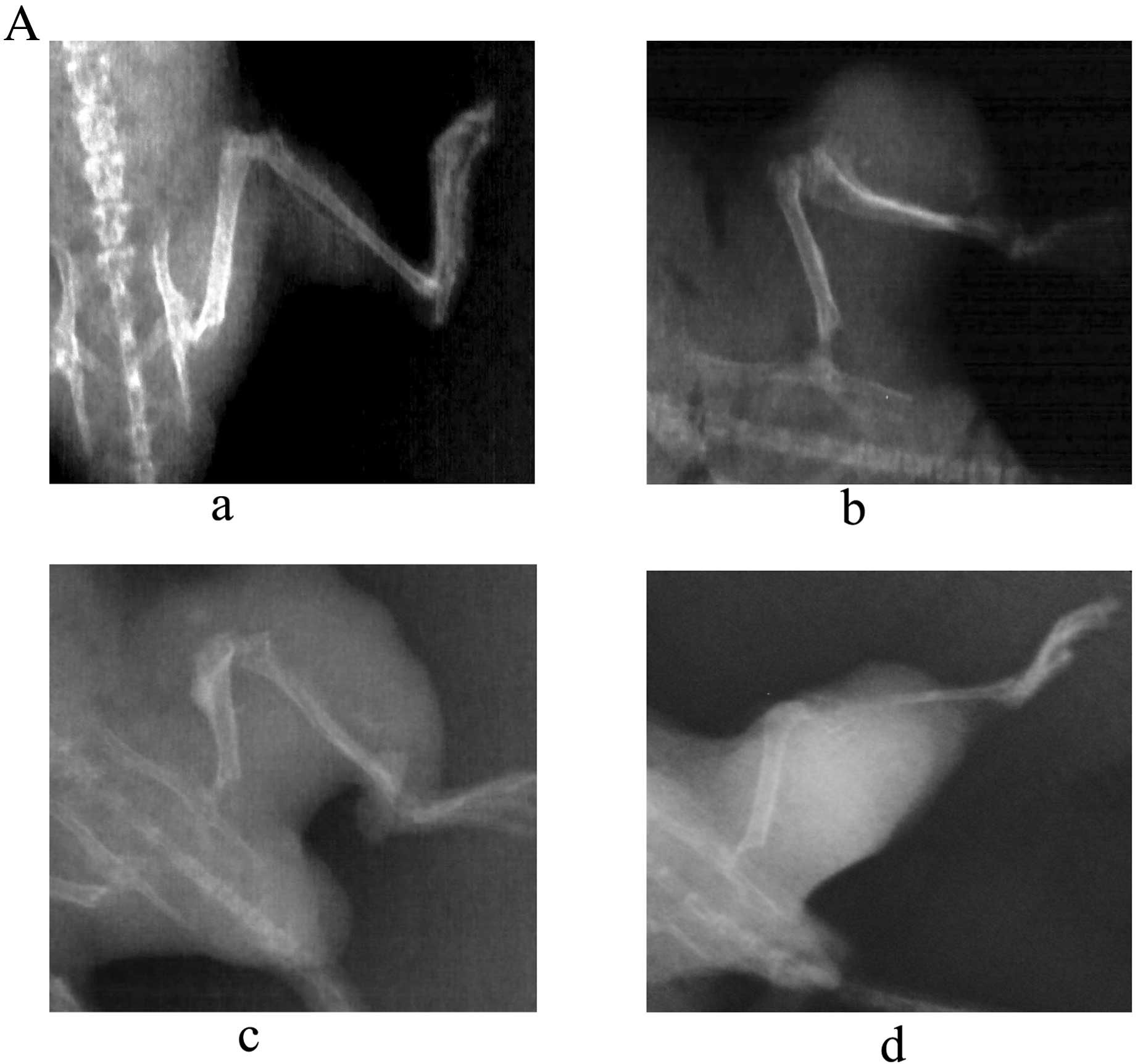
After ACHN-BO5 cell inoculation, all
animals developed aggressive osteolytic bone metastasis as
monitored by radiography with the endpoint at a mean of 6 weeks.
First lesions were observed by radiography 4 weeks after tumor cell
inoculation. Fig. 2A shows the time
course of the development of osteolytic lesions monitored weekly
from day 28 until day 56 by radiography. The determination of mean
lesion size from the X-ray images indicated a steady increase of
the osteolytic lesion area (Fig.
2B). The observed extensive bone destruction was similar to
those noted in clinical settings and mainly occurred in tibia,
femur, fibula, forelimbs, spine and sometimes in pelvis.
The proliferation, migration and invasion
of ACHN cells in vitro
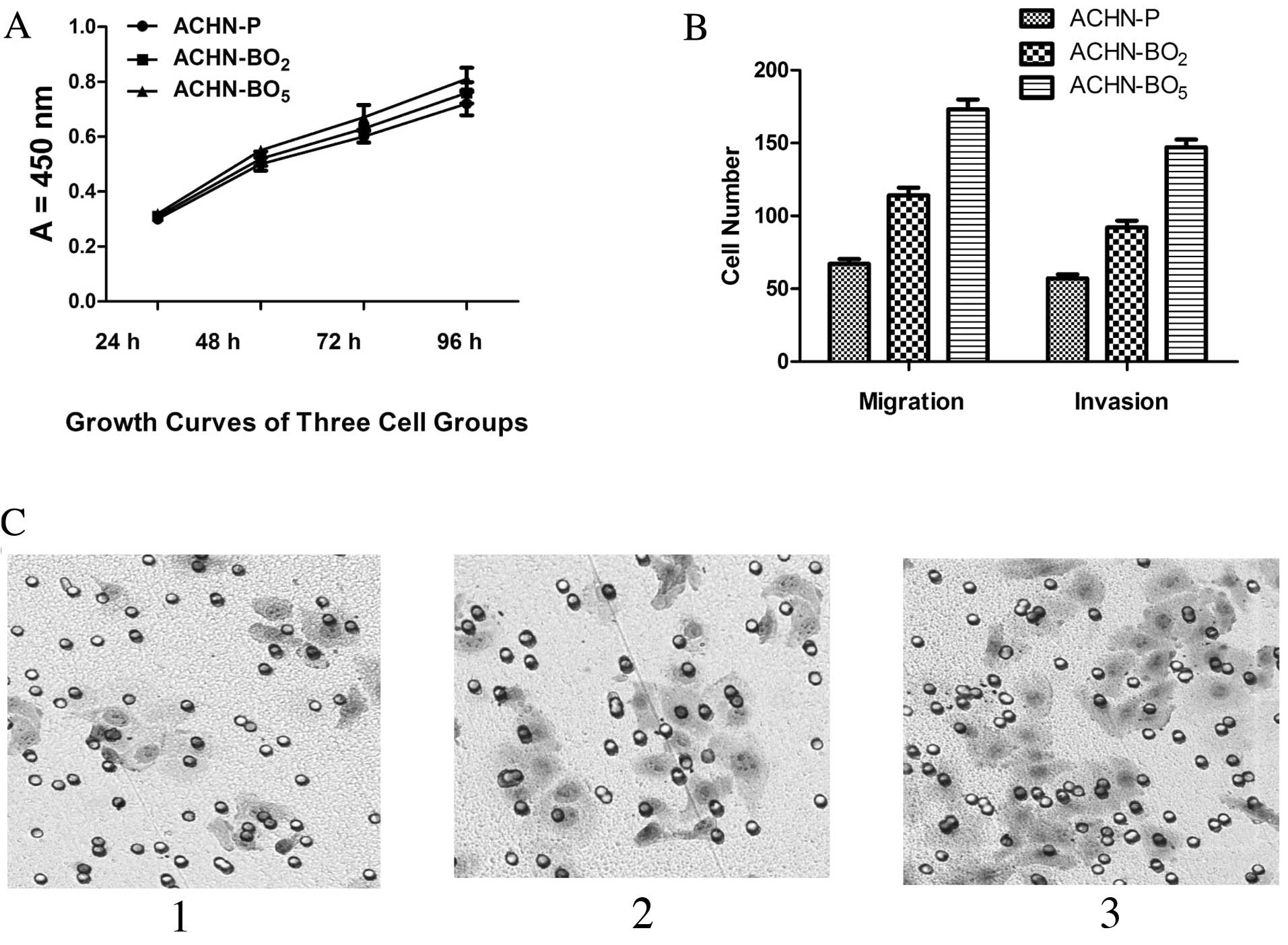
As shown in Fig. 3A,
compared with ACHN-P cell lines; the ACHN-BO exhibited increased
cell proliferation by 2.17% (ACHN-BO2) and 0.81%
(ACHN-BO5) for the first 48 h, respectively (P<0.05).
After 96 h, the proliferation was significantly increased by 8.97
and 4.72%, respectively (P<0.05). No difference in tumor cell
proliferation and in vivo tumor growth between the cell
lines could be observed (Fig. 3A).
We further evaluated whether the selected cell lines altered the
motility of ACHN cells across Transwell polycarbonate membranes. As
shown in Fig. 3B and C, compared
with ACHN-Ps, the cell migration and invasiveness of ACHN-BO were
reduced by 46.71% (ACHN-BO2) and 54.24%
(ACHN-BO5), respectively (P<0.05). Taken together,
these data suggest that the ACHN-BO cell line significantly
inreased the proliferation, migration and invasion of ACHN cells
in vitro.
Effects of in vivo selection on ACHN
apoptosis
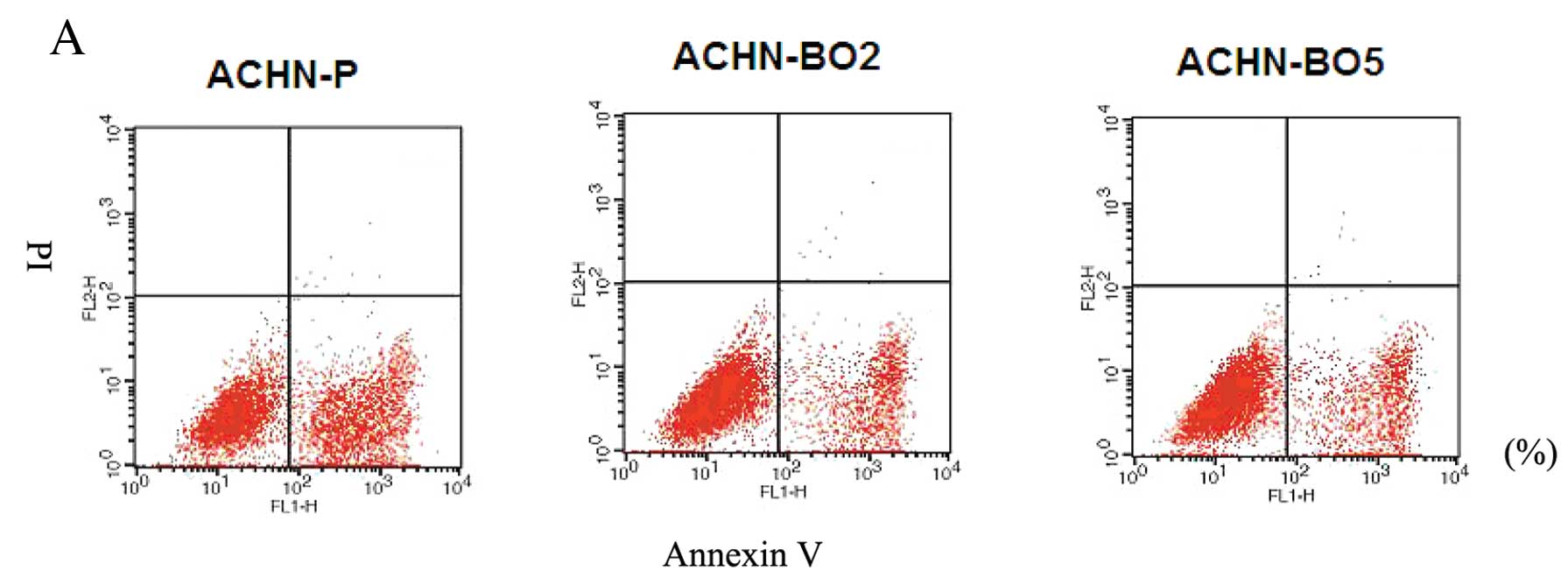
Cell apoptosis was measured using fluorescent dye
Annexin V-FITC, which binds to phosphatidylserine residues that are
redistributed from the inner to the outer leaflet of the cell
membrane as an early event in apoptosis. After loss of membrane
integrity, PI can enter the cell and intercalate into DNA. Annexin
V+/PI+ cells were considered necrotic cells,
whereas Annexin V+/PI− cells were counted as
apoptotic cells. Fig. 4 shows the
percentages of Annexin V-stained and PI-stained cells of ACHN cell
lines. The percentage of Annexin V+/PI− cells
was reduced to 10.4% compared with the static control value of
13.1% after 24 h and was reduced to 9.7% compared with the static
control value of 12.3% after 48 h. With a longer incubation (96 h),
the population of Annexin V+/PI+ cells was
much smaller than that of the static control (3.5 vs. 12.7%). These
data suggest that the ACHN- BO cell lines had a reduced cell
apoptosis compared to ACHN-P cell lines.
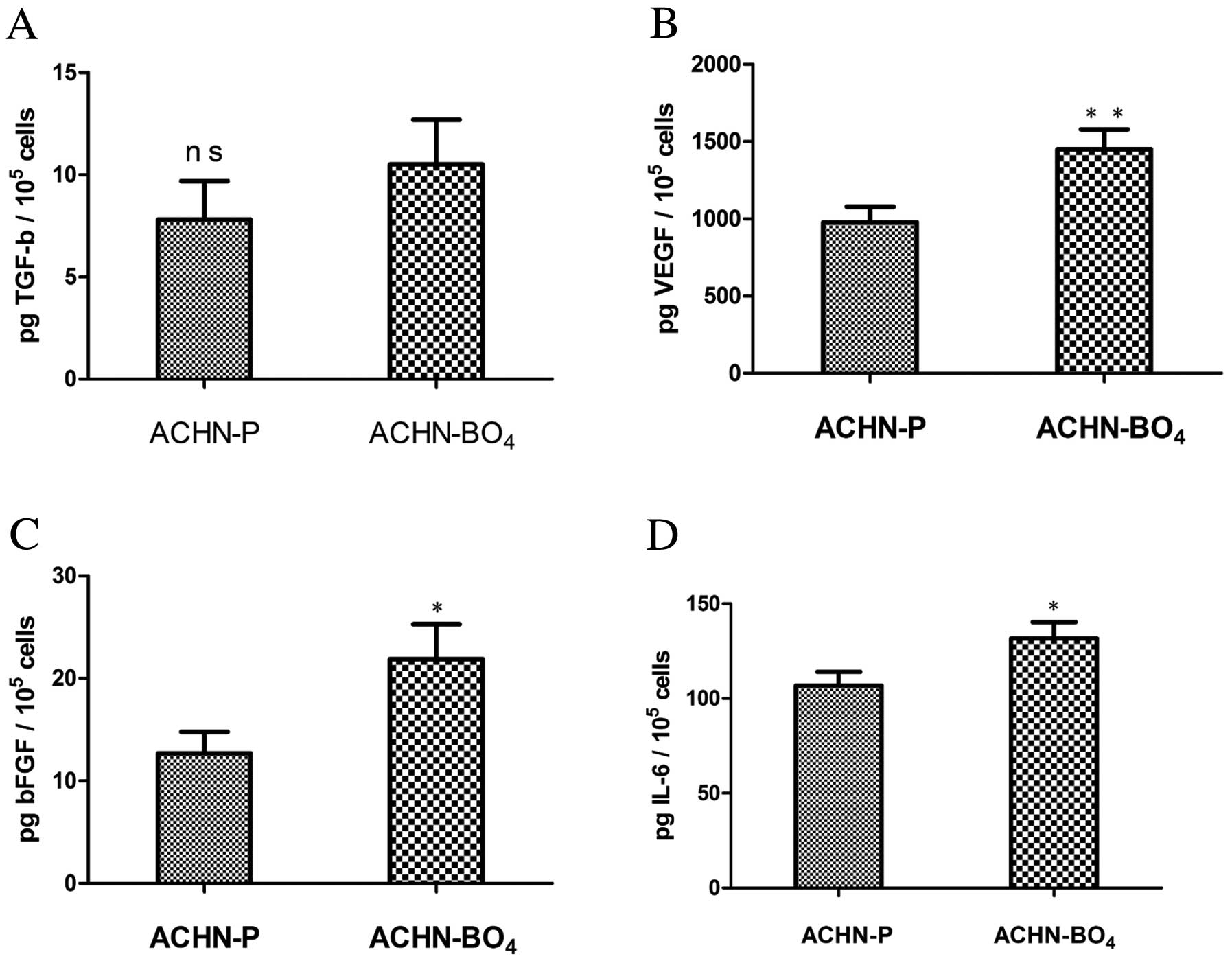
The expression of various growth factors
and cytokines
The subpopulation with high metastatic potential,
ACHN-BO4, showed higher or equivalent expression of all
analyzed growth factors and cytokines compared with the parental
cell line (Fig. 5). The secretion
of TGF-α was on the same level in the ACHN-BO4 cell line
(10.5±2.2 pg TGF-α/105 cells) as in the parental cell
line (7.8±1.9 pg TGF-α/105 cells, P=0.148) (Fig. 5A). Pro-angiogenic factors, such as
VEGF and bFGF, were significantly induced in the highly metastatic
ACHN-BO4 in vivo selected cell line compared to
the parental cell line. VEGF levels were significantly higher in
the ACHN-BO4 cell line, with a mean of 1450±129 pg
VEGF/105 cells compared with 978±101 pg
VEGF/105 cells in the parental cell line group
(P=0.009). bFGF secretion was ~2-fold higher in the highly
metastatic cell line with 21.9±3.4 pg bFGF/105 cells
compared with 12.7±2.1 pg bFGF/105 cells in the parental
cell line (P=0.026) (Fig. 5B and
C). Similarly, the level of IL-6 was also significantly
elevated in the highly metastatic cell line (131.7±8.6 pg
IL-6/105 cells) compared to parental cell line
(106.8±7.2 pg IL-6/105 cells; P=0.019) (Fig. 5D).
Discussion
In the present study, we validated a model of human
RCC bone metastasis as clinically relevant. It rapidly forms
growing osteolytic bone metastases after intravenous injection to
nude mice and displays distinguishing biological characteristics.
We showed that the ACHN-BO cell line has unique properties through
the in vivo body imaging and subpopulation cell line culture
in vitro.
Animal models of metastasis have supported drug
development and have been useful for identification of metastasis
suppressor and promoter genes as novel targets for therapies
(20,21). After tail vein injection of ACHN
cells, the development of osteolytic lesions were observed after 4
weeks. This nude mouse model is therefore very robust and properly
reflects the bone metastatic process observed in the clinic. Tumor
cell injection into the left heart ventricle is the standard
technique used to induce bone metastasis (22). Intratibial injection of tumor cells
is also a challenging procedures to induce bone metastasis
(23). Our model requires a more
straightforward route of injection. These cells are inoculated
intratibially and develop osteolytic lesions similar to those in
patients.
TGF-β is among the most abundant growth factor
stored in bone (24–26). TGF-β is released continually into
the bone marrow cavity in active form as a consequence of bone
resorption during physiological bone remodeling (27). Therefore, it is likely that the
growth of breast cancer cells metastasized in bone are under the
influence of bone derived TGF-β. TGF-β generally is known as a
tumor suppressor and inhibits the growth of most cancer cells in
culture (28–30). In this study, we have shown that the
anchorage-independent growth of the ACHN parental cells and the
bone-seeking clone is inhibited significantly by TGF-β. In
contrast, TGF-β failed to suppress the anchorage-independent growth
of the bone-seeking clone, suggesting that the bone-seeking clone
is resistant to the growth inhibitory effect of TGF-β. Our data
suggest that this resistance is unlikely because of changes in the
expression and activation of TGF-β signaling pathways such as type
I and type II receptors, Smad2, Smad3, Smad6, and Sma7. It is
possible that other mechanisms that have been shown recently to
cause the resistance of cancer cells to the growth inhibitory
effect of TGF-β [41–44] play a role (31–33).
As hypervascularity is associated with RCC bone
metastasis, we analyzed the pro-angiogenic factors VEGF and bFGF
which are reported to be crucial for angiogenesis, proliferation,
survival and spread of cancer cells (34). We were able to demonstrate that VEGF
and bFGF are induced in the highly metastatic ACHN in vivo
selected subpopulation compared to the parental ACHN cell line. As
reported, the induction of pro-angiogenic factors in the majority
of RCC cases is due to the inactivation of the von Hippel-Lindau
(VHL) tumor suppressor gene, which is also known to be missing in
ACHN cells. The loss of VHL leads to stabilization of
hypoxia-inducible factor-1α (HIF-1α) which in turn results in the
expression of many proangiogenic factors such as VEGF (35). VEGF is the most important angiogenic
cytokine and its overexpression contributes to the typical
hypervascular histology of clear cell RCC (36).
Elevated levels of IL-6, an autocrine tumor growth
factor produced by RCC cells, correlated with a poor outcome in
patients with metastatic RCC (37).
Serum levels of IL-6 were detectable in the majority of patients
with metastatic renal cell carcinoma and showed a significant
correlation to progression-free survival and overall survival
(38).
Taken together, the characterization of the ACHN
bone metastasis model and the consequent in vivo selection
approach are the basis for the identification of factors implicated
in RCC bone metastasis and provide a possibility to evaluate and
improve promising drug candidates. Characteristics of the
bone-seeking clones in vivo and in vitro compared
with the ACHN parental cells are distinguish. Our results suggest
that these capacities are at least necessary for RCC to develop and
accelerate bone metastases. However, it should be noted that these
capacities are not specifically attributable to bone metastasis of
breast cancer and that additional yet unknown properties are likely
involved. In this context, the bone-seeking clone we described
herein should provide a useful model for identification of novel
genes or molecules that are responsible for bone metastasis in
RCC.
References
|
1
|
Figlin RA: Renal cell carcinoma:
management of advanced disease. J Urol. 161:381–387. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zekri J, Ahmed N, Coleman RE and Hancock
BW: The skeletal metastatic complications of renal cell carcinoma.
Int J Oncol. 19:379–382. 2001.PubMed/NCBI
|
|
3
|
Jemal A, Tiwari RC, Murray T, et al:
Cancer statistics, 2004. CA Cancer J Clin. 54:8–29. 2004.
View Article : Google Scholar
|
|
4
|
Galasko CS: Bone metastases studied in
experimental animals. Clin Orthop Relat Res. 1981:269–285.
1981.
|
|
5
|
Durr HR, Maier M, Pfahler M, Baur A and
Refior HJ: Surgical treatment of osseous metastases in patients
with renal cell carcinoma. Clin Orthop Relat Res. 283–290.
1999.PubMed/NCBI
|
|
6
|
Iwai A, Fujii Y, Kawakami S, et al:
Down-regulation of vascular endothelial growth factor in renal cell
carcinoma cells by glucocorticoids. Mol Cell Endocrinol. 226:11–17.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi A, Sasaki H, Kim SJ, et al:
Markedly increased amounts of messenger RNAs for vascular
endothelial growth factor and placenta growth factor in renal cell
carcinoma associated with angiogenesis. Cancer Res. 54:4233–4237.
1994.
|
|
8
|
Motzer RJ and Russo P: Systemic therapy
for renal cell carcinoma. J Urol. 163:408–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber K, Doucet M and Kominsky S: Renal
cell carcinoma bone metastasis - elucidating the molecular targets.
Cancer Metastasis Rev. 26:691–704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi N, MacDonald BR, Hon J, et al:
Recombinant human transforming growth factor-alpha stimulates the
formation of osteoclast-like cells in long-term human marrow
cultures. J Clin Invest. 78:894–898. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kominsky SL, Doucet M, Brady K and Weber
KL: TGF-beta promotes the establishment of renal cell carcinoma
bone metastasis. J Bone Miner Res. 22:37–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mydlo JH, Michaeli J, Cordon-Cardo C,
Goldenberg AS, Heston WD and Fair WR: Expression of transforming
growth factor alpha and epidermal growth factor receptor messenger
RNA in neoplastic and nonneoplastic human kidney tissue. Cancer
Res. 49:3407–3411. 1989.PubMed/NCBI
|
|
13
|
Aebersold DM, Froehlich SC, Jonczy M, et
al: Expression of transforming growth factor-alpha, epidermal
growth factor receptor and platelet-derived growth factors A and B
in oropharyngeal cancers treated by curative radiation therapy.
Radiother Oncol. 63:275–283. 2002. View Article : Google Scholar
|
|
14
|
Pan J, Mestas J, Burdick MD, et al:
Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell
carcinoma metastasis. Mol Cancer. 5:562006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosol TJ, Tannehill-Gregg SH, LeRoy BE,
Mandl S and Contag CH: Animal models of bone metastasis. Cancer.
97:748–757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arguello F, Baggs RB and Frantz CN: A
murine model of experimental metastasis to bone and bone marrow.
Cancer Res. 48:6876–6881. 1988.PubMed/NCBI
|
|
17
|
Yoneda T, Sasaki A, Dunstan C, et al:
Inhibition of osteolytic bone metastasis of breast cancer by
combined treatment with the bisphosphonate ibandronate and tissue
inhibitor of the matrix metalloproteinase-2. J Clin Invest.
99:2509–2517. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Otsuka S, Hanibuchi M, Ikuta K, et al: A
bone metastasis model with osteolytic and osteoblastic properties
of human lung cancer ACC-LC-319/bone2 in natural killer
cell-depleted severe combined immunodeficient mice. Oncol Res.
17:581–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Chen A, Guo F and Yuan L: A
short-hairpin RNA targeting osteopontin downregulates MMP-2 and
MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett.
295:27–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelland LR: Of mice and men: values and
liabilities of the athymic nude mouse model in anticancer drug
development. Eur J Cancer. 40:827–836. 2004.PubMed/NCBI
|
|
21
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hawk ET, Umar A, Lubet RA, Kopelovich L
and Viner JL: Can animal models help us select specific compounds
for cancer prevention trials? Recent Results Cancer Res. 166:71–87.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaw TJ, Senterman MK, Dawson K, Crane CA
and Vanderhyden BC: Characterization of intraperitoneal,
orthotopic, and metastatic xenograft models of human ovarian
cancer. Mol Ther. 10:1032–1042. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hauschka PV, Mavrakos AE, Iafrati MD,
Doleman SE and Klagsbrun M: Growth factors in bone matrix.
Isolation of multiple types by affinity chromatography on
heparin-Sepharose. J Biol Chem. 261:12665–12674. 1986.PubMed/NCBI
|
|
25
|
Pfeilschifter J and Mundy GR: Modulation
of type beta transforming growth factor activity in bone cultures
by osteotropic hormones. Proc Natl Acad Sci USA. 84:2024–2028.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kretzschmar M, Doody J, Timokhina I and
Massague J: A mechanism of repression of TGFbeta/Smad signaling by
oncogenic Ras. Genes Dev. 13:804–816. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo K, Stroschein SL, Wang W, et al: The
Ski oncoprotein interacts with the Smad proteins to repress TGFbeta
signaling. Genes Dev. 13:2196–2206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Onichtchouk D, Chen YG, Dosch R, et al:
Silencing of TGF-beta signalling by the pseudoreceptor BAMBI.
Nature. 401:480–485. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stroschein SL, Wang W, Zhou S, Zhou Q and
Luo K: Negative feedback regulation of TGF-beta signaling by the
SnoN oncoprotein. Science. 286:771–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: a critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rini BI and Small EJ: Biology and clinical
development of vascular endothelial growth factor-targeted therapy
in renal cell carcinoma. J Clin Oncol. 23:1028–1043. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gorospe M, Egan JM, Zbar B, et al:
Protective function of von Hippel-Lindau protein against impaired
protein processing in renal carcinoma cells. Mol Cell Biol.
19:1289–1300. 1999.PubMed/NCBI
|
|
33
|
Semenza G: Signal transduction to
hypoxia-inducible factor 1. Biochem Pharmacol. 64:993–998. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iguchi H, Yokota M, Fukutomi M, et al: A
possible role of VEGF in osteolytic bone metastasis of
hepatocellular carcinoma. J Exp Clin Cancer Res. 21:309–313.
2002.PubMed/NCBI
|
|
35
|
Yang Q, McHugh KP, Patntirapong S, Gu X,
Wunderlich L and Hauschka PV: VEGF enhancement of osteoclast
survival and bone resorption involves VEGF receptor-2 signaling and
beta3-integrin. Matrix Biol. 27:589–599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hurley MM, Lee SK, Raisz LG, Bernecker P
and Lorenzo J: Basic fibroblast growth factor induces osteoclast
formation in murine bone marrow cultures. Bone. 22:309–316. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blay JY, Negrier S, Combaret V, et al:
Serum level of interleukin 6 as a prognosis factor in metastatic
renal cell carcinoma. Cancer Res. 52:3317–3322. 1992.PubMed/NCBI
|
|
38
|
Negrier S, Perol D, Menetrier-Caux C, et
al: Interleukin-6, interleukin-10, and vascular endothelial growth
factor in metastatic renal cell carcinoma: prognostic value of
interleukin-6 - from the Groupe Francais d’Immunotherapie. J Clin
Oncol. 22:2371–2378. 2004.PubMed/NCBI
|