Introduction
Virotherapy is a tumor-specific strategy, in which
viruses engineered to selectively kill tumor cells, through
targeted alterations in the cancer such as p53 mutation, viral
deletion, tissue-specific transcriptional control or tumor-specific
receptors, thus, representing a promising approach for the
treatment of neoplastic diseases refractory to conventional
therapies (1,2). Conditionally replicating adenoviruses
(CRAds) based on the native adenoviral serotype 5 (Ad5) are most
commonly used for generation of oncolytic agents that include
ONYX-015, a vector with a deletion in the E1B region, and the AdΔ24
vector, containing a 24-bp deletion in the E1A gene (3–5).
Recent generation of oncolytic adenoviruses have combined E1A and
E1B alterations in an effort to improve the selective killing of
cancer cells (6). Although their
selective and antitumor activity was demonstrated in numerous
experiments with different types of tumors in vitro and
in vivo, the CRAds based on Ad5 has a major limitation to be
solved owing to weak expression of coxsackie adenovirus receptor
(CAR) in tumor cells. This CAR deficiency makes cancer cells
resistant to Ad5 infection (7). To
overcome this problem, non-replicating adenoviral vectors
containing chimeric type 5 and type 35 or type 11 have been
successfully constructed, which permit CAR-independent infection of
tumor cell and are used for effective gene transfer (8,9). For
oncolytic adenoviral vector, we and others have recently introduced
Ad5/35 and Ad5/3 fiber chimeric replicating viruses, and
demonstrated their broad antitumor activity regardless of CAR
expression in cancer cells (10–13).
These data obtained from the strategy of adenoviral tropism
modification using alternate cellular attachment receptors predicts
an improved therapeutic index for the virotherapy of cancer.
The effect of oncolytic adenoviruses against cancer
cells could be significantly improved by combination with standard
chemotherapies (2). There are
several preclinical studies suggesting enhanced and even
synergistic cell killing and anticancer activity when oncolytic
adenoviruses and chemotherapeutic agents including cisplatin,
gemcitabine, epirubicin, cyclophosphamide, 5-fluorouracil,
docetaxel, and temozolomide have been combined (5,14–23).
Phase II clinic trails have been undertaken to evaluate the use of
ONXY-015 combined with cisplatin and 5-fluorouracil therapy in
patients with recurrent squamous cell carcinoma of the head and
neck. The results show an improved anticancer effect when compared
with historical data in patients treated with either ONXY-015 or
chemotherapy alone (24–26). In China, the addition of a modified
version of ONXY-015 (H101) to chemotherapy has already entered
Phase III trail with promising results (27). Together, these studies on
combination oncolytic adenovirotherapy and chemotherapy have shown
enhanced and even synergistic antitumor efficacy, suggesting the
combination therapy represents a promising strategy for the
treatment of cancer; however, the mechanism for the synergy between
CRAd virotherapy and chemotherapy is unclear.
In this study, we investigated the effects of SG511
(a new Ad5/11 fiber chimeric oncolytic adenovirus) on human cancer
cell lines. Next, we evaluated whether the combination treatment of
SG511 plus cisplatin perform robust synergistic killing in tumor
cells. We further studied the mechanism of enhanced cytotoxicity
induced by the combination therapy with attention to the alteration
of Bcl-2 family proteins, because pro- and anti-apoptotic Bcl-2
family proteins dictate the ultimate sensitivity or resistance of
cancer cells to various apoptotic stimuli (28).
Materials and methods
Cell lines and culture
HeLa (human cervical cancer cell line), HT-29 (human
colorectal cancer cell line), SW480 (human colon cancer cell line),
Panc-1 (human pancreatic carcinoma cell line), and L-02 (normal
human liver cell line) were purchased from the Shanghai Cell
Collection (Shanghai, China). Cells were maintained in RPMI-1640
medium (Hyclone Laboratories, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Life Technologies, Inc., Grand Island, NY,
USA) and 1% L-glutamine (Life Technologies). The cells were
cultured at 37°C in 5% CO2 humid atmosphere.
Human mesenchymal stem cell isolation and
culture
Bone marrow mononuclear cells were obtained from
bone marrow samples from healthy donors after informed consent, and
isolated by Ficoll density gradient and cultured in DMEM medium
(Gibco-BRL, Grand Island, NY, USA) containing 10% FBS. After 3
days, non-adherent cells were discarded and adherent cells were
replenished with fresh medium twice a week. When the culture
reached confluency, mesenchymal stem cells (MSCs) were detached and
identified by flow cytometry using the following mAbs: CD29-FITC,
CD90-FITC, CD44-PE (all from eBioscience Inc., San Diego, CA, USA),
and CD166-PE (from R&D Systems, Minneapolis, MN, USA). MSCs
were used for experiments between the 3rd and 4th passages.
Crystal violet assay
Cells were plated in 96-well or 24-well dishes at
1×105 cells/ml 12 h, and then treated with SG511,
cisplatin alone or SG511 combined with cisplatin at a ratio of
10:1. After incubation for 48 h, medium was removed, and dishes
were washed with phosphate-buffered saline (PBS) twice, and then
stained with 2% crystal violet for 5 min, followed by three rinses
with water. Air-dried dishes were photographed. For the detection
of OD value, 100 μl 33% acetic acid was applied per well to
decolorize. Absorbance was read at 595 nm with a Bio-Rad microplate
reader. The experiments were repeated at least three times, each
time in triplicate.
MTT assay
Cells (3×103) were seeded in 100 μl of
the growth medium in the presence or absence of increasing
concentrations of cisplatin or SG511 in 96-well plates, and
cultured at 37°C in 5% CO2 for 48 h. A
replication-deficient virus (Ad5/11) was used as a control. Ad5/11
and SG511 virus were kindly provided by Professor Qian (Second
Military Medical University, Shanghai, China). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed as previously described (29).
Evaluation of apoptosis
The treated or non-treated cells at
1×105/ml were washed with PBS, fixed in 4%
paraformaldehyde for 30 min, washed with PBS, and then stained with
4,6-diamidino-2-phenylindole (DAPI, 0.5 μg/ml) for 3 min in the
dark. After washed with PBS, cells were observed under a
fluorescent microscopy (Olympus, Japan) with a peak excitation
wavelength of 340 nm.
Mitochondrial membrane potential
measurement
HeLa cells treated with SG511, cisplatin, or
SG511/cisplatin combination were collected, and then washed,
stained with 5 μg/ml of Rhodamine 123 (Molecular Probes, Eugene,
USA) at 37°C for 15 min. Rhodamine 123 was excited with a 488 nm
argon ion laser, fluorescence emission was measured at 530 nm using
flow cytometry (Becton-Dickison).
Western blot analysis
Protein samples were diluted with sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading
buffer, boiled for 3 min before loading on a 12% SDS-PAGE gel, and
then transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Bedford, USA). The membranes were blocked with 5%
non-fat dry milk and then incubated with corresponding primary
antibodies. After incubation with peroxidase-conjugated secondary
antibodies (MultiSciences Biotech, Hangzhou, China),
immunodetection was done using an enhanced chemiluminescence
detection kit (KPL, Baltimore, USA). All primary antibodies were
purchased from Cell Signaling (Danvers, USA).
Statistics
Two-way analysis of variance (ANOVA) was used to
determined statistical significance. Results of combined treatment
were assessed by calculating combination index (CI) values using
CalcuSyn software (Biosoft, Cambridge, UK). According to this
method, synergy was expressed as log10 (CI) vs. fraction affected,
and log10 (CI) <0 indicates synergy; log10 (CI) = 0 indicates an
additive effect; and log10 (CI) >0 indicates antagonism.
Results
Characterization and infectivity of SG511
oncolytic virus
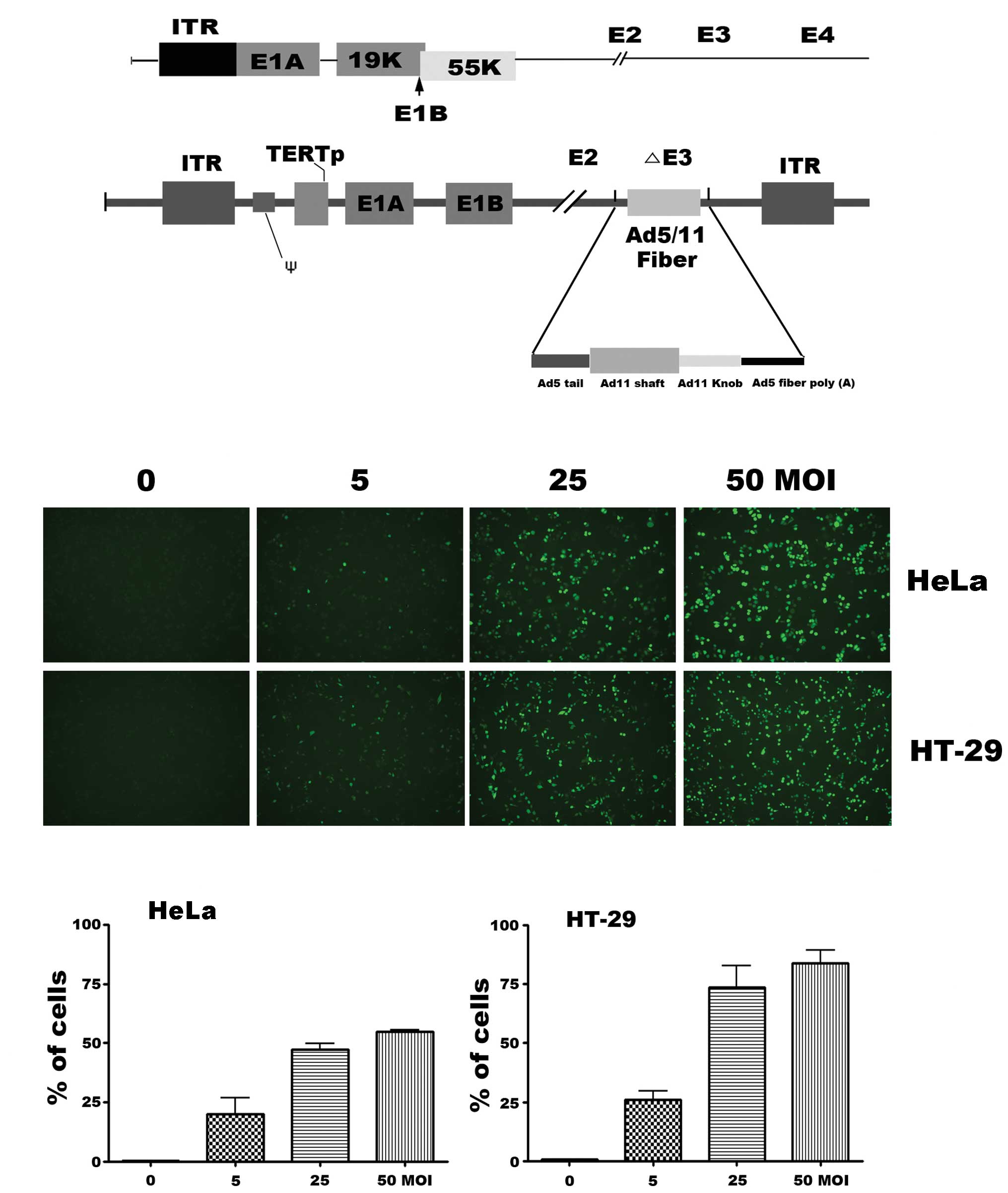
The oncolytic adenovirus, SG511, was engineered to
delete the E1B-55-kDa, which is similar to ONYX-015, and to have
Ad5/11 chimeric fiber (Fig. 1A).
Two established human cancer cell lines (HeLa, HT-29) were used to
evaluate the infectivity of SG511 vector. After infection with an
enhanced green fluorescent protein (EGFP) expressing SG511 virus
(SG511-GFP), green fluorescence was analyzed by fluorescence
microscopy. Results showed a dose-dependent shift in fluorescence
when the cells were treated with SG511-GFP at MOIs of 5, 25 and 50
(Fig. 1B). Next,
fluorescence-activated cell sorting analysis was used to quantitate
the percentage of GFP-positive cancer cells which indicate the
infective capacity of the virus. As seen in Fig. 1C, 53.9% of HeLa cells and 78.4% of
HT-29 expressed SG511-mediated GFP 12 h after infection at a MOI of
50.
Selective broad antitumor activity of
SG511
According to the mechanism of oncolytic virus, the
primary character of oncolytic adenovirus is its ability of
infecting cancer cells, selectively replicating within them and
inducing cell death. To detect the antitumor activity of SG511,
HeLa, SW480, Panc-1 and HT-29 cancer cell lines were infected with
SG511 at the indicated MOIs. At 48 h after viral infection, cells
were stained with crystal violet solution. Results showed that
SG511 killed all tested cancer cell lines effectively in a
dose-dependent way. Tumor cells were almost complete eliminated
when the virus was used at a MOI of 40 (Fig. 2A). Next, we compared the inhibitory
effect of SG511 to that of Ad5/11 (a fiber chimeric non-replicating
virus) by an MTT assay (Fig. 2B).
SG511 induced concentration-dependent cell death in HT-29 cells,
whereas Ad5/11 did not cause any detectable cytototoxic effect. We
examined effects of SG511 and cisplatin on human normal hepatic
cell line L-02. As shown in Fig.
2C, the treatment of cisplatin alone at 2 μg/ml and 4 μg/ml
elicited a marked growth inhibition. In contrast, SG511 did not
result in obvious cytotoxicity toward normal cells. Furthermore,
combined use of cisplatin and oncolytic virus SG511 had only
slightly greater cytotoxic effect compared with cisplatin alone. We
further investigated the cytotoxicity of SG511 against human normal
MSCs. As shown in Fig. 2D, SG511 at
the indicated dosage did not apparently affect the viability of
human MSCs. These data suggest that SG511 is an ideal
tumor-specific replicative adenovirus for virotherapy of
cancer.
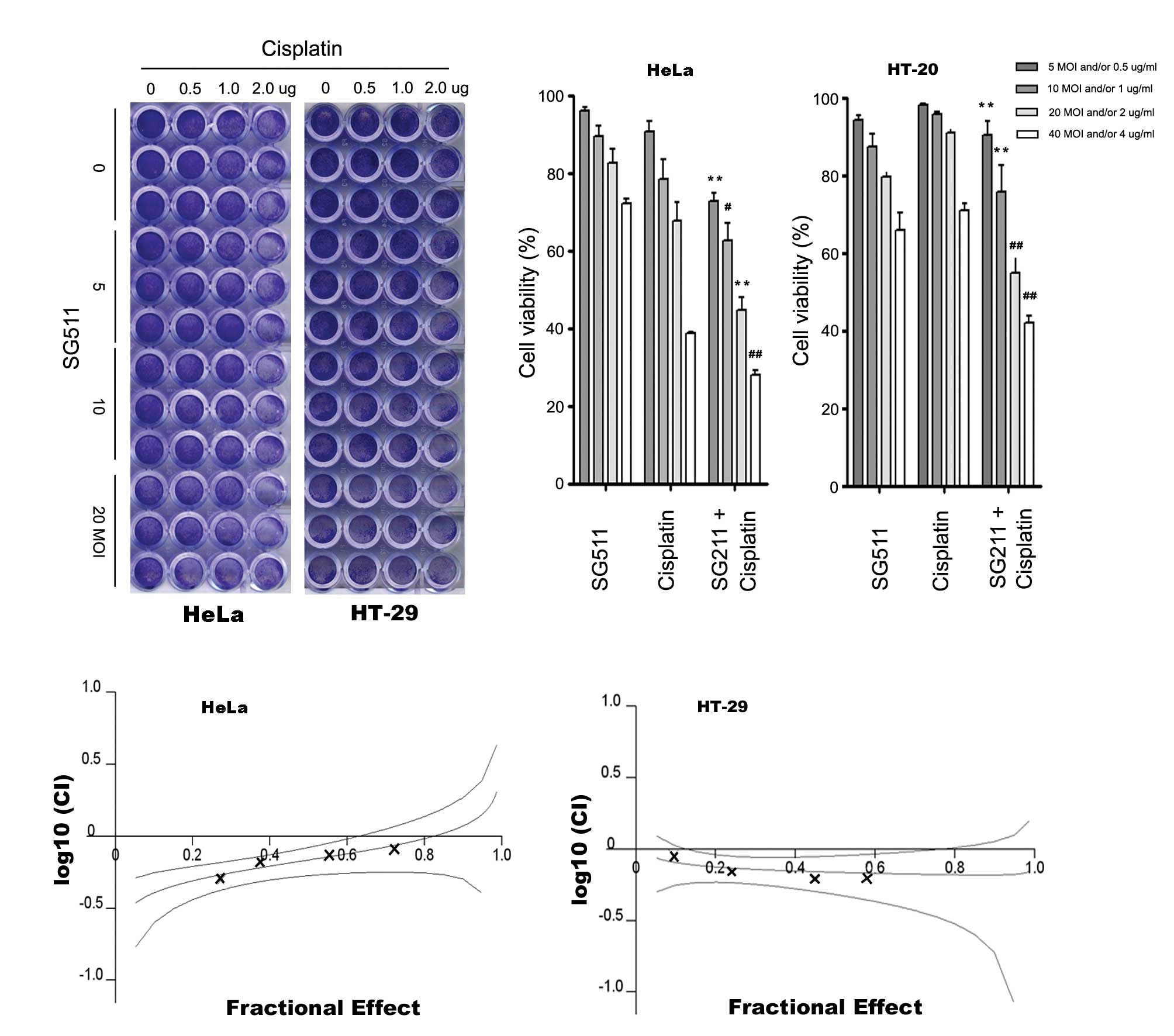
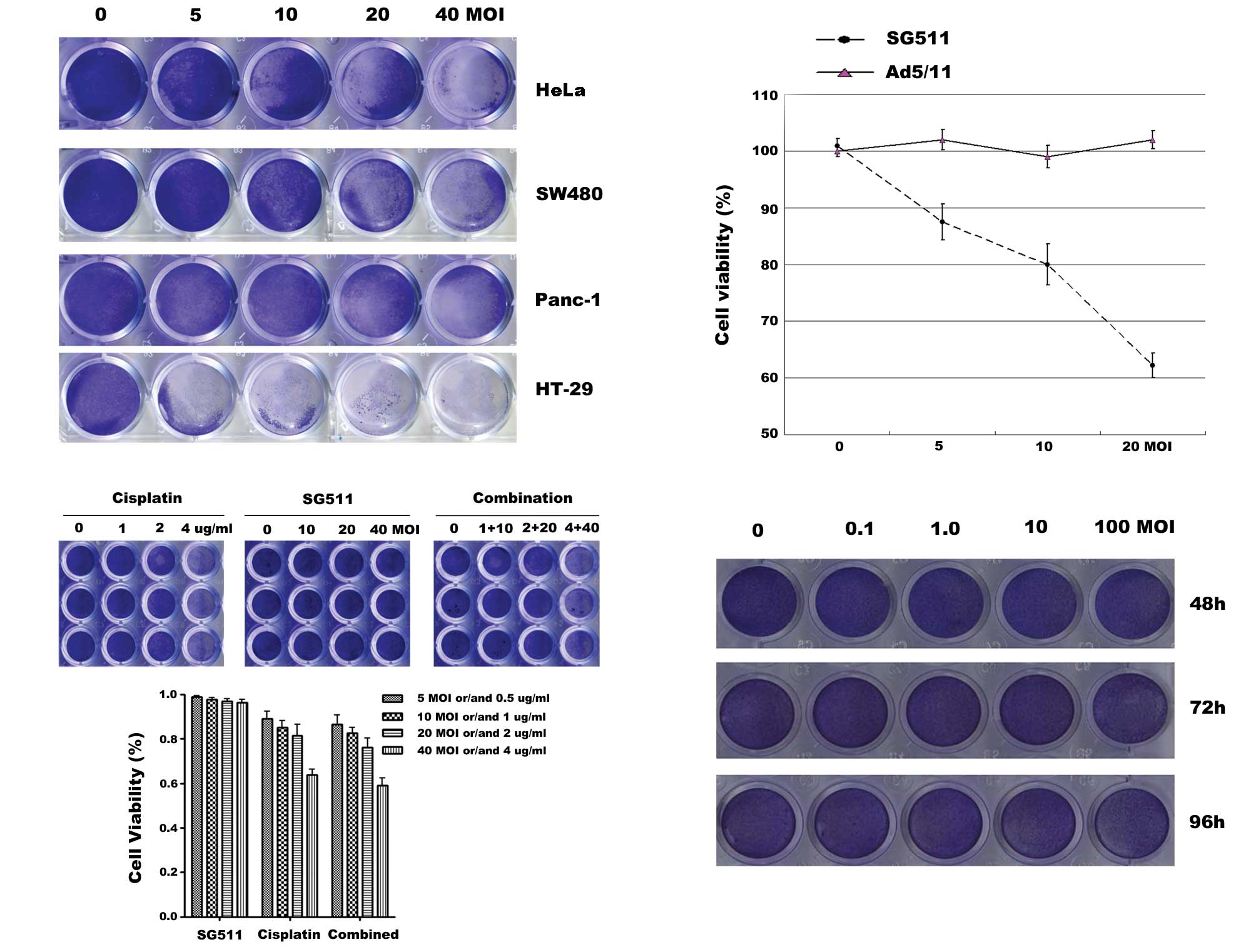 | Figure 2Anticancer activity of SG511 virus
and the cytotoxicity to human normal cells when SG511, cisplatin
was used alone or in combination. (A) HeLa, SW480, Panc-1, or HT-29
cells were seeded into 24-well plates and treated with the
indicated concentrations of SG511. After 48 h, cell viability was
assessed by a crystal violet assay. (B) HT-29 cells were treated
with SG511 and Ad5/11 vectors, respectively at the indicated MOIs
for 48 h. The cell viability was determined by an MTT assay. (C)
Human normal liver cells (L-02) were treated with cisplatin, SG511
alone, or two agents together at the dosage indicated for 48 h.
Cell viability was assessed by crystal violet staining. The means
and standard errors of results from three independent experiments
are shown (lower panel). (D) MSCs were infected with SG511 at the
indicated MOIs for 48, 72 and 96 h, respectively. Cytopathic
effects were evaluated by crystal violet assay. |
SG511 synergistically enhances
cisplatin-induced cancer cell death
We treated cancer cells with low concentrations of
cisplatin in combination with oncolytic virus SG511. HeLa cells and
HT-29 cells was infected with SG511 at MOI ranging from 5 to 40 in
96-well plates and then treated with various concentrations of
cisplatin (0.5–4 μg/ml). Cell survival was determined at 48 h by
crystal violet assay and MTT assay. As shown in Fig. 3A and B, cell death induced by SG511
in combination with cisplatin was significantly enhanced. In
particular, when used alone, cisplatin at 4 μg/ml induced 27.8%
cell death, SG511 at 40 MOI induced 21.3% cell death. However, when
4 μg/ml cisplatin was combined with 40 MOI SG511, the cell death
increased to 72.1% in HeLa cells. Similar results were observed in
HT-29 cells. This enhanced cytotoxicity of the combination therapy
was confirmed by MTT assay (data not shown). The CI at effective
concentration (ED50) were 0.68 for HeLa and 0.69 for HT-29 cells
(Fig. 3C), suggesting that SG511
and cisplatin cotreatment is highly synergistic.
Effect of the SG511/cisplatin combination
in cell apoptosis
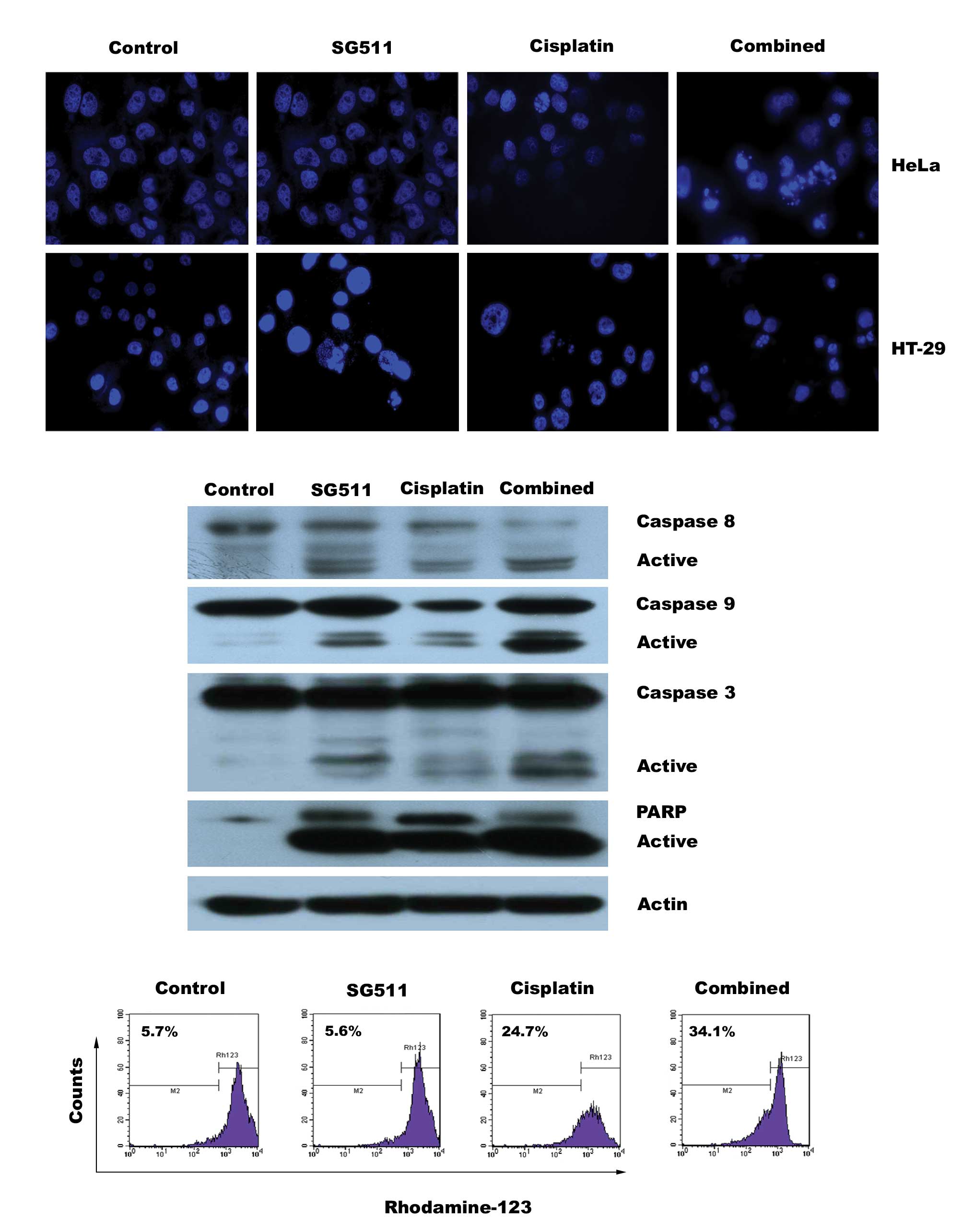
To study a possible mechanism that contributes to
the enhanced cytotoxicity induced by the combination of SG511 and
cisplatin, we evaluated whether this combination therapy results in
increased apoptosis in cancer cell lines. As shown in Fig. 4A, each agent administered
individually exhibited only slight apoptosis evidenced by
fluorescence microscopy analysis after DAPI staining, whereas a
higher number of condensed and/or fragmented nuclei in HeLa and
HT-29 cells was observed when SG511 was used in conjunction with
cisplatin. We then examined the effect of combined therapy on
caspase activation in HeLa cells. Consistent with the above
findings, combined but not individual treatment results in a
pronounced increase in cleavage of caspase-3 and caspase-9 and
degradation of poly (ADP-ribose) polymerase (Fig. 4B). Furthermore, increased loss of
mitochondrial membrane potential (ΔΨm) was observed in cells
treated with the combination, evaluated by flow cytometry after
staining with the Rhodamine 123 dye (Fig. 4C). Altogether, these findings
demonstrate that the combined treatment with low concentrations of
SG511 and cisplatin results in synergistic cytotoxicity by inducing
apoptosis of both the HeLa and HT-29 cancer cells.
Mechanism of enhanced apoptosis induced
by the combination therapy
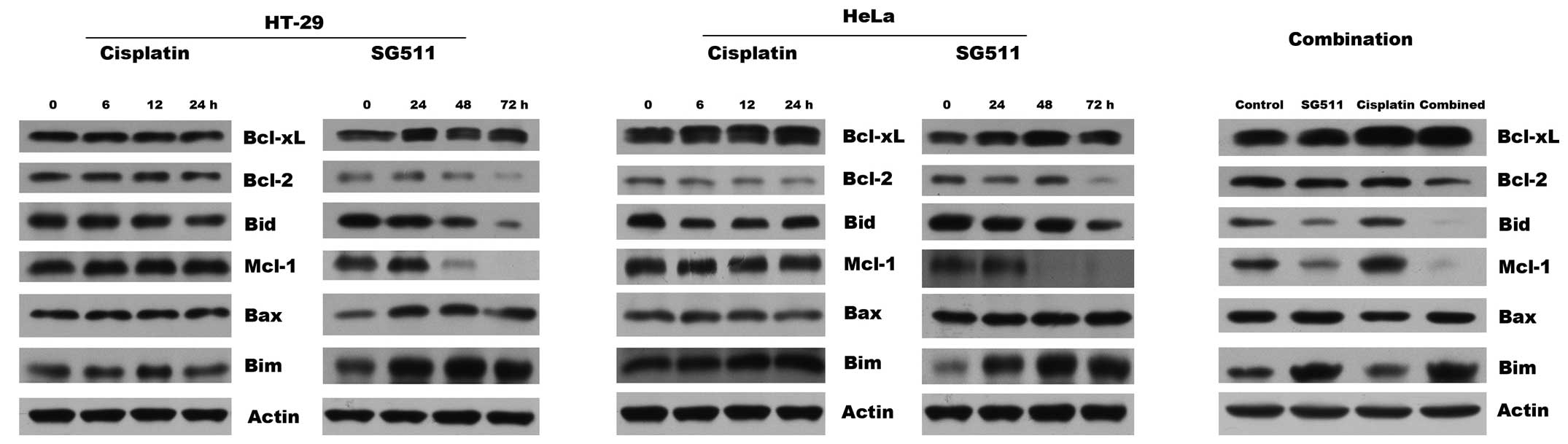
We investigated the potential molecular mechanism of
sensitization of cancer cells to SG511/cisplatin combination, by
examining possible alterations in the expression levels of
proapoptotic and antiapoptotic signaling molecules. As observed in
the western blots (Fig. 5),
individual treatment with cisplatin at 4 μg/ml did not induce
discernible changes in the expression of Bcl-2, Bid, Mcl-1, Bax,
and Bim in HT-29 and HeLa cells. In marked contrast, treatment with
SG511 at a MOI of 40 downregulated the levels of multidomain
anti-apoptotic proteins Bcl-2, and Mcl-1, and upregualted the level
of pro-apoptotic Bax. Cleavage of BH3-only pro-apoptotic protein
Bid and Bim accumulation was also observed in cells treated with
SG511. These conformational changes were also observed in HeLa
cells after treatment with SG511 combined with cisplatin. However,
these two agents, alone or in combination, had no effect on the
expression of Bcl-xL in the cancer cell lines.
Discussion
The antitumor potency of an oncolytic adenovirus is
largely dependent on the capacity of the virus to infect target
cells (7,30,31).
Recently, it has been demonstrated that chimeric Ad5 vector
possessing fiber proteins derived from group B adenoviruses such as
Ad35, Ad11 can efficiently enter tumor cells that are refractory to
Ad 5 infection by utilizing CD46 as a high-affinity primary
attachment receptor (32). Previous
studies suggest that CD46, a membrane regulator of complement
activation, is overexpressed on human malignancies including
colorectal, cervical, and pancreatic carcinomas compared with their
normal counterparts (33–36). In this study, we used human
colorectal, cervical, and pancreatic carcinoma cell lines, and
demonstrated that SG511, a new Ad5/11 fiber chimeric oncolytic
adenovirus, efficiently infected various tumor cell lines and led
to cytopathic killing of cancer cell in vitro, without
damage to human normal liver cells. Together, these results suggest
that SG511 is a new targeted oncolytic adenoviral vector with broad
antitumor activity.
Cisplatin, a well known DNA-damaging agent, has
broad spectrum of activity against epithelial cancers and has
become the foundation of curative regimens in testicular and
ovarian cancers. It also demonstrates significant activity against
cancers of the lung, head and neck, esophagus, bladder, cervix and
endometrium (37,38), however, drug-resistance and severe
toxicity present major hurdles associated with cisplatin therapy.
An excellent example to highlight this limitation is with ovarian
cancer, which generally responds well to cisplatin-based
chemotherapy. Unfortunately, the initial response is not durable,
and tumors in most patients become resistant to the drug (29,39).
Earlier clinical trails have indicated that oncolytic adenoviruse
therapy combined with cisplatin elicit greater antitumor efficacy
in the patients with head and neck or esophagus cancer, without an
increase in toxicity. Recently, some studies have shown that the
use of cisplatin conjugated with oncolytic adenoviral vectors
results in an apparent synergistic cytotoxicity in human
hepatocellular, nasopharyngeal, lung adenocarcinoma, and ovarian,
colorectal, cervical cancer cells (23,40–42).
Consistent with those results, our in vitro data showed that
SG511 alone has marked antitumor effects on HeLa, SW480, Panc-1,
and HT-29 cells at a MOI of 40, whereas modest to mild effects were
observed at a MOI of 20 or lower. Interestingly, SG511 did have a
sensitizing effect on HeLa cells to cisplatin. Similar sensitizing
effect was also observed in HT-29 cells that are relatively
resistant to cisplatin. Since classical apoptosis has been presumed
the mechanism of adenovirus-induced cell death (43), we investigated the role of apoptosis
in cytotoxicity resulting from the combination therapy. The data
showed that the nuclear morphology of cells treated with SG511
combined with cisplatin exhibited increased apoptosis characterized
by condensed and/or fragmented nuclei. SG511 in combination with
cisplatin elicited a more pronounced activation of caspase-9, -3,
and PARP. Furthermore, this combination therapy resulted in a high
level of ΔΨm loss. These data suggest that SG511 sensitizes cells
to cisplatin through activation of caspase-9 and induction of
apoptosis.
Apoptosis is regulated in part by the Bcl-2 family
of proteins which consist of both proapoptotic (Bax and Bak) and
antiapoptotic (Bcl-2, Bcl-xL, A1, Mcl-1 and Bcl-w) proteins
(44). In cancer, antiapoptotic
members are often overexpressed, rendering cancer cells resistant
to apoptosis (45). Our data show
that oncolytic virus SG-511 strongly sensitizes human cervical and
colorectal cancer cells to apoptosis induced by cisplatin.
Furthermore, treatment with SG511 alone or combined with cisplatin
induces increases in the levels of Bim and Bax, and actives Bid.
Direct activator BH3-only proteins, such as Bid and Bim, have been
reported to activate Bax and Bak at the outer mitochondrial
membrane leading to cytochrome c release which seems to be
primarily responsible for ΔΨm loss (46,47).
In this study, the combination therapy results in increased ΔΨm
loss and caspase-9 activation, supporting this view. Mcl-1 is a
multidomain antiapoptotic member of the Bcl-2 family that acts
through several mechanisms to block mitochondrial outer membrane
permeabilization and apoptosis (48). For example, Mcl-1 can interact with
truncated Bid and inhibits its ability to activate the
mitochondrial death pathway (49).
Our data show that cisplatin do not affect level of Mcl-1 in HeLa
and HT-29 cells. Whereas, SG511 produced a marked decrease in the
levels of Mcl-1, a short-lived protein critical for cancer cell
survival (50,51). It was reported that downregulation
of Mcl-1 induced by cycloheximide results in acceleration of
apoptosis in HeLa cells (52).
Moreover, previous studies indicate that overexpression of Mcl-1 in
human cervical and colorectal cancer tissues is associated with a
poorer prognosis (53). These data
suggest that Mcl-1 downregulation by SG511 virus contributed
significantly to potentiation of cisplatin lethality in these
cancer cells and to the resulting synergistic antitumor
interactions.
In summary, our results demonstrate that SG511, a
new fiber chimeric oncolytic adenovirus, can infect and kill
various cancer cell lines effectively. We show that SG511 combined
with cisplatin increases apoptosis and cell death in tumor cells
without enhanced lethality in normal cells. Additionally, SG511
effectively downregulate expression of Mcl-1 and Bcl-2 genes, which
may contribute to activation of the mitochondrial pathway for
apoptosis. Thus, we believe that the use of SG511 should be an
attractive strategy to improve the therapeutic results of
cisplatin-based chemotherapy of cancers.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China grants (no. 81070419), and the Zhejiang
Provincial Natural Science Foundation of China (no. R2090392).
Reference
|
1
|
Kirn D, Martuza RL and Zwiebel J:
Replication-selective virotherapy for cancer: biological
principles, risk management and future directions. Nat Med.
7:781–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu RL, Post DE, Khuri FR and Van Meir EG:
Use of replicating oncolytic adenoviruses in combination therapy
for cancer. Clin Cancer Res. 10:5299–5312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bischoff JR, Kirn DH, Williams A, et al:
An adenovirus mutant that replicates selectively in p53-deficient
human tumor cells. Science. 274:373–376. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganly I, Kirn D, Eckhardt G, et al: A
phase I study of Onyx-015, an E1B attenuated adenovirus,
administered intratumorally to patients with recurrent head and
neck cancer. Clin Cancer Res. 6:798–806. 2000.PubMed/NCBI
|
|
5
|
Conrad C, Miller CR, Ji Y, et al:
Delta24-hyCD adenovirus suppresses glioma growth in vivo by
combining oncolysis and chemosensitization. Cancer Gene Ther.
12:284–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukuda K, Abei M, Ugai H, Kawashima R, Seo
E, Wakayama M, Murata T, Endo S, Hamada H, Hyodo I and Yokoyama KK:
E1A, E1B double-restricted replicative adenovirus at low dose
greatly augments tumor-specific suicide gene therapy for
gallbladder cancer. Cancer Gene Ther. 16:126–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douglas JT, Kim M, Sumerel LA, Carey DE
and Curiel DT: Efficient oncolysis by a replicating adenovirus (ad)
in vivo is critically dependent on tumor expression of primary ad
receptors. Cancer Res. 61:813–817. 2001.PubMed/NCBI
|
|
8
|
Nilsson M, Ljungberg J, Richter J, et al:
Development of an adenoviral vector system with adenovirus serotype
35 tropism; efficient transient gene transfer into primary
malignant hematopoietic cells. J Gene Med. 6:631–641. 2004.
View Article : Google Scholar
|
|
9
|
Stone D, Ni S, Li ZY, et al: Development
and assessment of human adenovirus type 11 as a gene transfer
vector. J Virol. 79:5090–5104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawakami Y, Li H, Lam JT, Krasnykh V,
Curiel DT and Blackwell JL: Substitution of the adenovirus serotype
5 knob with a serotype 3 knob enhances multiple steps in virus
replication. Cancer Res. 63:1262–1269. 2003.PubMed/NCBI
|
|
11
|
Jin J, Liu H, Yang C, et al: Effective
gene-viral therapy of leukemia by a new fiber chimeric oncolytic
adenovirus expressing TRAIL: in vitro and in vivo evaluation. Mol
Cancer Ther. 5:1387–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Li G, Liu H, et al: E1B 55-kDa
deleted, Ad5/F35 fiber chimeric adenovirus, a potential oncolytic
agent for B-lymphocytic malignancies. J Gene Med. 11:477–485. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wohlfahrt ME, Beard BC, Lieber A and Kiem
HP: A capsid-modified, conditionally replicating oncolytic
adenovirus vector expressing TRAIL Leads to enhanced cancer cell
killing in human glioblastoma models. Cancer Res. 67:8783–8790.
2007. View Article : Google Scholar
|
|
14
|
Raki M, Särkioja M, Desmond RA, et al:
Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment
of orthotopic ovarian cancer. Gynecol Oncol. 108:166–172. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raki M, Kanerva A, Ristimaki A, et al:
Combination of gemcitabine and Ad5/3-Delta24, a tropism modified
conditionally replicating adenovirus, for the treatment of ovarian
cancer. Gene Ther. 12:1198–1205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu D, Kojima T, Ouchi M, et al:
Preclinical evaluation of synergistic effect of telomerase-specific
oncolytic virotherapy and gemcitabine for human lung cancer. Mol
Cancer Ther. 8:980–987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Qin X, Zhang Y, et al:
Combination of ZD55-MnSOD therapy with 5-FU enhances antitumor
efficacy in colorectal cancer. J Cancer Res Clin Oncol.
134:219–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon AR, Kim JH, Lee YS, et al: Markedly
enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in
combination with cisplatin. Hum Gene Ther. 17:379–390. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujiwara T, Kagawa S, Kishimoto H, et al:
Enhanced antitumor efficacy of telomerase-selective oncolytic
adenoviral agent OBP-401 with docetaxel: preclinical evaluation of
chemovirotherapy. Int J Cancer. 119:432–440. 2006. View Article : Google Scholar
|
|
20
|
Sagawa T, Yamada Y, Takahashi M, et al:
Treatment of hepatocellular carcinoma by AdAFPep/rep, AdAFPep/p53,
and 5-fluorouracil in mice. Hepatology. 48:828–840. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quirin C, Mainka A, Hesse A and Nettelbeck
DM: Combining adenoviral oncolysis with temozolomide improves cell
killing of melanoma cells. Int J Cancer. 121:2801–2807. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lun XQ, Jang JH, Tang N, et al: Efficacy
of systemically administered oncolytic vaccinia virotherapy for
malignant gliomas is enhanced by combination therapy with rapamycin
or cyclophosphamide. Clin Cancer Res. 15:2777–2788. 2009.
View Article : Google Scholar
|
|
23
|
Cheong SC, Wang Y, Meng JH, et al:
E1A-expressing adenoviral E3B mutants act synergistically with
chemotherapeutics in immunocompetent tumor models. Cancer Gene
Ther. 15:40–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khuri FR, Nemunaitis J, Ganly I, et al: A
controlled trial of intratumoral ONYX-015, a
selectively-replicating adenovirus, in combination with cisplatin
and 5-fluorouracil in patients with recurrent head and neck cancer.
Nat Med. 6:879–885. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lamont JP, Nemunaitis J, Kuhn JA, Landers
SA and McCarty TM: A prospective phase II trial of ONYX-015
adenovirus and chemotherapy in recurrent squamous cell carcinoma of
the head and neck (the Baylor experience). Ann Surg Oncol.
7:588–592. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galanis E, Okuno SH, Nascimento AG, et al:
Phase I-II trial of ONYX-015 in combination with MAP chemotherapy
in patients with advanced sarcomas. Gene Ther. 12:437–445. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia ZJ, Chang JH, Zhang L, et al: Phase
III randomized clinical trial of intratumoral injection of E1B
gene-deleted adenovirus (H101) combined with cisplatin-based
chemotherapy in treating squamous cell cancer of head and neck or
esophagus. Ai Zheng. 23:1666–1670. 2004.(In Chinese).
|
|
28
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar
|
|
29
|
Qian W, Liu J, Tong Y, et al: Enhanced
antitumor activity by a selective conditionally replicating
adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic
leukemia via induction of apoptosis. Leukemia. 22:361–369. 2008.
View Article : Google Scholar
|
|
30
|
Kanerva A, Zinn KR, Chaudhuri TR, et al:
Enhanced therapeutic efficacy for ovarian cancer with a serotype 3
receptor-targeted oncolytic adenovirus. Mol Ther. 8:449–458. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reddy PS, Ganesh S and Yu DC: Enhanced
gene transfer and oncolysis of head and neck cancer and melanoma
cells by fiber chimeric oncolytic adenoviruses. Clin Cancer Res.
12:2869–2878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hall K, Blair Zajdel ME and Blair GE:
Unity and diversity in the human adenoviruses: exploiting
alternative entry pathways for gene therapy. Biochem J.
431:321–336. 2010.PubMed/NCBI
|
|
33
|
Fishelson Z, Donin N, Zell S, Schultz S
and Kirschfink M: Obstacles to cancer immunotherapy: expression of
membrane complement regulatory proteins (mCRPs) in tumors. Mol
Immunol. 40:109–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thorsteinsson L, O’Dowd GM, Harrington PM
and Johnson PM: The complement regulatory proteins CD46 and CD59,
but not CD55, are highly expressed by glandular epithelium of human
breast and colorectal tumour tissues. APMIS. 106:869–878. 1998.
View Article : Google Scholar
|
|
35
|
Tuve S, Wang H, Ware C, et al: A new group
B adenovirus receptor is expressed at high levels on human stem and
tumor cells. J Virol. 80:12109–12120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stoff-Khalili MA, Stoff A, Rivera AA, et
al: Gene transfer to carcinoma of the breast with fiber-modified
adenoviral vectors in a tissue slice model system. Cancer Biol
Ther. 4:1203–1210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Go RS and Adjei AA: Review of the
comparative pharmacology and clinical activity of cisplatin and
carboplatin. J Clin Oncol. 17:409–422. 1999.PubMed/NCBI
|
|
38
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takakura M, Nakamura M, Kyo S, et al:
Intraperitoneal administration of telomerase-specific oncolytic
adenovirus sensitizes ovarian cancer cells to cisplatin and affects
survival in a xenograft model with peritoneal dissemination. Cancer
Gene Ther. 17:11–19. 2010. View Article : Google Scholar
|
|
41
|
Pan Q, Liu B, Liu J, Cai R, Wang Y and
Qian C: Synergistic induction of tumor cell death by combining
cisplatin with an oncolytic adenovirus carrying TRAIL. Mol Cell
Biochem. 304:315–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu YM, Zhang KJ, Yue XT, et al:
Enhancement of tumor cell death by combining cisplatin with an
oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol Sin.
30:467–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baird SK, Aerts JL, Eddaoudi A, Lockley M,
Lemoine NR and McNeish IA: Oncolytic adenoviral mutants induce a
novel mode of programmed cell death in ovarian cancer. Oncogene.
27:3081–3090. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Heiser D, Labi V, Erlacher M and Villunger
A: The Bcl-2 protein family and its role in the development of
neoplastic disease. Exp Gerontol. 39:1125–1135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuwana T, Bouchier-Hayes L, Chipuk JE, et
al: BH3 domains of BH3-only proteins differentially regulate
Bax-mediated mitochondrial membrane permeabilization both directly
and indirectly. Mol Cell. 17:525–535. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chipuk JE, Fisher JC, Dillon CP, Kriwacki
RW, Kuwana T and Green DR: Mechanism of apoptosis induction by
inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci
USA. 105:20327–20332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Green DR: At the gates of death. Cancer
Cell. 9:328–330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhuang J and Brady HJ: Emerging role of
Mcl-1 in actively counteracting BH3-only proteins in apoptosis.
Cell Death Differ. 13:1263–1267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang H, Guttikonda S, Roberts L, et al:
Mcl-1 is critical for survival in a subgroup of non-small cell lung
cancer cell lines. Oncogene. 30:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen W, Bai L, Wang X, Xu S, Belinsky SA
and Lin Y: Acquired activation of the Akt/cyclooxygenase-2/Mcl-1
pathway renders lung cancer cells resistant to apoptosis. Mol
Pharmacol. 77:416–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nijhawan D, Fang M, Traer E, et al:
Elimination of Mcl-1 is required for the initiation of apoptosis
following ultraviolet irradiation. Genes Dev. 17:1475–1486. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Henderson-Jackson EB, Helm J, Ghayouri M,
et al: Correlation between Mcl-1 and pAKT protein expression in
colorectal cancer. Int J Clin Exp Pathol. 3:768–774.
2010.PubMed/NCBI
|