Introduction
Colorectal cancer (CRC) is one of the leading causes
of worldwide cancer-associated morbidity and mortality (1). CRC is staged according to the extent
of primary organ involvement and metastatic spread to lymph nodes
or distant organs. The 5-year survival rates of CRC patients with
Dukes' stage B and C who underwent surgery were 75–80% and 65–70%,
respectively (2). Despite surgical
resection being highly effective for localized disease, a
significant proportion of these patients develop recurrence. Of
particular concern is the fact that it is not possible to
accurately differentiate between good and poor prognosis of Dukes'
stage B. Effective biomarkers for the detection of Dukes' stage B
patients who are at high risk are needed since the role of adjuvant
chemotherapy in these patients remains controversial (3–7).
The utility of circulating tumor cells (CTCs) as
biomarkers for predicting the clinical outcome of patients with
various cancers has been shown by many studies (8–11). The
detection of CTC in blood may not only provide the mechanism for
the early metastatic spreading of isolated cancer cells, but it can
also potentially indicate substantial predictive and prognostic
information on CRC patients (9,12–15).
In the detection of aggressive CTCs in blood, the possibility of a
cancer stem-like cell (CSCs) marker is currently attracting
attention (16). CSCs have been
defined as a unique subpopulation in tumors that possess the
ability to initiate tumor growth and sustain tumor self-renewal
(17,18). Accumulating evidence shows that CSCs
are associated with metastasis, resistance to chemotherapy and
radiotherapy, and recurrence. It is known that CSC markers are
frequently over-expressed in the CTC of patients with metastatic
breast cancer, and most CTCs have CSC phenotypes that are not
proliferating and resistant to chemotherapy (11,19).
These properties suggest that the founder cells of metastases may
arise from the CTC population. Recently, we demonstrated that
detection of CTC, including CSC (CTC/CSC) in PB, is a useful tool
for determining high risk for recurrence and poor prognosis in
patients with Dukes' stage B and C CRC (16).
Peripheral blood (PB) and tumor drainage vein blood
were used to obtain a CTC sample (9). As compared to the utility of CTCs in
PB, the property of CTCs in tumor drainage vein blood is still
unclear. It is known that the detection rate of CTCs in tumor
drainage vein blood is higher than that of PB (9). Therefore, the detection of CTC/CSC in
tumor drainage vein blood may show a high sensitivity for selection
of the high risk patients for recurrence in Dukes' B and C
patients. However, little is known about the clinical significance
of CTC/CSC in the tumor drainage vein blood of these patients.
In this study, we aimed to clarify the usefulness of
CTC/CSC in the tumor drainage vein blood as a prognostic biomarker
in CRC patients with Dukes' stage B and C. To detect the CTC/CSC,
we utilized the real-time RT-PCR method using multiple marker genes
consisting of CEA, CK19 and CK20 mRNA for the general
CRC-associated marker, and CD133 mRNA for the CSC marker.
Patients and methods
Patients
A total of 197 CRC patients (107 male and 90 female)
with Dukes' stage B and C who underwent surgery at Teikyo
University Hospital between 2000 and 2004 were enrolled in this
study. The observation period ranged from 22 to 60 months, and the
median follow-up period was 37 months. Study protocol conformed to
the guidelines of the ethics committee of each university. Written
informed consent was obtained from all patients. Their ages ranged
from 27–82 years, with a mean age of 68 years. The stages of the
tumors were determined according to the Dukes classification
system. As a follow-up, all patients were re-evaluated at 3-month
intervals during the first year, and then at 18 months, 24 months
and yearly thereafter. Each evaluation consisted of a pertinent
medical history, physical examination and repetition of imaging
studies, including CT scans of the abdomen.
Blood sampling and cDNA preparation
As controls, portal system blood samples collected
from 20 patients with benign diseases were prepared. In CRC
patients, blood samples were obtained from the mesenteric vein
draining the tumor immediately after laparotomy. These samples were
collected in PAXgene tubes, stored at −80˚C, and transferred to
Teikyo University for real-time RT-PCR assay. Total RNA of the
blood samples in PAXgene tubes was extracted using a PAXgene blood
RNA Kit (Qiagen K.K. GmbH, Germany). Extracted total RNA was
reverse transcribed into cDNA using oligo-p(dT)12–18
primers according to the manufacturer's protocol (Invitrogen Corp.,
CA, USA).
Quantitative real-time RT-PCR
The primer and probe sequences of CEA, CK19, CK20,
CD133 and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) have
been previously described (16).
GAPDH was utilized as an internal control. Real-time quantitative
RT-PCR of these transcripts was performed with the LightCycler
instrument (Roche Diagnostics, Mannheim, Germany) as previously
described. The expression levels of CEA CK19, CK20 and CD133 were
normalized by GAPDH, and the ratio of CEA, CK19, CK20 or CD133
copies to the GAPDH copies was calculated.
Statistical analysis
The correlation between the presence of CEA, CK19,
CK20 and CD133 mRNA in the blood and the various clinical
parameters was evaluated using the chi-squared test. Disease-free
survival (DFS) and overall survival (OS) were analyzed using the
Kaplan-Meier method, and the differences were examined using the
log-rank test. Univariate and multivariate analysis were performed
using Cox regression analysis. P-values <0.05 were considered
statistically significant.
Results
Cut-offs of markers and expression of
CEA/CK/CD133 mRNA
The CEA, CK19, CK20 and CD133 mRNA levels were
normalized with GAPDH mRNA levels in the portal system blood
samples from 20 patients with benign diseases prepared for the
control of tumor drainage blood to determine the cut-off levels.
Based on the range of CEA/GAPDH, CK19/GAPDH, CK20/GAPDH and
CD133/GAPDH, we determined the cut-off value by the 95% confidence
intervals (mean plus 1.96 standard deviation) of the control
groups. In the portal system blood samples, cut-off ratios were
5.7×10−6 in CEA/GAPDH and 9.5×10−5 in
CK19/GAPDH, 2.9×10−5 in CK20/GAPDH and
4.7×10−4 in CD133/GAPDH. The positive rates, sensitivity
and specificity of various combinations of genetic markers were
examined (data not shown). As previous reported, CEA+,
CK19+, CK20+, and/or CD133+
(CEA/CK/CD133) showed the highest positivity, sensitivity and
specificity in the multimarker groups (16). Therefore, we selected the
CEA/CK/CD133 group as representative of PCR positivity and used it
for the following prognostic analysis.
Relationship between CEA/CK/CD133 mRNA
and clinicopathological factors
The relationship between the expression of
CEA/CK/CD133 in the tumor drainage blood, and the
clinicopathological factors was examined. A significant correlation
was observed between CEA/CK/CD133 expression and the Dukes' stage
(Table I). These results suggest
that the presence of CTC in the tumor drainage blood correlated to
the tumor progression.
 | Table IRelationship between
clinicopathological factors and PCR positivity rates. |
Table I
Relationship between
clinicopathological factors and PCR positivity rates.
| Variables | No. of patients
(n=197) | PCR positive cases
(n=124) | Positive rate
(%) | p-value |
|---|
| Age (years) | 68±11 | | | |
| Sex |
| Male | 107 | 63 | 58.88 | 0.198 |
| Female | 90 | 61 | 67.78 | |
| Tumor size (cm) |
| <5 | 109 | 72 | 66.06 | 0.314 |
| ≥5 | 88 | 52 | 59.09 | |
| Histological
type |
| Well
differentiated | 138 | 92 | 66.67 | 0.098 |
| Poorly
differentiated | 59 | 32 | 54.24 | |
| Lymphatic
invasion |
| Negative | 130 | 83 | 63.85 | 0.715 |
| Positive | 67 | 41 | 61.19 | |
| Venous invasion |
| Negative | 84 | 50 | 59.52 | 0.391 |
| Positive | 113 | 74 | 65.49 | |
| Dukes' stage |
| B | 111 | 63 | 56.76 | 0.041a |
| C | 86 | 61 | 70.93 | |
Kaplan-Meier OS and DFS curve
analysis
The Kaplan-Meier survival curves show the OS and DFS
rates according to the CEA/CK/CD133 gene expression status in CRC
patients with Dukes' stage B and C. In this analysis, the average
follow-up time for OS was 36.1±20.7 months and that of DFS was
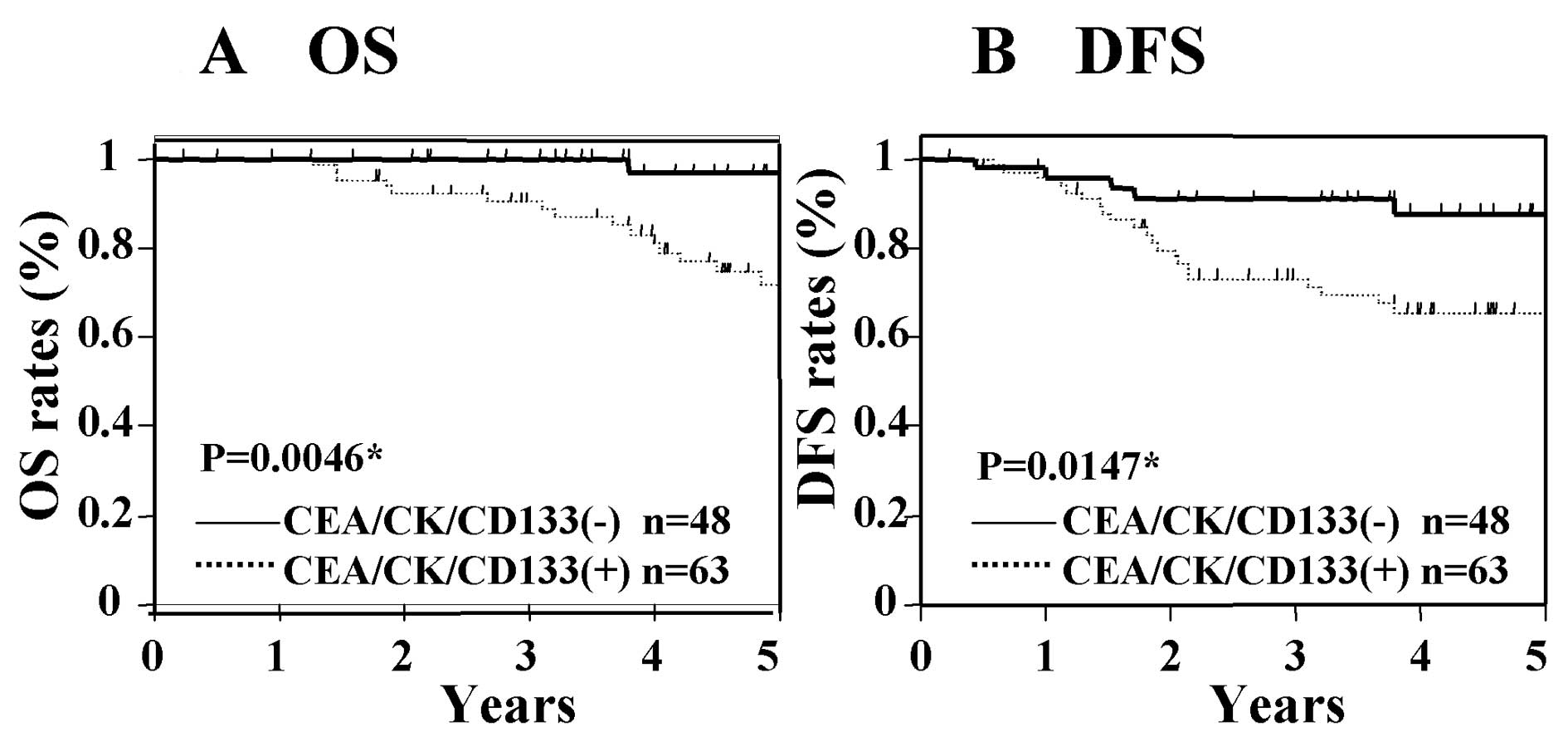
38.8±16.2 months. Fig. 1 shows the
OS and DFS in Dukes' stage B cancer according to the PCR status.
The OS and DFS of those patients with CEA/CK/CD133 positivity were
significantly worse than those who were negative for these markers
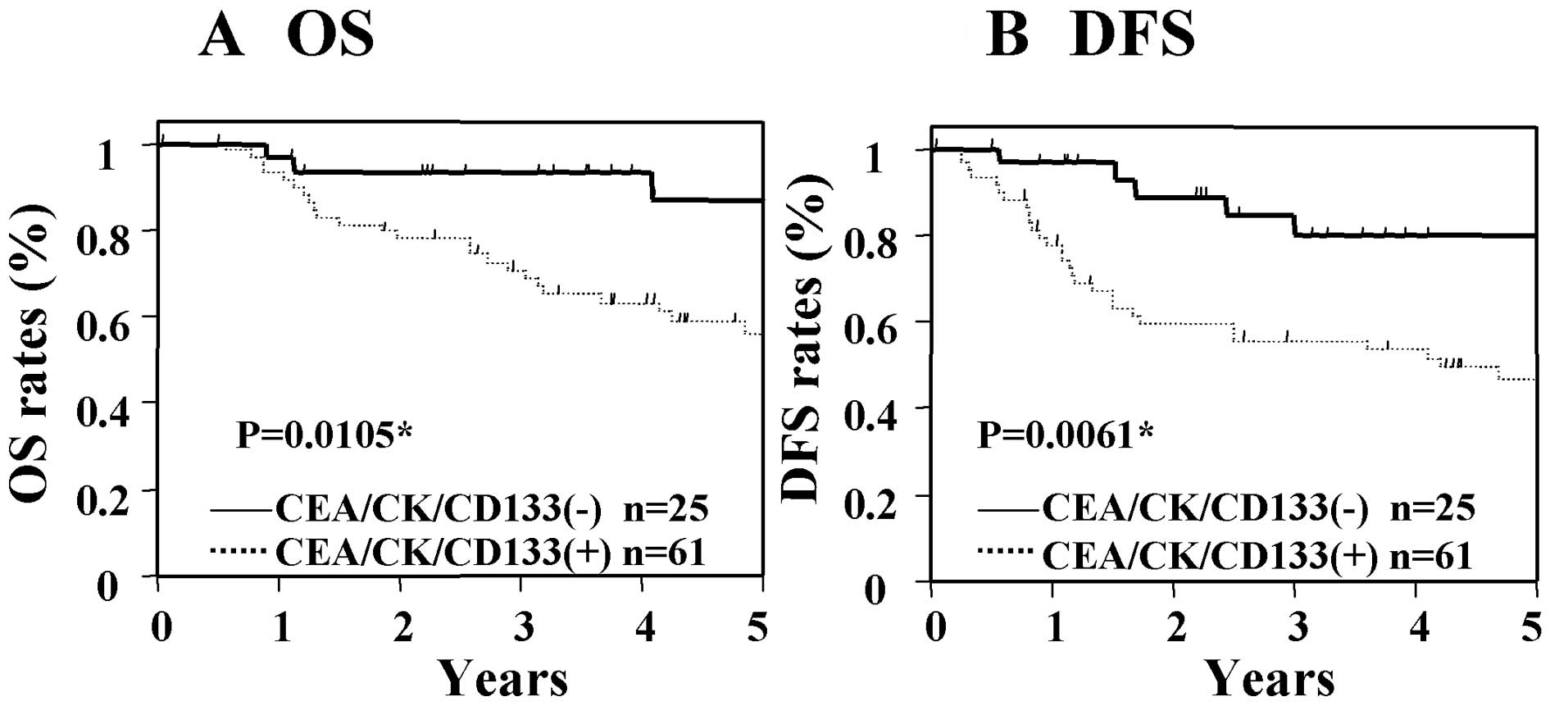
(Fig. 1). In patients with Dukes'
stage C cancer, the OS and DFS of the group with CEA/CK/CD133
positivity were significantly worse than those patients who were
negative for these markers (Fig.
2). These results suggest that the expression of CEA/CK/CD133
in tumor drainage blood is associated with poor prognosis in
patients with Dukes' stage B and C cancer.
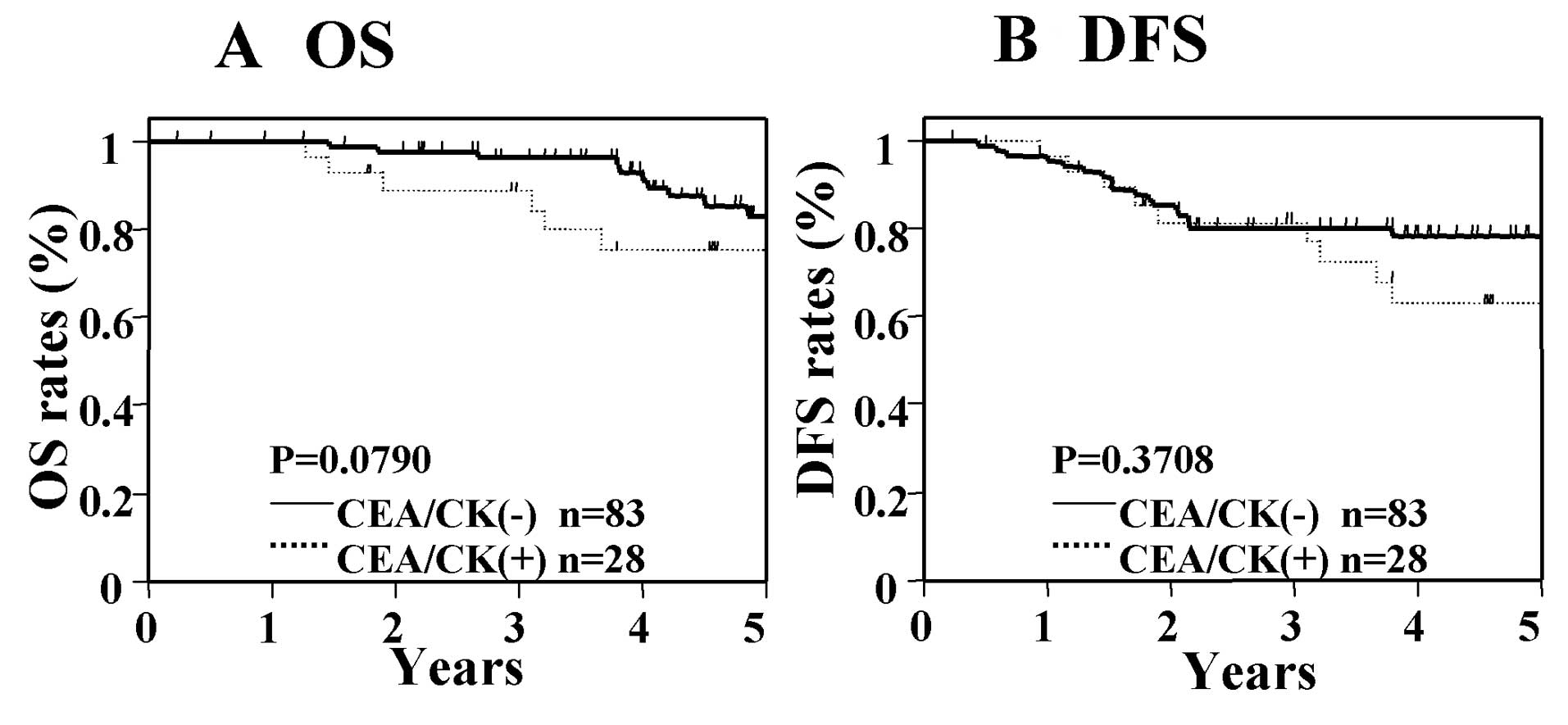
Next, we analyzed the OS and DFS in the general CTCs
markers (CEA, CK19, and/or CK20: CEA/CK), and in the CD133 single
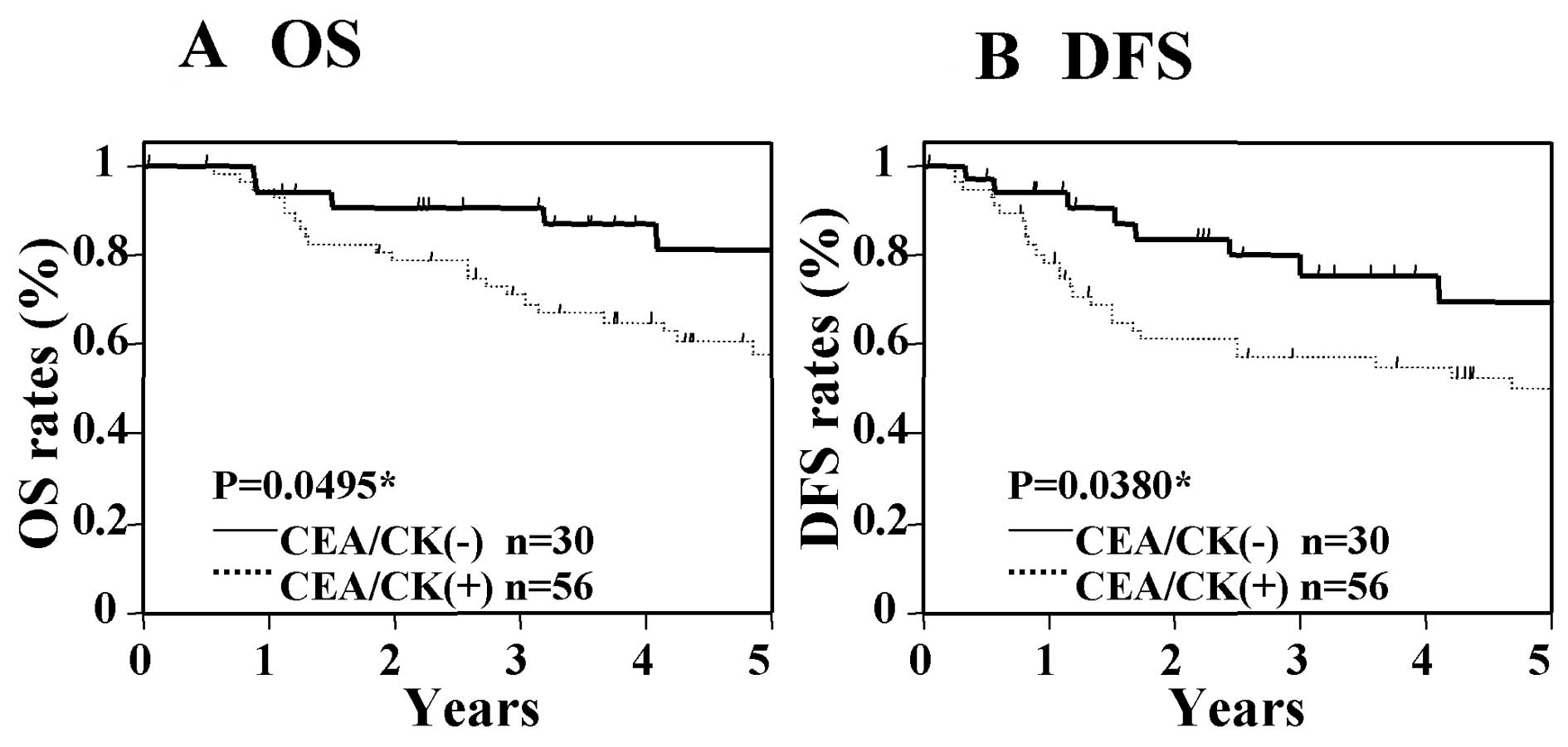
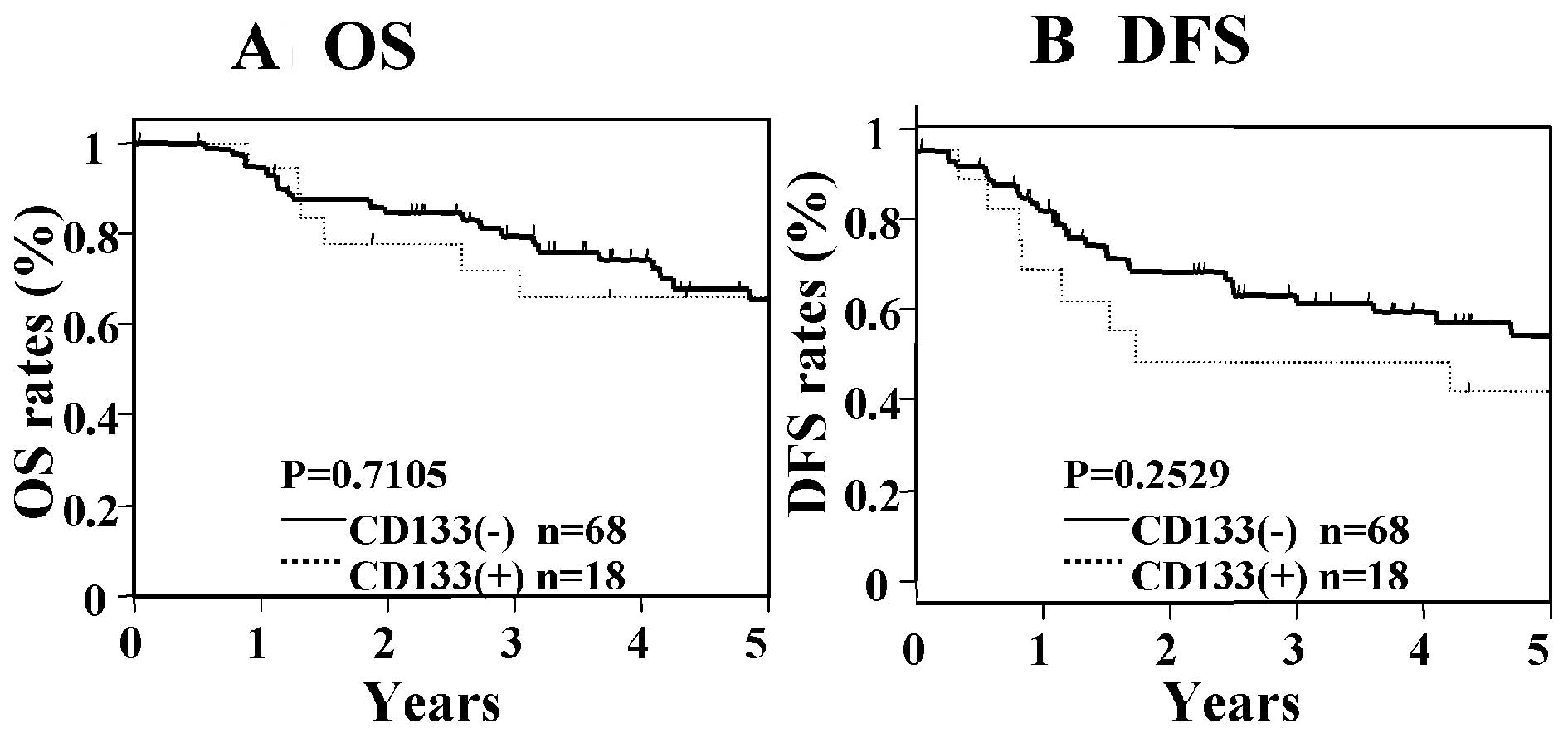
marker. In patients with Dukes' stage B cancer, significant
differences in OS and DFS were not seen between those who were
positive for CEA/CK and those who were negative for CEA/CK
(Fig. 3), nor were significant
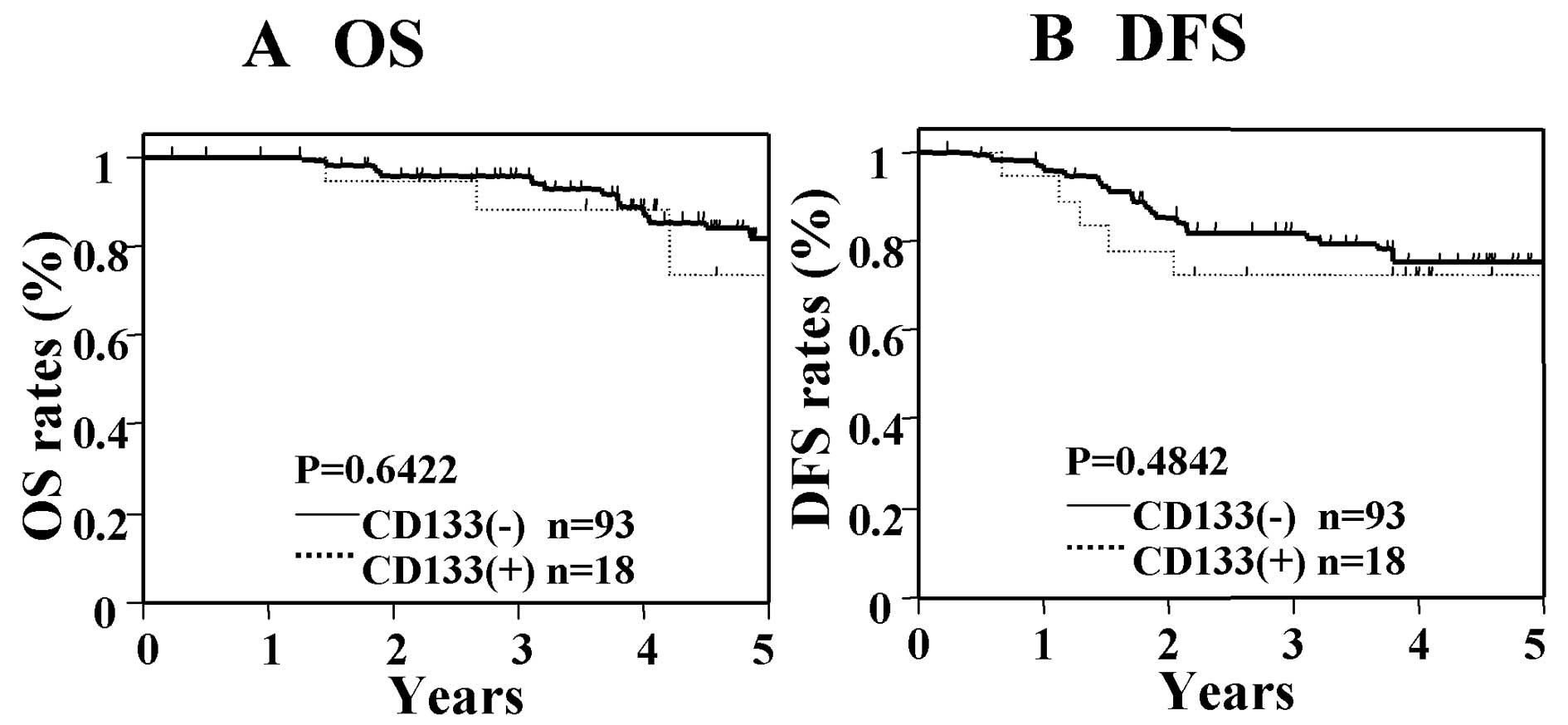
differences found between patients who were positive for CD133 as
compared with those who were negative for CD133 (Fig. 4). In contrast, in patients with
Dukes' stage C cancer, significant differences were seen in OS and
DFS between those who were positive for CEA/CK and those who were
negative for CEA/CK (Fig. 5).
However, in the CD133 single-marker analysis, significant
differences in OS and DFS were not found between patients who were
positive for CD133 and those who were negative for CD133 (Fig. 6). These results suggest that the
addition of CD133 to general CTCs markers is important for the
determination of patients at high risk of recurrence and poor
prognosis in Dukes' stage B cancer.
Cox univariate and multivariate analysis
of prognostic factors
Table II shows the
results of univariate and multivariate Cox analysis of various
factors for OS and DFS in patients with Dukes' stage B cancer. In
univariate analysis of these patients, venous invasion and the
CEA/CK/CD133 showed significance for OS and DFS. Then multivariate
analyses were evaluated in factors which showed significance in
univariate analyses. In these analyses, only CEA/CK/CD133 showed
significance for OS and DFS. Table
III show the results of univariate and multivariate Cox
analysis of various factors for OS and DFS in patients with Dukes'
stage C cancer. In univariate analyses of patients with Dukes'
stage C cancer, lymphatic invasion, venous invasion, serum CEA and
CEA/CK/CD133 showed significance for OS, and venous invasion, serum
CEA, serum CA19-9 and CEA/CK/CD133 showed significance for DFS. In
multivariate analyses, venous invasion and CEA/CK/CD133 showed
significances for OS and DFS. These results suggest that
CEA/CK/CD133 is an independent significant prognostic factor in
patients with Dukes' stage B and C cancer.
 | Table IIUnivariate and multivariate analysis
of prognostic factors for OS and DFS in Dukes' stage B
patients. |
Table II
Univariate and multivariate analysis
of prognostic factors for OS and DFS in Dukes' stage B
patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | Regression
coefficient | Hazard ratio (95%
CI) | P-value | Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| OS |
| Tumor size | 0.349 | 1.417
(0.521–3.858) | 0.488 | | (−) | |
| Lymphatic
invasion | 0.674 | 1.962
(0.665–5.303) | 0.209 | | (−) | |
| Venous
invasion | 1.109 | 3.030
(1.054–10.846) | 0.039a | 1.017 | 2.765
(0.961–9.905) | 0.060 |
| Histological
type | −0.235 | 0.791
(0.181–2.455) | 0.708 | | (−) | |
| Serum CEA | 0.744 | 2.105
(0.768–5.777) | 0.145 | | (−) | |
| Serum CA19-9 | 0.588 | 1.800
(0.394–6.235) | 0.409 | | (−) | |
| CEA/CK/CD133 | 2.347 | 10.456
(2.119–189.993) | 0.001a | 2.284 | 9.819
(1.987–177.568) | 0.002a |
| DFS |
| Tumor size | 0.053 | 1.055
(0.479–2.286) | 0.892 | | (−) | |
| Lymphatic
invasion | 0.570 | 1.768
(0.775–3.844) | 0.169 | | (−) | |
| Venous
invasion | 0.840 | 2.316
(1.040–5.645) | 0.040a | 0.619 | 1.857
(0.824–4.569) | 0.139 |
| Histological
type | 0.376 | 1.456
(0.597–3.242) | 0.389 | | (−) | |
| Serum CEA | 0.798 | 2.222
(0.823–4.901) | 0.064 | | (−) | |
| Serum CA19-9 | 0.240 | 1.271
(0.360–3.546) | 0.680 | | (−) | |
| CEA/CK/CD133 | 1.150 | 3.158
(1.286–9.462) | 0.011a | 1.282 | 3.602
(1.454–10.863) | 0.005a |
 | Table IIIUnivariate and multivariate analysis
of prognostic factors for OS and DFS in Dukes' stage C
patients. |
Table III
Univariate and multivariate analysis
of prognostic factors for OS and DFS in Dukes' stage C
patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | Regression
coefficient | Hazard ratio (95%
CI) | P-value | Regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| OS |
| Tumor size | −0.273 | 0.761
(0.326–1.654) | 0.498 | | (−) | |
| Lymphatic
invasion | 0.810 | 2.248
(1.048–5.004) | 0.038a | 0.647 | 1.910
(0.856–4.373) | 0.114 |
| Venous
invasion | 1.113 | 3.043
(1.243–9.110) | 0.013a | 1.156 | 3.178
(1.177–11.084) | 0.021a |
| Histological
type | 0.401 | 1.493
(0.655–3.229) | 0.328 | | (−) | |
| Serum CEA | 0.838 | 2.313
(1.006–5.958) | 0.048a | 0.560 | 1.751
(0.755–4.543) | 0.198 |
| Serum CA19-9 | 0.721 | 2.056
(0.900–4.613) | 0.086 | | (−) | |
| CEA/CK/CD133 | 1.439 | 4.215
(1.473–17.737) | 0.005a | 1.497 | 4.468
(1.537–18.948) | 0.004a |
| DFS |
| Tumor size | 0.316 | 1.372
(0.692–2.696) | 0.360 | | (−) | |
| Lymphatic
invasion | 0.271 | 1.311
(0.660–2.579) | 0.434 | | (−) | |
| Venous
invasion | 1.263 | 3.535
(1.562–9.476) | 0.002a | 1.172 | 3.228
(1.383–8.834) | 0.006a |
| Histological
type | 0.644 | 1.903
(0.934–3.777) | 0.075 | | (−) | |
| Serum CEA | 0.713 | 2.039
(1.008–4.373) | 0.047a | 0.555 | 1.742
(0.762–4.164) | 0.190 |
| Serum CA19-9 | 0.833 | 2.300
(1.133–4.622) | 0.022a | 0.291 | 1.337
(0.611–2.958) | 0.466 |
| CEA/CK/CD133 | 1.246 | 3.475
(1.466–10.222) | 0.003a | 1.253 | 3.502
(1.423–10.542) | 0.005a |
Discussion
Our study demonstrates that the detection of
CEA/CK/CD133 mRNA in tumor drainage vein blood samples has
prognostic significance in patients with Dukes' stage B and C
CRC.
Evidence is rapidly accumulating that cancers are
composed of heterogeneous populations of cancer cells. This
suggests that CTCs may contain CSCs which are more aggressive and
have invasive and metastatic capacity. We hypothesize that founder
cells at the metastatic site may be CSCs disseminated from the
primary tumor to a distant metastatic site. This hypothesis is
supported by the similarities between the properties of CTCs and
CSCs (19,20). It has been reported that CSCs or
tumor-initiating cell markers are frequently overexpressd in the
CTCs of patients with metastatic breast cancer, and most of them
have CSC phenotypes that are do not proliferate and resistant to
chemotherapy (19). Based on these
reports, we selected multimarkers which included the general CTCs
and the CSCs marker of CRC. Regarding the general marker of CRCs,
several studies support the usefulness of CEA, CK19 and CK20
(9,10,15,21).
In contrast, CSCs markers for CRC patients are still controversial
(17). Previously, several
interesting studies have demonstrated that CD133-positive cells of
CRC have high tumorigenic ability in nude mice (22–24).
Thereafter, it was reported that CD133-negative cells are capable
of initiating CRC growth. The expression of EpCAM, CD166 and CD44
in CRC is also associated with aggressive tumor phenotypes
(25). Thus far, several markers
such as CD133, CD44, CD166, CD24, CD29, leucine-rich
repeat-containing G-protein-coupled receptor 5 (Lgr5), and aldehyde
dehydrogenase 1 (ALDH1) have been reported (24). Although CD133 may have limitation
for CSCs, we elected the CD133, which is a key marker for CSCs. In
this study, we used five genetic markers consisting of CEA, CK19,
and CK20 for the general CTC markers, and CD133 for the CSC.
Next, we evaluated the prognostic value of CTC/CSC
in the tumor drainage vein blood of patients with Dukes' stage B
and C. Using general CTCs markers such as CEA and CK, many studies
have reported a significant correlation between the presence of
CTCs of PB and survival (10,21,26).
Previously, we demonstrated that CEA/CK20 in the tumor drainage
vein blood was an independent prognostic marker for OS and DFS when
using Kaplan-Meier and Cox multivariate analysis (9). In contrast, no studies have examined
the prognostic value of CTCs of tumor drainage vein blood in each
Dukes' stage. In the present study, we have demonstrated that the
detection of CEA/CK/CD133 in tumor drainage vein blood samples has
prognostic value in patients with Dukes' stage B and C. To the best
of our knowledge, our study is the first to demonstrate the
prognostic significance of CTC/CSC in the tumor drainage vein blood
of these patients.
Furthermore, to clarify the clinical value of CD133
addition in Dukes' stage B and C, we re-analyzed the prognostic
significance of the general CTC marker (CEA/CK) group and the CD133
group separately using the Kaplan-Meier survival curve analysis.
Our results demonstrated that CEA/CK/CD133, but not CEA/CK, is
prognostic factor in patients with Dukes' stage B. In contrast, not
only CEA/CK/CD133 but also CEA/CK showed significance in patients
with Dukes' stage C. These results were similar to ones we obtained
earlier when we examined PB samples (16). To date, the determination of
patients with Dukes' stage B who are at high risk for recurrence is
difficult, and efforts to find a tool to select them are ongoing.
In this study, we used the real-time RT-PCR method, and it was
difficult to examine whether individual cells express all markers
or not. We speculate that CTC/CSC, which includes the
CD133-positive cells, may enable identification of a certain
subgroup that has aggressive cancer stem-like cell properties and
that this may increase their prognostic value in Dukes' stage B and
C cancer. As for the reason why the CD133 single marker did not
show any prognostic value in the Dukes' B and C patients, we
speculate that it may be due to the potential limitation of CD133
as CSC marker.
In this study, we established that CEA/CK/CD133 mRNA
detection of tumor drainage vein blood is a useful tool for the
determination of high risk patients with Dukes' stage B and C who
are in need of postoperative adjuvant therapy.
Acknowledgements
We thank Ms. J. Tamura for her excellent technical
support and members of colorectal group for the sampling of
clinical specimens. This study was supported in part by the
Grant-in-Aid for Scientific Research, no. C21591734, from the
Ministry of Health, Labor and Welfare of Japan.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CTC
|
circulating tumor cells
|
|
CSC
|
cancer stem cells
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
CEA
|
carcinoembryonic antigen
|
|
CK20
|
cytokeratin 20
|
|
CK19
|
cytokeratin 19
|
|
GAPDH
|
glyceraldehyde-3-phosphate-dehydrogenase
|
References
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
2
|
Winawer S, Fletcher R, Rex D, et al:
Colorectal cancer screening and surveillance: clinical guidelines
and rationale-Update based on new evidence. Gastroenterology.
124:544–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Compton CC, Fielding LP, Burgart LJ, et
al: Prognostic factors in colorectal cancer. College of American
Pathologists Consensus Statement 1999. Arch Pathol Lab Med.
124:979–994. 2000.PubMed/NCBI
|
|
4
|
Guerra A, Borda F, Javier Jimenez F,
Martinez-Penuela JM and Larrinaga B: Multivariate analysis of
prognostic factors in resected colorectal cancer: a new prognostic
index. Eur J Gastroenterol Hepatol. 10:51–58. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hundt S, Haug U and Brenner H: Blood
markers for early detection of colorectal cancer: a systematic
review. Cancer Epidemiol Biomarkers Prev. 16:1935–1953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung KY and Kelsen D: Adjuvant therapy
for stage II colorectal cancer; who and with what? Cut Treat
Options Gastroenterol. 9:272–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Jatokoe T, Zhang Y, et al: Gene
expression profiles and molecular markers to predict recurrence of
Dukes' B colon cancer. J Clin Oncol. 22:1564–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engell HC: Cancer cells in the circulating
blood; a clinical study on the occurrence of cancer cells in the
peripheral blood and in venous blood draining the tumor area at
operation. Ugeskr Laeger. 117:822–823. 1995.
|
|
9
|
Iinuma H, Okinaga K, Egami H, et al:
Usefulness and clinical significance of quantitative real-time
RT-PCR to detect isolated tumor cells in the peripheral blood and
tumor drainage blood of patients with colorectal cancer. Int J
Oncol. 28:297–306. 2006.PubMed/NCBI
|
|
10
|
Peach G, Kim C, Zacharakis E, Purkayastha
S and Ziprin P: Prognostic significance of circulating tumour cells
following surgical resection of colorectal cancers: a systematic
review. Br J Cancer. 102:1327–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riethdorf S, Wikman H and Pantel K:
Biological relevance of disseminated tumor cells in cancer
patients. Int J Cancer. 123:1991–2006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tol J, Koopman M, Miller MC, et al:
Circulating tumour cells early predict progression-free and overall
survival in advanced colorectal cancer patients treated with
chemotherapy and targeted agents. Ann Oncol. 21:1006–1012. 2010.
View Article : Google Scholar
|
|
13
|
Yen LC, Yeh YS, Chen CW, et al: Detection
of KRAS oncogene in peripheral blood as a predictor of the response
to cetuximab plus chemotherapy in patients with metastatic
colorectal cancer. Clin Cancer. 15:4508–4513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Bono JS, Attard G, Adjei A, et al:
Potential applications for circulating tumor cells expressing the
insulin-like growth factor-I receptor. Clin Cancer. 13:3611–3616.
2007.PubMed/NCBI
|
|
15
|
Thorsteinsson M and Jess P: The clinical
significance of circulating tumor cells in non-metastatic
colorectal cancer: a review. Eur J Surg Oncol. 37:459–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iinuma H, Watanabe T, Mimori K, et al:
Clinical significance of circulating tumor cells, including cancer
stem-like cells, in peripheral blood for recurrence and prognosis
in patients with Dukes' stage B and C colorectal cancer. J Clin
Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumors:accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View
Article : Google Scholar
|
|
20
|
Balic M, Lin H, Young L, et al: Most early
disseminated cancer cells detected in bone marrow of breast cancer
patients have a putative breast cancer stem cell phenotype. Clin
Cancer. 12:5615–5621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sergeant G, Penninckx F and Topal B:
Quantitative RT-PCR detection of colorectal tumor cells in
peripheral blood: a systematic review. J Surg Res. 150:144–152.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumor
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Richardson GD, Robson CN, Lang SH, et al:
CD133, a novel marker for human prostatic epithelial stem cells. J
Cell Sci. 117:3539–3545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lugli A, Iezzi G, Hostettler I, et al:
Prognostic impact of the expression of putative cancer stem cell
markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer.
Br J Cancer. 103:382–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahbari NN, Aigner M, Thorlund K, et al:
Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|