Introduction
Type II endometrial carcinoma (EC) is one of the
most common gynecologic malignancies and is usually estrogen
receptor (ER) negative, poorly differentiated and high grade, with
a poor prognosis (1–3). Although great progress has made in the
development of therapies for type II EC, the current 5-year
survival rate remains <15%, and the molecular mechanisms
regulating the development and progression of type II EC are not
fully understood. The most important causes of treatment failure in
type II EC are metastasis, recurrence and the development of
chemotherapy resistance (1);
however, these mechanisms are poorly characterized.
Tumors consist of heterogeneous cell populations
with different biological properties, and recent evidence suggests
that the capacity for tumor formation and growth resides
exclusively within a small proportion of tumor cells, termed
carcinoma-initiating cells (CICs) (4–7). CICs
were first isolated from acute myeloid leukemia, in which they
express the cell-surface antigen CD34, but not CD38 (8,9). CICs
have also been isolated from primary tumors and cell lines using
flow cytometry on the basis of cell-surface antigen expression
(8,10–22).
For instance, the CIC population is defined as CD133+ in
brain (18), melanoma (20) and lung tumors (21); CD34+ or
EpCAMhigh/+/CD44+/CD166+ in colon cancer
(10–12);
CD44+/CD24low/-/lineage− in breast tumors
(10,14) and CD44+/CD133+
in ovarian tumors (8,12,13).
The observation that stem cells and some CICs share the common
defining features of an incompletely differentiated state and the
capacity for self-renewal has led to the cancer stem cell
hypothesis, which suggests that the proliferation of a small
sub-population of cells is responsible for the total tumor growth
(5–7,15).
Currently, clinical pathology characterizes type II
EC as consisting mostly of serous and clear cell carcinomas, which
typically arise in the atrophic endometrium via a mechanism
unrelated to estrogen exposure (1).
However, CICs have not yet been isolated from type II EC. In this
study, we aimed to sort sub-populations of CICs from human type II
EC cell lines, termed EC-CICs, with rates of high proliferation,
migration and multi-drug resistance. Consistent with previous
reports (4–22), we sorted a subpopulation of cells
overexpressing CD44 and CD133 at the cell surface
(CD44+/CD133+) from the ER-negative human
type II EC cell lines, KLE and AN3CA, using magnetic microbeads and
flow cytometry. We demonstrated that a subpopulation of
CD44+/CD133+ EC-CICs, which proliferate
rapidly and exhibit multi-drug resistance, exist in KLE and AN3CA
cells. Therefore, EC-CICs represent a potentially powerful in
vitro model to study metastasis, invasion and the self-renewal
of cancer cells, and to assess the effectiveness of novel
therapeutics for type II EC.
Materials and methods
Isolation and in vitro expansion of CD44
and CD133 phenotype cells by magnetic-activated cell sorting
system
CD44+ and CD133+ subpopulation
cells were isolated from human type II endometrial carcinoma cell
line KLE and AN3CA using 4 μl of the primary monoclonal antibodies
(mouse anti-human CD133-FITC, rabbit anti-human CD44-PE,
eBioscience) stored at 4°C in PBS for 30 min in a volume of 1 ml as
previously described (3,8,11,22).
After reaction, the cells were washed twice in PBS, and the
secondary monoclonal antibodies (goat anti-mouse or goat
anti-rabbit coupled to magnetic microbeads; Miltenyi Biotec,
Auburn, CA) added, incubated at 10°C in PBS for 15 min and then
washed twice in PBS. Single cells were plated at 1000 cells/ml in
DMEM: F12 (HyClone), supplemented with 10 ng/ml basic fibroblast
growth factor (bFGF), 10 ng/ml epidermal growth factor (EGF), 5
μg/ml insulin and 0.5% bovine serum albumin (BSA) (all from
Sigma-Aldrich). The CD44+/CD133+ cells were
cultured in above conditions as non-adherent spherical clusters,
EC-CICs, and CD44−/CD133− cells in KLE or
ANCA3 which was cultured under general conditions as adherent
clusters, EC-CCs. Cells were cultured on the same conditions until
passage 4 before carrying out the experiments.
Quantitative real-time PCR analysis of
stem cell marker expression
Total RNA from the cells was isolated using TRIzol
reagent (Invitrogen) according to the manufacturer’s protocol. The
RNA samples were treated with Dnase I (Sigma-Aldrich), quantified,
and reverse-transcribed into cDNA using the ReverTra Ace-α First
Strand cDNA Synthesis kit (Toyobo). Quantitative real-time PCR
(qRT-PCR) was conducted using a RealPlex4 real-time PCR detection
system from Eppendorf Co., Ltd. (Germany), with SyBR Green
real-time PCR Master MIX (Toyobo) used as the detection dye.
qRT-PCR amplification was performed over 40 cycles with
denaturation at 95°C for 15 sec and annealing at 58°C for 45 sec.
Target cDNA was quantified using the relative quantification
method. A comparative threshold cycle (Ct) was used to determine
gene expression relative to a control (calibrator) and steady-state
mRNA levels are reported as an n-fold difference relative to the
calibrator. For each sample, the gene Ct value was normalized using
the formula ΔCt = Ctmarkers - Ct18s rRNA. To
determine relative expression levels, the following formula was
used ΔΔCt = ΔCtCICs - ΔCtCCs. The values to
plot relative expressions of markers were calculated using the
expression 2−ΔΔCt. The mRNA levels were calibrated based
on levels of 18s rRNA. The cDNA of each stem cell markers was
amplified using primers as previously described (11).
Multi-chemodrugs resistant assay
The chemodrugs (cisplatin, paclitaxel, adriamycin
and methotrexate) resistant assay of the cells was performed as
previously described (11).
Western blot analysis
Protein extracts of the cell were resolved by 12%
SDS-PAGE and transferred on PVDF (Millipore) membranes. After
blocking, the PVDF membranes were washed 4 times for 15 min with
TBST at room temperature and incubated with primary antibody
(rabbit anti-human CD133, rabbit anti-human CD44, all from Santa
Cruz Biotechnology). Following extensive washing, membranes were
incubated with secondary peroxidase-linked goat anti-rabbit IgG
(Santa Cruz Biotechnology) for 1 h. After washing 4 times for 15
min with TBST at room temperature once more, the immunoreactivity
was visualized by enhanced chemiluminescence (ECL kit, Pierce
Biotechnology).
Immunofluorescence staining analysis of
relative protein expression
The cultured cells were washed 3 times with FCS and
fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA)
for 30 min. After blocking, the cells were incubated first with
rabbit anti-human Oct3/4 polyclonal antibody (1:200; Chemicon,
Temecula, CA, USA) and rabbit anti-human Nanog polyclonal antibody
(1:200; Chemicon, Temecula) overnight at 4°C, and then with
FITC-conjugated goat anti-rabbit IgG antibody (1:200; Abcam,
Cambridge, UK) and 5 μg/ml DAPI (Sigma-Aldrich) at room temperature
for 30 min. Then the cells were thoroughly washed with TBST and
viewed through a fluorescence microscope (DMI3000; Leica,
Allendale, NJ, USA).
Soft agar colony formation assay
All the steps were as previously described (2,23).
Soft Agar Assays were constructed in 6-well plates. The base layer
of each well consisted of 2 ml with final concentrations of 1×
media (DMEM+10% FBS) and 0.6% low melting point agarose. Plates
were chilled at 4°C until solid. Upon this, a 1.0-ml growth agar
layer was poured, consisting of 1×104 cells suspended in
1× media and 0.3% low melting point agarose. Plates were again
chilled at 4°C until the growth layer congealed. An additional 1.0
ml of 1× media without agarose was added on top of the growth layer
on Day 0 and again on Day 15 of growth. Cells were allowed to grow
at 37°C for 1 month and total colonies counted. Assays were
repeated a total of 3 times. Results were statistically analyzed by
paired t-test using the PRISM Graphpad program.
Transwell migration assay
All the steps were as previously described (2,23).
Cells (2×105) were resuspended in 200 μl of serum-free
medium and seeded on the top chamber of the 8.0-μm pore, 6.5 mm
polycarbonate transwell filters (Corning). The full medium (600 μl)
containing 10% FBS was added to the bottom chamber. The cells were
allowed to migrate for 24 h at 37°C in a humidified incubator with
5% CO2. The cells attached to the lower surface of
membrane were fixed in 4% paraformaldehyde at room temperature for
30 min and stained with 4,6-diamidino-2-phenylindole (DAPI) (C1002,
Beyotime Institute of Biotechnology, China), and the number of
cells on the lower surface of the filters was counted under the
microscope. A total of 5 fields were counted for each transwell
filter.
In vivo xenograft experiments
Cells (6×105) (EC-CICs oEC normal cell
lines) were inoculated s.c. in athymic nude mice, 6–7 weeks of age.
The animal studies were carried out at Tongji University with
Institutional Anminal Care and Use Committer approval in accordance
with institutional guidelines.
Statistical analysis
Each experiment was performed as least three times,
and data are shown as the mean ± SE where applicable, and
differences were evaluated using Student’s t-tests. The probability
of <0.05 was considered to be statistically significant.
Results
To determine whether CICs exist in human type II EC
cell lines, CD44 and CD133 were used as markers of CICs to isolate
endometrial CICs from two human type II EC cell lines, KLE and
ANCA3. Then, the stemness, proliferation, migration and multi-drug
resistance of CD44+/CD133+ cells (EC-CICs)
and CD44−/CD133− endometrial cancer cells
(EC-CCs) were assayed.
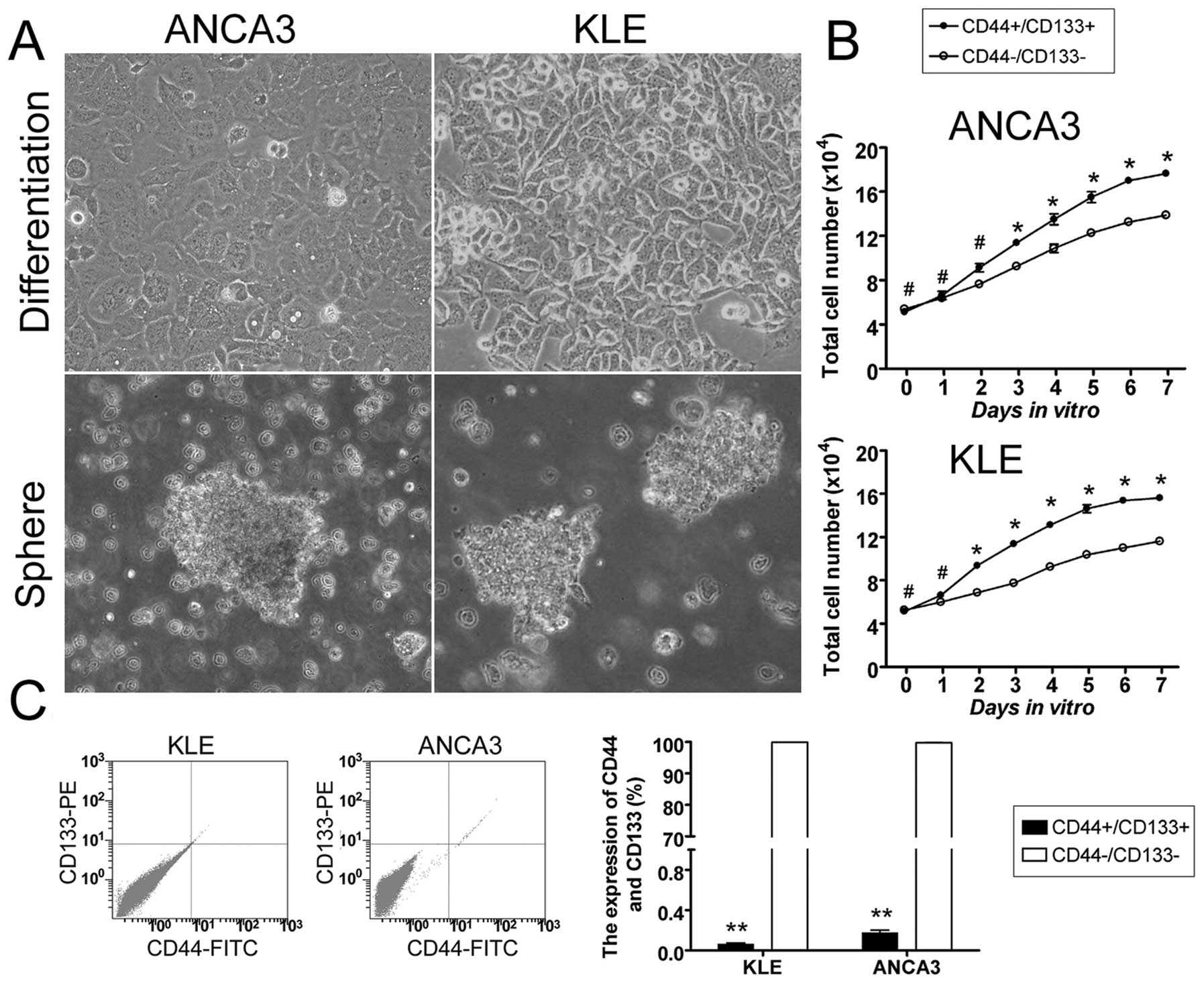
Isolation and enrichment of
CD44+ and CD133+ EC-CICs
Previous studies have suggested that the human
CD44+/CD133+ CIC subpopulation is relatively
small in many tumor types. Therefore, we used a magnetic-activated
cell sorting system to isolate and enrich the CD44 and CD133
subpopulation from two type II EC cell lines. After isolation, the
cells were quantified by flow cytometry (FCM).
CD44+/CD133+ cells represented 0.063±0.012%
and 0.177±0.024% of the total population in KLE and ANCA3 cell
lines, whereas CD44−/CD133− cells represented
99.897±0.009% and 99.743±0.041% of the total population,
respectively (Fig. 1). These
results demonstrated that CD44+/CD133+ cells,
although very exiguous, could be successfully enriched from human
type II EC cell lines using magnetic-activated cell sorting.
CD44 and CD133 EC-CIC proliferation
The proliferation rates of EC-CICs
(CD44+/CD133+ cells) and EC-CCs
(CD44−/CD133− cells) were examined on days
1–7 after passage. All measurements were repeated in triplicate.
There was no significant difference in the number of KLE EC-CICs
and EC-CCs on days 0 and 1. However, between days 2 and 7, KLE
EC-CICs divided significantly more rapidly than EC-CCs (p<0.05,
t-test). Similarly, there was no significant difference in the
number of ANCA3 EC-CICs and EC-CCs on days 0 and 2; however,
between days 3 and 7, ANCA3 EC-CICs divided significantly more
rapidly than EC-CCs (p<0.05, t-test).
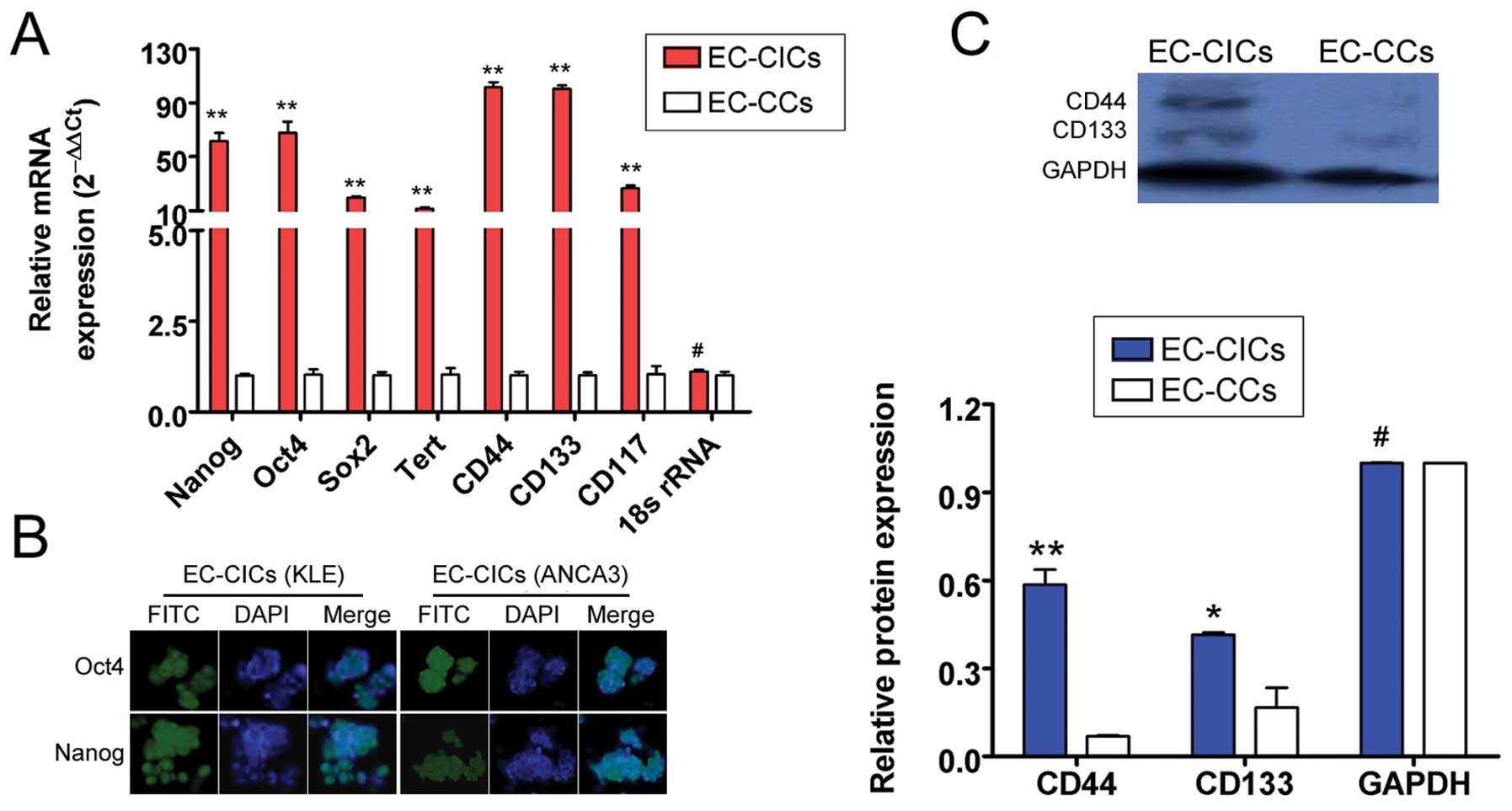
CD44+/CD133+
EC-CICs overexpress stem cell markers
Quantitative real-time polymerase chain reaction
(qRT-PCR) was used to compare the relative gene expression levels
of several stem cell markers in EC-CICs and EC-CCs, using 18s rRNA
as an internal control. The expression of Nanog,
Oct4, Sox2, Tert, ABCG2, CD44,
CD133 and CD117 were all significantly higher in
EC-CICs than EC-CICs (p<0.05; Fig.
2). Immunofluorescence staining (IF) confirmed that
CD44+/CD133+ EC-CICs expressed higher levels
of the stem cell markers Nanog and Oct4 than EC-CCs (Fig. 2). These results suggested that both
KLE and ANCA3 EC-CICs possess stem cell characteristics.
Additionally, expression of CD44 and CD133 were
evaluated in KLE and ANCA3 EC-CICs and EC-CCs using western blot
analysis. In EC-CICs, the levels of CD44 and CD133 were
0.586±0.051% and 0.415±0.008% of KLE and ANCA3, respectively
(Fig. 2). These values were
significantly higher than CD44 and CD133 in EC-CCs (0.068±0.003 and
0.168±0.068 of KLE and ANCA3, respectively). These results
confirmed that both CD44 and CD133 are expressed at high levels in
EC-CICs.
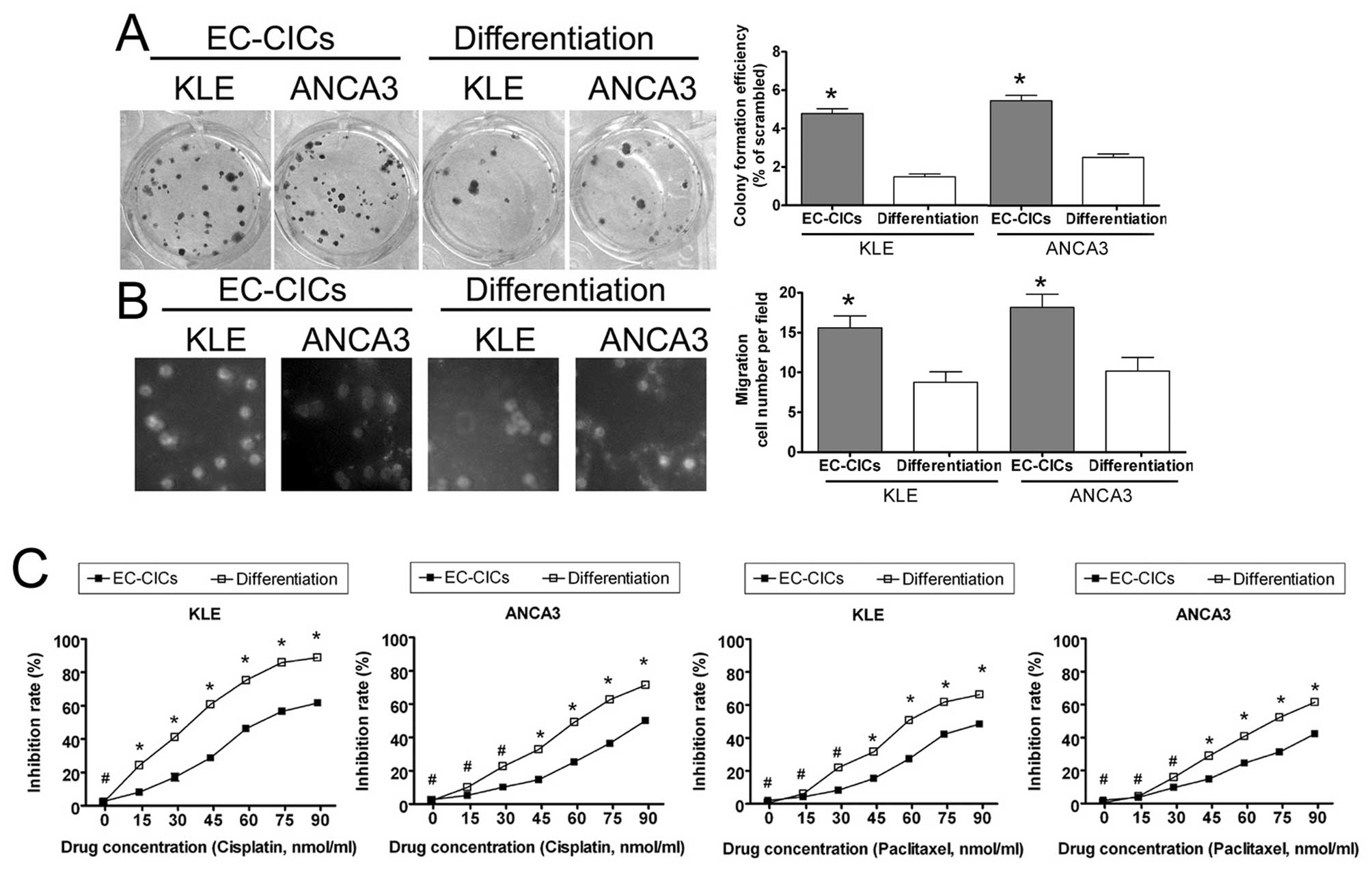
CD44+/CD133+
EC-CICs have increased migratory and invasive ability
The ability of EC-CICs and EC-CCs to migrate and
invade were determined using the transwell migration assay and soft
agar colony formation assay, respectively. The transwell migration
invasion assay showed that significantly fewer EC-CCs invaded,
compared to EC-CICs (invading cell numbers: KLE EC-CICs 16±2 vs.
EC-CCs 9±1, p<0.05; ANCA3 EC-CICs 18±2 vs. EC-CCs 10±2,
p<0.05; Fig. 3). Moreover, the
soft agar colony formation assay indicated that EC-CCs formed
substantially fewer colonies when plated at low density than
EC-CICs (colony formation efficiency: KLE EC-CCs 1.48±0.16% vs.
EC-CICs 4.78±0.25%, p<0.05; ANCA3 EC-CCs 2.52±0.18% vs. EC-CICs
5.48±0.29%, p<0.05; Fig. 3).
CD44+/CD133+
EC-CICs exhibit multi-drug resistance
In order to evaluate the multi-drug resistance of
EC-CICs and EC-CCs, the inhibitory rates of cisplatin and
paclitaxel (0, 15, 30, 45, 60, 75 and 90 nmol/ml) were measured
using the MTT proliferation assay. The growth of both EC-CICs and
EC-CCs were inhibited by cisplatin and paclitaxel; however, EC-CICs
were significantly less susceptible to the cytotoxic effects of the
drugs (Fig. 3). Thus,
CD44+/CD133+ EC-CICs were more resistant to
cisplatin and paclitaxel than CD44−/CD133−
EC-CCs, suggesting that the CD44+/CD133+
subpopulation may be resistant to a broad spectrum of
chemotherapeutics.
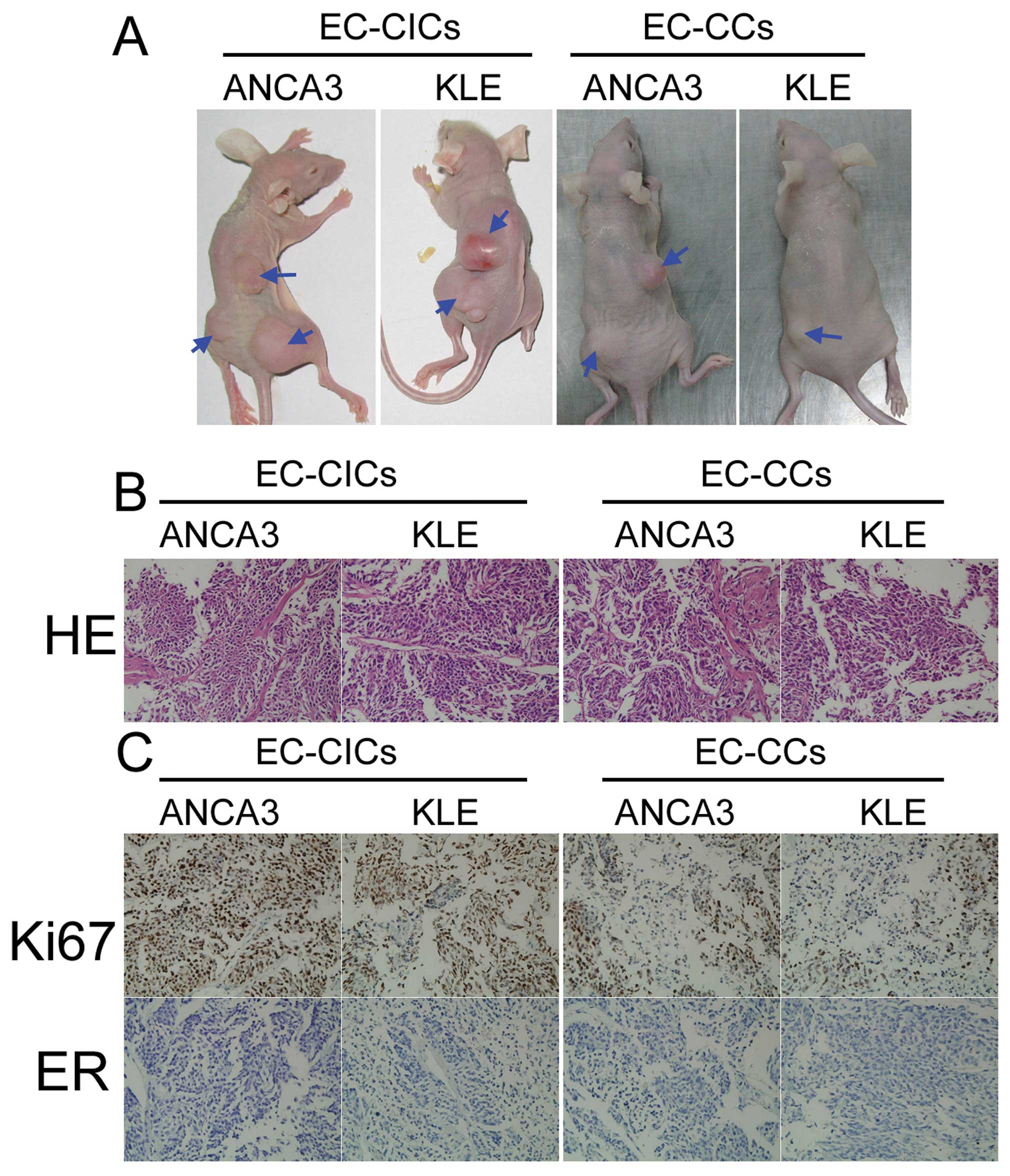
The CD44+/CD133+
subpopulation of EC-CICs induces tumor growth in vivo
In order to evaluate the tumorigenic capacity of
EC-CICs and EC-CCs, 7×104 EC-CICs or EC-CCs were
subcutaneously inoculated into athymic nude mice. Tumors were
visible in the EC-CIC-injected mice after 68 days; however,
EC-CC-injected mice did not have detectable tumors at this time
(Fig. 4). Very small tumors were
detected in EC-CC-injected mice after 94 days. When the mice were
sacrificed 110 days after injection, the tumors formed by EC-CICs
were significantly heavier than the tumors formed by EC-CCs. As
tumor growth is determined by the balance of cell proliferation and
programmed cell death, the cell proliferation-related protein Ki-67
was analyzed in the tumor sections using immunohistochemistry. The
tumors formed by EC-CICs displayed positive or strongly positive
Ki-67 staining, while the tumors formed by EC-CCs exhibited only
weak Ki-67 immunoreactivity (Fig.
4). Moreover, HE staining revealed cellular heterogeneity in
both the EC-CIC and EC-CC tumors. Taken together, the in
vivo xenograft model indicated that low numbers of
CD44+/CD133+ EC-CICs have the potential to
initiate tumor growth.
Discussion
Increasing numbers of studies have indicated the
presence of cancer-initiating cells (CICs, also known as cancer
stem cells) in most tumor types. CICs are thought to play an
important role in the recurrence, metastasis and multi-drug
resistance of cancer. CICs have several prominent characteristics,
including clonogenicity, the ability to self-renew and
differentiate in vitro to form organized spheroids in
suspension, and the expression of multipotency and tissue-specific
differentiation markers. CICs can also generate tumors in
vivo through self-renewal mechanisms, and undergo
differentiation in vivo to produce diseases similar to those
in human patients (11).
Additionally, the stem cell marker receptor, CD117 (also known as
c-kit), is expressed in various CICs, as well as by stem cells and
hematopoietic progenitor cells. However, CICs from human
endometrial carcinoma, especially type II EC, have not previously
been isolated. There is ample evidence to demonstrate the
importance of CD44 expression during the progression of many tumor
types, and CD44, a glycoprotein receptor which binds hyaluronan, is
expressed by many CICs (24). CD44
is encoded by a single gene and varies in size due to alternative
splicing of the extracellular domains and N-glycosylation or
O-glycosylation (24). In this
study, CD44 and the crucial stem cell marker CD133 were used as
markers to screen, isolate and enrich CICs from human type II EC
cell lines.
We identified a subpopulation of cells which express
high levels of both CD44 and CD133 on the cell membrane in the
human type II EC cell lines KLE and ANCA3. The
CD44+/CD133+ subpopulation overexpressed
several stem cell markers, including Nanog, Oct4,
Sox2, Tert and CD177.
CD44+/CD133+ cells proliferated at a higher
rate, and the transwell migration assay and soft agar colony
formation assay demonstrated that the
CD44+/CD133+ subpopulation had a increased
migratory and invasive ability, compared to
CD44−/CD133− cells. Additionally, the
CD44+/CD133+ subpopulation was more resistant
to the chemotherapeutic agents, cisplatin and paclitaxel, and
readily and rapidly formed xenografts in vivo from extremely
small numbers of cells. As the CD44+/CD133+
subpopulation exhibited the classical characteristics of stem
cells, we suggest that the CD44+/CD133+
subpopulation are endometrial carcinoma-initiating cells (EC-CICs).
Moreover, as EC-CICs possess common stem cell characteristics and
exhibit multi-drug resistance, these cells may serve as an
experimental platform to both study tumor cell physiology and
examine the effectiveness of clinical therapeutics for type II
EC.
In conclusion, CD44 and CD133 can be used to isolate
a subpopulation of cells from human type II EC cell lines. The
CD44+/CD133+ EC subpopulation displays the
proliferative, migratory, stem-cell and multi-drug resistance
characteristics of CICs, and may provide an important model for
future studies of therapeutic strategies in type II EC.
Acknowledgements
This study was supported by grant from Shanghai
Municipal Health Bureau Fund (No. 2010260) to Y.G.
References
|
1
|
Gehrig PA and Bae-Jump VL: Promising novel
therapies for the treatment of endometrial cancer. Gynecol Oncol.
116:187–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang F, Liu T, He Y, et al: MiR-125b
promotes proliferation and migration of type II endometrial
carcinoma cells through targeting TP53INP1 tumor suppressor in
vitro and in vivo. BMC Cancer. 11:4252011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marx J: Cancer research. Mutant stem cells
may seed cancer. Science. 301:1308–1310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mayol JF, Loeuillet C, Herodin F and Wion
D: Characterisation of normal and cancer stem cells: one
experimental paradigm for two kinds of stem cells. Bioessays.
31:993–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Xu F, Du X, et al: Establishment
and characterization of multi-drug resistant, prostate
carcinoma-initiating stem-like cells from human prostate cancer
cell lines 22RV1. Mol Cell Biochem. 340:265–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Lai D, Liu T, Cheng W and Guo L:
Cancer stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin
(Shanghai). 42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu T, Cheng W, Lai D, Huang Y and Guo L:
Characterization of primary ovarian cancer cells in different
culture systems. Oncol Rep. 23:1277–1284. 2010.PubMed/NCBI
|
|
14
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalerba P, Dylla SJ, Park IK, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
17
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou J, Pan M, Wen P, et al: Isolation and
identification of cancer stem-like cells from murine melanoma cell
lines. Cell Mol Immunol. 4:467–472. 2007.PubMed/NCBI
|
|
23
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zöller M: CD44: can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011.PubMed/NCBI
|