Introduction
Many people suffer from metastatic bone tumors
caused by a wide variety of cancers. A skeletal-related event
(SRE), such as a pathological fracture or spinal cord compression,
and the requirement for orthopedic surgery or palliative
radiotherapy reduce patients’ activity of daily living (ADL) and
quality of life (QOL) and worsen prognosis. Treatment for the
prevention of an SRE improves patients’ ADL and QOL. Osteolytic
change with bone metastasis leads to pain and pathological
fracture. When surgery, chemotherapy and radiotherapy are not
effective, bone destruction progresses. If we could inhibit
progression of osteolytic change and enhance osteogenic change more
than osteolytic change, it may be possible to prevent fracture and
pain and to maintain QOL. In addition, there is a need to develop
non-invasive treatment when considering patients’ general
condition. Low-intensity pulsed ultrasound (LIPUS) stimulation is
known as one of the methods for promoting bone formation in
orthopedics (1). LIPUS stimulation
is an established and widely used intervention for accelerating
fracture healing and bone maturation in distraction osteogenesis,
and delayed fracture union and nonunion in clinical settings
(1–4). To influence bone repair, LIPUS is
distinguished by being non-invasive and easy to apply, and the
LIPUS signal has a sufficiently low intensity to be considered
non-destructive (5). Because tumor
cells in metastatic bone tumors coexist with normal osteocytes in
impending fractures, osteoblasts and osteoclasts are stimulated by
tumor-induced bone destruction all the time. In this condition, we
hypothesized that LIPUS stimulates bone formation similar to normal
fracture healing and prevents progression of pathological
fractures. Effects of LIPUS on cell proliferation, vascularization,
and migration in the process of fracture healing have been reported
in many cases (6,7). Although the mechanisms involved have
not been elucidated, LIPUS stimulation has been reported to affect
osteoblast differentiation without increasing cell number of
osteoblast (8,9). LIPUS was reported to enhance the
effect of an anticancer drug on lymphoma and liver cancer cells
(10). However, there were no
reports of proliferation, vascularization and migration effects on
cancer cells. Therefore, there was a need to investigate the
effects of LIPUS on cancer cells because LIPUS might stimulate
tumor proliferation, vascularization, and migration. In this study,
we investigated the effects of LIPUS treatment on cell viability,
cell proliferation, vascularization, and migration in mouse
osteoblast, mouse and human osteosarcoma, human prostate cancer,
renal cancer, and lung cancer cell lines under the same conditions
as in clinical use.
Materials and methods
Cell culture
We used MC3T3-E1, a mouse osteoblast cell line, LM8,
a mouse osteosarcoma cell line, SaOS2, a human osteosarcoma cell
line, 786-O, a human renal cancer cell line, PC-3, a human prostate
cancer cell line, and A549, a human lung cancer cell line. MC3T3-E1
was maintained in alpha-minimum essential medium (α-MEM) with 10%
fetal calf serum (FCS) containing antibiotics (100 U/ml penicillin
G, 100 mg/ml streptomycin). LM8, SaOS2, 786-O, and A549 were
maintained in Dulbecco’s modified Eagle’s medium (DMEM) with FCS
containing antibiotics. PC-3 was maintained in RPMI-1640 with FCS
containing antibiotics. The cells were cultured in a humidified
atmosphere of 5% CO2 and 95% air at 37°C.
Ultrasound treatment
An ultrasound exposure system, which was made by
Medical Engineering Research Laboratories of Teijin Ltd. (Tokyo,
Japan), consisted of an array of six 2.5-cm-diameter PZT-4
(lead-zirconate titanate) transducers, specially designed for a
6-well tissue culture plate. This array was placed at the bottom of
a water tank, and the culture plate was located above the array.
The temporal average intensity was 30 mW/cm2 and the
frequency was 1.5 MHz with a 200-μs tone burst repeated at 1.0 KHz
(11,12). This setting is the same as in
clinical use. LIPUS was administered for 20 min every day for the
duration of this experiment.
Determination of cell number
MC3T3-E1 cells were seeded in a 6-well plate at a
density of 2.0×104 cells/cm2. LM8, SaOS2,
PC-3, 786-O, and A549 cells were seeded at a density of
1.0×104 cells/cm2. The cells were cultured in
the presence or absence of daily LIPUS stimulation for up to 72 h.
The cells were detached by gentle trypsinization and counted
microscopically using a trypan blue dye exclusion test.
Western blot analysis
MC3T3-E1, LM8, SaOS2, PC-3, 786-O, and A549 cells
were plated in a 6-well tissue cell culture plate at a density of
5.0×104 cells/cm2 and were harvested 5, 30
min, 1, 3, 6, 12, 24, and 48 h after LIPUS stimulation. The cells
were washed twice with PBS and lysed with RIPA buffer [20 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5%
sodium deoxycholate, 40 mM NaF, and protease inhibitor cocktail
(Sigma, St. Louis, MO, USA)]. The lysates were centrifuged at
15,000 rpm for 20 min. The supernatant lysate with sample buffer
[0.0625 M Tris-HCl (pH 6.8), 2% SDS, 5% glycerol, 5% 2-ME] was
boiled at 95°C for 5 min. The samples were separated on sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
then electroblotted onto a nitrocellulose membrane (Amersham
Biosciences, Tokyo, Japan). The membranes were saturated with 5%
(wt/vol) non-fat dry milk in Tris-buffered saline with Tween-20
(TBST) [25 mM Tris-HCl (pH 7.8), 140 mM NaCl, 0.1% (vol/vol)
Tween-20] and then incubated overnight with the following
antibodies (diluted 1:1,000 in TBST): extracellular
signal-regulated kinase (ERK1/2) (Cell Signaling Technology,
Beverly, MA, USA), phosphorylated ERK1/2 (pERK1/2) (Cell Signaling
Technology), Akt (Cell Signaling Technology), phosphorylated Akt
(p-Akt) (Cell Signaling Technology) and β-catenin
(Becton-Dickinson, Franklin Lakes, NJ, USA). The membranes were
washed thoroughly with TBST and incubated for 1 h with horseradish
peroxidase-conjugated anti-mouse or -rabbit IgG (Santa Cruz
Biotechnologies, Santa Cruz, CA, USA) (diluted 1:5,000 in TBST).
Detection was performed with enhanced chemiluminescence kits
(Amersham Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
analysis
MC3T3-E1, LM8, SaOS2, PC-3, 786-O, and A549 cells
were plated in a 6-well tissue cell culture plate at a density of
5.0×104 cells/cm2. After 48 h, each culture
medium was exchanged with 1 ml of serum-free medium. Twenty-four
hours after the medium exchange, the cells were treated with LIPUS.
The supernatants were harvested 0, 3, 6, 12, 24, and 48 h after
LIPUS stimulation. ELISA assays for vascular endothelial growth
factor (VEGF) were performed with the human and mouse VEGF ELISA
kit (Ray Biotech, Norcross, GA, USA).
Wound healing assay
MC3T3-E1, LM8, PC-3, SaOS2, 786-O, and A549 cells
were cultured until confluence. The confluent cell monolayer in a
6-well plate was wounded by manually scraping the cells with a
pipette tip. The cells were treated with or without LIPUS
stimulation. Cell migration into the wound surface was monitored by
microscopy at 0, 6, and 12 h after LIPUS stimulation. Quantitation
was carried out in terms of % wounded area filled [% wounded area
filled = 100 × (initial width of wounding − final width)/initial]
by measuring the distance of the wound edge of the migrating cells
from the start point to the migrated point from 3 independent
experiments (13).
Statistical analysis
The data are represented as the mean ± standard
deviation (SD). Statistical significance was determined using
Student’s t-test. P<0.05 was considered statistically
significant.
Results
Cell number
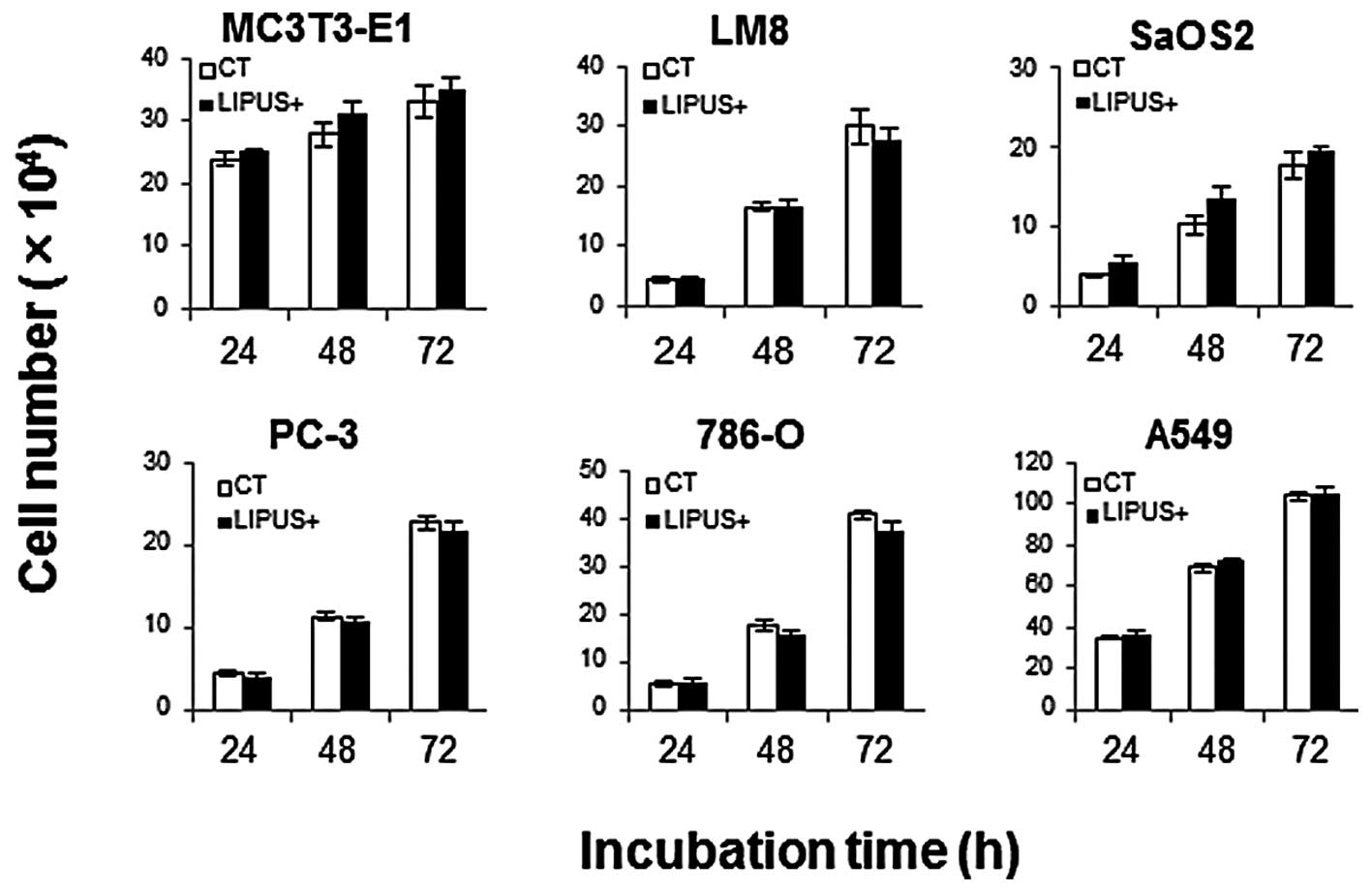
The cell number did not change regardless of the
presence or absence of daily LIPUS stimulation, up to 3 days
(Fig. 1).
Phosphorylation of ERK and Akt and
expression of β-catenin
ERK1/2: immunoreactive bands at 44 kDa (ERK1 and
phosphorylated ERK1 protein) and 42 kDa (ERK2 and phosphorylated
ERK2 protein) were observed. LIPUS stimulation on MC3T3-E1 and LM8
temporarily induced phosphorylation of ERK1/2 between 5 min and 1 h
after LIPUS stimulation. LIPUS stimulation on SaOS2 and A549
bimodally induced phosphorylation of ERK1/2 at 1 and 24 h. LIPUS
stimulation on PC-3 and 786-O did not induce phosphorylation of
ERK1/2 (Fig. 2A).
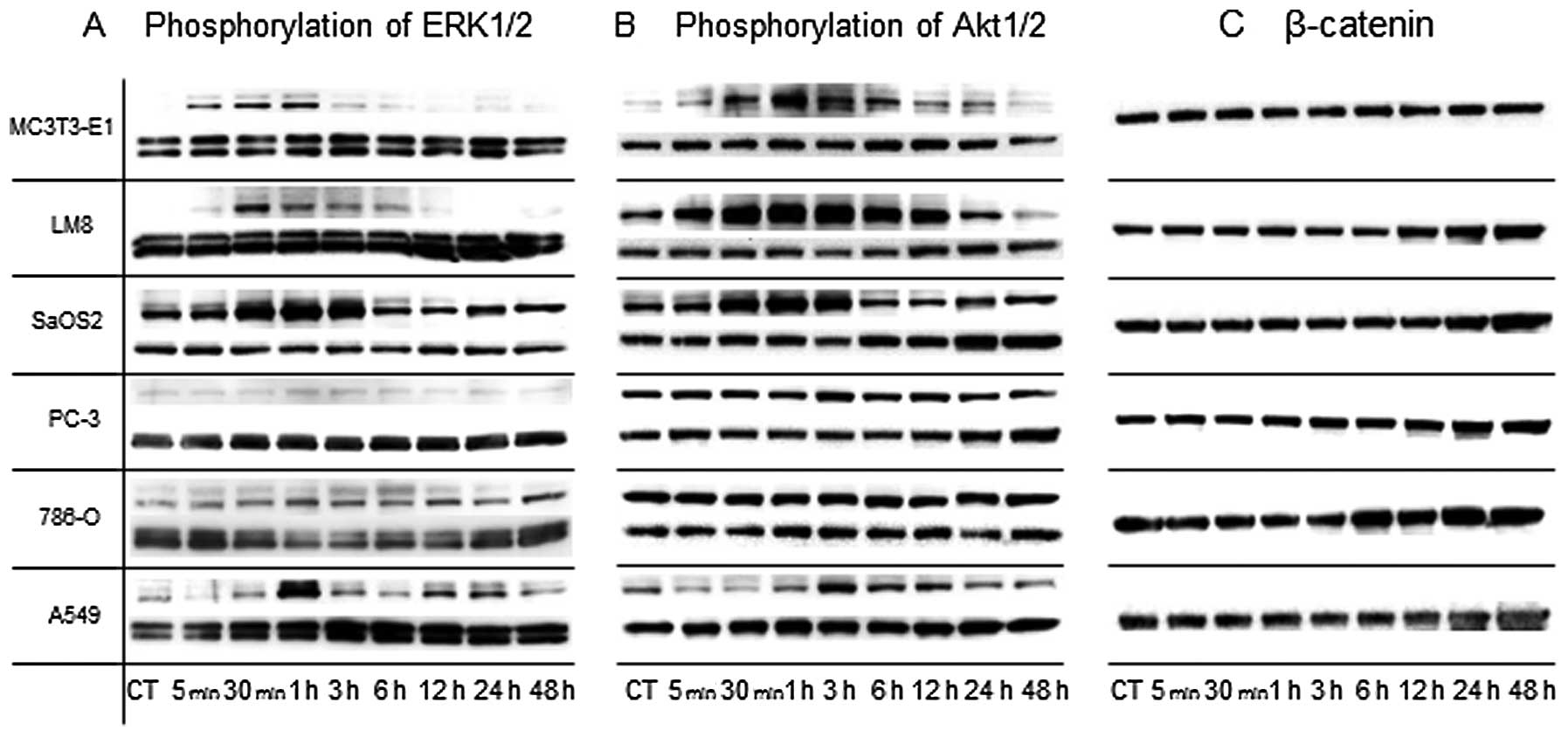 | Figure 2LIPUS stimulation on MC3T3-E1, LM8,
SaOS2, and A549 induced phosphorylation of ERK1/2 and Akt. Each
cell type was harvested between 5 min and 48 h after LIPUS
stimulation. The expressions of pERK1/2, ERK1/2, pAkt, Akt, and
β-catenin were analyzed by western blotting. (A) The expressions of
ERK1/2 and pERK1/2 were observed (upper, pERK; lower, ERK).
Phosphorylation of ERK1/2 on MC3T3-E1, LM8, SaOS2, and A549 was
induced by LIPUS stimulation, but not in PC-3 and 786-O. (B) The
expressions of Akt and pAkt were observed (upper, pAkt; lower,
Akt). Phosphorylations of Akt on MC3T3-E1, LM8, SaOS2, and A549
were induced by LIPUS stimulation, but not in PC-3 and 786-O. (C)
The expression of β-catenin was observed. β-catenin expression was
not increased by LIPUS stimulation. |
Akt: immunoreactive bands at 60 kDa (Akt and
phosphorylated Akt protein) were observed. LIPUS stimulation on
MC3T3-E1 temporarily induced phosphorylation of Akt between 30 min
and 24 h. LIPUS stimulation on LM8 temporarily induced
phosphorylation of Akt between 5 min and 12 h. LIPUS stimulation on
SaOS2 temporarily induced phosphorylation of Akt between 30 min and
3 h. LIPUS stimulation on A549 temporarily induced phosphorylation
of Akt at 3 h. LIPUS stimulation on PC-3 and 786-O did not induce
phosphorylation of Akt (Fig.
2B).
β-catenin: immunoreactive bands at 92 kDa (β-catenin
protein) were observed. LIPUS stimulation of the cells did not
significantly increase β-catenin expression (Fig. 2C).
Expression of VEGF protein
VEGF protein levels were assessed in the collected
culture media by sandwich-ELISA. VEGF protein levels in MC3T3-E1 at
12, 24, and 48 h after LIPUS stimulation were significantly
increased compared with those of the control, whereas those in LM8,
SaOS2, PC-3, 786-O, and A549 were not (Fig. 3).
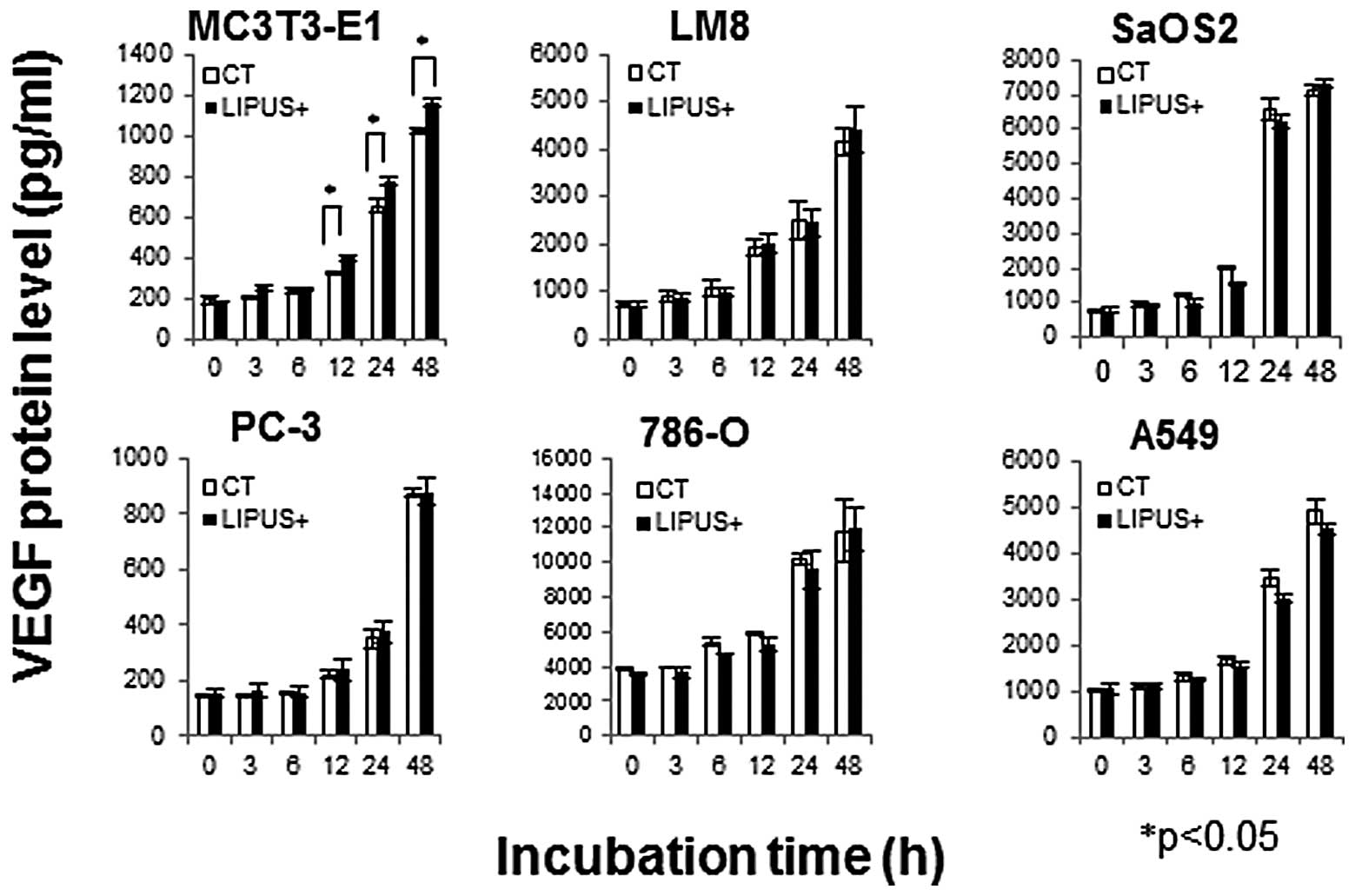 | Figure 3LIPUS stimulation increased VEGF
protein levels only in MC3T3-E1. Each cell type was cultured in the
presence (black bars, LIPUS+) or absence (white bars, CT) of daily
LIPUS stimulation for up to 48 h. The supernatants of MC3T3-E1,
PC-3, LM8, SaOS2, 786-O, and A549 cells were harvested between 0,
3, 6, 12, 24, and 48 h after LIPUS stimulation. VEGF protein levels
were assessed in the collected culture media by sandwich-ELISA.
VEGF protein levels in MC3T3-E1 were significantly increased
compared with those of the control and not significantly increased
in LM8, SaOS2, PC-3, 786-O, and A549 (*p<0.05). The
data are shown as the mean ± SD (bars, SD) of at least three
independent experiments. |
Cell migration
In order to investigate the effect of LIPUS
stimulation on cell migration, scratch wound healing assays were
performed. LIPUS stimulation resulted in significant promotion of
MC3T3-E1 cell migration. MC3T3-E1 cell migration increased by 8.7
and 9.4% at 6 and 12 h, respectively, compared with those of
control cells. LM8, SaOS2, PC-3, 786-O, and A549 cells were not
affected (Fig. 4).
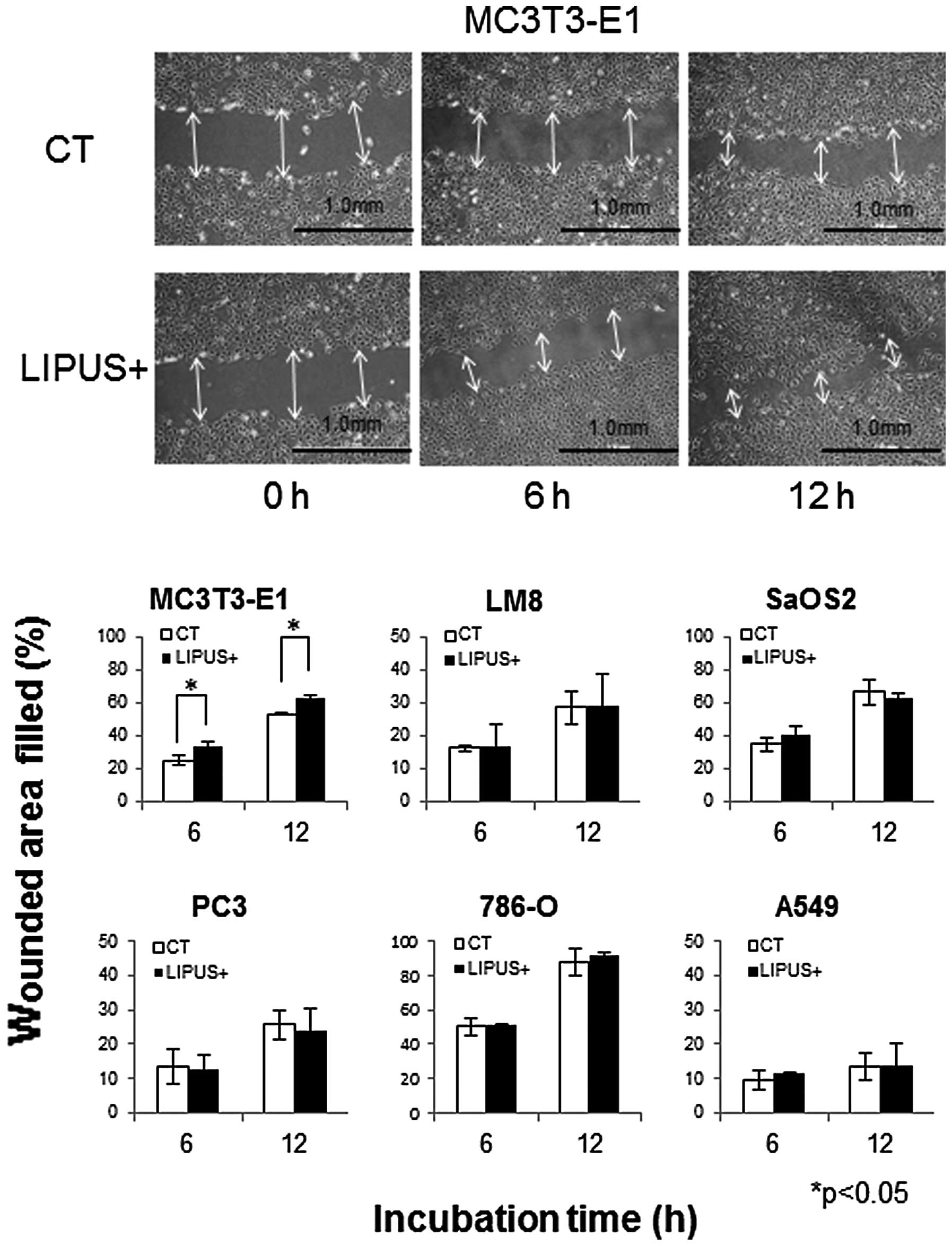 | Figure 4LIPUS stimulation promoted cell
migration only in MC3T3-E1. Each cell type was cultured in the
presence (black bars, LIPUS+) or absence (white bars, CT) of daily
LIPUS stimulation for up to 12 h. The cell migration of MC3T3-E1,
LM8, SaOS2, PC-3, 786-O, and A549 cells into the wound surface was
monitored by microscopy at 0, 6, and 12 h after LIPUS stimulation.
Quantitation was carried out in terms of % wounded area filled [%
wounded area filled = 100 × (initial width of wounding - final
width)/initial] measuring the distance of the wound edge of the
migrating cells from the start point to the migrated point. LIPUS
stimulation resulted in significant promotion of MC3T3-E1 cell
migration. SaOS2, PC-3, 786-O, and A549 cells were not affected
(*p<0.05). The data are shown as the mean ± SD (bars,
± SD) of at least three independent experiments. |
Discussion
Application of adequate mechanical stress to bone is
essential for maintaining bone mass and strength. Various types of
mechanical loading have been clinically tested for their bone
mass-promoting activity in the treatment of bone fractures. Among
them, LIPUS was reported to promote healing of bone fracture in
1983 (1). Then it was widely
reported that LIPUS reduced the time required for fracture healing
(14,15). Furthermore, LIPUS induced bone
maturation in distraction osteogenesis, and delayed fracture union
and nonunion in animal models as well as in clinical settings
(1–4). In vitro experiments have
demonstrated that LIPUS stimulation affects mouse and rat
osteoblast differentiation without influencing proliferation
(8,9). Although the mechanisms involved have
not been elucidated, LIPUS transmits signals into the cell via an
integrin that acts as a mechanoreceptor on the cell membrane
(16). The integrin/Ras/MAPK
pathway is known to be a general pathway involved in cell
proliferation. A previous study demonstrated that ERK
phosphorylation increased after LIPUS stimulation on MC3T3-E1,
starting at 5 min, reaching a maximum between 15 and 30 min, and
then gradually decreasing, and that LIPUS stimulation on human
pre-osteoblastic cells progressively increased ERK phosphorylation
in 1 h (17,18). The PI3K/Akt pathway, on the other
hand, is known to be involved in various functions such as cell
survival, proliferation, motility, control of cell size, and
metabolism. It was reported that LIPUS exposure in MC3T3-E1
increased Akt phosphorylation in a time-dependent manner and
maximal activation was detected 15 min after LIPUS stimulation
(17). In the present study, LIPUS
stimulation on MC3T3-E1 temporarily induced phosphorylation of
ERK1/2 between 5 min and 1 h and phosphorylation of Akt between 30
min and 24 h after LIPUS stimulation. These results are comparable
to those of a previous study.
Wnt/β-catenin signaling pathway has been reported to
play a crucial role in cell proliferation, differentiation, and
possibly apoptosis (19). There
have been no reports of studies investigating the effect of LIPUS
stimulation on β-catenin expression. Instead, mechanical loading in
mouse tibial bone increased expression of canonical Wnt pathway and
Wnt/β-catenin target genes (20).
Our experiment indicated that LIPUS stimulation did not
significantly increase β-catenin expression.
VEGF has a central role in the regulation of
vascularization. VEGF expression has been reported to be increased
by LIPUS stimulation in human fetal pre-osteoblastic cell and
rabbit bone-tendon junction (6,21).
Migration of osteoblasts has an important impact on fracture
healing. Our experiment indicated that LIPUS stimulation of
osteoblasts, MC3T3-E1, promoted cell migration. MC3T3-E1 cell
migration increased by 8.7 and 9.4% at 6 and 12 h, respectively,
compared with that of control cells. In addition, VEGF protein
levels in MC3T3-E1 at 12, 24, and 48 h after LIPUS stimulation were
significantly increased compared with those of the control. These
changes may help differentiation and formation of bone. However,
for malignant cells, these changes observed in osteoblastic cells
may favor tumor growth. Therefore, we next studied whether LIPUS
also affects malignant cells, viz., osteosarcoma cells, and other
visceral cancer cells.
Osteosarcoma is generally characterized by
exhibition of osteoblastic differentiation and production of
osteoid matrix. LIPUS effects on osteosarcoma cell lines such as an
osteoblastic cell line has been reported (9,22). In
the present study, LIPUS on LM8 and SaOS2 induced phosphorylation
of ERK1/2 and Akt and did not affect cell number, β-catenin
expression, VEGF protein expression, or cell migration. Although
phosphorylated ERK and Akt in osteosarcoma were generally
considered to activate the tumor cell proliferation, Cagnol and
Chambard reported that activation of ERK1/2 in osteosarcoma cells
induced apoptosis and autophagy (23). From our study, it was concluded that
LIPUS stimulation did not affect the cell number of osteosarcoma.
Moreover, angiogenic and migration effects were different from
those of osteoblast cells. It is tempting to speculate that LIPUS
stimulation of osteosarcoma cells might promote bone
differentiation and reduce the activity of a tumor, although our
results are limited.
Two types of cancer cells, 786-O and PC-3, were not
affected by LIPUS stimulation in any of the experiments. It may
thus be argued that LIPUS used for bone metastases from renal and
prostate cancer induces osteoblastic differentiation without
inducing cancer proliferation, vascularization, and migration. On
the other hand, the effect of LIPUS stimulation of A549 induced
phosphorylation of ERK1/2 and Akt and did not affect cell number,
β-catenin expression, VEGF protein expression, or cell migration.
Although activations of ERK and Akt were considered to induce
proliferation of lung cancer cells, it was reported that activation
of ERK is required for apoptosis in A549 lung cancer cells
(24,25). In our study, LIPUS stimulation did
not enhance proliferative activity of cancer cells, regardless of
the tissue of origin.
Generally, a part of intact bone is substituted with
malignant cells in metastatic bone tumor and malignant bone tumor.
Progression of tumor growth causes pain and bone destruction,
resulting in pathological bone fracture. Tumor growth stimulates
activation of osteoclasts leading to bone resorption whereby more
space is produced adequate for malignant cell proliferation.
Stimulation to osteoclasts essentially occurs in combination with
stimulation of osteoblasts. Metastatic bone tumor is similar to
bone fracture because both osteoclasts and osteoblasts are
stimulated. However, proliferative activity of malignant cells is
generally greater than that of osteoblasts. If we could enhance new
osteogenic activities in spite of malignant cell growth, it would
stop progression of the osteolytic change which may prevent bone
fracture. Patients could thus achieve relief of pain, prevention of
SRE, and finally satisfactory ADL and QOL.
This study model showed limited outcome because
in vitro experiments are different from in vivo
environments of metastatic bone tumor. The mechanisms by which
metastases are formed are complex, involving many types of cells
and steps that include angiogenesis, invasion, and proliferation in
the bone microenvironment. Tumor cells in the bone microenvironment
produce a large number of cytokines such as tumor-produced
parathyroid hormone-related protein (PTHrP), transforming growth
factor β, and interleukin-6 (26).
In conclusion, LIPUS did not significantly increase
cellular proliferation, migration or VEGF production of malignant
cells compared with that without LIPUS. In contrast, LIPUS induced
migration and VEGF production without proliferation in osteoblasts.
LIPUS when applied on metastatic bone tumors might be beneficial by
inducing osteoblast differentiation without cancer proliferation.
In the future, LIPUS stimulation might be one of the treatments of
metastatic bone tumor because of its non-invasiveness.
References
|
1
|
Duarte LR: The stimulation of bone growth
by ultrasound. Arch Orthop Trauma Surg. 101:153–159. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heckman JD, Ryaby JP, McCabe J, Frey JJ
and Kilcoyne RF: Acceleration of tibial fracture-healing by
non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg
Am. 76:26–34. 1994.PubMed/NCBI
|
|
3
|
Rutten S, Nolte PA, Guit GL, Bouman DE and
Albers GH: Use of low-intensity pulsed ultrasound for posttraumatic
nonunions of the tibia: a review of patients treated in the
Netherlands. J Trauma. 62:902–908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang SJ, Lewallen DG, Bolander ME, Chao
EY, Ilstrup DM and Greenleaf JF: Low intensity ultrasound treatment
increases strength in a rat femoral fracture model. J Orthop Res.
12:40–47. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dimitriou R and Babis GC: Biomaterial
osseointegration enhancement with biophysical stimulation. J
Musculoskelet Neuronal Interact. 7:253–265. 2007.PubMed/NCBI
|
|
6
|
Wang FS, Kuo YR, Wang CJ, et al: Nitric
oxide mediates ultrasound-induced hypoxia-inducible factor-1alpha
activation and vascular endothelial growth factor-A expression in
human osteoblasts. Bone. 35:114–123. 2004. View Article : Google Scholar
|
|
7
|
Tang CH, Lu DY, Tan TW, Fu WM and Yang RS:
Ultrasound induces hypoxia-inducible factor-1 activation and
inducible nitric-oxide synthase expression through the
integrin/integrin-linked kinase/Akt/mammalian target of rapamycin
pathway in osteoblasts. J Biol Chem. 282:25406–25415. 2007.
View Article : Google Scholar
|
|
8
|
Bandow K, Nishikawa Y, Ohnishi T, et al:
Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and
MIP-1beta expression in osteoblasts through the angiotensin II type
1 receptor. J Cell Physiol. 211:392–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki A, Takayama T, Suzuki N, Sato M,
Fukuda T and Ito K: Daily low-intensity pulsed ultrasound-mediated
osteogenic differentiation in rat osteoblasts. Acta Biochim Biophys
Sin. 41:108–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Yoshida T, Ogawa R, et al:
Low-intensity ultrasound adjuvant therapy: enhancement of
doxorubicin induced cytotoxicity and the acoustic mechanisms
involved. J Med Ultrasonics. 36:61–68. 2009. View Article : Google Scholar
|
|
11
|
Ito M, Azuma Y, Ohta T and Komoriya K:
Effects of ultrasound and 1,25-dihydroxyvitamin D3 on growth factor
secretion in co-cultures of osteoblasts and endothelial cells.
Ultrasound Med Biol. 26:161–166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwabuchi S, Ito M, Hata J, Chikanishi T,
Azuma Y and Haro H: In vitro evaluation of low-intensity pulsed
ultrasound in herniated disc resorption. Biomaterials.
26:7104–7114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tano K, Mizuno R, Okada T, et al: MALAT-1
enhances cell motility of lung adenocarcinoma cells by influencing
the expression of motility-related genes. FEBS Lett. 584:4575–4580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dyson M and Brookes M: Stimulation of bone
repair by ultrasound. Ultrasound Med Biol. 2:61–66. 1983.PubMed/NCBI
|
|
15
|
Kristiansen TK, Ryaby JP, McCabe J, Frey
JJ and Roe LR: Accelerated healing of distal radial fractures with
the use of specific, low-intensity ultrasound. A multicenter,
prospective, randomized, double-blind, placebo-controlled study. J
Bone Joint Surg Am. 79:961–973. 1997.
|
|
16
|
Takeuchi R, Ryo A, Komitsu N, et al:
Low-intensity pulsed ultrasound activates the phosphatidylinositol
3 kinase/Akt pathway and stimulates the growth of chondrocytes in
three-dimensional cultures: a basic science study. Arthritis Res
Ther. 10:R772008. View
Article : Google Scholar
|
|
17
|
Tang CH, Yang RS, Huang TH, Lu DY, Chuang
WJ, Huang TF and Fu WM: Ultrasound stimulates cyclooxygenase-2
expression and increases bone formation through integrin, focal
adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in
osteoblasts. Mol Pharmacol. 69:2047–2057. 2006. View Article : Google Scholar
|
|
18
|
Chen YJ, Wang CJ, Yang KD, et al:
Pertussis toxin-sensitive Galphai protein and ERK-dependent
pathways mediate ultrasound promotion of osteogenic transcription
in human osteoblasts. FEBS Lett. 554:154–158. 2003. View Article : Google Scholar
|
|
19
|
Almeida M, Han L, Bellido T, Manolagas SC
and Kousteni S: Wnt proteins prevent apoptosis of both uncommitted
osteoblast progenitors and differentiated osteoblasts by
beta-catenin-dependent and -independent signaling cascades
involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol
Chem. 280:41342–41351. 2005. View Article : Google Scholar
|
|
20
|
Robinson JA, Chatterjee-Kishore M,
Yaworsky PJ, et al: Wnt/beta-catenin signaling is a normal
physiological response to mechanical loading in bone. J Biol Chem.
281:31720–31728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu H, Qin L, Cheung W, Lee K, Wong W and
Leung K: Low-intensity pulsed ultrasound accelerated bone-tendon
junction healing through regulation of vascular endothelial growth
factor expression and cartilage formation. Ultrasound Med Biol.
34:1248–1260. 2008. View Article : Google Scholar
|
|
22
|
Borsje MA, Ren Y, de Haan-Visser HW and
Kuijer R: Comparison of low-intensity pulsed ultrasound and pulsed
electromagnetic field treatments on OPG and RANKL expression in
human osteoblast-like cells. Angle Orthod. 80:498–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh
TH and Huynh H: The role of activated MEK-ERK pathway in
quercetin-induced growth inhibition and apoptosis in A549 lung
cancer cells. Carcinogenesis. 25:647–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin CY, Moon DO, Lee JD, et al:
Sulforaphane sensitizes tumor necrosis factor-related
apoptosis-inducing ligand-mediated apoptosis through downregulation
of ERK and Akt in lung adenocarcinoma A549 cells. Carcinogenesis.
28:1058–1066. 2007. View Article : Google Scholar
|
|
26
|
Guise TA: Molecular mechanisms of
osteolytic bone metastases. Cancer. 88:2892–2898. 2000. View Article : Google Scholar : PubMed/NCBI
|