Introduction
Cisplatin (cis-diamminedichloroplatinum, DDP) is one
of the most potent and widely used chemotherapeutic agents for
treatment of a wide variety of solid tumors in clinic. By
interacting with DNA to form intra- and inter-strand adducts to
disrupt DNA replication and transcription, cisplatin activates
several cellular signal pathways including those involving ATR,
p53, p73 and MAPK, which results in apoptosis (1). However, cisplatin-induced apoptotic
cell death can be attenuated, and chemoresistance to cisplatin is a
major limitation of cisplatin-based chemotherapy. The molecular
mechanisms responsible for cisplatin resistance appear to be
multifactorial. Reduced drug uptake, increased drug inactivation
and increased DNA repair would limit the extent of
cisplatin-induced DNA damage. Moreover, mechanisms that inhibit
propagation of the DNA damage signal to the apoptotic machinery
have also been proposed, which include loss of damage recognition,
loss of p53 function, overexpression of HER-2/neu, activation of
the PI3-K/Akt pathway, overexpression of antiapoptotic Bcl-2, and
defects in apoptotic pathways (2,3).
Therefore, combination of cisplatin with other agents that could
modulate DNA damage and related signal pathways is a promising
strategy to overcome cisplatin resistance.
Because they are generally safe to humans, naturally
occurring compounds from diets or medicinal plants are good
candidates for increasing the cisplatin anticancer activity.
Wogonin (5,7-dihydroxy-8-methoxyflavone) is a flavonoid isolated
from the root of the medicinal herb Scutellaria baicalensis
Georgi, which has been shown to exert antioxidant,
anti-inflammatory, antiviral and anticancer activities in
vitro as well as in vivo(4–7).
Importantly, wogonin showed no significant toxicity to normal
peripheral blood T cells (8), and
was able to reduce etoposide-induced apoptotic cell death in normal
cells such as bone marrow cells and thymocytes (9). Therefore, wogonin is a good potential
sensitizer for cisplatin anticancer activity. Wogonin was found to
potentiate etoposide-induced apoptosis in cancer cells through
inhibition of P-glycoprotein (10),
to overcome IL-6-induced adriamycin resistance through suppressing
IL-6-mediated aldo-keto reductase (AKR) superfamily member
dihydrodiol dehydrogenases (AKR1C1/1C2) overexpression in human
non-small lung cancer cells (11),
and to enhance the cytotoxicity of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) through upregulating p53 and PUMA
(12). Recently, we found that
wogonin sensitizes cancer cells to tumor necrosis factor α
(TNF-α)-induced apoptosis by blocking TNF-induced NF-κB activation
(13). However, the effect of
wogonin on the anticancer activity of cisplatin, one of the most
widely used chemotherapeutics in clinic, has not been investigated.
In this study, we treated the non-small cell lung cancer cell line
A549 and the cervical cancer cell line HeLa with the combination of
wogonin and cisplatin and found for the first time that wogonin
potently sensitizes cisplatin-induced cancer cell apoptosis through
triggering intracellular reactive oxygen species (ROS)
accumulation, which added important new evidence supporting the
potential use of wogonin as adjuvant of cisplatin.
Materials and methods
Reagents
Wogonin was from National Institute of the Control
Pharmaceutical and Biological Products (Beijing, China). Cisplatin,
butylated hydroxyanisole (BHA) and N-acetyl-L-cysteine (NAC) were
purchased from Sigma (St. Louis, MO). Z-VAD-FMK was from Calbiochem
(La Jolla, CA). ROS-sensitive fluorescent dye
5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate
acetyl ester (CM-H2DCFDA) and dihydroethidium (DHE) were
purchased from Molecular Probes (Eugene, OR). Antibodies against
active caspase-3, poly (ADP-ribose) polymerase (PARP) were from BD
Bioscience (San Diego, CA). Anti-β-actin antibody was from Protein
Tech (Chicago, IL).
Cell culture and cell death assay
A549 (a non-small cell lung cancer cell line) and
HeLa (a cervical cancer cell line) were from American Type Culture
Collection (ATCC, Manassas, VA) and grown in RPMI-1640 supplemented
with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml
penicillin and 100 μg/ml streptomycin. The cultured cells were kept
in a 37°C humidified incubator with 5% CO2. For cell
death assay, cells were seeded in 96-well plate and after overnight
culture were then treated as indicated in each figure legend. Cell
death was assessed based on the release of lactate dehydrogenase
(LDH) using a cytotoxicity detection kit from Promega (Madison, WI)
as described previously (14). All
the experiments were repeated 3–5 times and the average is shown in
each figure.
Western blot analysis
Cells were treated as indicated in figure legend and
cell lysates were prepared by lysing cells with M2 buffer [20
mmol/l Tris-HCl (pH 7.6), 0.5% NP40, 250 mmol/l NaCl, 3 mmol/l
EDTA, 3 mmol/l EGTA, 2 mmol/l DTT, 0.5 mmol/l phenylmethylsulfonyl
fluoride, 20 mmol/l β-glycerophosphate, 1 mmol/l sodium vanadate
and 1 μg/ml leupeptin]. Cell lysates were then subjected to
SDS-PAGE and analyzed by western blotting using specific
antibodies. The proteins were seen by enhanced chemiluminescence
(Millipore, Billerica, MA) using Bio-Rad Image station (Hercules,
CA). Each experiment was repeated at least 3 times and
representative results are shown.
Apoptosis analysis by flow cytometry
Apoptosis was detected by flow cytometric analysis
using an Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen
Biotech, Nanjing, China). Cells were treated as indicated in the
figure legend, and then were double stained with Annexin V-FITC and
propidium iodide (PI) following manufacturer’s instruction. The
stained cells were analyzed by flow cytometry (Beckman Coulter,
Inc., Brea, CA). Cells that are in early apoptosis are Annexin
V-FITC positive and PI negative; and cells that are in late
apoptosis or already dead are both FITC Annexin V and PI
positive.
Detection of ROS
Cells were seeded in 12-well plates and after
overnight culture were then treated as indicated in each figure
legend. Thirty minutes before collecting cells,
H2O2-sensitive fluorescent dye
CM-H2DCFDA (5 μM) or superoxide-sensitive dye DHE (5 μM)
was added. ROS were detected by flow cytometry (Beckman Coulter,
Inc.) as reported previously (15).
Statistical analysis
All numerical data are expressed as mean ± standard
deviation (SD). Statistical significance was examined by Student’s
paired-sample t-test using the SPSS statistics software package
(IBM SPSS, Chicago, IL) and P<0.05 was used for
significance.
Results
Wogonin enhances cisplatin-induced cell
death in cancer cells
Aiming to overcome chemoresistance to cisplatin in
cancer cells, we first investigated whether wogonin is able to
enhance the anticancer activity of cisplatin. We treated the A549
cells with 10 μM of wogonin, 10 μM of cisplatin or both for 60 h
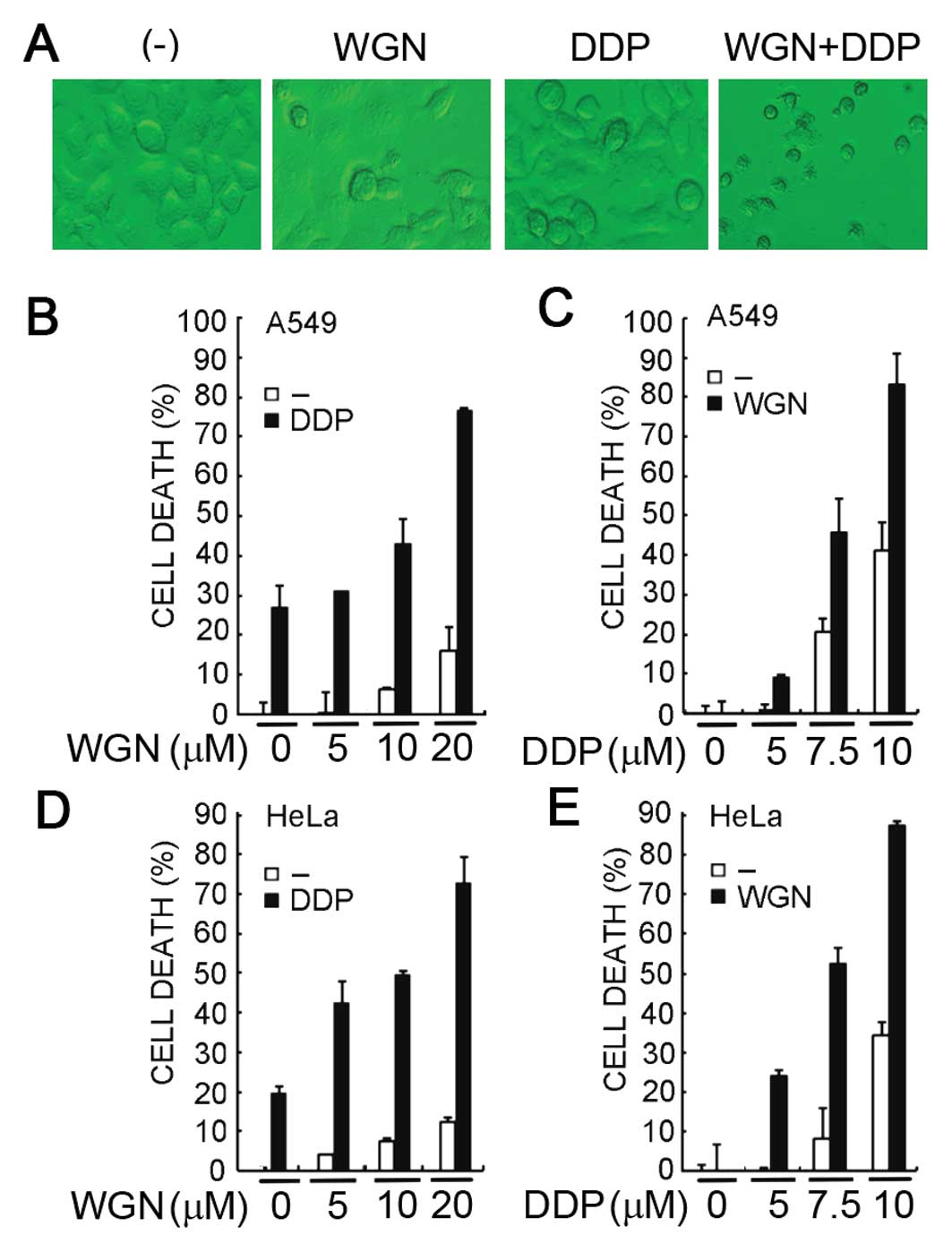
and cell death was observed microscopically. As shown in the
representative images (Fig. 1A),
while cisplatin or wogonin caused limited cell death, co-treatment
of these agents resulted in significantly enhanced cytotoxicity. To
more quantitatively measure cell death, A549 cells were treated
with increasing concentrations of wogonin (5–20 μM) and a fixed
concentration of cisplatin (7.5 μM) and cell death was detected by
LDH release assay. The results showed that while cisplatin alone
caused about 25% cell death in A549 cells, wogonin synergistically
sensitized A549 cells to cisplatin-induced cell death in a
dose-dependent manner (Fig. 1B).
The synergism that killed about 80% of cells was detected at the
highest dose of wogonin (20 μM), a concentration of wogonin alone
that only caused moderate cell death (~12%). Conversely, a similar
dose-dependent potentiation of cytotoxicity was detected when
increasing concentrations of cisplatin with a fixed wogonin dose
was used (Fig. 1C). The
sensitization of cisplatin’s anticancer activity was further
validated in the cervical cancer cell line HeLa. A similar
dose-dependent synergism either with fixed concentration of wogonin
or with fixed concentration of cisplatin was observed (Fig. 1D and E), suggesting wogonin is able
to sensitize cancer cells to cisplatin-induced cytotoxicity.
Wogonin enhances cisplatin-induced cancer
cell apoptosis
We next investigated whether wogonin potentiates
cisplatin-induced cell death through enhancing apoptosis. HeLa
cells were treated with wogonin, cisplatin alone or both. The cells
were stained with Annexin V-FITC and PI, apoptosis was analyzed by
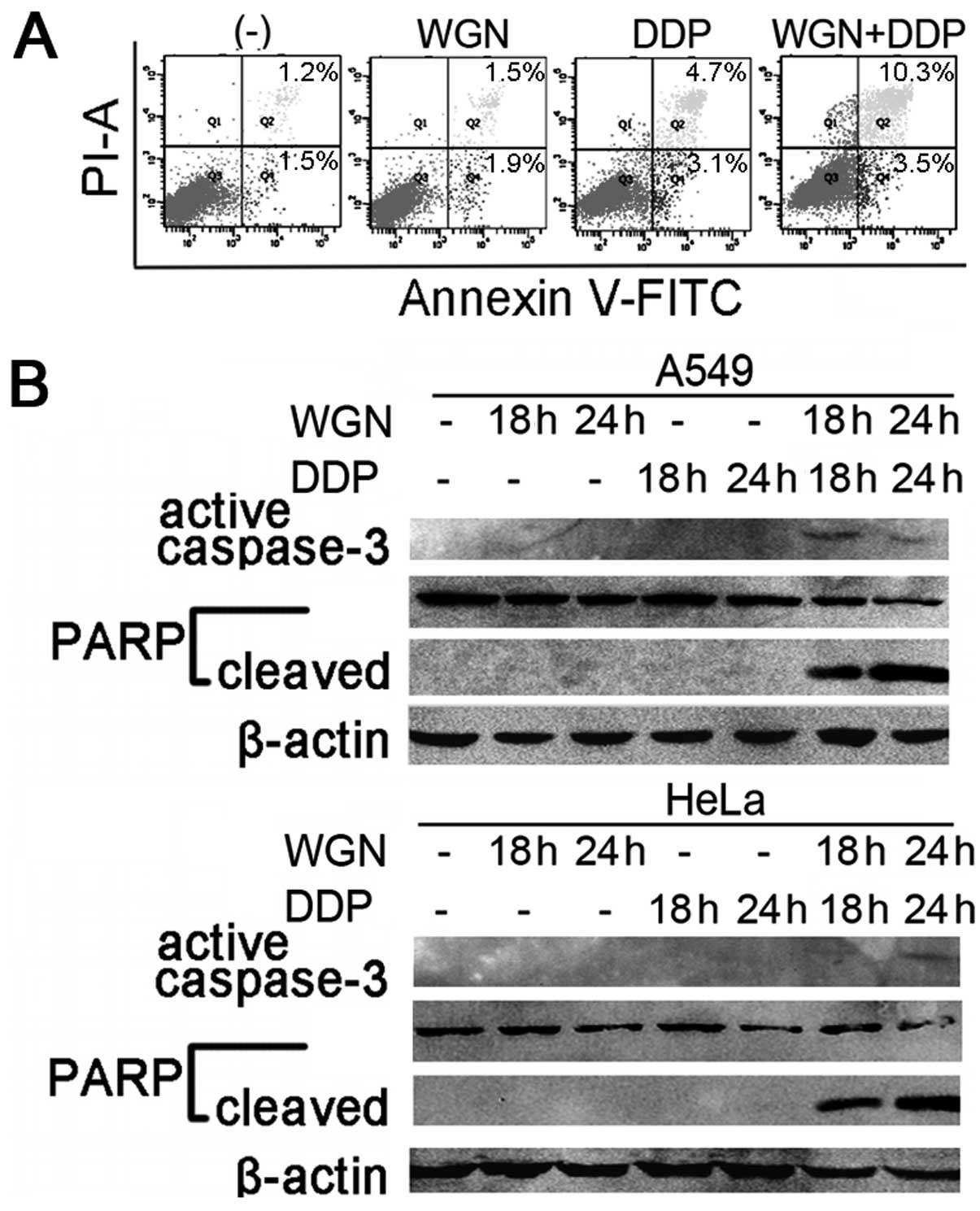
flow cytometry. As shown in Fig.
2A, both early apoptotic (Annexin V-FITC positive and PI
negative, 3.5%) and late apoptotic (both Annexin V-FITC and PI
positive, 10.3%) cell population were significantly increased in
wogonin and cisplatin co-treated cells compared to the samples
individually treated with cisplatin (3.1% and 4.7%) or wogonin
(1.9% and 1.5%), indicating that the enhanced cell death is mainly
through apoptosis. The activation of caspases was also detected by
western blotting. Whereas the activation of caspase cascade was
barely detected in wogonin or cisplatin alone treated cells, the
activation of caspase-3 and cleavage of the caspase-3 substrate
PARP were significantly enhanced in cisplatin and wogonin
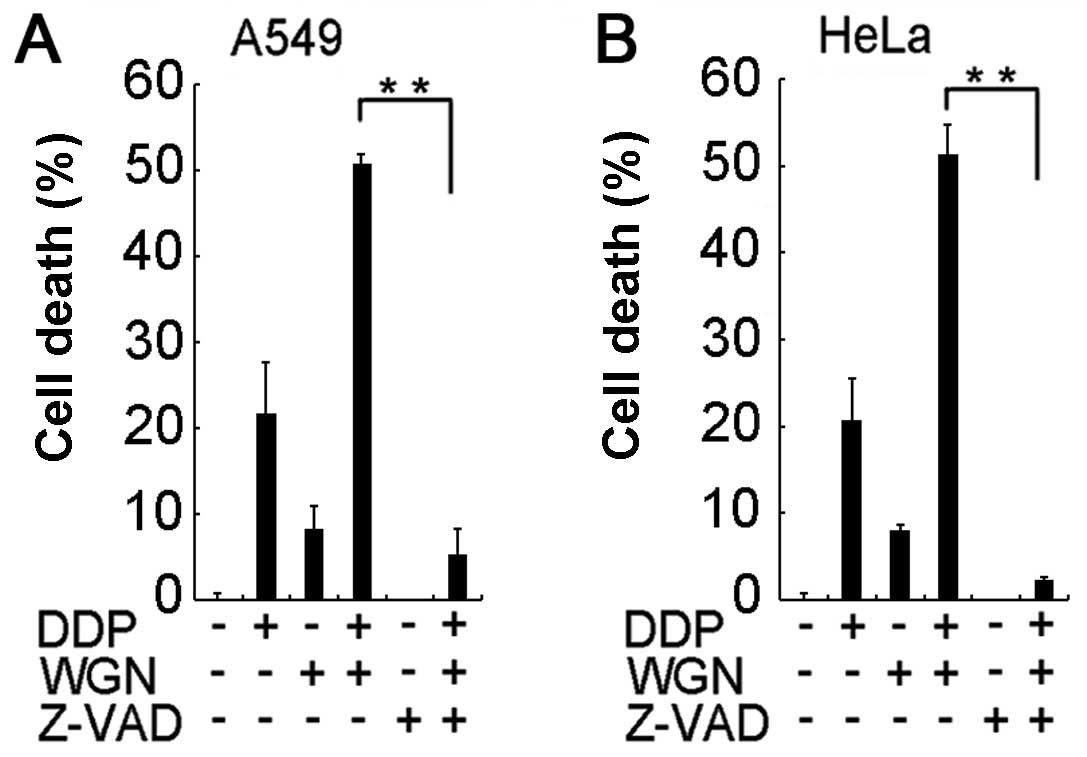
co-treated A549 cells and HeLa cells (Fig. 2B). The pan-caspase inhibitor
Z-VAD-FMK effectively suppressed the synergistic cytotoxicity
induced by wogonin and cisplatin co-treatment (Fig. 3), further confirming that the
sensitization to cisplatin by wogonin is through enhancement of
apoptosis in cancer cells.
Wogonin-induced intracellular
H2O2 accumulation contributes to the
synergistic cytotoxicity induced by wogonin plus cisplatin
It has been well established that ROS, a group of
reactive oxygen-containing species including superoxide, hydrogen
peroxide (H2O2) and hydroxyl radical, are
important signaling mediators for cell death pathways. Our previous
work has demonstrated that wogonin induces intracellular
accumulation of H2O2 in cancer cells, which
contributes substantially to the synergistic cytotoxicity induced
by wogonin plus TNF (13). The role
of ROS in wogonin plus cisplatin-induced synergistic cytotoxicity
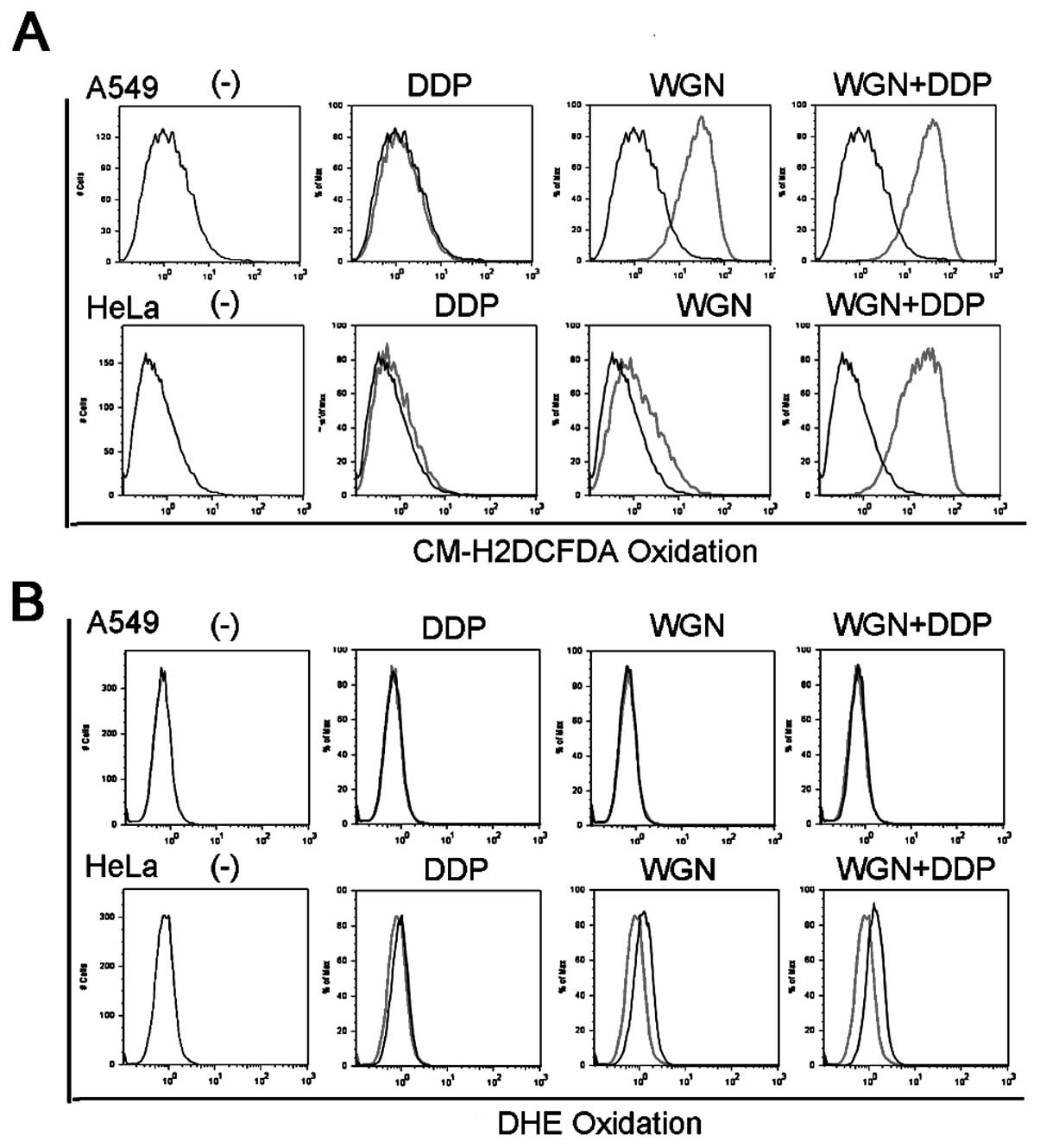
was thus investigated. Cells were treated with wogonin, cisplatin
or both, stained with two ROS-specific dyes, CM-H2DCFDA
that is specific for hydrogen peroxide (H2O2)
or DHE that is specific for superoxide, and then analyzed by flow
cytometry. As expected, wogonin induced strong intracellular
H2O2 accumulation in both A549 cells and HeLa
cells, as indicated by significant rightward shift of the peaks of
wogonin treated cells compared with that of the control cells
(Fig. 4A). The treatment with
wogonin plus cisplatin showed similar trend and even more striking
extent of H2O2 induction as treated by the
wogonin alone. On the contrary, wogonin and cisplatin had marginal
effect on cellular superoxide level in A549 and HeLa cells
(Fig. 4B), which is consistent with
our previous results (13). Then,
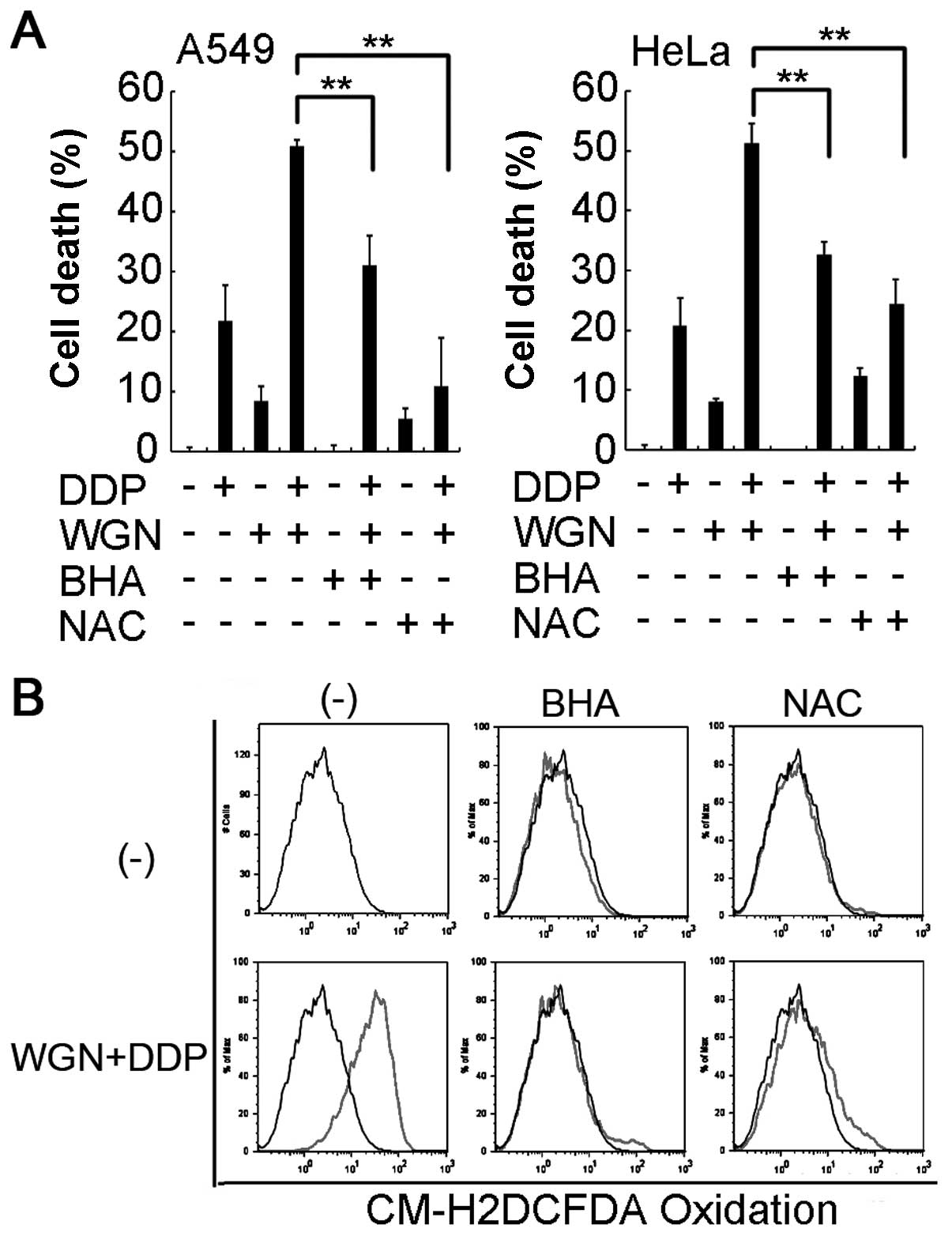
we treated cells with ROS scavengers BHA or NAC to remove
H2O2. As shown in Fig. 5A, these two scavengers effectively
suppressed the synergistic cytotoxicity induced by wogonin and
cisplatin co-treatment in both HeLa and A549 cells, which is
well-correlated to significant reduction of
H2O2 levels in the cells (Fig. 5B). Taken together, these results
strongly suggest that wogonin-induced H2O2
accumulation substantially contributes to the potentiated
cytotoxicity caused by cisplatin and wogonin co-treatment.
Discussion
In the current study, we demonstrate for the first
time that wogonin is able to sensitize cisplatin-induced apoptosis
through a ROS-dependent mechanism in the non-small cell lung cancer
cell line A549 and the cervical cancer cell line HeLa. First,
combination treatment of these two cell lines with wogonin and
cisplatin showed synergistic cytotoxicity in a dose-dependent
manner. Second, apoptosis was significantly enhanced in wogonin and
cisplatin co-treated cancer cells, which was indicated by the
potentiation of activation of caspase-3 and cleavage of the
caspase-3 substrate PARP in co-treated cells. Third, wogonin
robustly induced H2O2 accumulation in both
A549 and HeLa cells and two reactive oxygen species scavengers BHA
and NAC significantly suppressed the synergistic cytotoxicity
caused by wogonin and cisplatin co-treatment, indicating
H2O2 induced by wogonin substantially
contributes to the synergistic cytotoxicity.
Flavonoids are a group of naturally occurring
polyphenolic compounds found ubiquitously in plants and abundantly
present in human diets. Humans ingest significant quantities of
flavonoids in their diet because of their widespread distribution.
Scutellaria baicalensis Georgi was widely used as an
anti-inflammatory herbal remedy in traditional Chinese and Japanese
medicine. In this study, we tested the major flavonoids (wogonin)
in Scutellaria baicalensis Georgi and found that it
sensitized cisplatin-induced apoptosis through an
H2O2-dependent mechanism. Although flavonoids
are most commonly known for their antioxidant activity, previous
studies from our group and several other groups have demonstrated
that wogonin robustly induced intracellular
H2O2 accumulation in cancer cells, which
contributed to the wogonin anticancer activity (8,12,13,16).
We also found that wogonin induced H2O2
through suppression of catalase activity in cancer cells (13). Interestingly, wogonin induces
marginal H2O2 accumulation in normal
peripheral T cells and immortalized normal bronchial epithelial
cells, which may explain the selective cytotoxicity of wogonin on
malignant cells. Indeed, the pro-oxidant activity of other
flavonoids such as quercetin and luteolin has also been shown by
increasing number of reports (15,17,18).
It is believed that antioxidant and pro-oxidants behavior of
flavonoids may depend on the structure of flavonoids, the source of
the free radicals, and the context and microenvironment of the cell
such as the presence and concentration of Fe and Cu ions (19,20).
The pharmacological mechanisms of cisplatin and
etoposide are apparently different. Etoposide prevents re-ligation
of the DNA strands by forming a ternary complex with DNA and the
topoisomerase II enzyme, thus to cause errors in DNA synthesis and
promote apoptosis of cancer cells (21). Cisplatin disrupts DNA function and
induces apoptosis by interacting with DNA to form DNA adducts. It
is noteworthy that wogonin is able to sensitize cancer cells to
apoptosis induced by both etoposide and cisplatin, two widely used
frontline chemotherapeutics. Previously, the sensitizing effect of
wogonin on etoposide-induced apoptosis in cancer cells was reported
to involve inhibition of P-glycoprotein (10). Whereas the results from this study
strongly suggest that intracellular H2O2
accumulation contributes substantially to the enhanced apoptosis
observed in wogonin and cisplatin co-treated cancer cells, although
other mechanisms are not excluded. ROS are important modulator of
cellular signaling and can cause DNA-damage directly. The
underlying molecular mechanisms by which wogonin-induced
H2O2 sensitized cisplatin-induced apoptosis
are likely multifactorial and worthy further study. One possible
mechanism may involve H2O2
mediated-downregulation of Bcl-2 protein through dephosphorylation
and ubiquitination of the protein, which facilitates its
degradation by proteasome (22,23).
It is also possible that H2O2 oxidizes
important cellular components such as DNA to trigger DNA
damage-mediated apoptosis (24).
Nevertheless, our results clearly suggest that wogonin could be
used as a cisplatin sensitizer for cancer therapy.
Acknowledgements
This study was supported in part by grant 81172111
from National Natural Science Foundation of China, and also partly
supported by grant 2010JQ0012 from the Young Scientist Fund of
Science and Technology Department of Sichuan Province, China.
References
|
1
|
Cohen SM and Lippard SJ: Cisplatin: from
DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol.
67:93–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niedner H, Christen R, Lin X, Kondo A and
Howell SB: Identification of genes that mediate sensitivity to
cisplatin. Mol Pharmacol. 60:1153–1160. 2001.PubMed/NCBI
|
|
4
|
Chi YS, Lim H, Park H and Kim HP: Effects
of wogonin, a plant flavone from Scutellaria radix, on skin
inflammation: in vivo regulation of inflammation-associated
gene expression. Biochem Pharmacol. 66:1271–1278. 2003.PubMed/NCBI
|
|
5
|
Zhao Y, Li H, Gao Z, Gong Y and Xu H:
Effects of flavonoids extracted from Scutellaria baicalensis
Georgi on hemin-nitrite-H2O2 induced liver
injury. Eur J Pharmacol. 536:192–199. 2006.PubMed/NCBI
|
|
6
|
Ma SC, Du J, But PP, Deng XL, Zhang YW,
Ooi VE, Xu HX, Lee SH and Lee SF: Antiviral chinese medicinal herbs
against respiratory syncytial virus. J Ethnopharmacol. 79:205–211.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee DH, Kim C, Zhang L and Lee YJ: Role of
p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer
cells. Biochem Pharmacol. 75:2020–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumann S, Fas SC, Giaisi M, Muller WW,
Merling A, Gulow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCγ1- and Ca2+-dependent
apoptosis. Blood. 111:2354–2363. 2008.PubMed/NCBI
|
|
9
|
Enomoto R, Koshiba C, Suzuki C and Lee E:
Wogonin potentiates the antitumor action of etoposide and
ameliorates its adverse effects. Cancer Chemother Pharmacol.
67:1063–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee E, Enomoto R, Koshiba C and Hirano H:
Inhibition of P-glycoprotein by wogonin is involved with the
potentiation of etoposide-induced apoptosis in cancer cells. Ann NY
Acad Sci. 1171:132–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HW, Lin CP, Chiu JH, Chow KC, Kuo KT,
Lin CS and Wang LS: Reversal of inflammation-associated dihydrodiol
dehydrogenases (AKR1C1 and AKR1C2) overexpression and drug
resistance in non-small cell lung cancer cells by wogonin and
chrysin. Int J Cancer. 120:2019–2027. 2007. View Article : Google Scholar
|
|
12
|
Lee DH, Rhee JG and Lee YJ: Reactive
oxygen species up-regulate p53 and Puma; a possible mechanism for
apoptosis during combined treatment with TRAIL and wogonin. Br J
Pharmacol. 157:1189–1202. 2009. View Article : Google Scholar
|
|
13
|
Yang L, Zheng XL, Sun H, Zhong YJ, Wang Q,
He HN, Shi XW, Zhou B, Li JK, Lin Y, et al: Catalase
suppression-mediated H(2)O(2) accumulation in cancer cells by
wogonin effectively blocks tumor necrosis factor-induced NF-κB
activation and sensitizes apoptosis. Cancer Sci. 102:870–876
|
|
14
|
Wang X, Ju W, Renouard J, Aden J, Belinsky
SA and Lin Y: 17-Allylamino-17-demethoxygeldanamycin
synergistically potentiates tumor necrosis factor-induced lung
cancer cell death by blocking the nuclear factor-κB pathway. Cancer
Res. 66:1089–1095. 2006.PubMed/NCBI
|
|
15
|
Ju W, Wang X, Shi H, Chen W, Belinsky SA
and Lin Y: A critical role of luteolin-induced reactive oxygen
species in blockage of tumor necrosis factor-activated nuclear
factor-κB pathway and sensitization of apoptosis in lung cancer
cells. Mol Pharmacol. 71:1381–1388. 2007.PubMed/NCBI
|
|
16
|
Fas SC, Baumann S, Zhu JY, Giaisi M,
Treiber MK, Mahlknecht U, Krammer PH and Li-Weber M: Wogonin
sensitizes resistant malignant cells to TNFα- and TRAIL-induced
apoptosis. Blood. 108:3700–3706. 2006.PubMed/NCBI
|
|
17
|
Galati G and O’Brien PJ: Potential
toxicity of flavonoids and other dietary phenolics: significance
for their chemopreventive and anticancer properties. Free Radic
Biol Med. 37:287–303. 2004. View Article : Google Scholar
|
|
18
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao G, Sofic E and Prior RL: Antioxidant
and prooxidant behavior of flavonoids: structure-activity
relationships. Free Radic Biol Med. 22:749–760. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugihara N, Arakawa T, Ohnishi M and
Furuno K: Anti- and pro-oxidative effects of flavonoids on
metal-induced lipid hydroperoxide-dependent lipid peroxidation in
cultured hepatocytes loaded with α-linolenic acid. Free Radic Biol
Med. 27:1313–1323. 1999.PubMed/NCBI
|
|
21
|
Gordaliza M, Garcia PA, del Corral JM,
Castro MA and Gomez-Zurita MA: Podophyllotoxin: distribution,
sources, applications and new cytotoxic derivatives. Toxicon.
44:441–459. 2004.PubMed/NCBI
|
|
22
|
Hildeman DA, Mitchell T, Aronow B,
Wojciechowski S, Kappler J and Marrack P: Control of Bcl-2
expression by reactive oxygen species. Proc Natl Acad Sci USA.
100:15035–15040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Chanvorachote P, Toledo D, Stehlik
C, Mercer RR, Castranova V and Rojanasakul Y: Peroxide is a key
mediator of Bcl-2 down-regulation and apoptosis induction by
cisplatin in human lung cancer cells. Mol Pharmacol. 73:119–127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tenopoulou M, Doulias PT, Barbouti A,
Brunk U and Galaris D: Role of compartmentalized redox-active iron
in hydrogen peroxide-induced DNA damage and apoptosis. Biochem J.
387:703–710. 2005. View Article : Google Scholar : PubMed/NCBI
|