Introduction
Bladder cancer is the most common form of cancer in
developed countries. The vast majority of malignant tumors found in
the urinary bladder are transitional cell carcinoma (TCC) (1), which is a type that is intricately
associated with metastasis (2). The
knowledge of the cellular and molecular mechanisms of metastasis in
TCC of the bladder is essential for the potential application of
effective treatment.
The matrix metalloproteinase (MMP) family of
extracellular proteinases plays a central role in the migration and
invasion of tumor cells (3,4). Tumor cell migration requires a
degradation of the extracellular matrix (ECM) and basement membrane
by proteases such as MMP-2 (72 kDa) and MMP-9 (92 kDa) (3,4).
Previous results demonstrated that MMP-9 expression is associated
directly with tumor grade, invasion, migration, and metastasis in
the progression of bladder cancer (5–10).
MMP-9 is induced by several growth factors and cytokines in
different cell types (11–13). Accumulative studies showed that the
identification of the transcription factors, including NF-κB, Sp-1
and AP-1, was essential for the induction of MMP-9 in cancer cells
(11–13).
IL-5 is a T-cell replacing factor (TRF) that
stimulates the differentiation of B cells (14). Previous studies have demonstrated
the regulatory roles involved in the activation, proliferation and
survival of eosinophils (15). IL-5
binds at the cell surface of a receptor made up of heterodimer
complexes composed of 2 chains: ligand-specific receptor IL-5Rα and
the accessory receptor β-subunit (βc) (14,15).
The binding of IL-5 to IL-5Rα resulted in the activation of
Jak/Stat, MAPK and PI3K in B cells and eosinophils (14,15).
Although many studies have demonstrated the biological role of
IL-5, its exact regulatory mechanism in the process of tumor cell
migration remains unknown.
Here, we show the molecular and cellular mechanism
involved in cytokine IL-5-induced cell migration. In the present
study, the expression of both IL-5 and its receptor IL-5Rα was
observed in bladder cancer HT-1376 cells. This study is the first
to show the potent induction of cell migration by IL-5 in bladder
cancer cells. In addition, our results demonstrated the essential
role of ERK1/2-mediated MMP-9 expression in IL-5-induced migration
of bladder cancer cells.
Materials and methods
Materials
Polyclonal antibodies to ERK, phospho-ERK, p38MAPK,
phospho-p38MAPK, JNK and phospho-JNK were obtained from Cell
Signaling (Danvers, MA). U0126 was obtained from Calbiochem (San
Diego, CA). The polyclonal MMP-9 antibody was obtained from
Chemicon. Small interfering RNA (siRNA) oligonucleotides targeting
IL-5Rα (5′-GCAGAACGACCACTCACTA-3′) and scramble
(5′-CUGUCAGUCAGUCGUAGUAUU-3′) were designed and synthesized by
Genolution (Seoul, Korea).
Cell cultures
A human bladder carcinoma cell line (HT1376) was
obtained from the American Type Culture Collection. The cells were
maintained in DMEM (4.5 g glucose/liter) supplemented with 10%
fetal calf serum, L-glutamine and antibiotics (Biological
Industries, Beit Haemek, Israel) at 37°C in a 5% CO2
humidified incubator.
RNA extraction
Total RNA was isolated from tissue using TRIzol
reagent (Life Technologies, Grand Island, NY), according to the
manufacturer’s protocol. The quality and integrity of the RNA was
confirmed by agarose gel electrophoresis and ethidium bromide
staining, followed by visual examination under ultraviolet
light.
Real-time PCR
Real-time PCR assays using a Rotor-Gene 3000 PCR
system (Corbett Research, Mortlake, Australia) were performed in
the original and independent cohorts. GAPDH was analyzed in
parallel as an internal control. Real-time-PCR reactions containing
primers and SYBR Premix EX Taq (Takara Bio Inc., Otsu, Japan) were
carried out in micro-reaction tubes (Corbett Research). Spectral
data were captured and analyzed using Rotor-Gene Real-Time Analysis
software 6.0 Build 14 (Corbett Research). For amplification, IL-5
sense (5′-CATCCAGTGCTACTTGTGTT-3′), IL-5 anti-sense
(5′-ACTTCAGGTCGAAGTCAATC-3′), IL-5Rα sense
(5′-GCAGAACGACCACTCACTA-3′), and IL-5Rα anti-sense
(5′-GGTGCAGTGAAGGGAAACT-3′) primers were used. GAPDH was analyzed
in parallel as an endogenous RNA reference gene, and data were
normalized to the expression of GAPDH.
Immunoblot
Growth-arrested cells were treated with IL-5 in the
absence of 10% FBS for various durations at 37°C. The cells were
then washed twice with cold PBS and freeze-thawed in 250 μl lysis
buffer [containing, in mmol/l, HEPES (pH 7.5) 50, NaCl 150, EDTA 1,
EGTA 2.5, DTT 1, β-glycerophosphate 10, NaF 1,
Na3VO4 0.1, and phenylmethylsulfonyl fluoride
0.1 and 10% glycerol, 0.1% Tween-20, 10 μg/ml of leupeptin and 2
μg/ml of aprotinin), and then scraped into 1.5-ml tubes. The
lysates were placed on ice for 15 min and then centrifuged at
12,000 rpm for 20 min at 4°C. The protein concentration of the
supernatant was determined using a Bradford reagent method (Bio-Rad
Laboratories). Equal amounts of cellular proteins were resolved by
electrophoresis on a 0.1% SDS-10% polyacrylamide gel (SDS-PAGE)
under denaturing conditions. The proteins were transferred
electrophoretically to nitrocellulose membranes (Hybond; Amersham
Corp). After blocking in 10 mmol/l Tris-HCl (pH 8.0), 150 mmol/l
NaCl and 5% (wt/vol) non-fat dry milk, the membranes were treated
with primary antibodies for 90 min, followed by incubation with
peroxidase-conjugated secondary antibodies for 45 min. The
immunocomplexes were detected using a chemiluminescence reagent kit
(Amersham Corp). For the immunoblotting studies, the experiments
were repeated at least 3 times.
Wound healing migration assay
Cells were plated on 6-well dishes and grown to 90%
confluence in 2 ml of growth medium. The cells were damaged using a
2-mm-wide tip and were then treated with IL-5. They were allowed to
migrate, and photographs were taken through an inverted microscope
(original magnification, ×40).
Zymography
Conditioned medium was electrophoresed in a
polyacrylamide gel containing 1 mg/ml gelatin. The gel was then
washed at room temperature for 2 h with 2.5% Triton X-100 and
subsequently at 37°C overnight in a buffer containing 10 mM
CaCl2, 150 mM NaCl and 50 mM Tris-HCl, pH 7.5. The gel
was stained with 0.2% Coomassie blue and photographed on a light
box. Proteolysis was detected as a white zone in a dark blue field
(16).
Transfection
Cells were transfected with siRNA using
Lipofectamine 2000 transfection reagent according to the
manufacturer’s protocols (Invitrogen). After the indicated
incubation with IL-5, the cells were studied via immunoblot,
zymography, EMSA and wound healing migration.
Nuclear extracts and electrophoretic
mobility shift assay (EMSA)
Cultured cells were collected by centrifugation,
washed and suspended in a buffer containing 10 mM HEPES (pH 7.9),
10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT and 0.5 mM PMSF.
After 15 min on ice, the cells were vortexed in the presence of
0.5% Nonidet NP-40. The nuclear pellet was then collected by
centrifugation and extracted in a buffer containing 20 mM HEPES pH
7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and 1 mM PMSF for
15 min at 4°C.
The nuclear extract (10–20 μg) was preincubated at
4°C for 30 min with the 100-fold excess of an unlabeled
oligonucleotide spanning the -79 MMP-9 cis-element of interest. The
sequences were as follows: AP-1, CTGACCCCTGAGTCAGC ACTT; NF-κB,
CAGTGGAATTCCCCAGCC; Sp-1, GCCCA TTCCTTCCGCCCCCAGATGAAGCAG. The
reaction mixture was then incubated at 4°C for 20 min in a buffer
(25 mM HEPES buffer pH 7.9, 0.5 mM EDTA, 0.5 mM DTT, 0.05 M NaCl
and 2.5% glycerol) with 2 μg of poly dI/dC and 5 fmol
(2×104 cpm) of a Klenow end-labeled [32P-ATP]
30-mer oligonucleotide, which spanned the DNA binding site in the
MMP-9 promoter. The reaction mixture was electrophoresed at 4°C in
a 6% polyacrylamide gel using a TBE (89 mM Tris, 89 mM boric acid
and 1 mM EDTA) running buffer. The gel was rinsed with water, dried
and exposed to X-ray film overnight (16).
Statistical analysis
Where appropriate, data were expressed as the mean ±
SE. Data were analyzed by factorial ANOVA and Fisher’s least
significant difference test where appropriate. Statistical
significance was set at P<0.05.
Results
Expression of IL-5 and its receptor
IL-5Rα in bladder cancer HT1376 cells
To investigate whether the IL-5 and its receptor
IL-5Rα is expressed in bladder cancer HT1376 cells, we analyzed
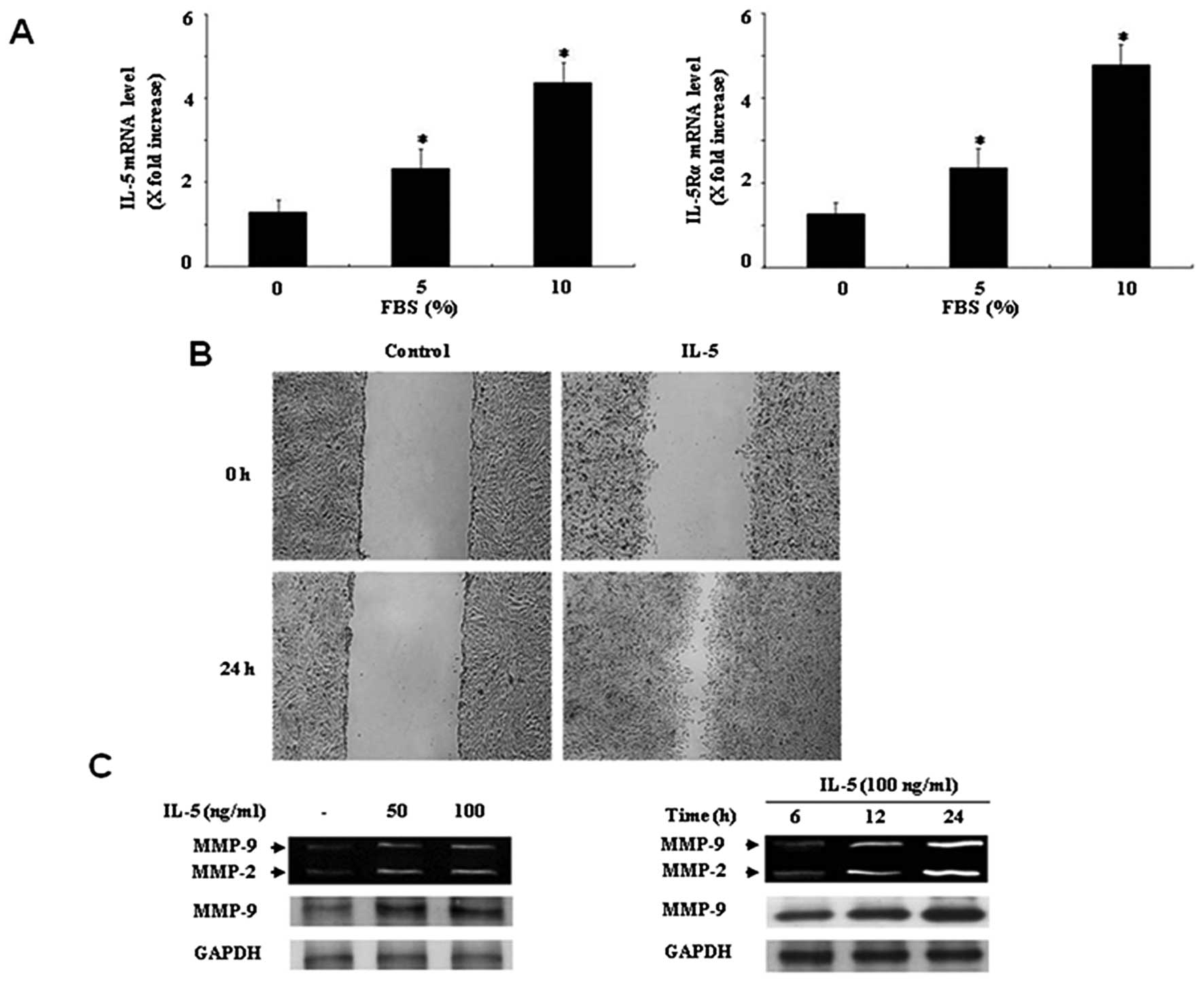
IL-5 and IL-5Rα mRNA expression. Real-time PCR analysis showed
detection of mRNA expression of IL-5 in HT1376 cells (Fig. 1A). Expression of IL-5Rα mRNA was
also observed in HT1376 cells (Fig.
1A). Moreover, both IL-5 and IL-5Rα mRNA expressions were
strongly enhanced by the addition of 10% FBS (Fig. 1A). These results indicate that
expression of IL-5 and its receptor IL-5Rα can be found in bladder
cancer HT1376 cells.
IL-5 induces wound healing migration and
MMP-9 expression in HT1376 cells
Previous studies have demonstrated the involvement
of cell migration in the development of bladder cancer (1,2). We
next examined the effect of IL-5 on bladder cancer cell migration
using a wound healing migration assay. Treatment of HT1376 cells
with IL-5 for 24 h induced the capacity of migration, as compared
with the control (Fig. 1B). To
explore the relationship between cell migration and MMP expression,
we performed gelatin zymographic assay in IL-5-treated HT1376
cells. IL-5 was added to HT1376 cells at various concentrations, in
order to determine the optimal dose. IL-5 significantly induced
both MMP-2 and MMP-9 expression at 100 ng/ml, as compared with the
control in HT1376 cells (Fig. 1C).
In addition, both MMP-2 and MMP-9 levels were increased in
IL-5-treated HT1376 cells in a time-dependent manner (Fig. 1C). The effect of IL-5 was confirmed
by immunoblot. Increase in expression levels of both MMP-2 and
MMP-9 were observed in HT1376 cells induced by IL-5 (Fig. 1C).
IL-5-induced MMP-9 expression is involved
in binding activities of NF-κB and AP-1 in HT1376 cells
Since the expression of MMP-9 is deeply associated
with the development of bladder cancer (5–10), we
focused our investigation on IL-5-induced MMP-9 expression. In
order to define transcription factors involved in the IL-5
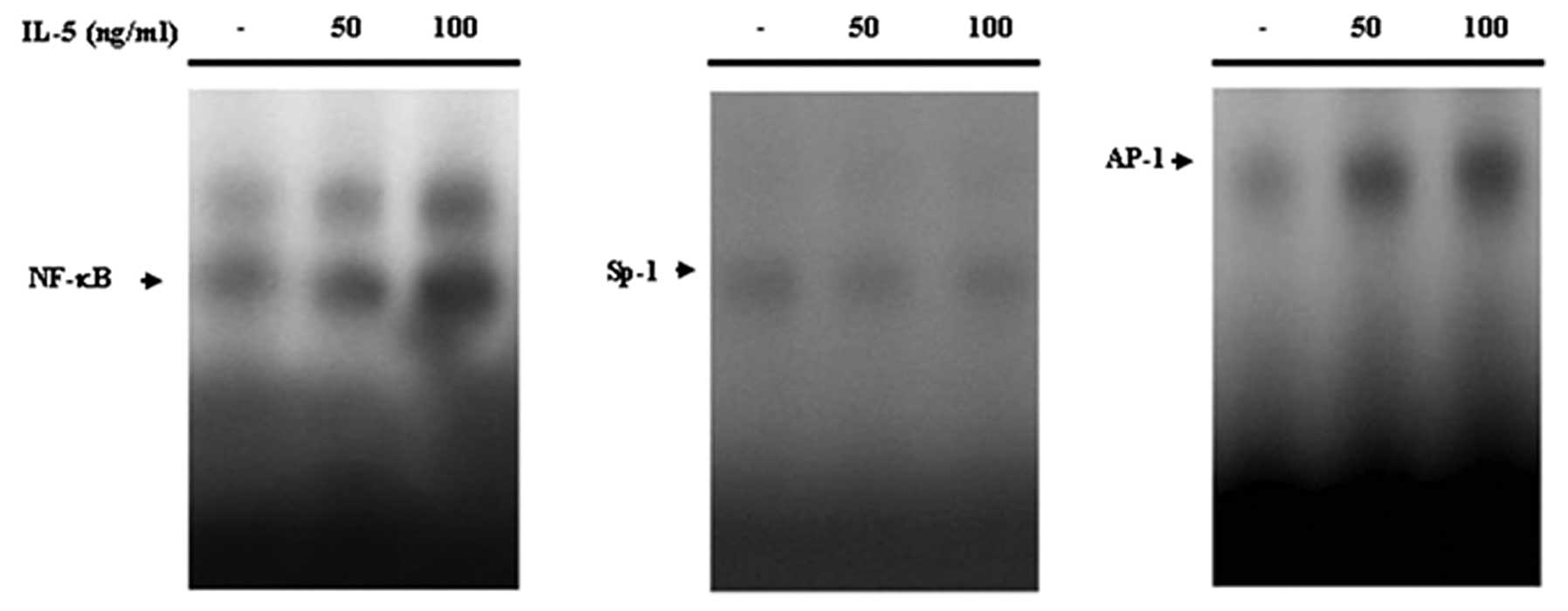
induction of MMP-9 in HT1376 cells, we performed a gel shift assay
(EMSA) using nuclear extract obtained from cells cultured for 24 h
in the presence or absence of IL-5 (100 ng/ml). IL-5 significantly
increased nuclear binding to NF-κB and AP-1 binding sites (Fig. 2). However, no significant Sp-1
levels were observed in IL-5-treated HT1376 cells (Fig. 2). These results suggest that
IL-5-induced MMP-9 expression might be mediated through the binding
activation of NF-κB and AP-1.
ERK1/2 inhibitor, U0126, decreases the
IL-5-induced migration in HT1376 cells
To examine the MAPK signaling pathway in
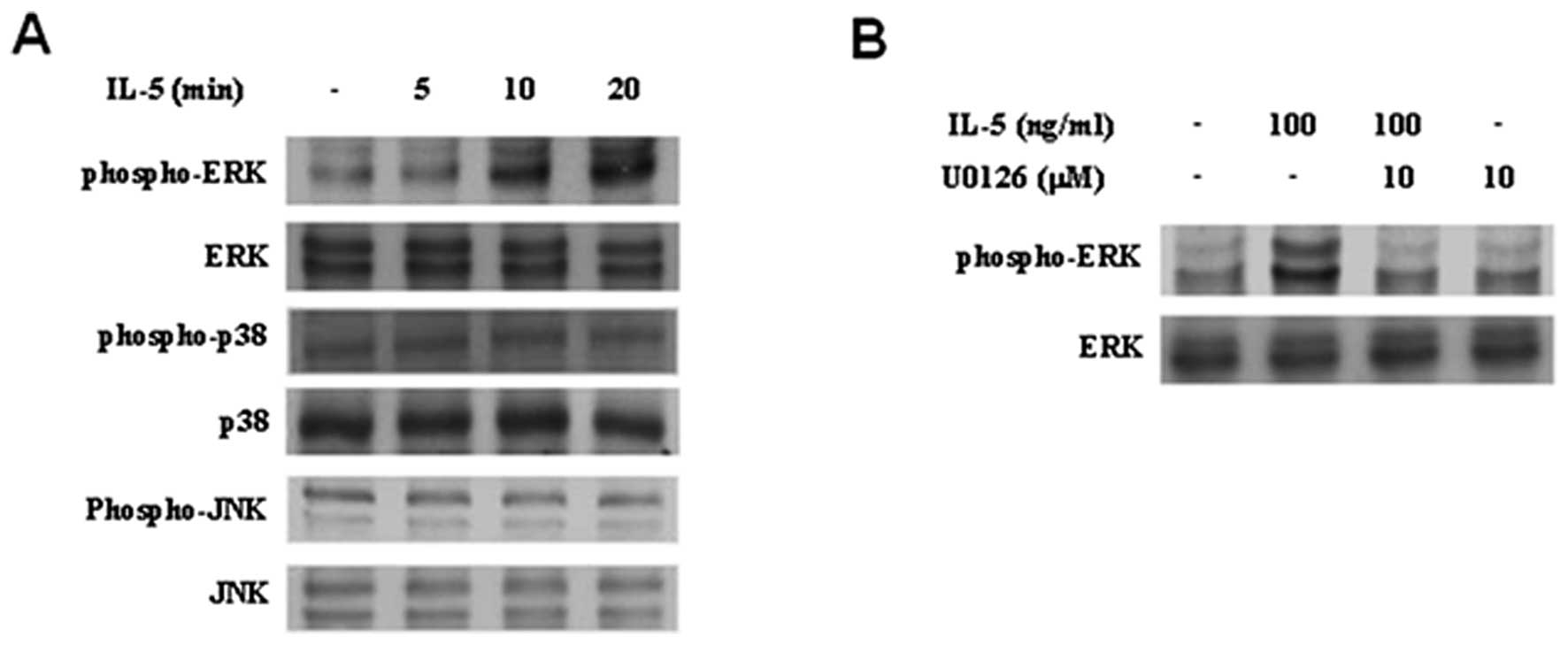
IL-5-treated HT1376 cells, we carried out immunoblot experiments.
Treatment with IL-5 resulted in a significant induction of ERK1/2
activation in HT1376 cells (Fig.
3A). IL-5-induced activation of ERK1/2 was suppressed by U0126,
ERK1/2-specific inhibitor (Fig.
3B). In contrast, treatment of HT1376 cells with IL-5 did not
lead to activation of JNK and p38MAPK (Fig. 3A). To further investigate the role
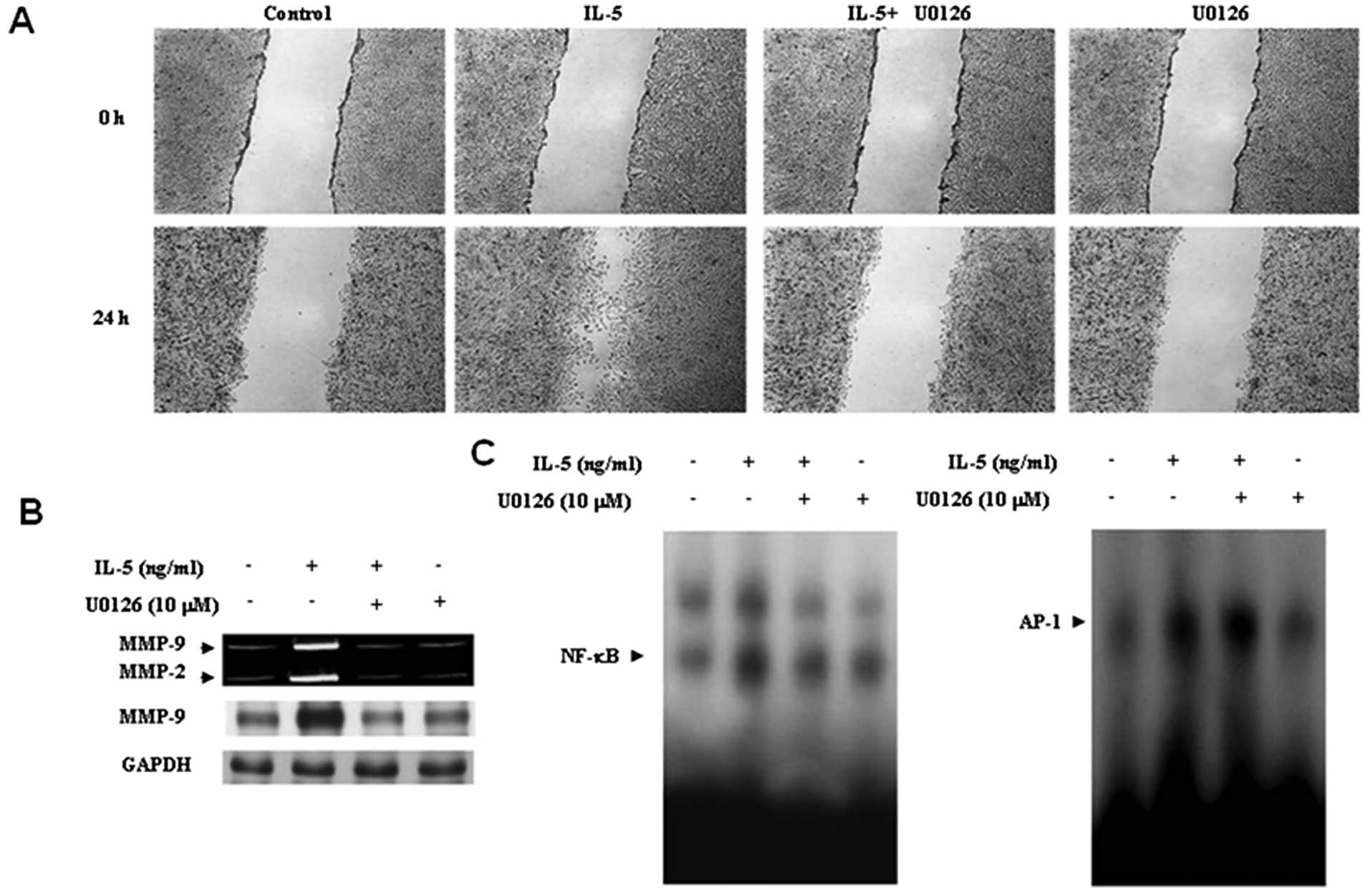
of ERK1/2 signaling in IL-5-treated HT1376 cells, cells were
pretreated with U0126. Blockage of ERK1/2 signaling significantly
reduced the migration of HT1376 cells induced by IL-5 (Fig. 4A). These results indicate that
ERK1/2 signaling plays an important role in the IL-5-induced
migration of bladder cancer cells.
Inhibition of ERK1/2 signaling abolishes
IL-5-mediated MMP-9 expression and NF-κB activation in HT1376
cells
The results of the present study showed that IL-5
regulates cell migration and MMP-9 expression (Fig. 1B and C). In addition, IL-5-mediated
migration of bladder cancer cells was suppressed by an ERK1/2
specific inhibitor U0126 (Fig. 4A).
Thus, to define the role of ERK1/2 signaling in IL-5-induced MMP-9
expression, HT1376 cells were pretreated with U0126 for 40 min,
followed by IL-5 treatment for 24 h. U0126 effectively blocked
increased MMP-9 expression (Fig.
4B). To verify the possible implication of ERK1/2 signaling in
transcription factors NF-κB and AP-1, which is associated with
IL-5-induced MMP-9 expression, we next performed a gel shift assay.
As shown in Fig. 4C, NF-κB DNA
binding activity was almost abolished by the addition of U0126. In
contrast, the inhibition of ERK1/2 had no effect on the
IL-5-induced binding activity of AP-1. These results suggest that
the ERK1/2 signaling pathway must be involved in IL-5-induced MMP-9
expression via activation of NF-κB in HT1376 cells.
Knockdown of IL-5Rα, ligand-specific IL-5
receptor, reduces IL-5-induced migration of HT1376 cells
To determine the regulatory mechanism of IL-5, we
used the siRNA-mediated knockdown of IL-5Rα in IL-5-treated HT1376
cells. The cells were transfected with si-IL-5Rα and scramble
siRNA, respectively, followed by treatment with IL-5. To evaluate
the transfection efficiency of siRNA in HT1376 cells, expression of
IL-5Rα was examined by immunoblotting. IL-5Rα was inhibited by
transfection of HT1376 cells with a specific siRNA against IL-5Rα
(si-IL-5Rα) (Fig. 5E). In addition,
transfection of scramble siRNA into cells remained unchanged for
protein levels of IL-5Rα (Fig. 5E).
The results suggested that the siRNA molecule was specific and
effective. The effect of si-IL-5Rα was then determined on
IL-5-induced migration. Our results showed that the inhibition of
IL-5Rα significantly reduced the migration of HT1376 cells induced
by IL-5, compare to siRNA control groups (Fig. 5A). These data suggest that IL-5
induced the migration of bladder cancer cells through its specific
IL-5 receptor, IL-5Rα.
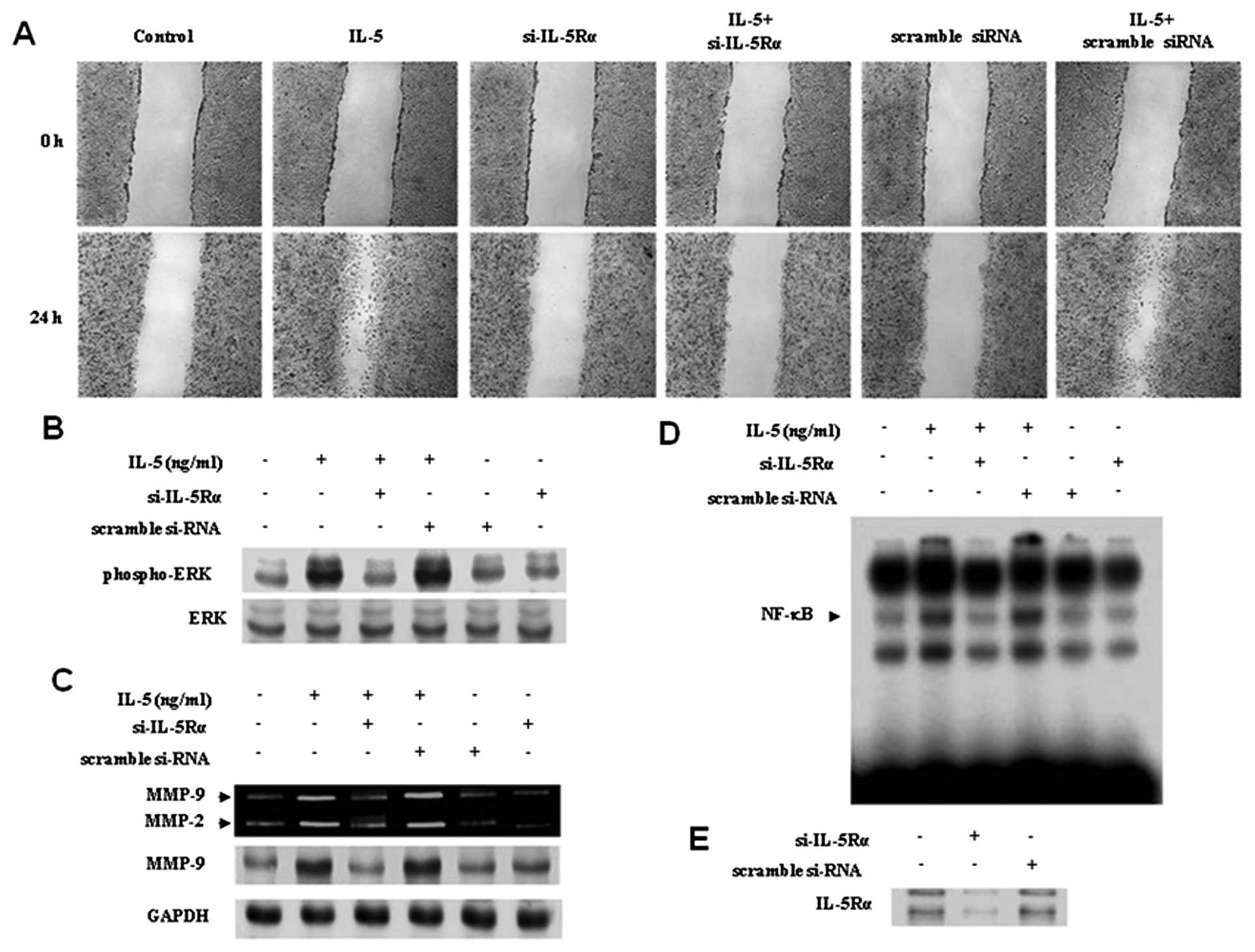 | Figure 5Blockage of IL-5Rα reversed increased
wound healing migration, ERK1/2 activation, MMP-9 expression, and
NF-κB binding activity in the IL-5-mediated stimulation of HT1376
cells. (A) Cells were transfected with either si-IL-5Rα or scramble
siRNA for 24 h, followed by stimulation with IL-5 (100 ng/ml), and
wound healing migration was measured after 24 h, as described in
Materials and methods. (B) Cells were transfected with either
si-IL-5Rα or scramble siRNA for 24 h, further stimulated with IL-5
(30 ng/ml) for 20 min, and the activation levels of ERK1/2 were
determined by immunoblotting. (C) Cells were transfected with
either si-IL-5Rα or scramble siRNA for 24 h, followed by
stimulation of IL-5. After 24 h, zymographic and immunoblot
analyses for MMP-9 were determined in the cultured medium and cell
lysates. (D) Nuclear extracts from the cells were analyzed by EMSA
for the binding activity of NF-κB using radiolabeled
oligonucleotide probes. (E) Effect of IL-5Rα silencing genes in
HT1376 cells. Cells were transfected with either si-IL-5Rα or
scramble siRNA. After 24 h, protein level of IL-5Rα was observed by
immublot analysis. |
Inhibition of IL-5Rα knockdown decreases
ERK1/2 activation, MMP-9 expression, and binding activity of NF-κB
in IL-5-treated HT1376 cells
We sequentially further investigated the effects of
si-IL-5Rα on the induction of ERK1/2 activation, MMP-9 expression,
and binding activity of NF-κB in IL-5-treated HT1376 cells. As
shown in Fig. 5B, transfection of
si-IL-5Rα significantly suppressed the activation of ERK1/2 in
response to IL-5. In addition, the blockade of IL-5Rα reversed
MMP-9 expression and NF-κB binding activity to control levels in
IL-5-treated HT1376 cells (Fig. 5C and
D). These results suggest that IL-5 enhanced ERK1/2 activation,
MMP-9 expression, and binding activity of NF-κB via IL-5Rα receptor
in HT1376 cells.
Discussion
Although the role of cytokine IL-5 in the biological
responses of immune cells is well established, the role and
mechanism involved in IL-5-induced migration of tumor cells remains
to be investigated. Previous studies proposed that IL-5 is a
regulatory cytokine supporting the growth and differentiation of
activated B cells (14). Subsequent
studies have shown the essential roles of IL-5 in the growth,
activation and survival of eosinophils (15). Some studies have indicated that IL-5
has an antitumor effect in mouse B cell lymphoma and colon tumor
cells (17,18). In contrast, the results of the
present study from our results showed that IL-5 plays a pivotal
role in the migration of bladder cancer cells.
The inflammatory process may be responsible for the
development and progression of cancer (19). Inflammation in the bladder is the
result of several pathological processes, which involve the
accumulation of immune cells and the release of cytokines (20,21).
We hypothesize that increased production of inflammatory cytokines
may contribute to an altered microenvironment in the bladder, which
leads to the progression of bladder cancer. In the first stage,
real-time PCR analysis revealed that the mRNA expression of IL-5
and its specific subunit of receptor IL-5Rα were detected in
bladder cancer HT1376 cells. These results suggest that IL-5 is
constitutively expressed in bladder cancer cells and might be an
important regulatory cytokine associated with the progression of
bladder cancer.
We next investigated the molecular regulation
involved in the development and progression of bladder cancer. In
the present study, we demonstrated that IL-5 enhanced wound healing
migration of bladder cancer cells. In addition, both MMP-2 and
MMP-9 expressions were induced by IL-5 treatment. MMPs have been
implicated in cell migration through the degradation of
extracellular matrix components (3,4). It is
well accepted that MMP-2 and MM-9 are particularly important
factors in cell migration (3,4). The
importance and role of MMP-9 in bladder tumor has been demonstrated
in in vivo orthotopic xenograft models (22,23),
preclinical evidence (5–9), and in the study of polymorphisms
(24). Therefore, we investigated
the transcription factors binding to human MMP-9 promoter regions.
Several proximal binding sites, including NF-κB, AP-1 and Sp-1,
that regulate MMP-9 promoter have been identified in tumor cells
(11–13). In 5637 bladder cancer cells
stimulated with TNF-α, NF-κB was shown to be essential for MMP-9
expression, but AP-1 and Sp-1 was not affected (25). Our EMSA results indicated that IL-5
increased the binding activity of NF-κB and AP-1, known binding
sites in the MMP-9 promoter, without detecting inducible levels of
Sp-1. We concluded that NF-κB and AP-1 sites may be responsible for
the IL-5-induced transcriptional activation of the MMP-9 in HT1376
bladder cancer cells.
Several signaling pathways have been identified in
the MMP-9 regulation of various cells (16,26–29).
Studies of MMP-9 in bladder cancer cells have focused mainly on
investigating the signaling pathways in response to various
factors. The ERK1/2 pathway mediates induction of MMP-9 via TNF-α
in HT1376 cells (30). The
involvement of p38MAPK has been associated with the regulation of
MMP-9 expression in bladder cancer HTB9, HTB5, 5637 and HT1376
cells (10,25,30).
In a recent study, Ras-induced MMP-9 expression was inhibited by
RhoA inhibitor Y-27632 (31).
However, the issue of the signaling pathways underlying the
induction of MMP-9 in IL-5-treated cancer cells has not yet been
clarified. In the present study, our result showed that IL-5
treatment induced activation of ERK1/2 in HT1376 cells. Our data
also show that U0126 (ERK1/2 inhibitor) decreases IL-5-induced
wound healing migration and MMP-9 expression via reductions in
NF-κB binding without altering AP-1 activation in HT1376 cells. The
results of the present study clearly show that ERK1/2 mediates
MMP-9 expression via activation of NF-κB binding, which results in
enhanced cell migration in bladder cancer HT1376 cells. It is
possible that NF-κB-mediated expression of MMP-9, regulated by
ERK1/2, may cooperate in the migration of bladder cancer cells.
IL-5 transduces signals through heterodimer receptor
complexes, a unique IL-5Rα and a common β-subunit (βc), leading to
the activation of different signaling pathways on target cells
(14,15). Previous reports have suggested that
the cytoplasmic region of the IL-5Rα subunit is critical for IL-5
signaling (14,15,32).
We therefore investigated whether IL-5Rα contributes to the
IL-5-mediated cell responses using IL-5Rα-specific siRNA
(si-IL-5Rα) in bladder cancer cells. The increased migration,
activation of ERK1/2, MMP-9 expression, and NF-κB binding activity
in response to IL-5 was suppressed by abolition of IL-5Rα,
suggesting that IL-5Rα is indispensable in the transmission of the
migratory cell signal of IL-5.
Our results disagree with those of previous reports
showing an antitumor effect of IL-5 on mouse B cell lymphoma and
colon tumor cells. Considering these opposing results, this may
explain the differences in cell responses by IL-5 within tumor cell
type species. Although an emerging amount of attention is being
paid to the opposing functions of inflammatory cytokines as
extrinsic suppressors of tumors and as pro-tumor growth
stimulators, the model remains to be controversial (19,33).
In the present study, we showed that IL-6 released by bladder
cancer cells could play a crucial role in tumor growth and
development. Further studies are required to investigate the role
of IL-5 in tumor growth using animal models.
In summary, our results are consistent with 3 major
results: i) both IL-5 and IL-5Rα are produced by bladder cancer
HT1376 cells; ii) IL-5-induced migration regulates ERK1/2-mediated
MMP-9 expression via the binding activity of NF-κB in HT1376 cells;
and iii) IL-5Rα receptor is essential for the migration of bladder
cancer HT1376 cells by IL-5, which may be mediated in part by
regulating the ERK1/2-associated MMP-9 expression via activation of
NF-κB binding. Collectively, these novel results point to the
potential use of IL-5 in future potential molecular therapy for
bladder cancer.
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education, Science and Technology
(2010-0001736) and the Korea Healthcare Technology R&D Project,
Ministry of Health & Welfare, Republic of Korea
(A100651-1011-0000200).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
2
|
Black PC and Dinney CP: Bladder cancer
angiogenesis and metastasis - translation from murine model to
clinical trial. Cancer Metastasis Rev. 26:623–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matrisian LM: Metalloproteinases and their
inhibitors in matrix remodeling. Trends Genet. 6:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liotta LA: Tumor invasion and
metastasis-role of extracellular matrix: Rhoads Memorial Award
Lecture. Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
5
|
Sier CF, Casetta G, Verheijen JH, et al:
Enhanced urinary gelatinase activities (matrix metalloproteinases 2
and 9) are associated with early-stage bladder carcinoma: a
comparison with clinically used tumor markers. Clin Cancer Res.
6:2333–2340. 2000.
|
|
6
|
Davies B, Waxman J, Wasan H, et al: Levels
of matrix metalloproteases in bladder cancer correlate with tumor
grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
7
|
Nutt JE, Durkan GC, Mellon JK and Lunec J:
Matrix metalloproteinases (MMPs) in bladder cancer: the induction
of MMP9 by epidermal growth factor and its detection in urine. BJU
Int. 91:99–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moses MA, Wiederschain D, Loughlin KR,
Zurakowski D, Lamb CC and Freeman MR: Increased incidence of matrix
metalloproteinases in urine of cancer patients. Cancer Res.
58:1395–1399. 1998.PubMed/NCBI
|
|
9
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinases
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
10
|
Kumar B, Koul S, Petersen J, et al: p38
mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion
of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer
Res. 70:832–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
12
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Busti C, Falcinelli E, Momi S and Gresele
P: Matrix metalloproteinases and peripheral arterial disease.
Intern Emerg Med. 5:13–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takatsu K and Nakajima H: IL-5 and
eosinophilia. Curr Opin Immunol. 20:288–294. 2008. View Article : Google Scholar
|
|
15
|
Adachi T and Alam R: The mechanism of IL-5
signal transduction. Am J Physiol. 275:C623–C633. 1998.PubMed/NCBI
|
|
16
|
Moon SK, Cha BY and Kim CH: ERK1/2
mediates TNF-alpha-induced matrix metalloproteinase-9 expression in
human vascular smooth muscle cells via the regulation of NF-kappaB
and AP-1: involvement of the ras dependent pathway. J Cell Physiol.
198:417–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu HK, Hirai H, Inamori K, Kitamura K and
Takaku F: Anti-tumor effects of interleukin-4 and interleukin-5
against mouse B cell lymphoma and possible mechanisms of their
action. Jpn J Cancer Res. 83:200–210. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masuda Y, Mita S, Sakamoto K, Ishiko T and
Ogawa M: Suppression of in vivo tumor growth by the transfection of
the interleukin-5 gene into colon tumor cells. Cancer Immunol
Immunother. 41:325–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tyagi P, Barclay D, Zamora R, et al: Urine
cytokines suggest an inflammatory response in the overactive
bladder: a pilot study. Int Urol Nephrol. 42:629–635. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michaud DS: Chronic inflammation and
bladder cancer. Urol Oncol. 25:260–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dinney CP, Fishbeck R, Singh RK, et al:
Isolation and characterization of metastatic variants from human
transitional cell carcinoma passaged by orthotopic implantation in
athymic nude mice. J Urol. 154:1532–1538. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mian BM, Dinney CP, Bermejo CE, et al:
Fully human anti-interleukin 8 antibody inhibits tumor growth in
orthotopic bladder cancer xenografts via down-regulation of matrix
metalloproteases and nuclear factor-kappaB. Clin Cancer Res.
9:3167–3175. 2003.PubMed/NCBI
|
|
24
|
Kader AK, Liu J, Shao L, et al: Matrix
metalloproteinase polymorphisms are associated with bladder cancer
invasiveness. Clin Cancer Res. 13:2614–2620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SJ, Park SS, Lee US, Kim WJ and Moon
SK: Signaling pathway for TNF-alpha-induced MMP-9 expression:
mediation through p38 MAP kinase, and inhibition by anti-cancer
molecule magnolol in human urinary bladder cancer 5637 cells. Int
Immunopharmacol. 8:1821–1826. 2008. View Article : Google Scholar
|
|
26
|
Cho A, Graves J and Reidy MA:
Mitogen-activated protein kinases mediate matrix
metalloproteinase-9 expression in vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol. 20:2527–2532. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiehler S, Cuvelier SL, Chakrabarti S and
Patel KD: p38 MAP kinase regulates rapid matrix metalloproteinase-9
release from eosinophils. Biochem Biophys Res Commun. 315:463–470.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao J, Xiong S, Klos K, et al: Multiple
signaling pathways involved in activation of matrix
metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast
cancer cells. Oncogene. 20:8066–8074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Genersch E, Hayess K, Neuenfeld Y and
Haller H: Sustained ERK phosphorylation is necessary but not
sufficient for MMP-9 regulation in endothelial cells: involvement
of Ras-dependent and -independent pathways. J Cell Sci.
113:4319–4330. 2003.PubMed/NCBI
|
|
30
|
Lee SJ, Park SS, Cho YH, et al: Activation
of matrix metalloproteinase-9 by TNF-alpha in human urinary bladder
cancer HT1376 cells: the role of MAP kinase signaling pathways.
Oncol Rep. 19:1007–1013. 2008.PubMed/NCBI
|
|
31
|
Chang HR, Huang HP, Kao YL, et al: The
suppressive effect of Rho kinase inhibitor, Y-27632, on oncogenic
Ras/RhoA induced invasion/migration of human bladder cancer TSGH
cells. Chem Biol Interact. 183:172–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takaki S, Murata Y, Kitamura T, Miyajima
A, Tominaga A and Takatsu K: Reconstitution of the functional
receptors for murine and human interleukin 5. J Exp Med.
177:1523–1529. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:12–22. 2004.
View Article : Google Scholar
|