Introduction
Colorectal cancer is the third leading cause of
cancer-related death in the world and is a multistep and multigene
process. Several studies have shown that the aberrant expression of
the cyclooxygenase-2 (Cox-2) gene is related to colorectal cancer
(1,2). Cox-2 is an inducible enzyme that
converts arachidonic acid to prostaglandins. Through the production
of prostaglandins, Cox-2 induces carcinogenesis by promoting cell
proliferation, inhibiting apoptosis, stimulating angiogenesis, and
mediating immune suppression (3–8).
Studies have revealed that the Cox-2 gene is upregulated in human
colorectal adenomas and adenocarcinoma (9,10). Our
previous studies found that a high expression level of Cox-2 is
related to the metastasis of colorectal cancer. Interestingly, we
observed that Cox-2 was expressed in the glandular cavity of
colorectal cancer and in the surrounding interstitial tissues
(11). The usefulness of the Cox-2
protein as a colorectal carcinoma diagnostic marker needs to be
explored. Non-steroidal anti-inflammatory drugs (NSAIDs) could
inhibit the enzymatic activity of Cox-2 and suppress the growth of
cancer cells (12–17). Furthermore, the long-term use of
NSAIDs was associated with a reduction in colorectal cancer risk.
In animal models, Cox-2 inhibitors can reduce the incidence of
colon cancer in APC knockout mice treated with chemical carcinogens
(18). However, more studies are
needed to demonstrate whether the Cox-2 gene can be used as a
colorectal carcinoma gene therapy target.
In this study, we sought to establish an imageable
metastasis model of colorectal cancer and to evaluate whether the
Cox-2 gene can be used as a gene therapy target for colorectal
cancer. A new, improved immunobead-PCR technique was used to detect
Cox-2 in the serum using the imageable metastasis model of
colorectal cancer to evaluate whether serum Cox-2 is a useful
diagnostic serum marker.
Materials and methods
Cell lines and animals
The human colorectal cancer cell line SW480 was
purchased from the American Type Culture Collection (ATCC). The
human colorectal cancer cell line SW480/EGFP, which stably
expresses the EGFP protein, was established from the SW480 cells by
the transfection of the pEGFP-N1 plasmid and was cultured in
RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum
(FBS) and 100 U/ml penicillin/streptomycin in a 5% CO2
humidified atmosphere at 37°C.
The 293FT cells were cultured in Dulbecco's modified
Eagle's medium (Gibco). Four-week-old female/male athymic BALB/c
nu/nu mice were purchased from the Central Laboratory of Animal
Science at Southern University and maintained in laminar-flow
cabinets under specific pathogen-free conditions.
Preparation of lentiviral vectors
Three different shRNA sequences targeting the Cox-2
gene were selected using the Block-iT™ RNAi Designer (Invitrogen,
Carlsbad, CA) (http://rnaidesigner.invitrogen.com/rnaiexpress/rnaiExpress.jsp)
(Table I). The human Cox-2 short
hairpin RNA (shRNA) lentiviral-expressing vectors were used with
the Block-iT™ Lentiviral RNAi Expression system (catalog no.
K4944-00; Invitrogen) following the manufacturer's instructions. In
brief, 3 pLenti6/Cox-2 shRNA expression vectors containing the
human Cox-2 shRNA-expressing cassette and 1 scramble control vector
were constructed. The replication-incompetent lentivirus was
produced by cotransfection of the pLenti6/Cox-2 shRNA expression
vector and ViraPower packaging mix (Invitrogen) containing an
optimized mixture of three packaging plasmids: pLP1, pLP2 and
pLP/VSVG, into 293FT cells. The viral supernatant was harvested 48
h after transfection, filtered through a 0.45-μm cellulose acetate
filter, and frozen at −70°C.
 | Table IThe Cox-2 specific shRNA
sequences. |
Table I
The Cox-2 specific shRNA
sequences.
| shRNA | Sequence |
|---|
| shRNA1 |
5′-CACCGCTGGGAAGCCTTCTCTAACGAATTAGAGAAGGCTTCCCAGC-3′ |
|
5′-AAAAGCTGGGAAGCCTTCTCTAATTCGTTAGAGAAGGCTTCCCAGC-3′ |
| shRNA2 |
5′-CACCGCTTTATGCTGAAGCCCTACGAATAGGGCTTCAGCATAAAGC-3′ |
|
5′-AAAAGCTTTATGCTGAAGCCCTATTCGTAGGGCTTCAGCATAAAGC-3′ |
| shRNA3 |
5′-CACCGCTGTCCCTTTACTTCATTCGAAAATGAAGTAAAGGGACAGC-3′ |
|
5′-AAAAGCTGTCCCTTTACTTCATTTTCGAATGAAGTAAAGGGACAGC-3′ |
| Ctrl-shRNA |
5′-CACCGCTACCTTCGCCCATATGGAATTTTGCCGTACCAAACGGAGC-3′ |
|
5′-AAAAGCTCCGTTTGGTACGGCAAAATTCCATATGGGCGAAGGTAGC-3′ |
Construction of the stable
Cox-2-silencing cell line
The SW480/EGFP cells were transduced with the most
effective Cox-2 shRNA lentiviral vectors or the negative control
lentiviral vectors. The cells were selected for stable integration
by culturing in complete medium containing blasticidin.
Real-time PCR
The total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), and the cDNA was synthesized using
SuperScript reverse transcriptase (Takara). The real-time PCR was
performed using a Mx3000P real-time PCR system (Stratagene, La
Jolla, CA) and Brilliant SYBR-Green QPCR Master Mix kit
(Stratagene) following the manufacturer's protocol. The human Cox-2
and GAPDH primers were as follows: Cox-2, forward primer: 5′-AAGTCC
CTGAGCATCTACG-3′ and the reverse primer: 5′-TTCCTAC CACCAGCAACC-3′.
GAPDH, the forward primer: 5′-ATC TCTGCCCCCTCTGCTGA-3′ and reverse
primer: 5′-GATG ACCTTGCCCACAGCCT-3′.
Western blot analysis
The cells were washed twice with cold
phosphate-buffered saline (PBS) and lysed on ice in RIPA buffer
with an added cocktail of protease inhibitors. The protein lysates
were resolved on a 10% SDS polyacrylamide gel, electrotransferred
to PVDF membranes (Millipore) and blocked in 5% non-fat dry milk in
Tris-buffered saline. The membranes were immunoblotted overnight at
4°C with an anti-Cox-2 monoclonal antibody (Sigma) or an
anti-tubulin antibody (Sigma) followed by their respective
horseradish peroxidase-conjugated secondary antibodies. The signals
were detected by enhanced chemiluminescence (Pierce).
Immunocytochemistry
The cells were plated on a cover glass slide in
6-well plates with medium for 24 h until cells were 30–50%
confluent. The cells on the cover glass were fixed with cold
methanol for 2 min and incubated for 1 h with the Cox-2 primary
antibody (Sigma) at 37°C. The cells were then incubated with the
secondary antibody (goat anti-mouse IgG conjugated to peroxidase,
Dingguo, China) for 1 h at 37°C. The cells were DAB substrate
stained and then counterstained with hematoxylin for 10 sec. All of
the steps were interspersed by rigorous rinsing with PBS 3 times.
The staining was evaluated under a microscope.
MTT assay
The cells were seeded into 96-well culture plates at
1×103 cells per well. After they were cultured for 24,
48 or 72 h, the cells in each well were incubated with 20 μl of 5
mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT, Promega) for 4 h. The supernatant was removed, and 150 μl of
dimethyl sulfoxide (DMSO Sigma) was added and incubated for 15 min.
The absorbance value (OD) of each well was measured using a
microplate reader set at 570 nm. All of the experiments were
performed in triplicate.
Plate clone formation assay
Approximately 1×102 cells were added to
each well of a 6-well culture plate, and each group contained three
wells. Following incubation at 37°C for 12 days, the cells were
washed twice with PBS and stained with Giemsa solution. The number
of colonies containing ≥50 cells was counted under a microscope.
The plate clone formation efficiency = (number of colonies/number
of cells inoculated) × 100%. All of the experiments were performed
in triplicate.
Invasion assay
Warm serum-free medium was added to the top chamber
of the cell invasion chamber (Chemicon) to rehydrate the Matrigel
layer for 2 h at room temperature. A total of 1×105
cells were seeded in the top chamber in serum-free medium (300 μl
containing 1×105 cells). The bottom chamber was prepared
with 10% FBS as a chemoattractant. After a 48-h incubation, the
membrane were fixed with methanol and stained with hematoxylin. The
cells were counted under a microscope in five predetermined fields
at ×200. All of the experiments were performed in triplicate.
In vivo metastasis assays
The method of colon surgical orthotopic implantation
was previously described (19).
Animal experiments were conducted in accordance with
the Animal Research Committee Guidelines of Southern Medical
University [SCXK (Yue) 2006-0015, 2006B023]. The whole-body optical
images (Lighttools, Encinitas) were used to observe the real-time
primary tumor growth and the formation of metastatic lesions. Two
months later, before the mice were euthanized, blood samples from
all of the nude mice were collected. The blood samples were
immediately frozen at −80°C for analysis. All of the mice were then
euthanized, individual organs were excised, and metastases were
checked by hematoxylin-eosin (H&E) staining.
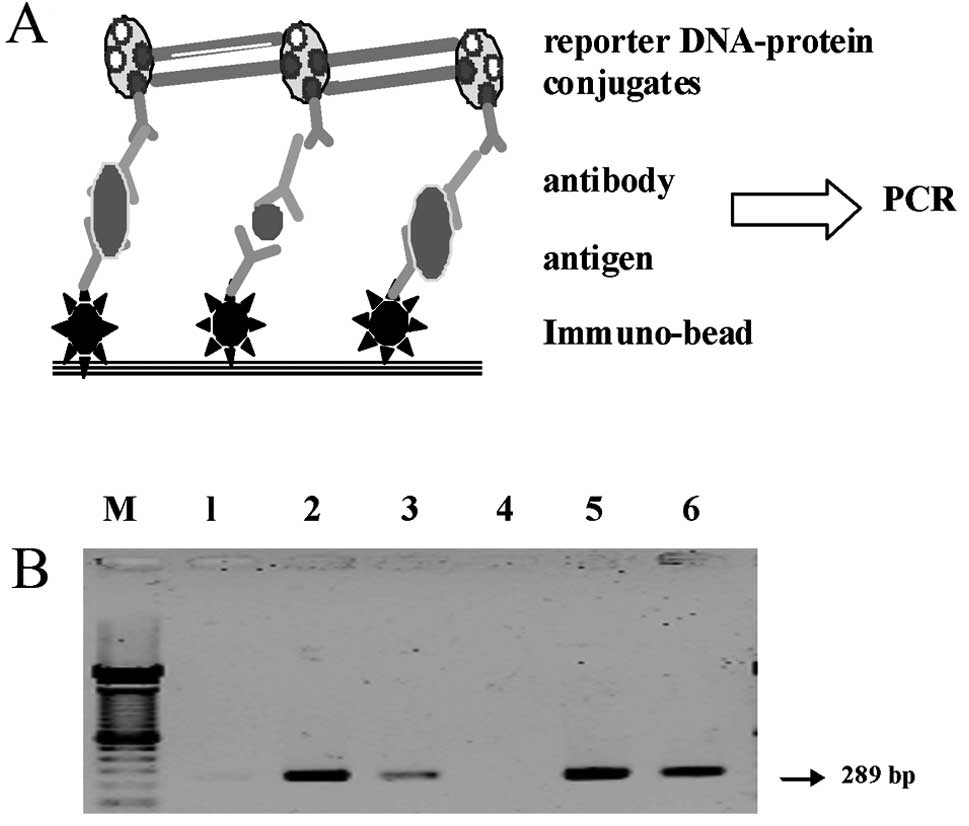
Immuno-PCR assay
The Cox-2 protein in the serum samples of the
metastasis models of colorectal carcinoma were detected using our
new, improved immuno-PCR assay. The assay for detecting very low
concentrations of Cox-2 protein in the serum is described as
follows (Fig. 5A). First,
biotinylated 289 bp reporter DNA fragment originating from the
pEGFP-N1 plasmid was amplified using PCR with the following
primers: forward primer, biotin-5′-CAGTGCTT CAGCCGCTACCC-3′, and
reverse primer, biotin-5′-AGTT CACCTTGATGCCGTTCTT-3′ (Sangon,
Shanghai). The PCR product was purified using a QIAquick PCR
purification kit (Qiagen). In the second step, the preparation of
reporter DNA-protein conjugates was performed. This reporter
DNA-protein conjugate was made by conjugating recombinant
streptavidin and the biotinylated dsDNA fragment and biotinylated
antibody (goat anti-mouse IgG, Sigma). The biotinylated DNA (1 μM)
was mixed with 1 μM recombinant streptavidin (Roche) and 1 μM
biotinylated antibody in Tris-buffered saline, incubated for 30 min
at room temperature and filtered and purified using Pall Microsep
100K ultrafiltration equipment (with Omega PES membrane inside,
Millipore). The purified reporter DNA-protein conjugates were
frozen at −20°C until further analysis. This complex can be
conjugated to antigen and captured by immunobeads to be used as the
template for PCR.
Third, signal amplification was performed by
conventional PCR. The Dynabeads M-280 immunobead (labeled by sheep
anti-rabbit IgG, Dynal), rabbit anti-Cox-2 antibody (Sigma), serum
samples and mouse anti-Cox-2 antibody (Sigma) were added into PCR
tubes in this order. The Cox-2 protein in the serum samples will be
captured by the immunobeads and the Cox-2 antibody. The PCR tubes
were thoroughly washed with PBS by magnetic force rinse on the
Dynal magnetic platform. The super-molecule complex was then added.
The antibody in the complex (biotinylated goat anti-mouse IgG,
Sigma) combines with the mouse anti-Cox-2 antibody, which is bound
to the serum Cox-2 protein specifically. The reporter DNA in the
complex serves as the amplification template of immuno-bead PCR.
The complex was then washed in the PCR tube repeatedly by magnetic
force rinse to remove the uncombined impurities. Conventional PCR
was performed using a thermocycler (Biometra). The PCR mixture
contained 3 μl of the template DNA, 200 μM of each deoxynucleoside
triphosphate, 15 pmol of the primers designed for the reporter DNA
(forward primer: 5′-CAGTGCTTCAGCCGCTACCC-3′, reverse primer:
5′-AGTTCACCTTGATGCCGTTCTT-3′), 3 μl of 10-fold-concentrated
polymerase synthesis buffer, 1.5 mM MgCl2, and 0.65 U of
Taq DNA polymerase. The thermal cycling conditions were as follows:
95°C for 5 min and 30 cycles at 95°C for 30 sec, followed by 56°C
for 45 sec and 72°C for 60 sec. Aliquots (15 μl) of the PCR
products were analyzed by electrophoresis on a 1.5% agarose gel and
visualized using ethidium bromide staining. The positive controls
and negative controls were performed simultaneously. Identical 289
bp DNA bands in the samples and the positive controls, but not in
the negative controls, indicated that there was Cox-2 protein in
the serum samples.
Statistical analysis
The SPSS software package, version 13.0 (Abbott
Laboratories), was used to perform the statistical comparisons
between the samples. The data are expressed as the mean ± standard
deviation (SD). Statistical significance was determined using the
Student's t-test and ANOVA; the differences were considered
significant at a P-value of <0.05.
Results
Establishment of the Cox-2 shRNA
colorectal cancer cell line
To establish a stable Cox-2 knockdown colorectal
cancer cell line, we developed three Cox-2 shRNA lentiviral vectors
(pLenti6/Cox-2 shRNA1, pLenti6/Cox-2 shRNA2 and pLenti6/Cox-2
shRNA3) and a control shRNA lentiviral vector. The pLenti6/Cox-2
shRNA vector and the ViraPower packaging mix were co-transfected,
and the lentivirus in the supernatant was collected and
concentrated 48 h later. The SW480/EGFP cells were transduced with
pLenti6/Cox-2 shRNA virus for 18 h and then selected with
blasticidin for 48 h. Ten days later, the cells were analyzed for
Cox-2 expression using real-time PCR and western blot analysis. The
real-time PCR and western blot analysis revealed that the
pLenti6/Cox-2 shRNA 3 lentiviral vector was the most effective at
blocking Cox-2 expression. Subsequently, we transduced the
pLenti6/Cox-2 shRNA 3 into the SW480/EGFP cells and established
blasticidin-resistant Cox-2 knockdown SW480-EGFP-Cox-2-shRNA
clones. The SW480-EGFP-Cox-2-shRNA clones were expanded and
examined using real-time PCR, western blotting and
immunocytochemical staining. The real-time PCR and western blot
analysis revealed that there were no changes in Cox-2 mRNA and
protein expression in the control lentivirus-infected cell clone.
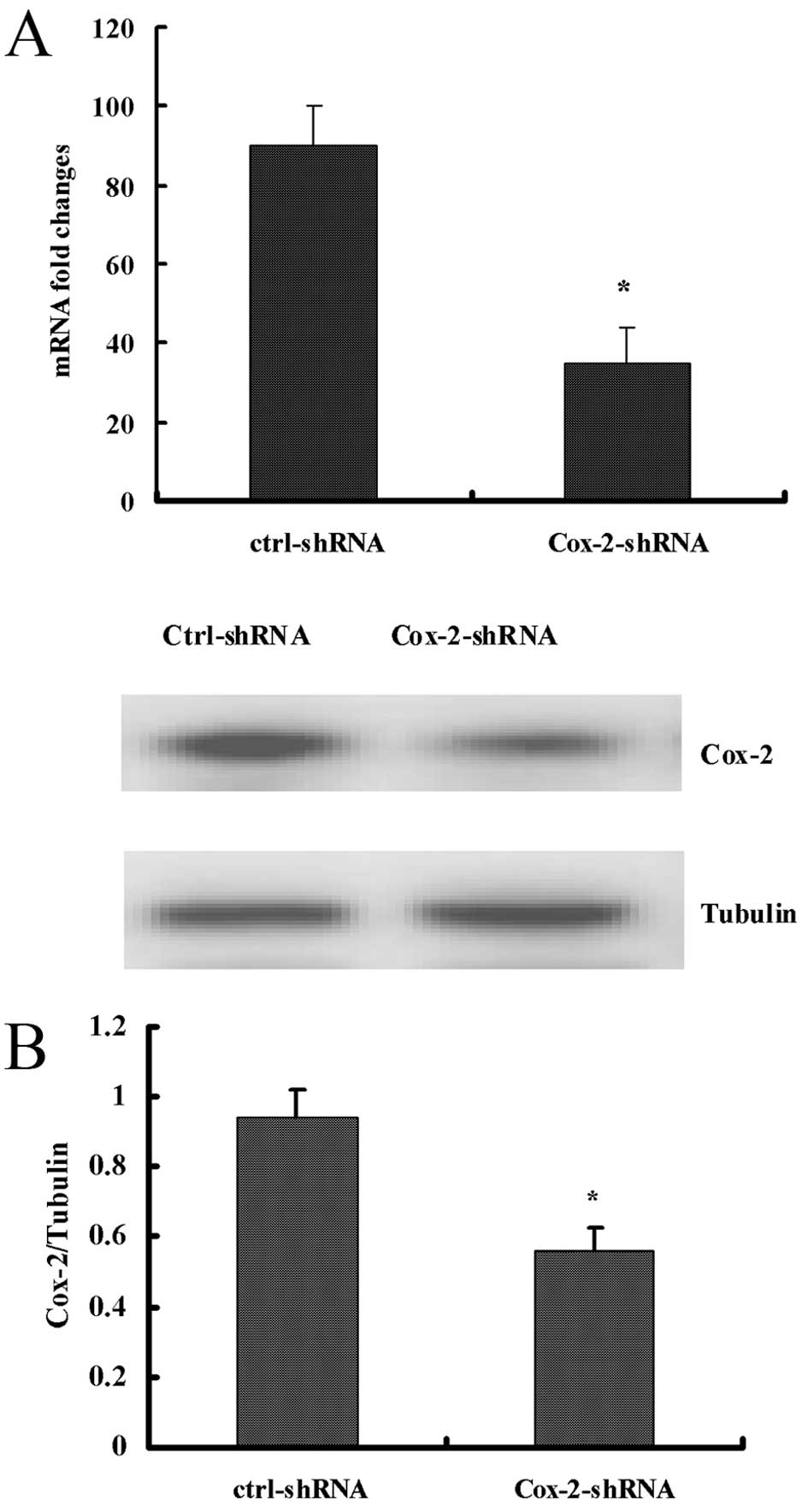
Compared with the control lentivirus infected cell clone, the Cox-2
mRNA and protein expression in the SW480-EGFP-Cox-2-shRNA cells
were reduced by 71.2 and 47.2%, respectively (Fig. 1).
Knockdown of the Cox-2 gene suppresses
cell proliferation in vitro
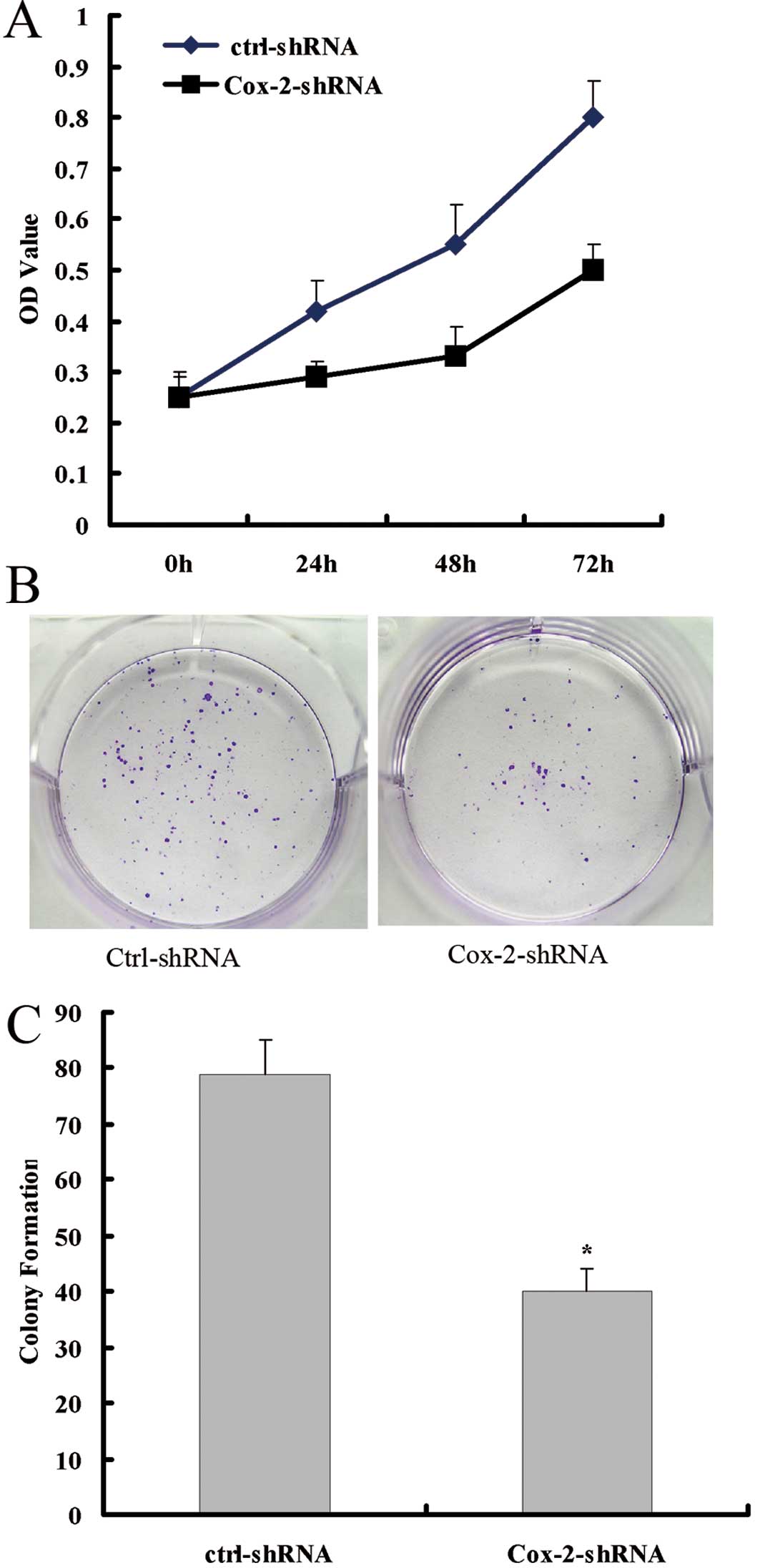
The effect of Cox-2 protein reduction on the
proliferation of colorectal cancer cells was analyzed using the MTT
and plate clone formation assays. The MTT assay showed that the
SW480-EGFP-Cox-2-shRNA had reduced growth ability compared with
that of the control cells (Fig.
2A). The colony formation assay showed that the ability to form
colonies of SW480-EGFP-Cox-2-shRNA cells was significantly reduced
compared with that of the control cells (Fig. 2B and C).
Knockdown of the Cox-2 gene suppresses
cell invasion in vitro
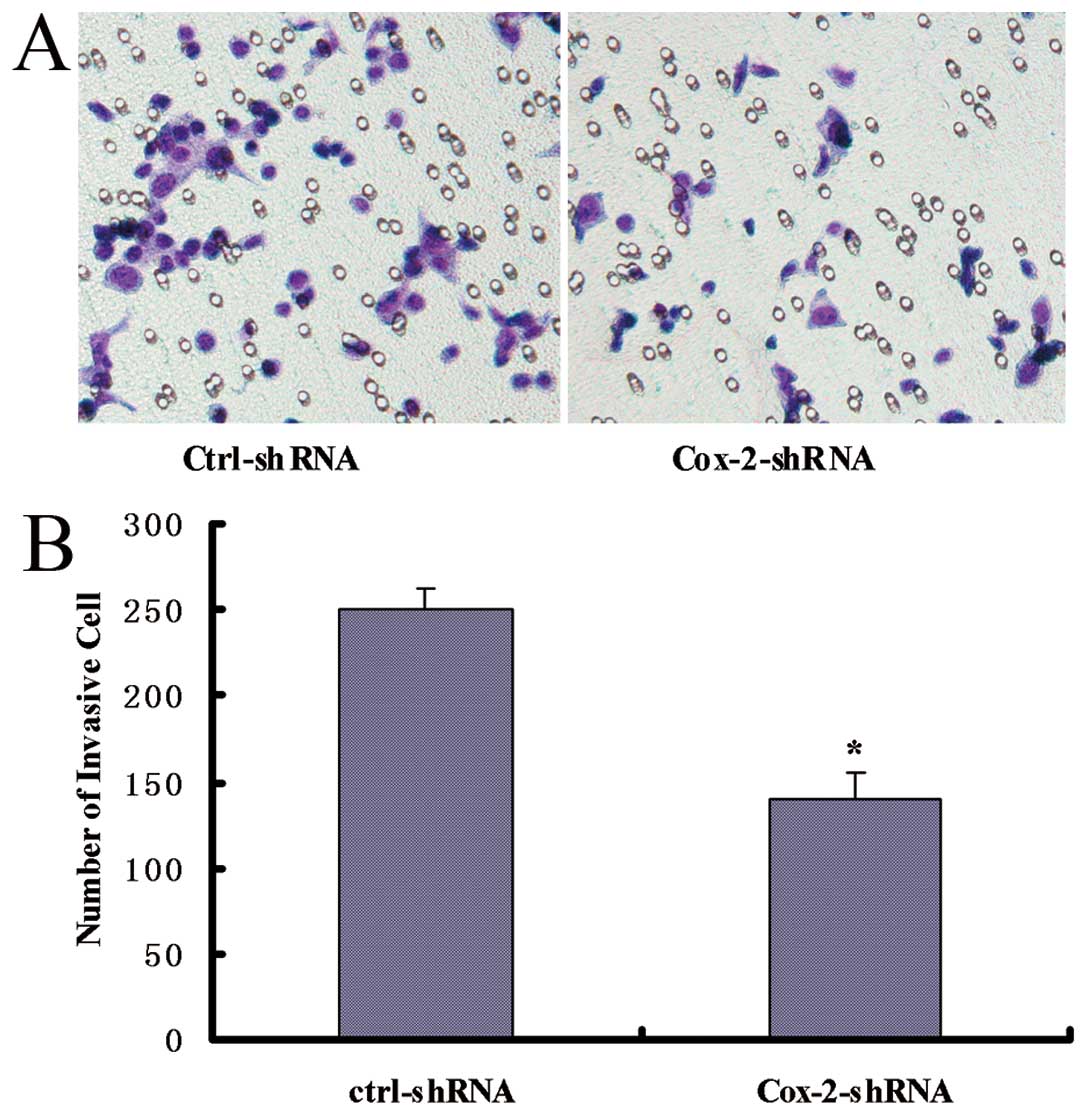
To measure the effect of Cox-2 gene knockdown on
cell invasion, a matrigel invasion assay was performed. Compared
with the control cells, the SW480-EGFP-Cox-2-shRNA cells displayed
decreased invasion (P<0.05) (Fig.
3). These results demonstrated that the invasive ability of
colorectal cancer cells was correlated with Cox-2 expression and
that the Cox-2 gene knockdown effectively attenuates the invasion
of colorectal cancer cells.
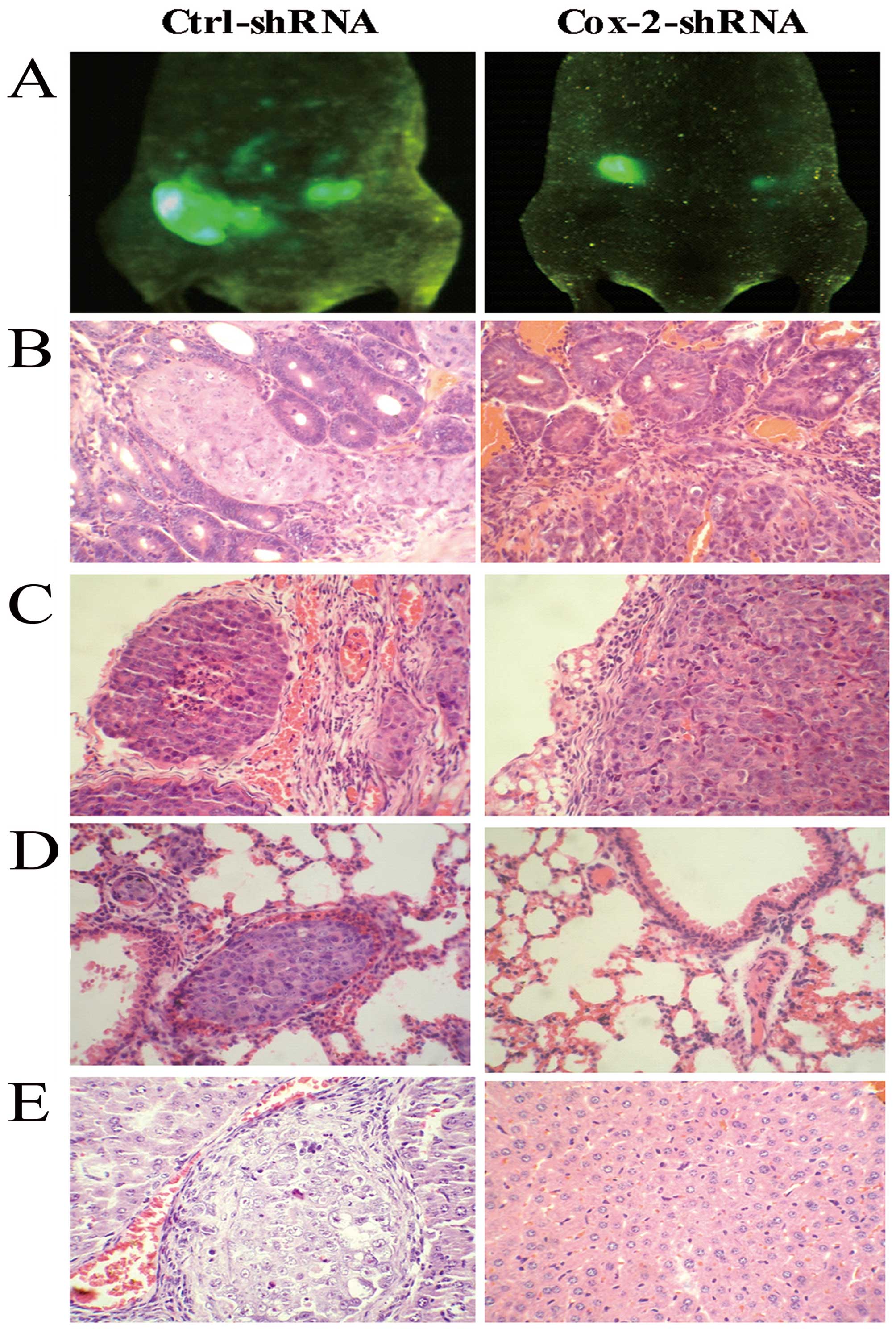
Cox-2 gene knockdown reduces the
metastatic tumor formation
To evaluate the effect of the Cox-2 gene knockdown
on colorectal cancer metastasis, an in vivo metastasis assay
of the surgical orthotopic implantation of colorectal cancer cells
was performed. The whole-body optical images were used to observe
the real-time primary tumor growth and the formation of metastatic
lesions. Two months after the surgical orthotopic implantation of
colorectal cancer cells into the nude mouse colon, the fluorescent
images were used to compare the formation of metastatic lesions.
The results showed that there were fewer metastatic lesions formed
in the Cox-2-shRNA group compared with the control group. A high
number of fluorescence signals was observed on the peritoneum and
abdominal organs using the whole-body optical imaging system
(Fig. 4A). The mice were sacrificed
and autopsied following blood collection. The incidence of
metastasis in the liver, lungs, or other organs was determined by
macroscopic and histological examinations. In the control group,
87.5% of the mice developed peritoneal metastases; however, in the
SW480-Cox-2shRNA group, only 37.5% of the animals exhibited
peritoneal metastases. The incidence of hepatic metastasis and lung
lesions in the mice from the control group was 37.5% (3 of 8) and
12.5% (1 of 8), respectively. The SW480-Cox-2shRNA group did not
produce detectable tumors in the liver or other organs (Fig. 4B-E). These results indicate that
Cox-2 silencing was sufficient to decrease the metastasis of
colorectal cancer cells.
Cox-2 gene knockdown reduces the positive
detection incidences of Cox-2 protein in the serum of an imageable
metastasis model of colorectal cancer
To detect very low concentrations of serum Cox-2
protein, an improved immunobead PCR protocol was established and
utilized. Our data show that the 289 bp amplified DNA bands were
found in the positive control, and no amplified DNA band was seen
in the negative control (Fig. 5B).
The positive detection rate for the Cox-2 protein in the
SW480-shRNA group and SW480-Ctrl-shRNA group was 12.5 and 87.5%,
respectively. The positive detection rate for the Cox-2 protein in
the samples from the SW480-shRNA group was significantly lower than
that in the SW480-Ctrl-shRNA group (P<0.05). These results
suggest that the Cox-2 protein can be detected in the serum of a
metastatic colorectal cancer nude mouse model and that Cox-2 can be
used as a colorectal cancer metastasis marker.
Discussion
Cox-2 is an inducible enzyme that converts
arachidonic acid to prostaglandins. Cox-2 catalyzes the oxidation
of arachidonic acid, which produces prostaglandins that may
accelerate the carcinogenesis and metastasis process. It is
possible that the increased levels of Cox-2 serve to lower the
intracellular level of free arachidonic acid and, thereby, prevent
apoptosis by the depletion of the apoptotic signal (20). Data have shown that the
overexpression of Cox-2 is related to colorectal cancer metastasis
(12). Our data also show that the
expression of Cox-2 is related to colorectal cancer metastasis.
Additionally, we found that Cox-2 can be overexpressed in the
glandular tube of colorectal cancer and in the surrounding
inflammatory tissue (11). Because
Cox-2 can be secreted into the glandular tube of colorectal cancer
and the surrounding inflammatory tissue, whether it can be detected
in the serum has not been determined.
In previous studies of azoxymethane-induced colon
carcinogenesis in rats, treatment with a selective Cox-2 inhibitor
(NS-398) has been shown to reduce the tumor size and multiplicity
(21,22). The clinical relevance of these
findings was shown in patients with germ line mutations in the APC
gene and the autosomal dominant inherited syndrome of familial
adenomatous polyposis. In these patients, treatment with the NSAID
has been shown to regress existing colorectal adenomas.
Furthermore, the NSAID treatments aimed at inhibiting Cox-2 have
been proven to be chemopreventive, reducing both the polyp number
and the polyp burden following clinical trials in colon cancer
patients (23). The side-effects of
some of the Cox-2 inhibitors that have emerged following long-term
treatment have caused much concern.
The use of RNA interference (RNAi) technology to
reduce gene expression has been become widely utilized. RNA
interference (RNAi) is a gene regulatory system in which small RNA
molecules silence genes that have a similar sequence to the small
RNAs. Short-hairpin RNA has been widely used to reduce the
expression of many target genes due to their high specificity and
apparent non-toxicity (24–27). The published data show that a
reduction in the expression of Cox-2 in a colorectal cancer cell
line by RNA interference (RNAi) technology resulted in decreased
cell proliferation, invasion and metastasis (28); these data were obtained using a cell
model and not from suitable animal model. Additionally, the
previous experiments used a routine transfect reagent to
transiently transfect shRNA vector plasmid into the cells and did
not generate a stable Cox-2 gene knockdown cell line. In the
current study, we used a lentiviral vector-based RNAi expression
system in which the expression of shRNAs is driven by a U6 promoter
to stably knockdown the expression of Cox-2 mRNA in the colorectal
cancer SW480-EGFP cell line. The lentiviral expression system
offers several advantages over other transfection reagents. The
lentiviral system can easily infect over 90% of cultured cells,
which is sufficient for studying the effects of RNAi on endogenous
gene expression (29). Lentivirus
is relatively easy to generate, and thus, it has been applied to
large-scale, high-throughput RNAi assays for studying gene
functions (30). Finally, the
lentiviral system has the advantage of the long-term stable
expression of a transgene to establish stable transfected cell
lines (31). Using this system, we
generated a Cox-2 gene stable knockdown colorectal cell line, the
SW480-EGFP-Cox-2-shRNA cell line, and used this cell line to
generate an imageable metastasis model of colorectal cancer.
In this study, we found that the knockdown of Cox-2
in the SW480-EGFP-Cox-2shRNA cells abrogated the cell's ability to
proliferate and invade and strongly impaired colon xenograft
formation. We found that the knockdown of Cox-2 expression appeared
to have an inverse correlation with tumorigenesis. The experiments
also demonstrate that a reduction in Cox-2 expression in colorectal
cancer cells decreased angiogenesis. The whole-body optical imaging
system allows for the continuous visual monitoring of malignant
tumor growth within intact animals. Our data show that the reduced
expression of Cox-2 in SW480-EGFP-Cox-2shRNA cells abrogated their
ability to metastasize to lymph nodes, lungs or liver. In the
orthotopic xenograft model, the data showed that the reduced
expression of Cox-2 in the SW480-EGFP-Cox-2shRNA cells abrogated
their ability to develop lung and hepatic metastases. These results
support the concept that Cox-2 may be a positive regulator of tumor
growth in colorectal cancer. A stable lentivirus-based Cox-2
knockdown mediated by RNAi impaired the invasive and metastatic
ability of colorectal cancer cells. Our study confirms that the
Cox-2 gene may be an effective target for cancer therapies. The
endogenous Cox-2 gene can be specifically downregulated by RNAi in
a colorectal cancer cell line. All of these data show that the
inhibition of Cox-2 can reduce the risk of colorectal cancer
development and metastasis. The results reported here indicate an
easy yet powerful and highly selective lentivirus-based method to
knockdown Cox-2 expression in a stable and long-lasting manner.
Furthermore, we propose the possibility of an in vivo
application of this anti-Cox-2 lentiviral vector to establish a
stable human cancer metastasis model system.
Because Cox-2 is a key gene and pays an important
role in colorectal cancer development and metastasis, it is
frequently highly expressed in colorectal cancer. The ability of
Cox-2 to infiltrate into the serum has not been investigated, and
whether it can be used as a serum marker of colorectal cancer still
needs to be explored. As we have found that Cox-2 can be detected
in the glandular cavity of colorectal cancer and in the surrounding
inflammatory tissue by immunohistochemistry, we speculated that the
Cox-2 protein can be secreted into the interstitial liquid and then
cycled into the blood at a very low concentration. Thus, in this
study, we generated a new, improved immunobead-PCR method to detect
very low concentrations of the tumor marker in serum samples.
Initially, we used Dynabeads® M-280 sheep
anti-rabbit IgG immunobeads, rabbit anti-Cox-2 antibody and a mouse
anti-Cox-2 antibody to capture low concentrations of Cox-2 protein
in serum samples. The Dynabeads® M-280 sheep anti-rabbit
IgG are uniform, superparamagnetic, polystyrene beads with
affinity-purified sheep anti-rabbit IgG covalently bound to the
bead surface. These polyclonal antibodies bind both heavy and light
chain rabbit IgG. They are designed as a solid support for the
simple and efficient binding of target molecules. These beads allow
for the isolation and subsequent handling of target molecules in a
highly specific manner. The beads are added directly to the sample
containing the target antibody/antigen (Cox-2 antibody/Cox-2
protein). After a short incubation that allows for the affinity
capture of the target, the beads are pulled to the side of the test
tube by a magnet, thus allowing for the aspiration of the unbound
material. The magnetic separation facilitates the easy washing and
concentration of the isolated target molecule. The target molecule
can be eluted off of the beads with conventional elution methods.
Second, this assay utilizes the self-assembly capabilities of
semi-synthetic DNA-protein conjugates (32,33).
In particular, the covalent ssDNA-STV conjugates are employed as
molecular adapters for the effective DNA-directed immobilization of
the captured antibodies on solid supports containing complementary
oligonucleotides. The capture antibodies are used for the selective
capture of the protein antigen, similar to the conventional
sandwich ELISA. Subsequently, immuo-PCR is employed as a
high-sensitivity detection method, taking advantage of the
conjugates produced by the self-assembly of the STV, biotinylated
dsDNA and antibodies directed against the protein. This assay
allowed us to detect very low amounts of antigen, typically
100–1,000-fold less than is detectable by conventional sandwich
ELISAs (34). In addition, this
immunoassay can be performed in a single step by simultaneously
tagging the protein with capture and detection reagents, thereby
significantly reducing handling time. In our experiment, a false
positive result occasionally occurred because of the sensitivity of
this assay. Therefore, strict positive and negative controls are
needed and the procedure should be performed carefully each time
this sensitive assay is used. Using this improved immunobeads PCR
assay, we found that the Cox-2 protein infiltrated into the serum.
Additionally, we found that the positive detection incidence of the
Cox-2 protein in samples from the SW480-shRNA group was
significantly lower than that in the control group with
metastasis.
Taken together, our results indicate that the
knockdown of Cox-2 expression by RNAi suppressed the proliferation
of colorectal cancer both in vitro and in vivo. This
study also demonstrated that targeting Cox-2 in vivo reduced
the metastatic potential of colorectal cancer cells. Thus, Cox-2 is
a promising marker for the diagnosis of colorectal metastasis, and
it is also a promising target for therapeutic intervention.
Acknowledgements
This study was supported by the major projects of
the National Natural Science Foundation of China (no.
81090422/H1606), the National Natural Science Foundation of China
(nos. 30770976, 81071735, 81172054), the National Basic Research
Program of China (973 Program, no. 2010CB529403), the Science and
technology projects in Guangdong Province (no. 2010B031500012), the
Guangdong Provincial Key Science and Technology Innovation Fund for
Higher Education (no. GXZD1016), the Innovative Research Team
Foundation in University (no. IRT0731) and the Universities in
Guangdong Province 211 key construction projects.
References
|
1
|
Masunaga R, Kohno H, Dhar DK, et al:
Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.
|
|
2
|
Wendum D, Masliah J, Trugnan G and Fléjou
JF: Cyclooxygenase-2 and its role in colorectal cancer development.
Virchows Arch. 445:327–333. 2004.
|
|
3
|
Möbius C, Stein HJ, Spiess C, et al: COX2
expression, angiogenesis, proliferation and survival in Barrett's
cancer. Eur J Surg Oncol. 31:755–759. 2005.
|
|
4
|
Wang Q, Takei Y, Kobayashi O, Osada T and
Watanabe S: Cyclooxygenase 2 modulates killing of cytotoxic T
lymphocytes by colon cancer cells. J Clin Biochem Nutr. 45:163–170.
2009.
|
|
5
|
Boland GP, Butt IS, Prasad R, Knox WF and
Bundred NJ: Cox-2 expression is associated with an aggressive
phenotype in ductal carcinoma in situ. Br J Cancer. 90:423–429.
2004.
|
|
6
|
Singh B, Berry JA, Shoher A, Ramakrishnan
V and Lucci A: Cox-2 overexpression increases motility and invasion
of breast cancer cells. Int J Oncol. 26:1393–1399. 2005.
|
|
7
|
Wang W, Bergh A and Damber JE:
Cyclooxygenase-2 expression correlates with local chronic
inflammation and tumor neovascularization in human prostate cancer.
Clin Cancer Res. 11:3250–3256. 2005.
|
|
8
|
Petersen S, Haroske G, Hellmich G, Ludwig
K, Petersen C and Eicheler W: Cox-2 expression in rectal carcinoma:
immunohistochemical pattern and clinical outcome. Anticancer Res.
22:1225–1230. 2002.
|
|
9
|
Eberhart CE, Coffey RJ, Radhika A,
Giardiello FM, Ferrenbach S and DuBois RN: Up-regulation of
cyclooxygenase 2 gene expression in human colorectal adenomas and
adenocarcinomas. Gastroenterology. 107:1183–1188. 1994.
|
|
10
|
Kim JY, Lim SJ and Park K:
Cyclooxygenase-2 and c-erbB-2 expression in colorectal carcinoma
assessed using tissue microarrays. Appl Immunohistochem Mol
Morphol. 12:67–70. 2004.
|
|
11
|
Li ZG, Liu TF, Xie WB, Zhou J, Yu L and
Ding YQ: Association of abnormal cyclooxygenase-2 gene expression
with colorectal carcinoma metastasis. Nan Fang Yi Ke Da Xue Xue
Bao. 26:1408–1411. 2006.(In Chinese).
|
|
12
|
Dempke W, Rie C, Grothey A and Schmoll HJ:
Cyclooxygenase-2: a novel target for cancer chemotherapy. J Cancer
Res Clin Oncol. 127:411–417. 2001.
|
|
13
|
Narayanan BA, Narayanan NK, Pttman B and
Reddy BS: Adenocarcina of the mouse prostate growth inhibition by
celecoxib: downregulation of transcription factors involved in
COX-2 inhibition. Prostate. 66:257–265. 2006.
|
|
14
|
Yao M, Kargman S, Lam EC, et al:
Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth
and metastatic potential of colorectal carcinoma in mice. Cancer
Res. 63:586–592. 2003.
|
|
15
|
Bottone FG Jr, Martinez JM, Collins JB,
Afshari CA and Eling TE: Gene modulation by the cyclooxygenase
inhibitor, sulindac sulfide, in human colorectal carcinoma cells:
possible link to apoptosis. J Biol Chem. 278:25790–25801. 2003.
|
|
16
|
Dubé C, Rostom A, Lewin G, et al: The use
of aspirin for primary prevention of colorectal cancer: a
systematic review prepared for the U.S. Preventive Services Task
Force. Ann Intern Med. 146:365–375. 2007.
|
|
17
|
Chan AT, Ogino S and Fuchs CS: Aspirin and
the risk of colorectal cancer in relation to the expression of
Cox-2. N Engl J Med. 356:2131–2142. 2007.
|
|
18
|
Oshima M, Murai N, Kargman S, et al:
Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by
rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res.
61:1733–1740. 2001.
|
|
19
|
Rashidi B, Gamagami R, Sasson A, Sun FX,
Geller J, Moossa AR and Hoffman RM: An orthotopic mouse model of
remetastasis of human colon cancer liver metastasis. Clin Cancer
Res. 6:2556–2561. 2000.
|
|
20
|
Cao Y, Pearman AT, Zimmerman GA, McIntyre
TM and Prescott SM: Intracellular unesterified arachidonic acid
signals apoptosis. Proc Natl Acad Sci USA. 97:11280–11285.
2000.
|
|
21
|
Yoshimi N, Kawabata K, Hara A, Matsunaga
K, Yamada Y and Mori H: Inhibitory effect of NS-398, a selective
cyclooxygenase-2 inhibitor, on azoxymethane-induced aberrant crypt
foci in colon carcinogenesis of F344 rats. Jpn J Cancer Res.
88:1044–1051. 1997.
|
|
22
|
Yoshimi N, Shimizu M, Matsunaga K, Yamada
Y, Fujii K, Hara A and Mori H: Chemopreventive effect of
N-(2-cyclohexyloxy-4-nitrophenyl)methane sulfonamide (NS-398), a
selective cyclooxygenase-2 inhibitor, in rat colon carcinogenesis
induced by azoxymethane. Jpn J Cancer Res. 90:406–412. 1999.
|
|
23
|
Steinbach G, Lynch PM, Phillips RK, et al:
The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial
adenomatous polyposis. N Engl J Med. 342:1946–1952. 2000.
|
|
24
|
Caplen NJ, Parrish S, Imani F, Fire A and
Morgan RA: Specific inhibition of gene expression by small
double-stranded RNAs in invertebrate and vertebrate systems. Proc
Natl Acad Sci USA. 98:9742–9747. 2001.
|
|
25
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001.
|
|
26
|
Soutschek J, Akinc A, Bramlage B, Charisse
K, et al: Therapeutic silencing of an endogenous gene by systemic
administration of modified siRNAs. Nature. 432:173–178. 2004.
|
|
27
|
Lu PY, Xie F and Woodle MC: In vivo
application of RNA interference: from functional genomics to
therapeutics. Adv Genet. 54:117–142. 2005.
|
|
28
|
Charames GS and Bapat B: Cyclooxygenase-2
knockdown by RNA interference in colon cancer. Int J Oncol.
28:543–549. 2006.
|
|
29
|
Bartosch B and Cosset FL: Strategies for
retargeted gene delivery using vectors derived from lentiviruses.
Curr Gene Ther. 4:427–443. 2004.
|
|
30
|
Bailey SN, Ali SM, Carpenter AE, Higgins
CO and Sabatini DM: Microarrays of lentiviruses for gene function
screens in immortalized and primary cells. Nat Methods. 3:117–122.
2006.
|
|
31
|
Abbas-Terki T, Blanco-Bose W, Déglon N,
Pralong W and Aebischer P: Lentiviral-mediated RNA interference.
Hum Gene Ther. 13:2197–201. 2002.
|
|
32
|
Niemeyer CM, Wacker R and Adler M:
Combination of DNA-directed immobilization and immuno-PCR: very
sensitive antigen detection by means of self-assembled DNA-protein
conjugates. Nucleic Acids Res. 31:e902003.
|
|
33
|
Grinde B, Jonassen T and Ushijima H:
Sensitive detection of group A rotaviruses by immunomagnetic
separation and reverse transcription-polymerase chain reaction. J
Virol Methods. 55:327–338. 1995.
|
|
34
|
Xiang CQ, Shen CL, Wu ZR, Qin YQ, Zhang
YY, Liu CZ, Chen JG and Zhang SN: Detection of mutant p53 protein
in workers occupationally exposed to benzidine. J Occup Health.
49:279–284. 2007.
|