Introduction
Cervical carcinoma is the second most common
malignancy of female reproductive tract and ranks second among
female deaths caused by cancers. More than 50 million people
worldwide suffer from this disease each year. Although early
diagnosis of cervical cancer has made great progress, the incidence
and mortality of this disease still remains high, and the onset age
is becoming younger, seriously threatening women's health and
lives. The treatments mainly depend on surgery and radiotherapy,
supplemented with chemotherapy. Five-year survival rate of
terminally-ill patients is only 50% although therapeutic techniques
have been improved greatly in the past years (1). It was reported that the poor effects
of chemotherapy on advanced stage of cervical cancer may be
directly due to obstacles in apoptosis of tumor cells (2).
Indole-3-carbinol (I3C), a type of compound derived
from cruciferous vegetables (3),
was demonstrated to exhibit significant anticancer efficacy both
in vivo and in vitro by inducing cell apoptosis and
cell cycle arrest in G1 phase (4,5),
affecting DNA damage repair (6–8), as
well as resisting angiogenesis, metastasizing and invasion of
cancer cells (9,10). 3,3′-diindolylmethane (DIM), an
important polymer converted from I3C under pH 5.0–7.0 (11), was characterized with
anti-proliferative and pro-apoptotic activities in cancer cells
(12,13). Chinnakannu et al(13) reported that a formulated DIM (B-DIM)
could inactivate NF-κB signaling and induce apoptosis through
inhibiting proteasome activity in S phase of LNCaP and C4–2B cells,
two types of prostate cancer cells, suggesting that B-DIM could be
a potent agent for prevention and/or treatment of both
hormone-sensitive and hormone-refractory prostate cancers. I3C-6, a
derivative of I3C, was proven to significantly inhibit the growth
and migration of HeLa cells (adenocarcinoma type) (14). However, there are no reports on
anti-tumor effects of DIM in cervical carcinoma cells, or
comparisons of its effects on two different types of cervical
cancer cells, including HeLa cells (adenocarcinoma type) and SiHa
cells (squamous carcinoma). The present study aimed to investigate
the potential anticancer effects of DIM in cervical carcinoma cells
in vitro and compare its different influence on HeLa cells
and SiHa cells, thus providing useful information for clinical
therapies of cervical carcinoma.
Materials and methods
Reagents
Cell Counting kit-8 (CCK-8) was purchased from
Dongji (Japan). All antibodies were purchased from Cell Signaling
Technology (USA), including p-p38, t-p38, p-ERK, t-ERK, p-JNK,
t-JNK, PI3K p85, PI3K p110α, PI3K p110β, p-Akt, t-Akt, p-PDK1,
p-c-Raf and GAPDH. Annexin V FITC/PI Apoptosis Detection kit was
from Lianke (China).
Cell cultures
The HeLa cells and SiHa cells, kindly provided by
the Type Culture Collection of Chinese Academy of Sciences, were
maintained in RPMI-1640 medium (Sigma, USA) supplemented with 10%
fetal bovine serum (FBS) (Hyclone, USA) and 20 μg/ml antibiotics
(ampicillin and kanamycin). The cultivations were performed in a
humidified atmosphere of 5% CO2 at 37°C. The medium was
changed at 48-h intervals and culture transfers were performed
every 4 days when the culture almost reached confluence.
Measurement of cell proliferation
Cells were harvested by trypsinization at their
logarithmic growth phases and plated in 96-well microwell plates in
100 μl RPMI-1640 medium with 10% FBS at a density of
1×103 cells/well. Cells were treated with DIM at
different concentrations (25, 50 and 100 μM) after 24 h of
incubation and continuously cultured for 0, 24, 48 and 72 h before
proliferation analysis. Cell proliferation assays were performed
using the WST-8 based Colorimetric Assay Cell Counting kit-8
(CCK-8). In addition, proliferation was also measured in parallel
negative groups without DIM treatment and the blank groups without
cells in the wells. Six repeats were prepared for each group.
Quantification of viable cells was performed by measuring the
absorbance at 450 nm (A450) using a microplate reader (Beckman,
USA). The following formula was used to calculate the inhibitory
rate of DIM on cell proliferation: inhibitory rate (%) = [A450
(negative group) − A450 (treated group)] / [A450 (negative group) −
A450 (blank group)] × 100%.
Apoptosis analysis
After DIM treatment for 48 h, HeLa and SiHa cells
were harvested by trypsinization (≥1×104) and washed
twice with cold phosphate-buffered saline (PBS). The cells
resuspended in 200 μl annexin-V binding buffer were incubated in 5
μl of annexin-V fluorescein isothiocyanate (FITC) solution and 10
μl of propidium iodide (PI) solution for 15 min in the dark at room
temperature. The fluorescences of each group were analyzed on a
FACSCanto™II spectrometer (BD Biosciences, San Jose, CA, USA)
within 1 h and the cells stained with FITC/PI were counted as
apoptotic cells.
Western blot analysis
Proteins were extracted from the cultured cells and
loaded onto a polyacrylamide gel (10%). Then electrophoresis was
performed at room temperature at 50 V for 30 min and then 200 V for
1.5 h, followed by transferring to nitrocellulose membranes. The
membranes were incubated with blocking solution for 2 h at room
temperature, and then incubated with different primary antibodies
including p-p38, t-p38, p-ERK, t-ERK, p-JNK, t-JNK, PI3K p85, PI3K
p110α, PI3K p110β, p-Akt, t-Akt, p-PDK1, p-c-Raf and GAPDH (Cell
Signaling) for 12 h at 4°C. After washing for 1 h with 20 mM
Tris·HCl and 0.5 M NaCl (TBS) with 0.05% Tween-20 (TTBS), membranes
were incubated with the secondary antibody conjugated to
horseradish peroxidase for 30 min at room temperature and washed
again for 1 h. GAPDH was used as an internal reference. Protein
bands were finally colored with enhanced chemiluminescence (ECL)
reagent (Thermo, USA) and exposed to X-ray.
Statistical analysis
Data are expressed as mean ± SD. Difference in the
effects of DIM on HeLa and SiHa cells were compared with one-way
ANOVA (SPSS statistical software, SPSS Inc., Chicago, IL, USA).
P<0.05 (two-tailed) was considered statistically
significant.
Results
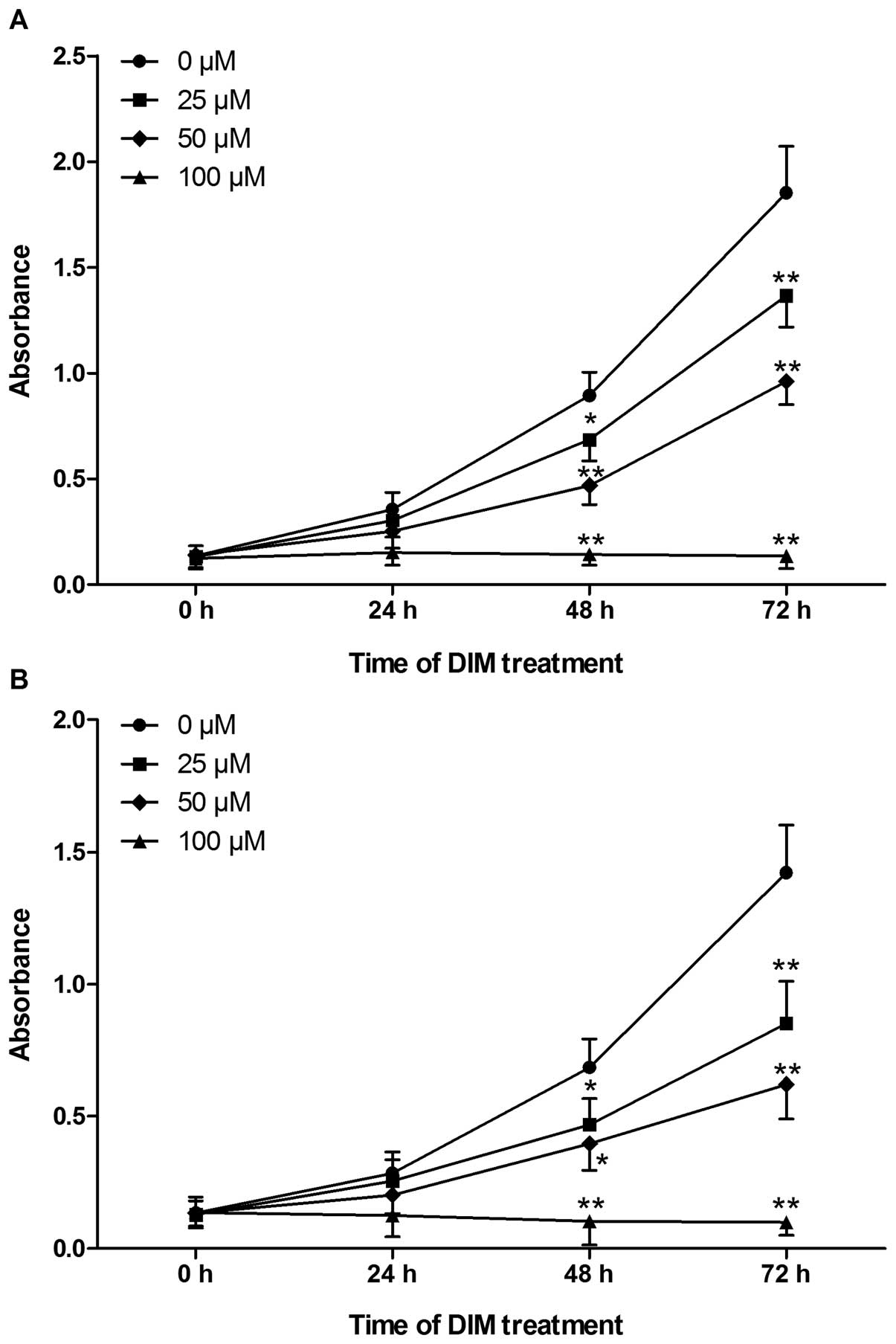
Effects of DIM on the proliferations of
HeLa and SiHa cells
HeLa and SiHa cells were treated with various
concentrations (0, 25, 50 and 100 μM) of DIM for 0–72 h. Cell
proliferation was assessed by the CCK-8 method and represented by
absorbance value at 450 nm. DIM showed a dose- and time-dependent
anti-proliferative effect on the growth of HeLa and SiHa cells
(Fig. 1).
DIM showed a profound dose-dependent inhibition of
the growth of HeLa and SiHa cells with the inhibitory rates ranging
from 14.61 to 93.03 (Table I). It
was noticeable that the inhibitory effects of DIM on SiHa cells
were much stronger than that on HeLa cells after 48 h of treatment
in each concentration group (except for 50 μM group after 48 h of
treatment). In addition, after DIM treatment for 48 h, the
calculated IC50 of DIM for HeLa and SiHa cells were 46.8
and 44.44 μM respectively, suggesting a much more sensitive
response of SiHa cells to DIM than that of HeLa cells.
 | Table IInhibitory rates of DIM on the
proliferation of HeLa and SiHa cells. |
Table I
Inhibitory rates of DIM on the
proliferation of HeLa and SiHa cells.
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|---|
| DIM (μM) | HeLa | SiHa | HeLa | SiHa | HeLa | SiHa |
|---|
| 25 | 14.61 | 10.18 | 23.38 | 31.58 | 26.19 | 40.04 |
| 50 | 29.21 | 28.77 | 47.54 | 42.11 | 48.06 | 56.30 |
| 100 | 57.02 | 56.14 | 84.00 | 84.94 | 92.66 | 93.03 |
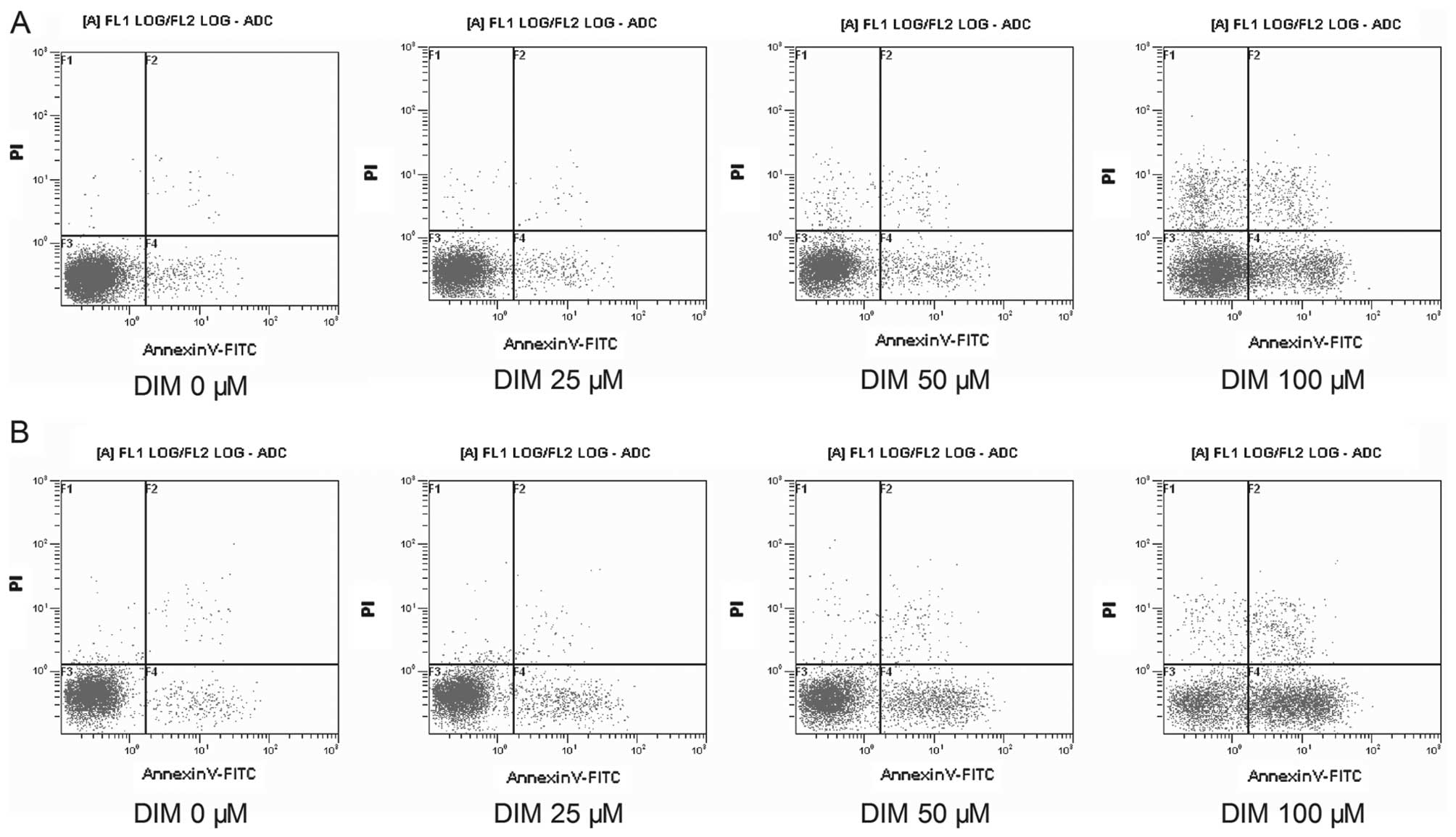
Effects of DIM on the apoptosis of HeLa
and SiHa cells
Flow cytometry was performed to detect the apoptosis
of HeLa cells and SiHa cells treated with various concentrations of
DIM for 48 h. As DIM concentrations increased from 0 μM (negative
control group) to 100 μM, both HeLa cells and SiHa cells showed the
following tendencies (Fig. 2): i)
The cells distributed in the 3rd region (normal zone) gradually
decreased, indicating that the number of living cancer cells were
reduced by DIM treatment. ii) The cells distributed in the 4th
region gradually increased, suggesting the number of cells at early
stage of apoptosis were increased after DIM treatment. iii) The
number of cells with broken nuclei, which would be distributed in
the 2nd region (advanced apoptosis cells), also increased with the
increment of DIM concentrations.
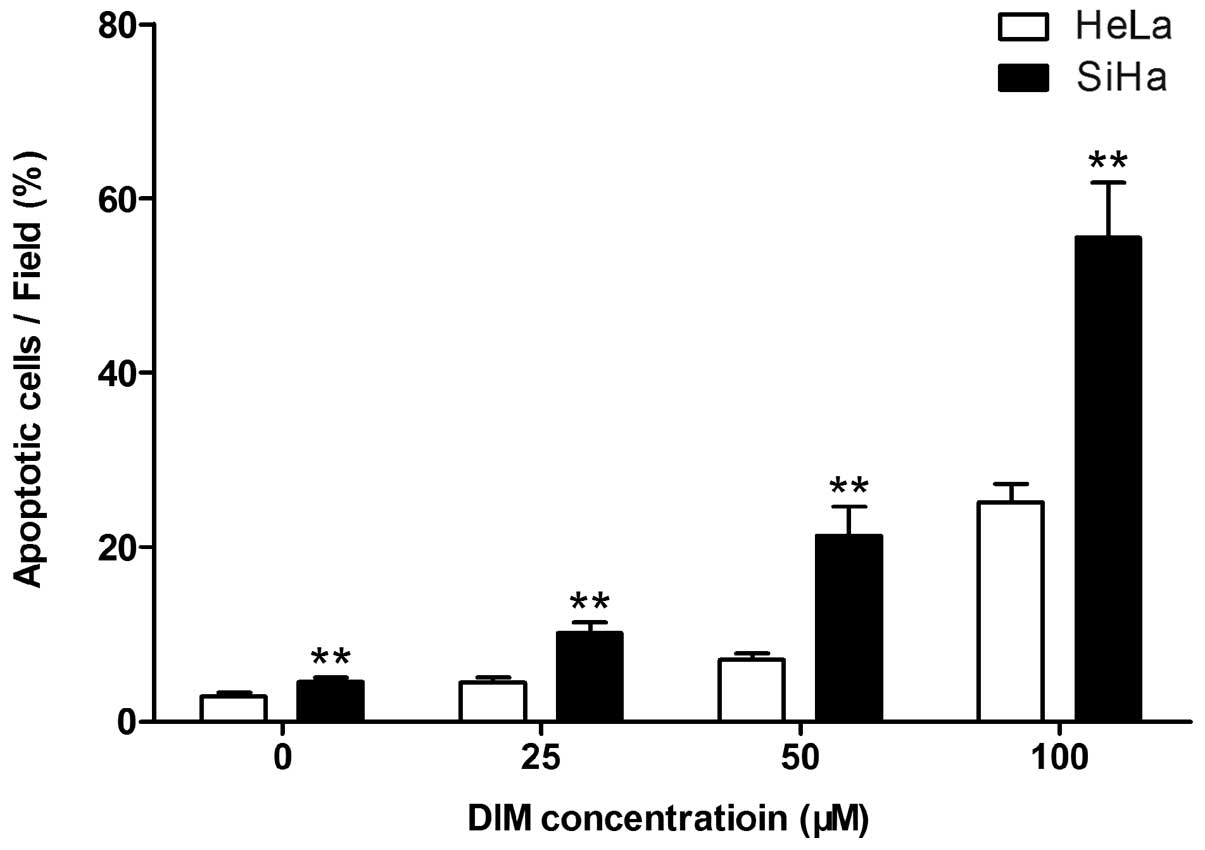
DIM showed a significantly stronger pro-apoptotic
effect on SiHa cells compared with HeLa cells (Fig. 3). Apoptosis was induced in
(4.45±0.56), (7.11±0.67) and (25.16±2.12)% of HeLa cells at 25, 50
and 100 μM DIM respectively, while the apoptotic ratio in SiHa
cells were (10.09±1.32), (21.11±3.36) and (55.46±6.33)% at each
concentration (all P<0.01).
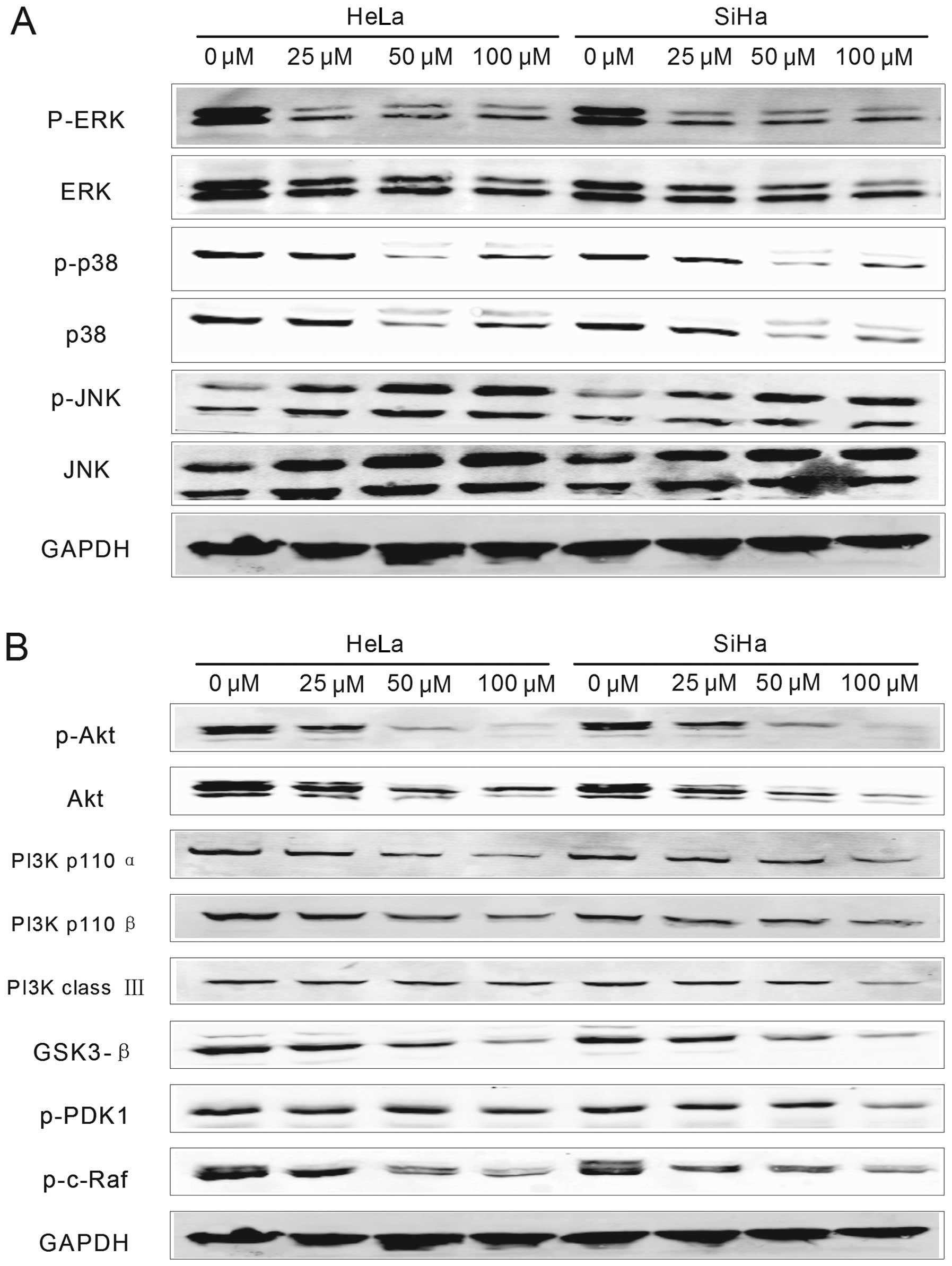
Effects of DIM on the expressions of
proteins involved in apoptosis
Among many apoptosis pathways, the activation of
mitogen-activated protein kinases (MAPK) and phosphoinositide
3-kinases (PI3K) signaling pathways drive a significant percentage
of human cancer and serve as the targets for drug development.
Western blottting was performed to determine the expression changes
of proteins involved in these two pathways. The results showed that
expression levels of different proteins involved were more or less
influenced by DIM treatment. As shown in Fig. 4, GAPDH, the internal reference,
remained stable in the two types of cells before and after DIM
treatment. For MAPK pathway (Fig.
4A), the expression levels of extracellular signal regulated
kinase (ERK), as well as the levels of phosphorylated ERK (p-ERK),
were all decreased in HeLa and SiHa cells in accordance with the
increase of DIM concentration. p38 and p-p38, also proteins
involved in MAPK pathway, were downregulated with relatively lower
DIM concentrations (25 and 50 μM), but then upregulated when the
concentration reached 100 μM. Moreover, the expression levels of
p38 and p-p38 in SiHa cells treated with 100 μM DIM were lower than
those in HeLa cells. Another protein involved in MAPK pathway,
c-Jun N-terminal kinase (JNK), was upregulated by DIM treatment in
a dose-dependent manner, accompanied with the increase of
phosphorylated JNK (p-JNK) and there were no evident differences
between HeLa and SiHa cells.
For proteins involved in PI3K pathway (p110α, p110β,
class III, Akt, GSK3-β, p-PDK1 and p-c-Raf) (Fig. 4B), their expressions and
phosphorylation levels were all downregulated in both HeLa and SiHa
cells by DIM treatment with decreasing the drug concentration. In
addition, the expression levels of Akt, PI3K class III, GSK3-β, and
p-PDK1 of SiHa cells treated with 100 μM DIM were significantly
lower than those of HeLa cells.
Disscussion
There is well documented evidence that I3C, a
natural anticancer compound extracted from cruciferous vegetables,
exerted anti-proliferative effects on many cancers in vitro,
including breast, colon and prostate cancers (12,13).
While DIM, the polymer converted from I3C under acidic conditions,
was suggested to be the real actor during anticancer process
induced by I3C (15). In the
present study we investigated the effects of DIM on the
proliferation and apoptosis of HeLa and SiHa cells, as they are two
different typical types of cervical cancer cells.
In the present study it was demonstrated that DIM
inhibited proliferation and induced apoptosis in HeLa and SiHa
cells in a time- and dose-dependent manner, consistent with other
reports concerning the anti-proliferative and pro-apoptotic
ablities of DIM (12,13). Interestingly, SiHa cells, a type of
squamous carcinoma cells, seemed to be more sensitive to DIM
treatment than the adenocarcinoma cells (HeLa cells), contrary to
the effects of many other anti-cancer compounds. According to Zhou
et al(16), artesunate
inhibited the growth of HeLa cells more efficiently than SiHa
cells, and it also increased the radio-sensitivity of HeLa cells
but not SiHa cells. Cisplatin, a widely-used drug in cancer
chemotherapy, was demonstrated to be more effective in HeLa cells
compared with SiHa cells (17).
However, Han et al(18)
found that extracts from shiitake (Lentinula edodes) mycelial
culture broth, by an organic solvent ethyl acetate, inhibited the
proliferation of cultured tumor cells, including Caski, SiHa, HeLa,
HP-1 and A375 cells. Among them, HeLa cells showed lower
sensitivity to the extracts than SiHa cells, not only in cell
growth, but also in fragmentation of DNA, as well as the activity
of caspase-3 in cancer cell extracts. In the present study a
similar phenomenon for DIM treatment was observed.
Mitogen-activated protein kinases (MAPK), including
ERK, JNK, and p38 MAPK (19), are
ubiquitous serine/threonine protein kinases that have been
implicated in many cellular processes such as proliferation,
differentiation, and apoptosis (20,21).
Human MAPK pathway was often deregulated in cancer cells (22) and thus considered as potential
therapeutic targets (23). Elevated
ERK activity was frequently observed in human tumors and suggested
to be a good indicator of tumor progression and evaluation of the
efficacy of therapeutic agents (24–26).
Studies have shown that targeting ERK1/2 using siRNAs effectively
reduced lung metastasis and sensitized tumor cells to
chemotherapeutic agents such as cisplatin (25,27).
The activation of p38 was also reported to be elevated in human
non-small cell lung cancer (28)
and the inhibition and reduction of p38 by inhibitor or RNAi could
restrain the invasion and movement of cancer cells (29–31),
suggesting the important roles of p38 in oncogenesis, tumor
development, tumor invasion and metastasis. Several reports also
provided evidence on JNK's function as proapoptotic kinase in
response to various stimuli (32–37).
The inhibitory effect of DIM on the proliferation of cervical
cancer cells, as well as the induction of apoptosis in the present
study, were demonstrated to be accompanied by downregulation of
ERK, P38, and upregulation of JNK, indicating that MAPK pathway was
involved in the pro-apoptotic effects of DIM treatment on HeLa and
SiHa cells, which was consistent to reports on other cancer cells.
However, it remained unclear why expression of p38 and p-p38 was
reduced at relatively lower concentration of DIM treatment, but
then increased at the concentration of 100 μM. This might be
explained by a compensatory mechanism triggered at the advanced
apotosis stage since the main function of p38 was to protect cells
from apoptosis (38).
PI3K, a major signaling hub downstream of HER2
(human epidermal growth factor receptor 2) and other receptor
tyrosine kinases (RTKs), activates Akt, SGK, PDK1, mTOR, and
several other molecules involved in cell cycle progression and
survival (39). Three classes of
PI3K enzymes have been defined and class I is the most intensely
studied which includes p110α, β, γ, and δ catalytic isoforms
(40). As a direct downstream
target of PI3K, Akt is also a key oncogenic survival factor and can
phosphorylate and inactivate a panel of critical proapoptotic
molecules, including Bad, caspase 9, GSK3-β, cell cycle inhibitors
p21 and p27, and tumor suppressor TSC2 (41–45).
The PI3K pathway is an important regulator in cell survival,
proliferation, and apoptosis. Moreover, it is one of the most
frequently altered networks in cancer (46). Overexpression of PI3K/AKT was
observed recently in various malignant tumors, such as cervical
carcinoma (47), prostate cancer
(48), as well as breast cancer
(49). Furthermore, PI3K p110
expression was detected to be positive in all stages of cervical
lesions except normal cervical tissue (50). The inhibitors of PI3K or Akt,
curcumin (51), celecoxib (52), perifosine (53–55)
and CMEP (56), were all reported
to attenuate the growth and proliferation of cancer cells and
induce cell apoptosis. In the present study, downregulation of
proteins associated with PI3K/Akt signaling pathway in HeLa and
SiHa cells suggested that this pathway was also involved in the
apoptosis of cervical cancer cells due to DIM treatment. In
addition, different expression changes of p38, p-p38, Akt, PI3K
classIII, GSK3-β, and p-PDK1 after DIM treatment between SiHa cells
and HeLa cells might explain their different sensitivities to DIM
treatment.
In conclusion, according to our results in the
present study, DIM showed evident anti-proliferative and
pro-apoptotic effects on cervical carcinoma cells in time and
dose-dependent manner, and SiHa cells presented a stronger
sensitivity than HeLa cells. MAPK and PI3K signaling pathways were
proven to be involved in the pro-apoptotic effects of DIM on
cervical cancer cells. Given that DIM exerted the above anti-tumor
effects, especially on SiHa cells, it might be helpful for the
development of novel therapeutic compounds for cervical cancers.
Specific therapies for different types of cervical carcinoma are
necessary since they react differently to different drugs.
References
|
1
|
Newall AT, Beutels P, Wood JG, Edmunds WJ
and MacIntyre CR: Cost-effectiveness analyses of human
papillomavirus vaccination. Lancet Infect Dis. 7:289–296. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka T, Bai T, Yukawa K and Umesaki N:
Optimal combination chemotherapy and chemoradiotherapy with
etoposide for advanced cervical squamous cancer cells in vitro.
Oncol Rep. 15:939–947. 2006.PubMed/NCBI
|
|
3
|
Sarkar FH, Li Y, Wang Z and Kong D:
Cellular signaling perturbation by natural products. Cell Signal.
21:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brew CT, Aronchik I, Hsu JC, et al:
Indole-3-carbinol activates the ATM signaling pathway independent
of DNA damage to stabilize p53 and induce G1 arrest of human
mammary epithelial cells. Int J Cancer. 118:857–868. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chinni SR, Li Y, Upadhyay S, Koppolu PK
and Sarkar FH: Indole-3-carbinol (I3C) induced cell growth
inhibition, G1 cell cycle arrest and apoptosis in prostate cancer
cells. Oncogene. 20:2927–2936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman KM, Aranha O, Glazyrin A, Chinni SR
and Sarkar FH: Translocation of Bax to mitochondria induces
apoptotic cell death in indole-3-carbinol (I3C) treated breast
cancer cells. Oncogene. 19:5764–5771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cover CM, Hsieh SJ, Tran SH, et al:
Indole-3-carbinol inhibits the expression of cyclin-dependent
kinase-6 and induces a G1 cell cycle arrest of human breast cancer
cells independent of estrogen receptor signaling. J Biol Chem.
273:3838–3847. 1998. View Article : Google Scholar
|
|
8
|
Fan S, Wang J, Yuan R, et al: BRCA1
inhibition of estrogen receptor signaling in transfected cells.
Science. 284:1354–1356. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng Q, Qi M, Chen DZ, et al: Suppression
of breast cancer invasion and migration by indole-3-carbinol:
associated with up-regulation of BRCA1 and E-cadherin/catenin
complexes. J Mol Med. 78:155–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang X, Tou JC, Hong C, et al:
3,3′-Diindolylmethane inhibits angiogenesis and the growth of
transplantable human breast carcinoma in athymic mice.
Carcinogenesis. 26:771–778. 2005.
|
|
11
|
Safe S, Papineni S and Chintharlapalli S:
Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane
and synthetic analogs. Cancer Lett. 269:326–338. 2008.
|
|
12
|
Khwaja FS, Wynne S, Posey I and Djakiew D:
3,3′-diindolylmethane induction of p75NTR-dependent cell death via
the p38 mitogen-activated protein kinase pathway in prostate cancer
cells. Cancer Prev Res. 2:566–571. 2009.
|
|
13
|
Chinnakannu K, Chen D, Li Y, et al: Cell
cycle-dependent effects of 3,3′-diindolylmethane on proliferation
and apoptosis of prostate cancer cells. J Cell Physiol. 219:94–99.
2009.
|
|
14
|
Ge C, Xu J and Jiao Y: New derivatives
I3C-6 of indole-3-carbinol in cervical cancer HeLa cells. Suzhou
University J Med Sci. 29:838–834. 2009.
|
|
15
|
Chang YC, Riby J, Chang GH, Peng BC,
Firestone G and Bjeldanes LF: Cytostatic and antiestrogenic effects
of 2-(indol-3-ylmethyl)-3,3′-diindolylmethane, a major in vivo
product of dietary indole-3-carbinol. Biochem Pharmacol.
58:825–834. 1999.PubMed/NCBI
|
|
16
|
Zhou Y, Feng Y and Zhang X: Study on the
effect of artesunate combined with irradiation on DNA damage of
HeLa and SiHa cells of human cervical cancer. J Radiat Res Radiat
Process. 29:33–36. 2011.
|
|
17
|
Wang H, Zhang Y and Jiang K: Enhancement
of photodynamic therapy sensitivity by cisplatin in human cervical
carcinoma cell lines. J Xian Jiaotong University (Med Sci ).
31:625–630. 2010.
|
|
18
|
Han B, Toyomasu T and Shinozawa T:
Induction of apoptosis in cultured cells by extracts from shiitake
(Lentinula edodes) mycelial culture broth. Mycoscience. 41:623–631.
2000. View Article : Google Scholar
|
|
19
|
Fecher LA, Amaravadi RK and Flaherty KT:
The MAPK pathway in melanoma. Curr Opin Oncol. 20:183–189. 2008.
View Article : Google Scholar
|
|
20
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
21
|
Cobb MH: MAP kinase pathways. Prog Biophys
Mol Biol. 71:479–500. 1999. View Article : Google Scholar
|
|
22
|
Meira DD, de Almeida VH, Mororo JS, et al:
Combination of cetuximab with chemoradiation, trastuzumab or MAPK
inhibitors: mechanisms of sensitisation of cervical cancer cells.
Br J Cancer. 101:782–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inamdar GS, Madhunapantula SV and
Robertson GP: Targeting the MAPK pathway in melanoma: why some
approaches succeed and other fail. Biochem Pharmacol. 80:624–637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lopez-Bergami P, Huang C, Goydos JS, et
al: Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell.
11:447–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mirmohammadsadegh A, Mota R, Gustrau A, et
al: ERK1/2 is highly phosphorylated in melanoma metastases and
protects melanoma cells from cisplatin-mediated apoptosis. J Invest
Dermatol. 127:2207–2215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flaherty KT: Sorafenib: delivering a
targeted drug to the right targets. Expert Rev Anticancer Ther.
7:617–626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma A, Tran MA, Liang S, et al:
Targeting mitogen-activated protein kinase/extracellular
signal-regulated kinase kinase in the mutant (V600E) B-Raf
signaling cascade effectively inhibits melanoma lung metastases.
Cancer Res. 66:8200–8209. 2006. View Article : Google Scholar
|
|
28
|
Greenberg AK, Basu S, Hu J, et al:
Selective p38 activation in human non-small cell lung cancer. Am J
Respir Cell Mol Biol. 26:558–564. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MS, Lee EJ, Kim HR and Moon A: p38
kinase is a key signaling molecule for H-Ras-induced cell motility
and invasive phenotype in human breast epithelial cells. Cancer
Res. 63:5454–5461. 2003.PubMed/NCBI
|
|
30
|
Sossey-Alaoui K, Ranalli TA, Li X, Bakin
AV and Cowell JK: WAVE3 promotes cell motility and invasion through
the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res.
308:135–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X, Chen S, Xu L, et al: Genistein
inhibits p38 map kinase activation, matrix metalloproteinase type
2, and cell invasion in human prostate epithelial cells. Cancer
Res. 65:3470–3478. 2005.PubMed/NCBI
|
|
32
|
Zwang Y and Yarden Y: p38 MAP kinase
mediates stress-induced internalization of EGFR: implications for
cancer chemotherapy. EMBO J. 25:4195–4206. 2006. View Article : Google Scholar
|
|
33
|
Lin A: A five-year itch in TNF-alpha
cytotoxicity: the time factor determines JNK action. Dev Cell.
10:277–278. 2006.PubMed/NCBI
|
|
34
|
Bowen C, Birrer M and Gelmann EP:
Retinoblastoma protein-mediated apoptosis after gamma-irradiation.
J Biol Chem. 277:44969–44979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li T, Dai W and Lu L: Ultraviolet-induced
junD activation and apoptosis in myeloblastic leukemia ML-1 cells.
J Biol Chem. 277:32668–32676. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouwens DM, Gomes de Mesquita DS, Dekker J
and Maassen JA: Hyperosmotic stress activates the insulin receptor
in CHO cells. Biochim Biophys Acta. 1540:97–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stadheim TA and Kucera GL: c-Jun
N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is
required for mitoxantrone- and anisomycin-induced apoptosis in
HL-60 cells. Leuk Res. 26:55–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao Y and Hung MC: Regulation of the
activity of p38 mitogen-activated protein kinase by Akt in cancer
and adenoviral protein E1A-mediated sensitization to apoptosis. Mol
Cell Biol. 23:6836–6848. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garrett JT, Chakrabarty A and Arteaga CL:
Will PI3K pathway inhibitors be effective as single agents in
patients with cancer? Oncotarget. 2:1314–1321. 2011.PubMed/NCBI
|
|
40
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blain SW and Massague J: Breast cancer
banishes p27 from nucleus. Nat Med. 8:1076–1078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inoki K, Li Y, Zhu T, Wu J and Guan KL:
TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR
signalling. Nat Cell Biol. 4:648–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brugge J, Hung MC and Mills GB: A new
mutational AKTivation in the PI3K pathway. Cancer Cell. 12:104–107.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma JL, Lee SJ, Duong JK and Stern DF:
Activation of the checkpoint kinase Rad53 by the phosphatidyl
inositol kinase-like kinase Mec1. J Biol Chem. 281:3954–3963. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu J, Gao M, Fan S, et al: Effect of Akt
inhibition on scatter factor-regulated gene expression in DU-145
human prostate cancer cells. Oncogene. 26:2925–2938. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Junttila TT, Akita RW, Parsons K, et al:
Ligand-independent HER2/HER3/PI3K complex is disrupted by
trastuzumab and is effectively inhibited by the PI3K inhibitor
GDC-0941. Cancer Cell. 15:429–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie L, Chen X and Liu Q: Study of PI3K
p110α expression in cervical lesions. Pract Prev Med. 18:2011.
|
|
51
|
Shankar S and Srivastava RK: Involvement
of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and
mitochondrial p53 in curcumin (diferulolylmethane)-induced
apoptosis in prostate cancer. Int J Oncol. 30:905–918. 2007.
|
|
52
|
Barnes NL, Warnberg F, Farnie G, et al:
Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling
and lymphangiogenesis in a xenograft model of breast cancer. Br J
Cancer. 96:575–582. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de Marsh RW, Rocha Lima CM, Levy DE,
Mitchell EP, Rowland KM Jr and Benson AB III: A phase II trial of
perifosine in locally advanced, unresectable, or metastatic
pancreatic adenocarcinoma. Am J Clin Oncol. 30:26–31. 2007.
|
|
54
|
Cirstea D, Hideshima T, Rodig S, et al:
Dual inhibition of akt/mammalian target of rapamycin pathway by
nanoparticle albumin-bound-rapamycin and perifosine induces
antitumor activity in multiple myeloma. Mol Cancer Ther. 9:963–975.
2010. View Article : Google Scholar
|
|
55
|
Floryk D and Thompson TC: Perifosine
induces differentiation and cell death in prostate cancer cells.
Cancer Lett. 266:216–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang M, Fang X, Liu H, Wang S and Yang D:
Blockade of AKT activation in prostate cancer cells with a small
molecule inhibitor, 9-chloro-2-methylellipticinium acetate (CMEP).
Biochem Pharmacol. 73:15–24. 2007. View Article : Google Scholar : PubMed/NCBI
|