Introduction
Autophagy refers to the highly regulated and
evolutionarily conserved process of turnover and maintenance of
cellular components that is required for cellular homeostasis
(1). It is a multistep process
where portions of the cytoplasm and organelles are engulfed in
double-membrane vesicles called autophagosomes. These structures
then fuse with lysosomes, resulting in the destruction of their
contents by the acid hydrolases provided by the lysosome. There are
three main autophagic pathways: macroautophagy, microautophagy, and
chaperone-mediated autophagy (2).
Autophagy is controlled by autophagy-related genes, many of which
are involved in autophagosome formation. This process features
conjugation systems that are well-conserved among eukaryotes:
Atg12-Atg5 and Atg8 (microtubule-associated protein 1 light chain
3, LC3)-phosphatidylethanolamine systems (3).
Numerous studies have demonstrated a variety of
physiological and pathophysiological roles in autophagy, such as
adaptation to nutrient deprivation, intracellular clearance of
protein and organelles, development, antiaging, elimination of
microorganisms, cell death, tumor suppression, and antigen
presentation (4–6). In tumor cells, autophagy has been
recognized as an important regulator of the promotion of tumor cell
survival and development and restriction of necrosis (7–9).
However, recent studies suggest that the role of autophagy is more
complicated, and may have diametrically opposite consequences for
the tumor, depending on the circumstances (10).
Here, we demonstrate a contradictory role for
autophagy in HepG2 hepatoma cells, which was induced by knockdown
of the expression of transmembrane protein 192 (TMEM192), a
lysosome membrane protein.
Materials and methods
Cell lines and cell culture
If not stated otherwise, LO2 and HepG2 cells were
grown in DMEM (Dulbecco’s modified Eagle’s medium; Gibco Life
Technologies) supplemented with 10% fetal calf serum (Gibco Life
Technologies) and 1% penicillin/streptomycin (Sigma, St. Louis, MO,
USA) under 5% CO2 atmosphere at 37°C.
Western blotting
Cells were harvested in lysis buffer and subjected
to western blot analysis. Signals were detected using
HRP-conjugated secondary antibodies and an ECL detection reagent.
Details are provided in a previous study (11). The primary antibodies included
rabbit anti-human TMEM192 (Sigma-Aldrich), anti-LAMP1 (Santa Cruz
Biotecnhology, Santa Cruz, CA, USA), rabbit anti-human caspase-12
(Biotime, Haimen, Zhejiang, China), rabbit anti-caspase-3,
anti-P-p38 MAPK and β-actin, rat anti-human Bax and caspase-8
(Biotime). The LC3 antibody was a gift of Dr Yi Wang (Shanghai Jiao
Tong University).
RNA interference
Cells were transfected with a non-targeted control
siRNA (small interfering RNA) or target-specific siRNAs directed to
TMEM192 sequences (Shanghai Sangon, China). Briefly, 30 nM
TMEM192 siRNA (5′-AATCTTCTGTGGTTTATTCUU-3′) was transfected
into cells using Oligofectamine (Invitrogen). Transfected
non-targeted siRNA (20 nM, 5′-AATATCTCGTTCGTTTGTTUU-3′) cells and
untransfected cells were mocked as controls. After 24 h, cells were
re-transfected with the indicated siRNAs for additional 24 h
intervals. In the experiments of 1–5 days, cells were harvested for
further analysis. The Atg7 gene was silenced by the same
procedure. The siRNA targeting Atg7 mRNA comprised the
27-base RNA nucleotide sequence 5′-AACTTAGCCCAGTACCCTGGATGGCCT-3′.
That of the control siRNA was
5′-AATAGCTCCACCTCCATGAGGGTCGCTUU-3′.
Cell proliferation assay
For growth curves, 106 cells were
collected and transferred to a 24-well plate, and were seeded in
10% serum. Monolayers were washed and the medium was replaced by
siRNA transfections as above, carried out on Days 1 and 2. Fresh
medium was added on Day 4. Cell survival and growth were determined
using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) on different days, as previously described (12).
Apoptosis cell analysis by flow cytometry
(FCM)
To measure the quantity and the ratio of apoptotic
cells, the Annexin V-FITC Apoptosis Detection kit (BD Biosciences,
Franklin Lakes, NJ, USA) was utilized. The cells were treated as
described above. Cells were harvested, fixed and stained with PI
and Annexin V-FITC as described in the kit instructions. Finally,
the stained cells were analyzed immediately on a FACScalibur flow
cytometer (BD Biosciences).
Immunoflurorescence staining
For cells grown on coverslips in 24-well plates, the
monolayer was washed twice with PBS (phosphate-buffered saline).
Then the cells were fixed with 4% PFA (paraformaldehyde) for 10–30
min. After washing twice with PBS, cells were permeabilized/blocked
by treatment with SS-PBS (0.2% saponin containing 10% bovine serum
albumin in PBS) for 30 min. For double immunostaining, primary and
secondary antibodies were overlaid on coverslips in SS-PBS for 1 h
and 30 min separately, followed by 3 washes with PBS. Coverslips
were mounted onto 1-mm glass slides using fluorescent mounting
medium. All steps were performed at room temperature. Samples were
analyzed using a Leica TCS SP2 confocal microscope. In the case of
double staining with LysoTracker Red, cells were labeled with 50 nM
LysoTracker Red for 2 h in serum-free DMEM and washed with PBS,
then fixed with 4% PFA for 20 min at room temperature.
Quantitative real-time PCR and
RT-PCR
Using 5 μg of total RNA as template, reverse
transcription was performed with PrimeScript II first strand cDNA
Synthesis kit (Takara). Measurement of the relative quantity of the
cDNA of interest was carried out using SYBR® Premix Ex
Taq™ (Takara), 300 nM of the appropriate forward and reverse
primers, and 1 μl of the cDNA mixture. The sense and antisense
primers used for TMEM192 were 5′-GCCATCTTGGCACTGGAAC-3′ and
5′-GCTTACTCAGCAGCGCATT-3′ (59°C, 245 bp), respectively. The primers
for mouse homologous TMEM192 were 5′-GTATGGATGACGACCCGCTTCT-3′ and
5′-TGGGCACTTGTCTTCAGTTGGAT-3′ (63°C, 200 bp). Real-time PCR assays
were performed in the Applied Biosystems 7500 Real-Time PCR System
using thermal cycling parameters recommended by the manufacturer
(40 cycles of 15 sec at 95°C and 1 min at 60°C). PCR product purity
was determined by melting curve analysis. Within each plate, we
included triplicates of each sample. Data were analyzed with SDS2.1
software (v.2.1.0.3, Applied Biosystems). At least three separate
experiments per subject were performed. Values exceeding two
standard deviations were excluded.
Statistical analysis
The data are expressed as means ± SD. Tests for
significance of differences were performed by ANOVA or Student’s
t-test as appropriate. P<0.05 was considered statistically
significant.
Results
Lysosomal localization of TMEM192 by
immunofluorescence
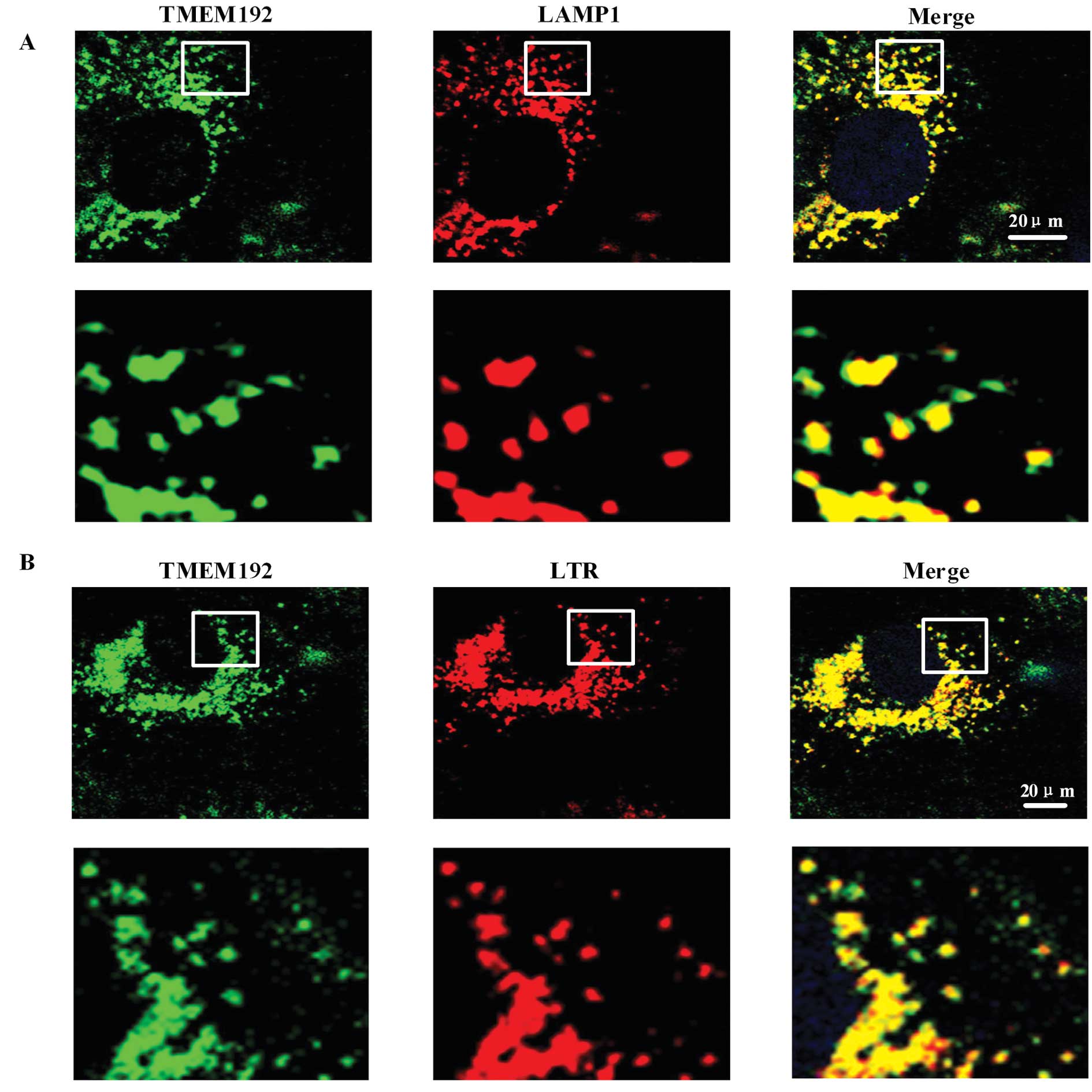
The lysosomal localization of TMEM192 was confirmed
by immunofluorescence. Using specific anti-TMEM192 antibody,
TMEM192 staining in HLF-I cells showed small punctuate patterns
throughout the cytoplasm, predominantly in the perinuclear region.
No signal was detected at the plasma membrane or in the nucleus.
This suggested that TMEM192 protein was localized in an
intracellular compartment (green fluorescence in Fig. 1A). Double staining with antibody
against LAMP1, a marker protein for lysosomes (red fluorescence in
Fig. 1A), showed colocalization
with TMEM192. Double staining with the lysosome tracker red (LTR),
a marker of the lysosome, also showed that it was colocalized with
TMEM192 (Fig. 1B).
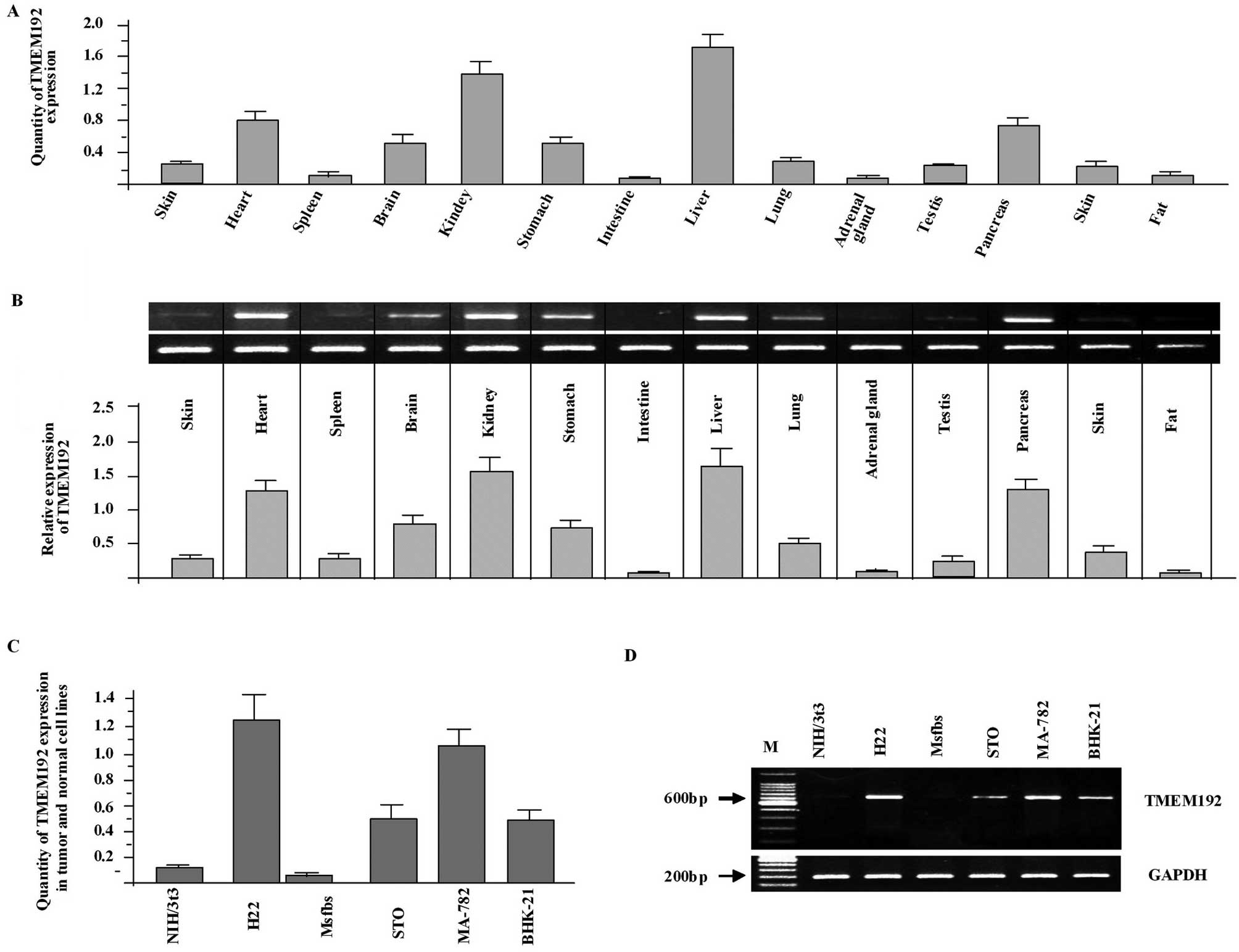
Tissue distribution of the TMEM192
Tissue expression analysis provides important
information about the function of TMEM192. Therefore, we analyzed
the expression of TMEM192 in mouse tissues and different cell
lines. Using real-time PCR, we found that TMEM192 was a
widely-expressed protein. Its mRNA was abundant in the kidney and
liver while little or no expression was detected in other tissues
in the mouse, such as the spleen and intestine (Fig. 2A and B). Interestingly and
importantly, TMEM192 was highly expressed in tumor cell lines while
little or low expression was detected in normal cell lines
(Fig. 2C). The differential
expression of TMEM192 was further confirmed by RT-PCR analysis
(Fig. 2D).
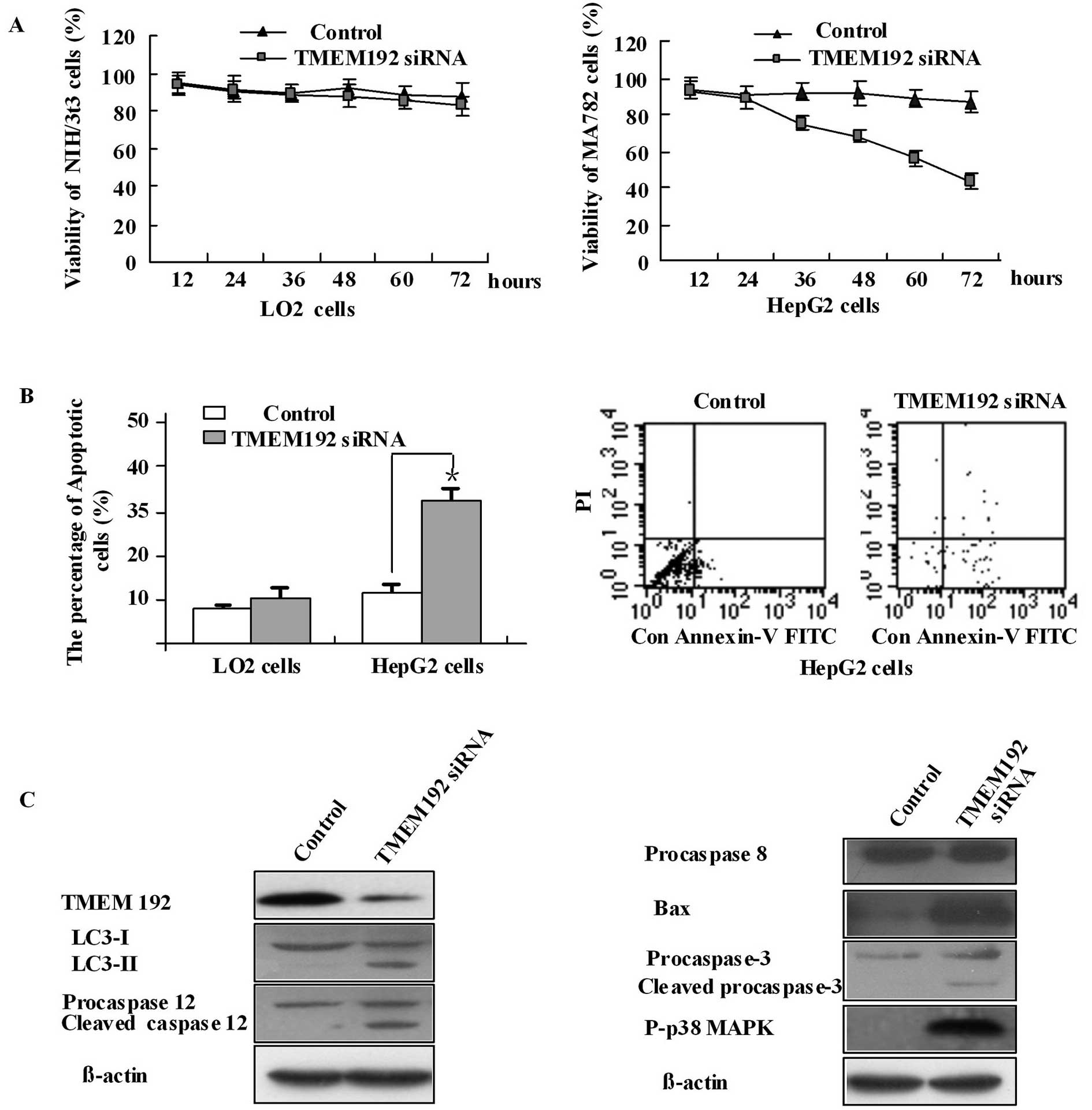
TMEM192 siRNA induces apoptosis in HepG2
tumor cell lines by upregulating Bax, caspase-3 and p38 MAPK and
activating autophagy
According to the experiment above, TMEM192 is highly
expressed in tumor cells. To elucidate the potential molecular
function of TMEM192, we analyzed the effect of TMEM192 deficiency
in HepG2 hepatoma cells and normal LO2 cell lines, by silencing
TMEM192 expression with specific siRNA targeting the
TMEM192 gene. RT-PCR with specific primers demonstrated a
marked reduction in the levels of TMEM192 mRNA in cells
transfected with the target siRNA (data not shown). This was
consistent with the protein expression data (Fig. 3C). Our results using MTT and FCM
have shown that TMEM192 deficiency in HepG2 cells induced growth
inhibition and increased apoptosis while LO2 cells were unaffected
(Fig. 3A and B). Intracellular
TMEM192 has been shown to localize in the lysosome, and may have a
function in the lysosomes. In the present experiments, HepG2 cells
transfected with TMEM192 siRNA were shown to activate autophagy by
detecting the LC3 protein (Fig.
3C). LC3 exists in two forms: the free mature form (LC3-I) and
the faster lapidated LC3 (LC3-II). The presence of LC3-II band
confirms that the reaction of LC3 conjugation to phospholipids was
activated. Caspase-12 is an ER stress-related protein. These cells
also showed the presence of an activated degraded caspase-12
fragment (48 kDa, Fig. 3C).
Caspase-3, normally present as a zymogen (32 kDa), was detected in
its activated 17 kDa form, while caspase-8 was detected only as the
zymogen. Synthesis of Bax protein was activated 48 h after
transfection. In addition, we found that p38-MAPK was
phosphorylated (Fig. 3C). These
results indicate that TMEM192 deficiency induces autophagy and
apoptosis of HEPG2 cells. It is likely that the apoptosis occurred
by upregulating the expression of Bax and caspase-3, as well as the
phosphorylation of p38-MAPK.
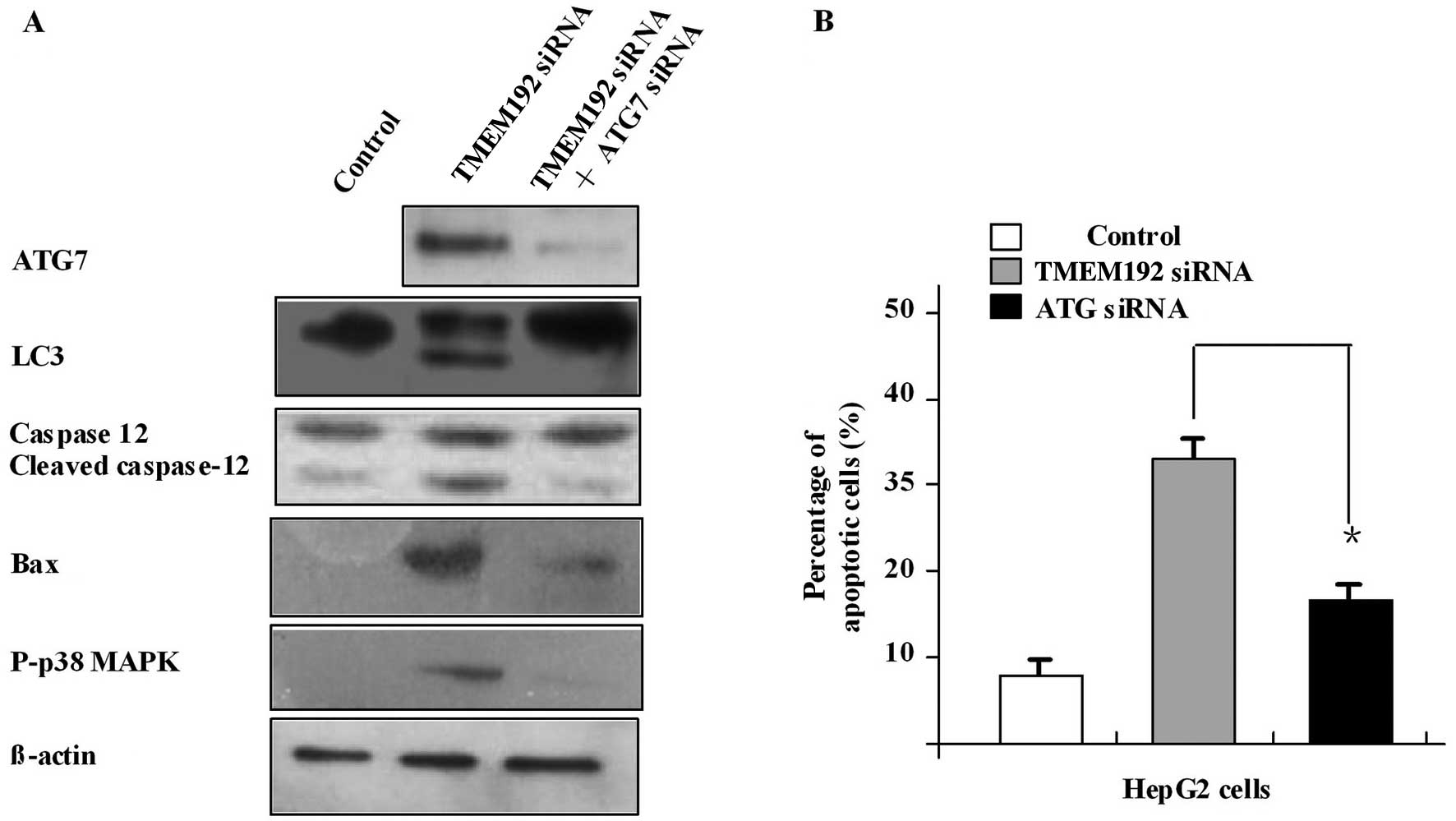
Blocking autophagy inhibits apoptosis of
HEPG2 cells induced by TMEM192 deficiency and downregulates
caspase-12, capase-3 and Bax
Atg7 is a crucial autophagy gene. We used Atg7 siRNA
to investigate whether blocking the autophagy would affect the
apoptosis of HepG2 cells induced by TMEM192 deficiency. In this
experiment, the Atg7 mRNA was silenced successfully using Atg7
siRNA (Fig. 4A). In
TMEM192-deficient HepG2 cells, autophagy and apoptosis were
inhibited after transfection with Atg7 siRNA. Blocking of the
activation of caspase-12, Bax and caspase-3 was also observed
(Fig. 4B). These results indicate
that TMEM192 siRNA induced apoptosis in HepG2 cells through
autophagy and the ER pathway, and this effect was closely related
to the mitochondrial pathway, all of which can be suppressed by
downregulation of autophagy by silencing the Atg7 gene.
Discussion
In previous proteomic analyses, TMEM192 was
identified in lysosomal membranes (13). The exogenous and endogenous
localization of TMEM192 was further verified (14), which suggest that TMEM192 is a
lysosomal membrane protein. In the present study, using
immunofluorescence staining approaches, we also demonstrated that
TMEM192 colocalized with the lysosomal marker protein, LAMP1, and
the lysosomal tracker, LTR. TMEM192 has tissue-specific expression.
In mouse, its ortholog is highly expressed in the liver and brain
but shows little or low expression in spleen and other tissues.
Importantly, TMEM192 was highly expressed in tumor cell lines,
whereas lower expression was detected in normal cell lines. This
distribution may suggest the important function of TMEM192. In
order to investigate the potential role of TMEM192 in tumor cell
line proliferation and growth, we obtained TMEM192-deficient HepG2
cells using RNAi. HepG2 cells deficient in TMEM192 showed growth
inhibition.
Autophagy refers to the highly regulated and
evolutionarily conserved process of turnover and maintenance of
cellular components that is required for cellular homeostasis
(1). Autophagy and apoptosis are
partners in cell life and death. They crosstalk, interact and share
common regulatory pathways (15).
In this study, our results show that TMEM192 siRNA activated
autophagy accompanied by an increased expression of LC3-II. In
addition, apoptosis was induced as evidenced by caspase-12
activation. Autophagy and apoptosis were inhibited after treatment
with Atg7 siRNA, which indicates that the autophagy pathway plays a
critical role in apotosis of HepG2 cells.
Studies have shown that caspase activation through
the death receptor pathway, the mitochondrial pathway, and the ER
pathway causes cell apoptosis. Caspase-8 is the primary initiator
of the death receptor pathway, while caspase-3 is the main caspase
effector in the mitochondrial pathway (16,17).
Bax plays an important role in the mitochondrial apoptosis pathway
(18,19). In HepG2 cells, we found that RNAi of
TMEM192 increased the expression of Bax and caspase-3, as well as
the phosphorylation of p38 MAPK, without affecting the levels of
caspase-8. When Atg7 siRNA was used to block the autophagy,
however, expression of Bax and caspase-3 was significantly
downregulated. Since caspase-12 is a key factor in ER stress, we
therefore, hypothesized that TMEM192 siRNA induces ER stress in
HepG2 cells by activating caspase-12, and further activates Bax and
the phosphorylation of p38 MAPK. In turn this induces a
translocation of Bax from the cytosol into the mitochondria
promoting the release of cytochrome c, which finally activates
caspase-3 resulting in tumor cell apoptosis.
Autophagy is controlled by autophagy-related genes,
many of which are involved in autophagosome formation. This process
features conjugation systems that are well-conserved among
eukaryotes: Atg5–12 and Atg8 (LC3)-phosphatidylethanolamine
systems. In this study, we have shown that TMEM192 deficiency can
induce autophagy concurrently with apoptosis in HepG2 hepatoma
cells. Previous studies have shown that there is crosstalk between
apoptosis and autophagy (15).
Although the two processes are markedly different, several pathways
regulate both the autophagic and the apoptotic machinery and
autophagy can cooperate with apoptosis. For example, alterations in
the mitochondrial membrane potential-induced Bax/Bak ratio can
signal to both apoptosis and mitochondrial autophagy (20). Indeed, earlier studies have shown
that mitochondria that have sustained stress-induced damage can be
removed by selective mitochondrial autophagy (21–24).
There is a consensus that autophagy itself is usually a survival
mechanism (25). However, it is
possible that autophagy can contribute to the induction of some
death responses, such as apoptosis, in light of the fact that the
coordinate activation of both autophagic and apoptotic signals were
induced by TMEM192 siRNA. By knockdown of the Atg7 expression, we
found that autophagy was inhibited by the reduction of LC3-II
expression. Interestingly, caspase-12 and Bax-independent apoptosis
was reversed. The impact of autophagy on apoptosis is highly
context-dependent, with compelling evidence that autophagy can
either inhibit or promote apoptosis, depending on the system. In
tumor cells, the relations between autophagy and apoptosis are more
complicated (10,26).
Lysosomal membrane proteins have been associated
with autophagy. For example, deficiency of mice or cells in
lysosomal protein, such as LAMP1 and LAMP2, resulted in increased
autophagy, while other proteins, such as LAPTM4B, exhibited
promotion of autophagy. In the tumor cells, TMEM192 deficiency can
also activate the autophagy signaling pathway which can promote
apoptosis independently of the caspase-12 and Bax pathway. None of
these results were found in normal cells (data not shown). In
summary, this study offers proof of crosstalk between autophagy and
apoptosis. TMEM192 promotion of autophagy may be a new route for
tumor therapy.
Acknowledgements
The authors would like to gratefully acknowledge the
contributions of Professor Xu Ra and Professor Chen Xiao.
Abbreviations:
|
ER
|
endoplasmic reticulum
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
HRP
|
horseradish peroxidase
|
|
LAMP
|
lysosome-associated membrane
protein
|
|
siRNA
|
small interfering RNA
|
|
TMEM192
|
transmembrane protein 192
|
References
|
1
|
Mizushima N: Autophagy: process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar
|
|
2
|
Yang W, Monroe J, Zhang Y, George D,
Bremer E and Li H: Proteasome inhibition induces both pro- and
anti-cell death pathways in prostate cancer cells. Cancer Lett.
243:217–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yokoyama T, Miyazawa K, Naito M, et al:
Vitamin K2 induces autophagy and apoptosis simultaneously in
leukemia cells. Autophagy. 4:629–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: a double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi JJ, Liu KY and Yang YP: Autophagy in
the pathogenesis of Parkinson’s disease. Sheng Li Ke Xue Jin Zhan.
40:67–71. 2009.(In Chinese).
|
|
6
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Degenhardt K, Mathew R, Beaudoin B, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abedin MJ, Wang D, McDonnell MA, Lehmann U
and Kelekar A: Autophagy delays apoptotic death in breast cancer
cells following DNA damage. Cell Death Differ. 14:500–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauvy C, Gane P, Arico S, Codogno P and
Ogier-Denis E: Autophagy delays sulindac sulfide-induced apoptosis
in the human intestinal colon cancer cell line HT-29. Exp Cell Res.
268:139–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen T, Guo J, Yang M, et al: Cyclosporin
A impairs dendritic cell migration by regulating chemokine receptor
expression and inhibiting cyclooxygenase-2 expression. Blood.
103:413–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oka M, Maeda S, Koga N, Kato K and Saito
T: A modified colorimetric MTT assay adapted for primary cultured
hepatocytes: application to proliferation and cytotoxicity assays.
Biosci Biotechnol Biochem. 56:1472–1473. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lubke T, Lobel P and Sleat DE: Proteomics
of the lysosome. Biochim Biophys Acta. 1793:625–635. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schroder B, Wrocklage C, Hasilik A and
Saftig P: Molecular characterisation of ‘transmembrane protein 192’
(TMEM192), a novel protein of the lysosomal membrane. Biol Chem.
391:695–704. 2010.
|
|
15
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oubrahim H, Chock PB and Stadtman ER:
Manganese(II) induces apoptotic cell death in NIH3T3 cells via a
caspase-12-dependent pathway. J Biol Chem. 277:20135–20138. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: Apoptosis: mechanisms and relevance in cancer. Ann
Hematol. 84:627–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and
cytotoxicity by amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao XX, Mohuiddin I, Chada S, et al:
Adenoviral transfer of mda-7 leads to BAX up-regulation and
apoptosis in mesothelioma cells, and is abrogated by
over-expression of BCL-XL. Mol Med. 8:869–876. 2002.PubMed/NCBI
|
|
20
|
Yee KS, Wilkinson S, James J, Ryan KM and
Vousden KH: PUMA- and Bax-induced autophagy contributes to
apoptosis. Cell Death Differ. 16:1135–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elmore SP, Qian T, Grissom SF and
Lemasters JJ: The mitochondrial permeability transition initiates
autophagy in rat hepatocytes. FASEB J. 15:2286–2287.
2001.PubMed/NCBI
|
|
22
|
Zhu JH, Horbinski C, Guo F, Watkins S,
Uchiyama Y and Chu CT: Regulation of autophagy by extracellular
signal-regulated protein kinases during
1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol.
170:75–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colell A, Ricci JE, Tait S, et al: GAPDH
and autophagy preserve survival after apoptotic cytochrome c
release in the absence of caspase activation. Cell. 129:983–997.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue L, Fletcher GC and Tolkovsky AM:
Autophagy is activated by apoptotic signalling in sympathetic
neurons: an alternative mechanism of death execution. Mol Cell
Neurosci. 14:180–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimmelman AC: The dynamic nature of
autophagy in cancer. Genes Dev. 25:1999–2010. 2011. View Article : Google Scholar
|