Introduction
Lung cancer is currently the leading cause of cancer
death in Japan and other industrialized countries (1). Although surgery is the most effective
and definitive therapeutic modality, especially for patients with
early-stage non-small cell lung cancer (NSCLC), their postoperative
survival rate remains poor. To improve the postoperative prognosis,
definite predictive biomarkers should be used to detect the poor
prognosis cohort and intensive postoperative therapy should then be
performed on them.
Ki-67, which is known as a representative cell
proliferative marker, has been used widely for various malignancies
including lung cancer to evaluate the malignant potential or the
proliferative activity of the tumor (2–6). In
addition, we have focused on the cell cycle-related proteins as
tumor proliferative biomarkers and revealed that some of these
proteins are useful for the prognostic prediction of patients with
lung adenocarcinoma (7,8). There have also been various other
studies on the prognostic markers of NSCLC. However, these have not
actually contributed to improving the prognosis of patients with
early-stage NSCLC. Although almost all of these previous studies
described the prognostic significance of a single biomarker, recent
investigations have suggested that prognostic prediction is more
reliable when multiparameter analysis with several biomarkers is
performed than with analysis with a single one (9–11).
There are significant interactions between numerous
molecules around the S phase as the DNA replication period and the
M phase as the cell mitotic period. Among these molecules, the
minichromosome maintenance families (MCM2-7) and Geminin play
crucial roles in the DNA replication licensing pathway, which
restricts the replication of the chromosome to only once per cell
cycle (12). Briefly, MCM proteins
(MCM2-7) assemble on the origin of replication with other initiator
proteins and facilitate DNA unwinding by acting as a DNA helicase.
After DNA replication is initiated during the cell cycle, Geminin
inhibits re-uploading of the MCM proteins onto chromatin, thus
preventing DNA re-replication in the same cell cycle (13). It has been confirmed that these
proteins are useful biomakers that reflect the proliferative
activity of the tumor cells and predict the survival of patients
with various types of cancer (5–11,14–18).
Aurora A, known as a member of the serine/threonine kinase family,
controls a subset of critical mitotic events including centrosome
maturation and separation, as well as chromosome orientation and
segregation (19). In addition,
histone H3 is as substrate for the Aurora kinases and is
phosphorylated on serine 10 only in mitosis, producing a
phosphohistone (H3S10ph) (20).
Gene amplification and protein overexpression of Aurora kinases may
lead to centrosome function disorder, chromosomal instability, and
carcinogenesis (21). Moreover,
there are some reports concerning significant correlations between
Aurora A and the tumor malignant grade or the prognosis of patients
with various types of cancer including NSCLC (9,10,22–28).
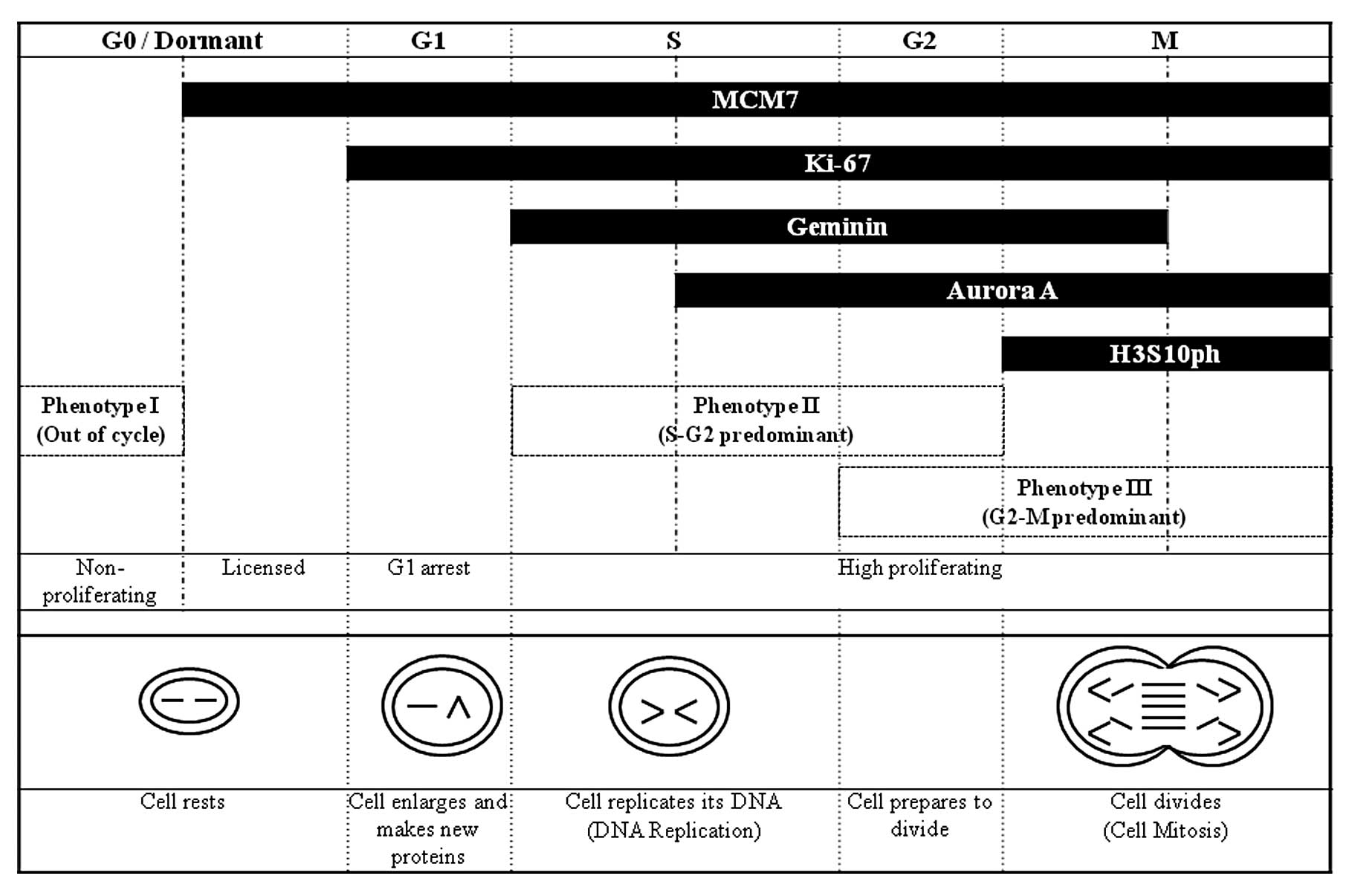
These cell cycle-related proteins (MCM7, Ki-67,
Geminin, Aurora A and H3S10ph) can also serve as markers for the
cell cycle phase distribution on immunohistochemistry, as shown in
Fig. 1. Therefore,
immunohistochemical multiparameter analysis using these biomarkers
allows a detailed evaluation of the kinetics of complex dynamic
tumor cell populations (29). In
the present study, we classified small (3 cm or less in diameter)
invasive lung adenocarcinomas [pure bronchioloalveolar carcinomas
(BACs) were not included] into three phenotypes based on the
dominant cell cycle phase of the tumor cell population. In
addition, we evaluated whether these phenotypes were associated
with clinicopathological factors and patient survival.
Materials and methods
Patients and surgical specimens
This study enrolled a total of 102 patients with
lung adenocarcinoma who underwent curative resection at Tottori
University Hospital between January 1997 and December 2006. All the
102 tumors were diagnosed as invasive lung adenocarcinoma (pure
BACs were not included) with a maximum diameter of ≤3 cm (pT1).
Routinely, neutral buffered formalin (pH 7.4)-fixed and
paraffin-embedded tumor tissue samples were sectioned in 3 μm
serial slices. The sections were stained using hematoxylin and
eosin (HE) and Elastic van Gieson (EvG) stain.
The patients included 53 males and 49 females, with
a mean age of 67.8±11.2 (SD) years (range, 26–85 years).
Histological specimens were reviewed by the first author and
qualified pathologists (K.S. and H.I.) and assessed for
histological subtype and tumor grade according to the World Health
Organization (WHO) criteria (1).
These 102 tumors consisted of 7 acinar, 20 papillary, 11 solid with
mucin and 64 adenocarcinoma with mixed subtypes. Well-, moderately,
and poorly differentiated adenocarcinomas were present in 28, 59,
and 15 cases, respectively. Tumor stage of the disease at
pathological diagnosis was determined according to the UICC
guidelines (6th edition) of the TNM classification of malignant
tumor (30). The pathological stage
of lung cancer was I in 83 patients, II in 5 and III in 14. Pleural
invasion was classified as pl0, pl1, pl2 and pl3; pl0 included
tumor with no pleural involvement or reaching the visceral pleura
but not extending beyond its elastic pleural layer; pl1 included
tumor reaching visceral pleural elastic layer but not exposed on
the pleural surface; pl2 included tumor exposed on the pleural
surface; and pl3 included tumor invading parietal pleura or chest
wall. We defined pl0 as pleural invasion-negative and pl1-3 as
-positive. As for lymphatic and vascular invasion, we determined
the status of invasion-positive or -negative on the basis of
whether or not tumor cells were identifiable in the lymphatic lumen
or blood vessel lumen, respectively (31). The slides of EvG stain were used
supplementarily for evaluation of lymphatic and vascular invasion.
Among all the 102 subjects, pleural invasion was negative in 70
patients and positive in 32, lymphatic invasion was negative in 37
and positive in 65, and vascular invasion was negative in 62 and
positive in 40. The median follow-up period was 56.4 months (range,
2–146 months). The study protocol was approved by the institutional
review board, and informed consent was obtained from all patients
for tumor sample collection.
Immunohistochemistry
Tissue sections were de-waxed in xylene, rehydrated
through a graded series of ethanol solution, rinsed in distilled
water for 5 min, and then immersed in 0.3% hydrogen peroxide
(H2O2) in methanol for 30 min to block
endogenous peroxidase. For antigen retrieval, sections were
microwaved in 0.01 mol/l sodium citrate-buffered saline (pH 6.0)
for 20 min at 95°C using a microwave processor model MI-77
(Azumaya, Tokyo, Japan). After being rinsed in PBS for 5 min, the
slides were pre-blocked with a solution of 2% FBS at room
temperature for 20 min and incubated at 4°C overnight with the
antibodies. A subsequent reaction was initiated by the
streptavidin-biotin-peroxidase complex technique (SAB method) using
a Histofine SAB-PO (M) immunohistochemical staining kit (Nichirei,
Tokyo, Japan). The immunoreactions were visualized with 0.2 mg/ml
3,3′-diaminobenzidine and 20 μl/dl hydrogen peroxide in 0.05 M
Tris-HCl buffer (pH 7.6). Finally, the slides were counterstained
with 0.1% hematoxylin and then dehydrated and mounted.
Antibodies
We used the following primary antibodies for
immunohistochemistry: mouse anti-MCM7 antibody (1:100 dilution;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-Ki-67
antibody (1:50 dilution; Dako, Glostrup, Denmark), rabbit
anti-Geminin antibody (1:100 dilution; Santa Cruz Biotechnology),
mouse anti-Aurora A antibody (1:100 dilution; Novocastra,
Newcastle, UK), and rabbit polyclonal antibody to Histone H3
(phospho S10) (1:100 dilution; Abcam, Cambridge, UK).
Evaluation of immunohistochemical
findings
To evaluate MCM7, Ki-67, Geminin, and H3S10ph
expression, positively stained tumor cell nuclei were counted.
Counts were performed in high-magnification fields using the FLOVEL
Image Filling System FlvFs (FLOVEL Inc., Tachikawa, Japan). Both
positive and negative cells within the fields were counted and any
stromal or inflammatory cells were excluded. For MCM7, Ki-67,
Geminin, and H3S10ph, at least 500 tumor cells were counted in
areas showing a high frequency of cells with nuclei positive for
such expression, and these labeling indices (LIs) were calculated
using the following formula: LI = number of positive cells/total
number of cells × 100. On the other hand, the immunohistochemical
staining of Aurora A was scored into the following four grades on
the basis of the expression levels in the tumor cell cytoplasm: 3+
(strongly positive), 2+ (intermediately positive), 1+ (weakly
positive), and 0 (negative). The pathological evaluation was
performed by three authors (T.H., K.S. and H.I.) independently. In
reference to the average value of each LI, we decided that the
cut-off values of MCM7, Ki-67, Geminin, and H3S10ph were 15, 10, 5,
and 3%, respectively. As for Aurora A, tumors with scores of 2+ and
3+ were considered to be positive. We chose and determined these
cut-off values and scores through trial and error. To confirm the
specificity of the immunostaining results, sections immunoreacted
without the primary antibodies were used as negative controls.
Statistical analysis
Data were analyzed using StatView version 5.0 (SAS
Inc., Cary, NC, USA). Kruskal-Wallis test and χ2 test
were used in evaluation of the relationship between cell-cycle
phenotypes and clinicopathological parameters. The survival rates
were estimated with the Kaplan-Meier method and statistical
analyses were carried out using the log-rank test. A P-value
<0.05 was considered to be significant in statistical analyses.
Univariate and multivariate Cox regression analyses were used to
evaluate the contribution of various factors to the overall
survival of all patients.
Results
Expression of cell cycle biomarkers in
lung adenocarcinoma
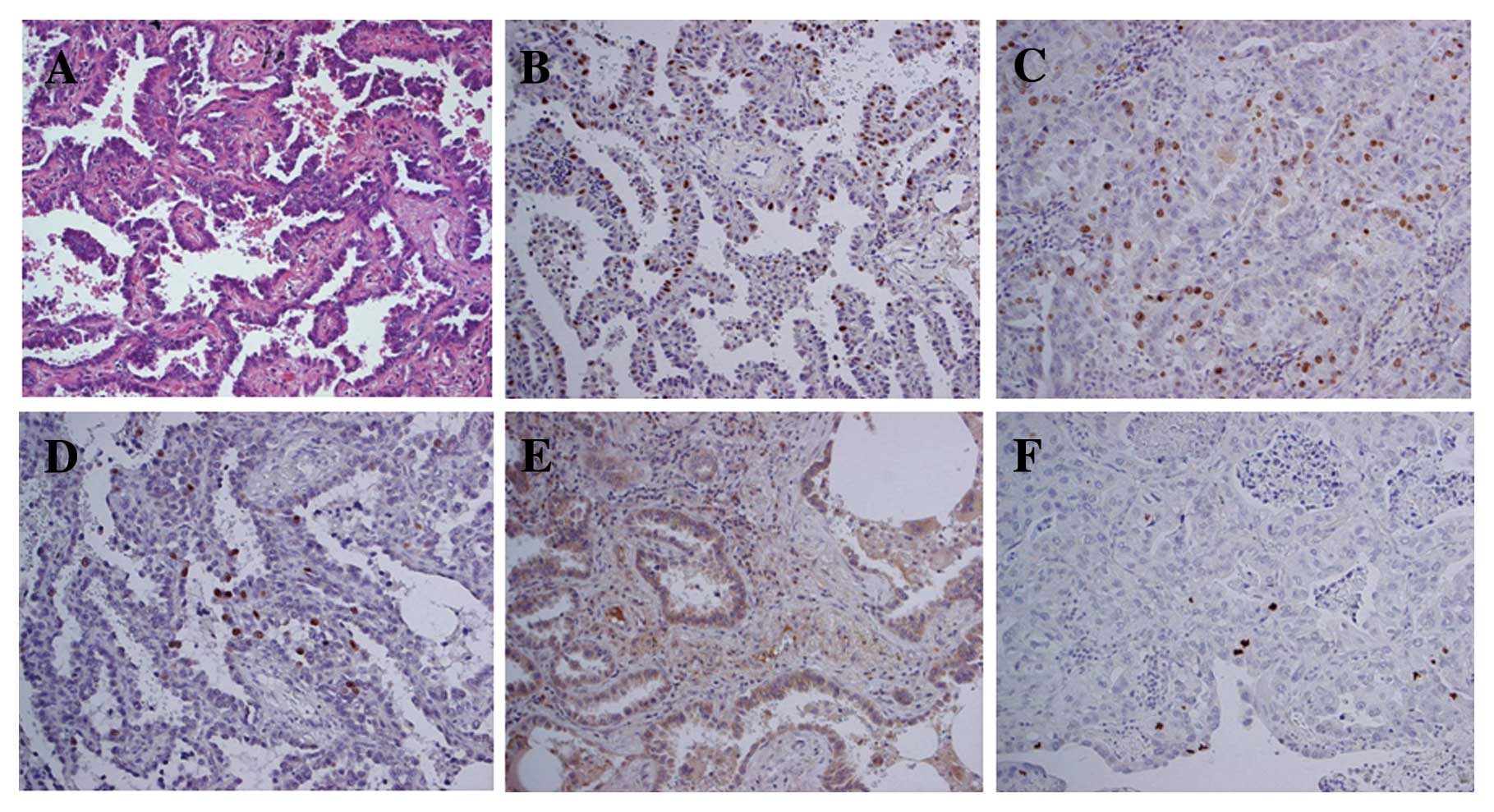
Fig. 2 shows
representative cases of immunohistochemical staining positive for
MCM7, Ki-67, Geminin, Aurora A, and H3S10ph (Fig. 2). Immunoreactivity of MCM7, Ki-67,
Geminin and H3S10ph was mainly observed in the nuclei of tumor
cells, whereas that of Aurora A was noted in the cytoplasm of tumor
cells.
Cell cycle phase algorithm in lung
adenocarcinoma
The cell cycle biomarker proteins, MCM7, Ki-67,
Geminin, Aurora A, and H3S10ph, provide information on their
specific cell cycle phase distribution: MCM7 protein is expressed
throughout the cell cycle (G1-S-G2-M phases) but is tightly
downregulated during exit into the out-of-cycle quiescent phase (G0
phase), corresponding to a differentiated or senescent state
(11). Ki-67 is expressed during
all phases of the cell cycle except for G0, while Geminin is
expressed during the S-G2-M phases but not in G0 and G1 phases
(17). Aurora A accumulates during
S phase and reaches a peak in G2-M phase, followed by rapid
degradation at the end of mitosis, and phosphohistone (H3S10ph)
represents a biomarker of the only M-phase transition (10) (Fig.
1).
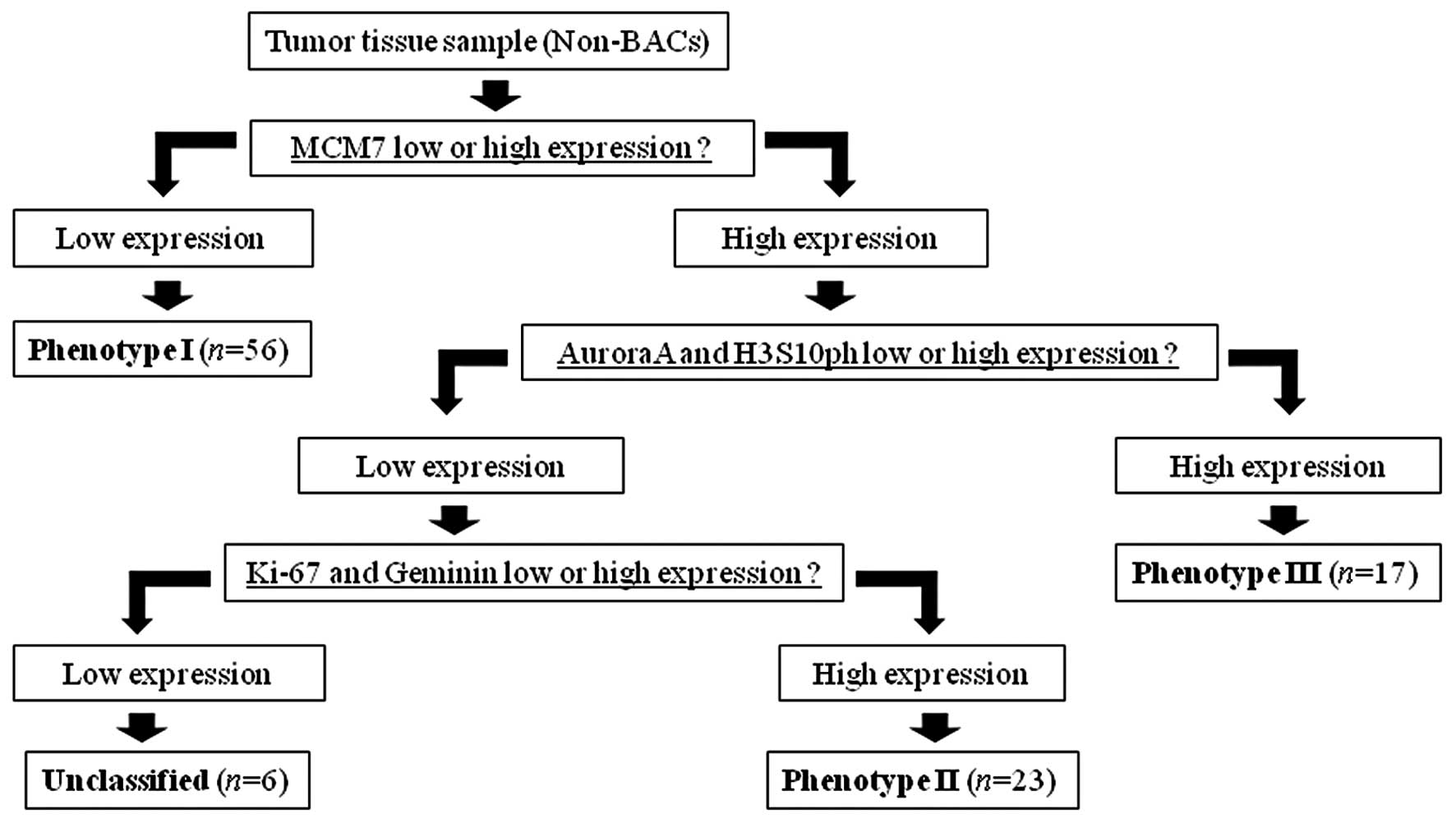
In this study, we defined the cell cycle phase
algorithm with cell cycle biomarkers for lung adenocarcinomas by
reference to the above cell cycle distribution (Fig. 3). On the basis of this algorithm, we
classified the subjects (102 tumors) into the following phenotypes:
i) phenotype I (n=56), MCM7-negative (out-of-cycle) tumors; ii)
phenotype II (n=23), MCM7-, Ki67-, and Geminin-positive (S-G2 phase
dominant) tumors; iii) phenotype III (n=17), MCM7-, Aurora A-, and
H3S10ph-positive (G2-M phase dominant) tumors; iv) unclassified
(n=6), MCM-positive, but Ki-67-, Geminin-, Aurora A-, and
H3S10ph-negative tumors.
Relationship between cell cycle
phenotypes and clinicopathological parameters
We evaluated the correlations between some
clinicopathological parameters and the above phenotypes. The
phenotypes were significantly correlated with histological grade
(P<0.01), tumor size (P=0.01), lymph node metastasis
(P<0.01), and pathological stage (P<0.01), but not with age
(P=0.19), gender (P=0.42), and pleural invasion (P=0.08) (Table I).
 | Table IRelationship between cell-cycle
phenotype and clinicopathological parameters. |
Table I
Relationship between cell-cycle
phenotype and clinicopathological parameters.
| Phenotype Ia n=56 (%) | Phenotype IIb n=23 (%) | Phenotype IIIc n=17 (%) | P-value |
|---|
| Age (years) |
| Mean ± SD | 66.6±12.0 | 71.0±8.8 | 67.4±11.9 | 0.19d |
| Gender |
| Male | 26 (46) | 14 (61) | 10 (59) | 0.42e |
| Female | 30 (54) | 9 (39) | 7 (41) | |
| Histological
grade |
| Well | 24 (43) | 4 (17) | 0 (0) | <0.01e |
| Moderately | 30 (53) | 10 (44) | 13 (76) | |
| Poorly | 2 (4) | 9 (39) | 4 (24) | |
| Tumor size (mm) |
| Mean ± SD | 18.7±5.7 | 22.2±4.6 | 22.1±4.1 | 0.01d |
| Pleural invasion |
| Negative | 43 (77) | 16 (70) | 10 (59) | 0.08e |
| Positive | 13 (23) | 7 (30) | 7 (41) | |
| Lymphatic
invasion |
| Negative | 28 (50) | 4 (17) | 3 (18) | <0.01e |
| Positive | 28 (50) | 19 (83) | 14 (82) | |
| Vascular
invasion |
| Negative | 44 (79) | 11 (48) | 4 (24) | <0.01e |
| Positive | 12 (21) | 12 (52) | 13 (76) | |
| Lymph node
metastasis |
| N0 | 53 (95) | 16 (69) | 10 (59) | <0.01e |
| N1 | 0 (0) | 2 (9) | 2 (12) | |
| N2 | 3 (5) | 5 (22) | 5 (29) | |
| Pathological
stage |
| I | 53 (95) | 16 (69) | 10 (59) | <0.01e |
| II | 0 (0) | 2 (9) | 2 (12) | |
| III | 3 (5) | 5 (22) | 5 (29) | |
Analysis of prognostic significance
Next, the cumulative overall survivals of those with
the three phenotypes were analyzed using the Kaplan-Meier method
and log-rank test. For all patients, the 5-year survival rates of
those with phenotypes I, II, and III tumors were 89.8, 55.4, and
38.6%, respectively, with a statistically significant difference
(P<0.01; log-rank test) (Fig.
4A). Of patients in stage I, the 5-year survival rates were
91.3, 61.4 and 48.0%, respectively, with a significant difference
between them (P<0.01; log-rank test) (Fig. 4B). Next, we performed analyses to
evaluate the contribution of potential prognostic markers to the
overall survival in the 79 stage I patients. A multivariate
analysis of prognostic factors using a Cox proportional hazard
model confirmed that phenotype II [P=0.02, Hazard ratio (HR), 5.01;
95% CI, 1.34–18.7] and phenotype III (P=0.01, HR, 5.50; 95% CI,
1.39–21.8) were significant factors to predict poor survival in all
subjects in this study (Table
II).
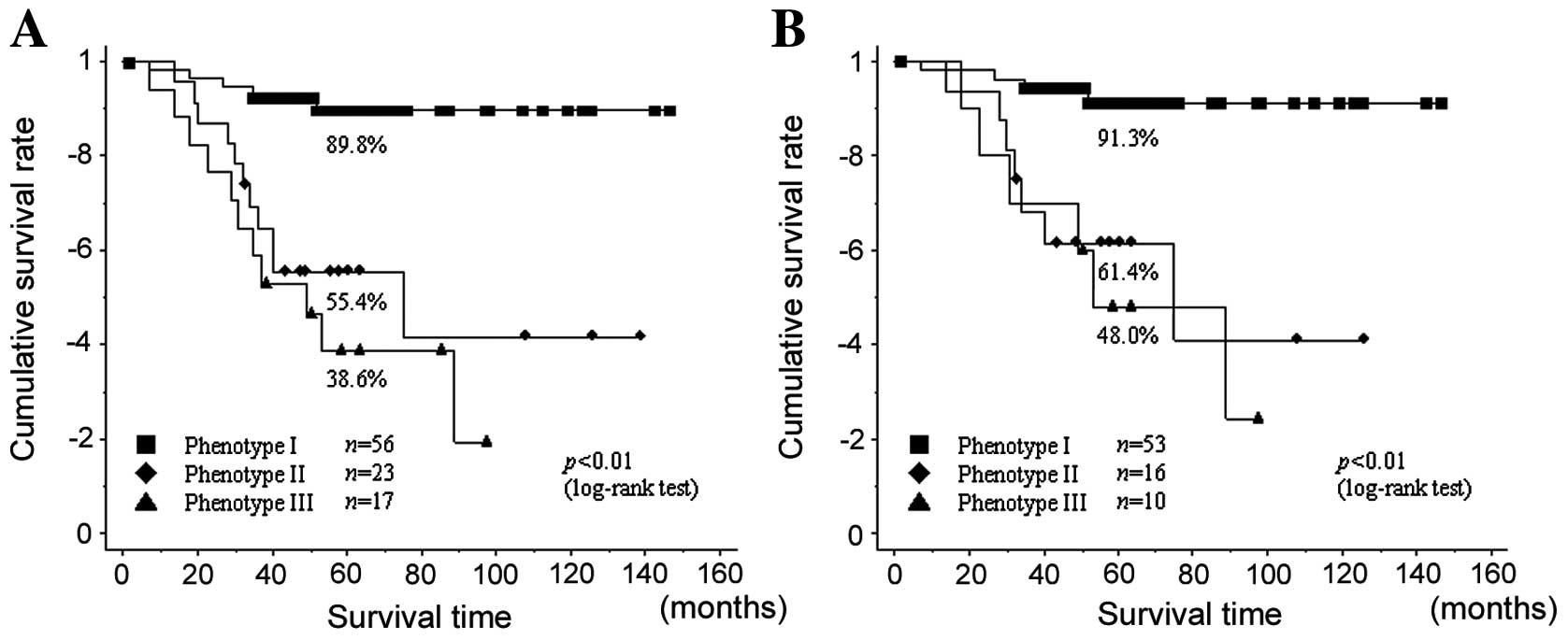 | Figure 4(A) The 5-year survival rates of all
patients with phenotypes I, II, and III were 89.8, 55.4 and 38.6%,
respectively, with a statistically significant difference
(P<0.01; log-rank test). (B) Of patients in stage I, the 5-year
survival rates for phenotypes I, II and III were 91.3, 61.4 and
48.0%, respectively, which were also significantly different
(P<0.01; log-rank test). |
 | Table IIUnivariate and multivariate analysis
for prognostic factors (79 patients with stage I). |
Table II
Univariate and multivariate analysis
for prognostic factors (79 patients with stage I).
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Parameters | P-value | P-value | Hazard ratio | 95% CI |
|---|
| Age |
| Young (≤68) | 0.05 | | | |
| Old (>68) | | | | |
| Gender |
| Female | 0.04 | 0.35 | Ref | |
| Male | | | 1.77 | 0.53–5.88 |
| Histological
grade |
| Well | | | Ref | |
| Moderate | 0.04 | 0.73 | 1.37 | 0.23–8.09 |
| Poorly | 0.01 | 0.83 | 1.26 | 0.16–10.1 |
| Tumor size
(mm) |
| Small (≤20) | 0.3 | | | |
| Large (>20,
≤30) | | | | |
| Pleural
invasion |
| Negative | 0.07 | | | |
| Positive | | | | |
| Lymphatic
invasion |
| Negative | 0.01 | 0.17 | Ref | |
| Positive | | | 2.63 | 0.66–10.5 |
| Vascular
invasion |
| Negative | 0.02 | 0.65 | Ref | |
| Positive | | | 1.29 | 0.42–3.94 |
| Phenotype |
| I | | | Ref | |
| II | <0.01 | 0.02 | 5.01 | 1.34–18.7 |
| III | <0.01 | 0.01 | 5.5 | 1.39–21.8 |
Discussion
In this study, we have demonstrated that phenotype
classification by immmunohistochemical multiparameter analysis
using cell cycle-related biomarkers is a useful procedure to
predict the degree of tumor malignant behavior and the prognosis of
patients with small-size lung adenocarcinoma.
Our data suggest that there was a highly significant
association between the phenotypes and histological grade, tumor
local invasiveness including lymphatic and vascular invasion, and
lymph node metastasis. Phenotype I, which consists of MCM7-negative
tumors, was frequently noted in the well- or
moderately-differentiated tumors and less frequently in the locally
invasive tumors compared with phenotypes II and III. MCM7, one of
the replication licensing factors, is expressed throughout all cell
cycle phases (G1-S-G2-M), but is downregulated during exit into
out-of-cycle states (29,32). The repression of licensing
contributes to replication arrest and loss of proliferative
capacity, as cells exit the mitotic cycle into the out-of-cycle
state. This allows a functional distinction between the
proliferative state and the non-proliferative out-of-cycle state
(33). Recent investigations
suggest that the detection of MCM proteins is a powerful tool for
assessing the proliferative potential of the cell. These unique
biomarkers can clearly distinguish between active cycling cells and
those in the out-of-cycle state in malignant disorders (29,34,35).
It is thought that phenotype I tumors may consist of a tumor cell
population that is almost out of cycle, which might provide the
lower tumor invasiveness and the good histological differentiation.
From the perspective of cell cycle kinetics, we suggest that MCM7
is the most useful biomarker to distinguish the cell-cycle
phenotypes firstly by means of immunohistochemical analysis.
Both phenotype II and III tumors might be
characterized as having higher proliferative activity defined as
MCM7, Ki-67, and Geminin positivity (S-G2 phase dominant phenotype)
and MCM7, Aurora A and H3S10ph positivity (G2-M phase dominant
phenotype), respectively. These phenotypes were relatively well
associated with the histologically poor degree of tumor
differentiation and tumor local invasiveness. Geminin, which is
also one of the DNA replication licensing factors, is expressed
during the S, G2, and M phases but not in G0 and G1 phases, and is
degraded at M phase without playing a specific role in cell
division control (36). It has been
suggested that Geminin might provide useful information about tumor
proliferation activities that is equal or superior to that of MCM
proteins or Ki-67. Moreover, there have been many studies
demonstrating that immunohistochemical analysis with the
combination of MCM proteins, Ki-67, and Geminin may be useful for
predicting tumor proliferating activities or patient prognosis in
various malignancies (5,11,14,15,17,37).
These findings are consistent with the present study, in which
tumors that are positive for all of MCM7, Ki-67, and Geminin
possess high proliferation potential, resulting in a high ratio of
poorly differentiated and locally invasive tumors in phenotype
II.
Aurora A, a family member of Aurora kinases,
regulated G2-M transition by phosphorylation of histone H3, a key
molecule in the conversion of the relaxed interphase chromatin to
mitotic condensed chromosomes (20,38).
In the cell cycle, Aurora A is expressed during S-G2-M phases and
its level is reduced rapidly after mitosis. It has been suggested
that this mitotic kinase is associated with the development of
malignant tumors and malignant alteration, and there have been some
studies that showed a significantly positive correlation between
Aurora A overexpression and aggressive tumor behavior, such as poor
differentiation and nodal metastasis (39,40).
The present study was supportive of the biological mechanism by
which Aurora A dysregulation at an early point during tumorigenesis
might contribute to genetic instability, resulting in aggressive
and local invasiveness in phenotype III tumors (9).
For survival analysis, the 5-year overall survival
rates significantly differed among the phenotypes: the prognoses of
the patients with phenotypes II and III were more unfavorable than
those with phenotype I. Furthermore, multivariate analysis revealed
that phenotypes II and III were independent prognostic factors in
the 79 patients with stage I lung adenocarcinoma. Considering these
results, it appears that this multiparameter analysis using cell
cycle biomarkers provides more reliable and definite information
about the prognostic implication of small-size lung adenocarcinoma
when compared with the prognostic analysis with a single
biomarker.
Cell cycle profiling by multiparameter analysis may
have more useful features for determining cancer therapeutic
significance. Loddo and colleagues (10) suggested that the cell cycle
profiling of tumors has potential as a predictor of treatment
response to cell cycle phase-specific chemotherapeutic agents,
including small molecule inhibitors targeting the cell cycle
machinery. For example, tegafur/uracil (UFT) and taxanes are
representative and commonly used anticancer drugs, especially in
Japan, among various chemotherapeutic agents for NSCLC. The
therapeutic target of UFT is thymidylate synthase (TS), especially
in tumor cells in S phase, and its mechanism of action is
ribonucleotide depletion, which leads to the arrest of tumor cells
at the G1-S phase of the cell cycle. On the other hand, the target
of taxanes is tubulin of tumor cells, especially those in M phase,
and their antitumor effects result mainly from interference with
the normal function of microtubules and the blockage of cell cycle
progression in later G2-M phases via prevention of mitotic spindle
formation (41). Other almost
chemotherapeutic agents also have their specific acting phase in
the cell cycle. These agents might work more effectively for tumor
cells in a stage of cell cycle phase distribution where the target
molecules exist. It might be possible to select these
chemotherapeutic agents more effectively by multiparameter analysis
to demonstrate the predominant cell cycle phase distribution of
tumor cells.
Acknowledgements
We thank Mr. Itaki, Ms. Yamasaki, Ms. Iwatani and
Ms. Tokuoka for excellent technical assistance.
References
|
1
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumors. Pathology and Genetics of Tumors of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon: pp. 12–44. 2004
|
|
2
|
Louis DN, Edgerton S, Thor AD and
Hedley-Whyte ET: Proliferating cell nuclear antigen and Ki-67
immunohistochemistry in brain tumors: a comparative study. Acta
Neuropathol. 81:675–679. 1991.
|
|
3
|
Mehdi SA, Etzell JE, Newman NB, Weidner N,
Kohman LJ and Graziano SL: Prognostic significance of Ki-67
immunostaining and symptoms in resected stage I and II non-small
cell lung cancer. Lung Cancer. 20:99–108. 1998.
|
|
4
|
Haga Y, Hiroshima K, Iyoda A, et al: Ki-67
expression and prognosis for smokers with resected stage I
non-small cell lung cancer. Ann Thorac Surg. 75:1727–1732.
2003.
|
|
5
|
Dudderidge TJ, Stoeber K, Loddo M,
Atkinson G, Fanshawe T, Griffiths DF and Williams GH: Mcm2,
Geminin, and Ki67 define proliferative state and are prognostic
markers in renal cell carcinoma. Clin Cancer Res. 11:2510–2517.
2005.
|
|
6
|
Vargas PA, Cheng Y, Barrett AW, Craig GT
and Speight PM: Expression of Mcm-2, Ki-67 and geminin in benign
and malignant salivary gland tumours. J Oral Pathol Med.
37:309–318. 2008.
|
|
7
|
Fujioka S, Shomori K, Nishihara K, et al:
Expression of minichromosome maintenance 7 (MCM7) in small lung
adenocarcinomas (pT1): prognostic implication. Lung Cancer.
65:223–229. 2009.
|
|
8
|
Hashimoto K, Araki K, Osaki M, Nakamura H,
Tomita K, Shimizu E and Ito H: MCM2 and Ki-67 expression in human
lung adenocarcinoma: prognostic implications. Pathobiology.
71:193–200. 2004.
|
|
9
|
Kulkarni AA, Loddo M, Leo E, et al: DNA
replication licensing factors and aurora kinases are linked to
aneuploidy and clinical outcome in epithelial ovarian carcinoma.
Clin Cancer Res. 13:6153–6161. 2007.
|
|
10
|
Loddo M, Kingsbury SR, Rashid M, et al:
Cell-cycle-phase progression analysis identifies unique phenotypes
of major prognostic and predictive significance in breast cancer.
Br J Cancer. 100:959–970. 2009.
|
|
11
|
Kayes OJ, Loddo M, Patel N, et al: DNA
replication licensing factors and aneuploidy are linked to tumor
cell cycle state and clinical outcome in penile carcinoma. Clin
Cancer Res. 15:7335–7344. 2009.
|
|
12
|
Nishitani H and Lygerou Z: Control of DNA
replication licensing in a cell cycle. Genes Cells. 7:523–534.
2002.
|
|
13
|
Pitulescu M, Kessel M and Luo L: The
regulation of embryonic patterning and DNA replication by geminin.
Cell Mol Life Sci. 62:1425–1433. 2005.
|
|
14
|
Tamura T, Shomori K, Haruki T, et al:
Minichromosome maintenance-7 and geminin are reliable prognostic
markers in patients with oral squamous cell carcinoma:
immunohistochemical study. J Oral Pathol Med. 39:328–334. 2010.
|
|
15
|
Torres-Rendon A, Roy S, Craig GT and
Speight PM: Expression of Mcm2, geminin and Ki67 in normal oral
mucosa, oral epithelial dysplasias and their corresponding
squamous-cell carcinomas. Br J Cancer. 100:1128–1134. 2009.
|
|
16
|
Nishihara K, Shomort K, Fujioka S, et al:
Minichromosome maintenance protein 7 in colorectal cancer:
implication of prognostic significance. Int J Oncol. 33:245–251.
2008.
|
|
17
|
Nishihara K, Shomori K, Tamura T, Fujioka
S, Ogawa T and Ito H: Immunohistochemical expression of geminin in
colorectal cancer: implication of prognostic significance. Oncol
Rep. 21:1189–1195. 2009.
|
|
18
|
Tokuyasu N, Shomori K, Nishihara K, et al:
Minichromosome maintenance 2 (MCM2) immunoreactivity in stage III
human gastric carcinoma: clinicopathological significance. Gastric
Cancer. 11:37–46. 2008.
|
|
19
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001.
|
|
20
|
Crosio C, Fimia GM, Loury R, et al:
Mitotic phosphorylation of histone H3: spatio-temporal regulation
by mammalian Aurora kinases. Mol Cell Biol. 22:874–885. 2002.
|
|
21
|
Bischoff JR, Anderson L, Zhu Y, et al: A
homologue of Drosophila aurora kinase is oncogenic and
amplified in human colorectal cancers. EMBO J. 17:3052–3065.
1998.
|
|
22
|
Jeng YM, Peng SY, Lin CY and Hsu HC:
Overexpression and amplification of Aurora-A in hepatocellular
carcinoma. Clin Cancer Res. 10:2065–2071. 2004.
|
|
23
|
Kurahashi T, Miyake H, Hara I and Fujisawa
M: Significance of Aurora-A expression in renal cell carcinoma.
Urol Oncol. 25:128–133. 2007.
|
|
24
|
Lam AK, Ong K and Ho YH: Aurora kinase
expression in colorectal adenocarcinoma: correlations with
clinicopathological features, p16 expression, and telomerase
activity. Hum Pathol. 39:599–604. 2008.
|
|
25
|
Ogawa E, Takenaka K, Katakura H, et al:
Perimembrane Aurora-A expression is a significant prognostic factor
in correlation with proliferative activity in non-small cell lung
cancer (NSCLC). Ann Surg Oncol. 15:547–554. 2008.
|
|
26
|
Shang X, Burlingame SM, Okcu MF, et al:
Aurora A is a negative prognostic factor and a new therapeutic
target in human neuroblastoma. Mol Cancer Ther. 8:2461–2469.
2009.
|
|
27
|
Tanaka E, Hashimoto Y, Ito T, et al: The
clinical significance of Aurora-A/STK15/BTAK expression in human
esophageal squamous cell carcinoma. Clin Cancer Res. 11:1827–1834.
2005.
|
|
28
|
Zhang XH, Rao M, Loprieato JA, et al:
Aurora A, Aurora B and survivin are novel targets of
transcriptional regulation by histone deacetylase inhibitors in
non-small cell lung cancer. Cancer Biol Ther. 7:1390–1399.
2008.
|
|
29
|
Williams GH and Stoeber K: Cell cycle
markers in clinical oncology. Curr Opin Cell Biol. 19:672–679.
2007.
|
|
30
|
Sobin LH: UICC International Union Against
Cancer: TNM classification of malignant tumours. 6th edition. John
Wiley & Sons; New York: 2002
|
|
31
|
Suzuki K, Yokose T, Yoshida J, Nishimura
M, Takahashi K, Nagai K and Nishiwaki Y: Prognostic significance of
the size of central fibrosis in peripheral adenocarcinoma of the
lung. Ann Thorac Surg. 69:893–897. 2000.
|
|
32
|
Stoeber K, Tlsty TD, Happerfield L, Thomas
GA, Romanov S, Bobrow L, Williams ED and Williams GH: DNA
replication licensing and human cell proliferation. J Cell Sci.
114:2027–2041. 2001.
|
|
33
|
Blow JJ and Hodgson B: Replication
licensing - defining the proliferative state? Trends Cell Biol.
12:72–78. 2002.
|
|
34
|
Freeman A, Morris LS, Mills AD, Stoeber K,
Laskey RA, Williams GH and Coleman N: Minichromosome maintenance
proteins as biological markers of dysplasia and malignancy. Clin
Cancer Res. 5:2121–2132. 1999.
|
|
35
|
Gonzalez MA, Tachibana KK, Laskey RA and
Coleman N: Control of DNA replication and its potential clinical
exploitation. Nat Rev Cancer. 5:135–141. 2005.
|
|
36
|
Montanari M, Macaluso M, Cittadini A and
Giordano A: Role of geminin: from normal control of DNA replication
to cancer formation and progression? Cell Death Differ.
13:1052–1056. 2006.
|
|
37
|
Gonzalez MA, Tachibana KE, Chin SF, et al:
Geminin predicts adverse clinical outcome in breast cancer by
reflecting cell-cycle progression. J Pathol. 204:121–130. 2004.
|
|
38
|
Marumoto T, Zhang DW and Saya H: Aurora-A:
a guardian of poles. Nat Rev Cancer. 5:42–50. 2005.
|
|
39
|
Tong T, Zhong Y, Kong J, et al:
Overexpression of Aurora-A contributes to malignant development of
human esophageal squamous cell carcinoma. Clin Cancer Res.
10:7304–7310. 2004.
|
|
40
|
Royce ME, Xia WY, Sahin AA, et al:
STK15/Aurora-A expression in primary breast tumors is correlated
with nuclear grade but not with prognosis. Cancer. 100:12–19.
2004.
|
|
41
|
Johnson KR, Wang L, Miller MC III, et al:
5-Fluorouracil interferes with paclitaxel cytotoxicity against
human solid tumor cells. Clin Cancer Res. 3:1739–1745. 1997.
|