Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies in the whole world. Each year more than 360,000
new cases are diagnosed with HCC in China and the incidence is
continuing to rise (1). It is known
that surgical resection is the primary curative treatment for HCC
in clinic, however, the major treatment of patients with
unresectable or metastatic hepatocellular carcinoma (HCC) is
chemotherapy (2). Though numerous
patients with HCC could be successfully induced into first
remission by chemotherapy, most of them ultimately experienced a
relapse within 3–5 years. In addition, chemotherapy is often
accompanied with substantial side effects for the reason of
non-specific cytotoxicity. Therefore, it is imperative to develop
novel or other therapy approaches or agents for clinical HCC
therapy. Targeted therapy by antibody-mediated therapy has emerged
as a novel approach for the effective and innovative treatment of
HCC (3,4).
Gelatinase (including matrix metalloproteinase
(MMP)-2 and MMP-9), a member of MMPs, plays an important role in
tumor growth and metastasis, and overexpression of these molecules
is strongly correlated with poor prognosis in a variety of
malignant tumors (5,6). It is known that the gelatinases are
abundantly presented in certain hepatocellular carcinoma (7,8),
therefore, gelatinases is a possible target for cancer therapy or
cancer interference. Antibody targeting therapy of hepatoma is a
new approach. To augment the antitumor efficiency, a potent
cytotoxic drug conjugated with antibody has been attempted and
already obtained distinct clinical outcome (9).
The enediyne family of antibiotics is among the most
toxic antitumor compounds described to date (10). Lidamycin (LDM, also called C-1027)
is an enediyne antibiotic with potent cytotoxicity causing DNA
strand breaks at very low concentration (11). Therefore, lidamycin is a potential
moiety or ‘warhead’ to conjugate with the antibody. It has been
documented that antibody-lidamycin conjugate showed very good
antitumor efficacy in vitro and in vivo (12–14).
Our previous results demonstrated that a tandem scFv format
conjugated with lidamycin (dFv-LDP-AE) improved the antitumor
efficacy compared with the scFv-lidamycin conjugate, and showed
excellent tumor targeting capability to certain tumor cell lines,
which indicated that the dFv-LDP-AE is a promising anticancer agent
for development (15). As HCC is a
leading cause of cancer death in China, developing new therapeutic
strategies is warranted.
In this study, we observed the potential application
of the tandem scFv-LDM conjugate (dFv-LDP-AE) in HCC. To evaluate
the antitumor efficacy of dFv-LDP-AE, ELISA, immunofluorescene,
cell cycle arrest and apoptosis experiments were performed, the
inhibition of tumor growth and the targeting capability in
vivo was also investigated.
Materials and methods
Materials
The preparation of the dFv-LDP fusion protein and
its enediyne-energized product dFv-LDP-AE were described in our
previous report (15). MTT and FITC
were purchased from Sigma Chemical Co. (USA). Other chemical agents
used were of analytical grade.
Cell culture
Human hepatoma Bel-7402 cells, and HepG 2 cells were
stored in our lab and cultured at 37°C in DMEM medium supplemented
with 10% fetal bovine serum, penicillin G (100 U/ml) and
streptomycin (100 μg/ml). All cell lines were passaged every 3 days
and maintained in exponential growth to ~80% confluence for
experiments.
Binding specificity of dFv-LDP with
cancer cells by immunofluorescence
The human hepatoma Bel-7402 cells were grown on
slides and fixed with ice-cold 70% methanol for 30 min at 4°C.
Non-specific binding was blocked with 3% BSA-PBS. After washing
with PBS, the cells were incubated with dFv-LDP. The cells were
then overlaid with mouse anti-His-Tag monoclonal antibodies after
being washed with PBS. Then, the slides were mounted with
fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse
antibody and then re-stained with propidium iodide (PI). Following
a final washing step with PBS, the fluorescence images were
captured by a fluorescence microscope.
Binding affinity assay
To quantitatively determine the binding affinity of
fusion protein dFv-LDP to hepatoma cells, the cell-based ELISA was
used. Briefly, serial dilutions of re-folded dFv-LDP or Fv-LDP in
1% BSA-PBS were added into Bel-7402 and HepG 2 tumor cells in
pre-coated plates, incubated and washed. Then, the plate was
incubated with anti-His-tag HRP-conjugate and washed,
3,3′,5,5′-tetramethylbenzidine was used as the chromogen for the
color development, absorbance values at 450 nm were measured on
microplate reader (Bio-Rad Laboratories).
MTT assay
Cells were detached by trypsinization, seeded at
3,000 cells/well in a 96-well plate (Costar, Cambridge, MA)
overnight. Then different concentrations of LDM were added and
incubated for an additional 48 h. The effect on cell growth was
examined by MTT assay. Briefly, 20 μl of MTT solution (5 mg/ml) was
added to each well and incubated at 37°C for 4 h. The supernatant
was removed, and the MTT formazan formed by metabolically viable
cells was dissolved in 150 μl of DMSO, and then monitored with a
microplate reader (Bio-Rad Labortories) at a wavelength of 570 nm.
Survival ratio was calculated according to the following formula:
Survival ratio =
(Atest-Ablank)/(Acontrol-Ablank)
× 100%.
Cell cycle analysis
Cells were treated with dFv-LDP-AE (0.01 and 0.1 nM)
up to 48 h. Floating and adherent cells were harvested and
centrifuged at 500 × g for 5 min. Then cells were fixed in ice-cold
70% ethanol and stored at −20°C for 24 h before analysis. For cell
cycle analysis, cells were washed twice in PBS and stained with 50
mg/ml propidium iodide and 200 μg/ml RNase A for 30 min. The
samples were analyzed on a fluorescence-activated cell sorter
(Becton-Dickinson).
FITC-Annexin V/PI apoptosis assay
Cells were collected and resuspended in 200 μl
binding buffer. Then 10 μl FITC-labeled enhanced Annexin V and 100
ng propidium iodide were added. Upon incubation in the dark (15
min, room temperature or 30 min at 4°C), the samples were diluted
with 500 μl binding buffer. Flow cytometry was carried out on a
FACScan instrument (Becton-Dickinson) and data were processed by
WinMDI/PC-software.
In vivo antitumor experiments
The antitumor experiments were carried out using two
hepatoma cancer cell lines, the mouse hepatoma 22 (H22) and human
hepatoma Bel-7402. Kunming (KM) mice and the male athymic nude mice
(Balb/c nu/nu) were purchased from the institute of animal
research, Chinese academy of medical science, and allowed to
acclimatize in the institutional animal house for >5 days before
use. The study protocols were in accordance with the regulations of
Good Laboratory Practice for non-clinical laboratory studies of
drugs issued by the National Scientific and Technologic Committee
of People's Republic of China.
The mouse H22 animal experiment was performed with
7-week-old female Kunming mice. H22 cells suspended in saline were
inoculated subcutaneously (Day 0) at the right axilla of the mouse
with 1.5×106 cells/0.2 ml/mouse. The mice were
randomized into seven groups with 10 mice in each group. On Day 2,
dFv-LDP-AE was administered at doses of 0.75, 1.0 and 1.25 mg/kg of
body weight. LDM, and dFv-LDP fusion protein was administered at
doses of 0.06, and 1.25 mg/ kg, respectively. All treatments were
administered by intravenous injection into the tail vein in 0.2 ml
of sterile PBS.
To further evaluate the antitumor efficacy of
dFv-LDP-AE, a second animal experiment was performed with
16–18-week-old nude mice. Exponentially growing human hepatoma
Bel-7402 cells were implanted into two male athymic nude mice by
the subcutaneous injection of 5×106 cells on the right
flank. Tumors resulting after 2 weeks in donor animals were
aseptically dissected and mechanically minced. Pieces of tumor
tissue (2 mm3 in size) were transplanted
(subcutaneously) by a trocar needle into nude mice. When tumors
reached about 100 mg in size, the mice were randomized into
treatment groups (n=6 per group). Then the treatments were started
(intravenously injection twice approximately on Day 7 and 14 after
tumor implantation), the nude mice were injected in the tail vein
with dFv-LDP-AE at different doses (0.4, 0.6 and 0.8 mg/kg), and
with LDM (0.05 mg/kg), dFv-LDP (0.8 mg/kg), respectively. Control
nude mice were injected with saline. Tumor growth was measured with
a caliper, and tumor volumes were calculated with the following
formula: V=0.5a × b2, where a and b are the long and the
perpendicular short diameters of the tumor, respectively (15). The data are presented as the mean ±
SD. Tumor growth curves were plotted of time against the mean tumor
volume ± SD.
The inhibitory rates of tumor growth were calculated
according to the weight of excised tumor. The Student's t-test was
used to determine statistically significant differences. P<0.05
was considered significant.
Fluorescein isothiocyanate (FITC)
labeling of the dFv-LDP and the small animal optical imaging
FITC were purchased from Sigma and was dissolved in
dimethylsulfoxide. The protein dFv-LDP was dialyzed against 0.1 M
sodium bicarbonate (pH 9.6) three times. Then the protein and FITC
solutions were mixed in a final volume of 2.5 ml and incubated for
8 h at 4°C in the dark with constant shaking, after that 25 μl
ammonium chloride (5 mol/l) was added to terminate the reaction for
another 2 h. The FITC-labeled dFv-LDP were separated from free FITC
on a Sephade× G-25 column equilibrated with phosphate-buffered
saline (PBS). The protein-FITC ratio was measured with the formula
3.1 × A495/(A280 - 0.31 × A495) and the concentration of labeled
protein was determined by the formula (A280 - 0.31 × A495)/1.4
(16). The small animal optical
imaging was performed with an IVIS Imaging System (Xenogen)
comprised of a highly sensitive, cooled CCD camera mounted in a
light-tight specimen box. Images and measurements of fluorescence
signals were acquired and analyzed using Living Image software
(Xenogen). Ten to 15 min prior to in vivo imaging, animals
received the FITC-labeled dFv-LDP at 40 mg/kg by tail vein and were
anesthetized using 1–3% isoflurane. Animals were placed onto the
warmed stage inside the camera box and received continuous exposure
to 1–2% isoflurane to sustain sedation during imaging. The imaging
time was 20 sec and two to three mice were imaged at a time. The
light emitted from the FITC was detected in vivo by the IVIS
Imaging System, digitized and electronically displayed as a
pseudocolor overlay onto a gray scale animal image.
Statistical analysis
Significant difference between two values was
determined with the Student's t-test. P<0.05 was considered
statistically significant.
Results
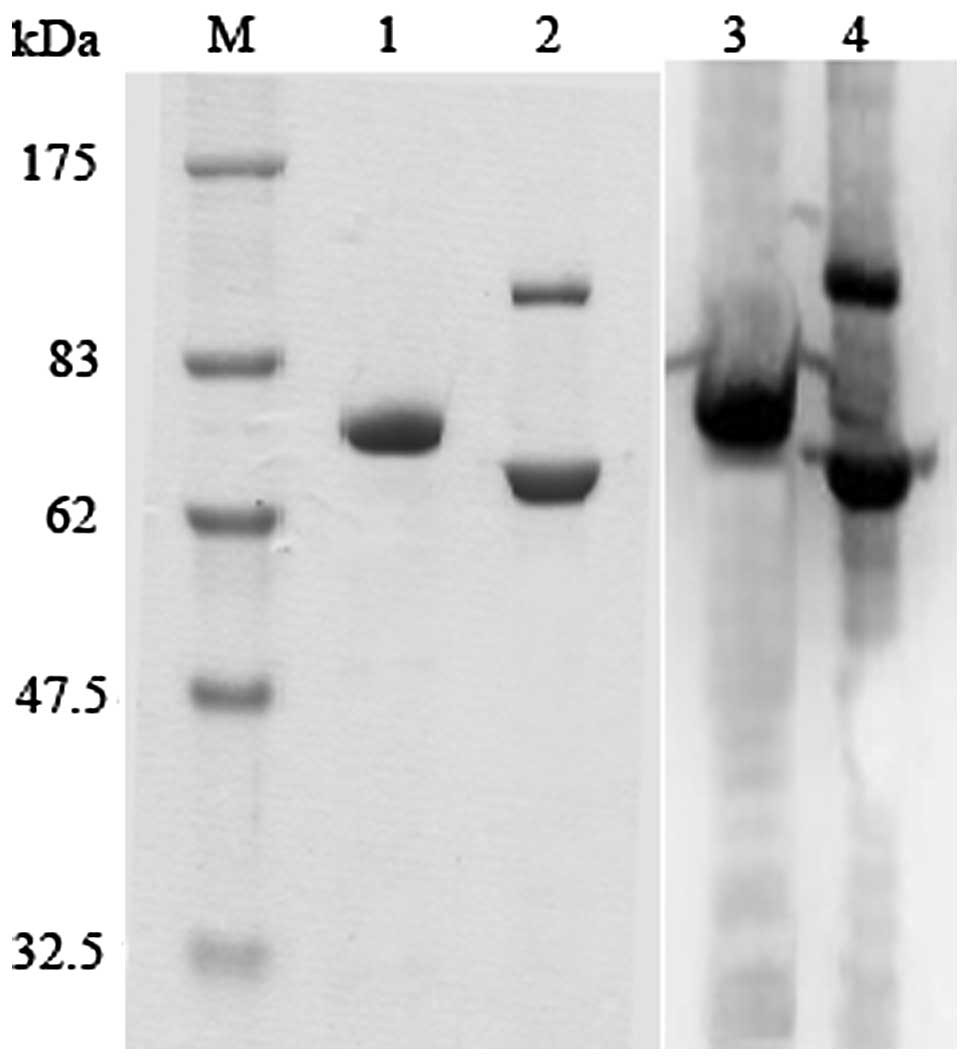
The preparation of dFv-LDP fusion
protein
The fusion protein dFv-LDP was produced by E.
coli and expressed mainly as inclusion body. After purification
and refolding as reported previously (15). In this study, the PAGE was used to
analyze the fusion protein dFv-LDP under reduced and non-reduced
conditions. As shown in Fig. 1,
there is a tiny band under the non-reduced condition, which
indicates that a small percent of dFv-LDP exists as a dimer.
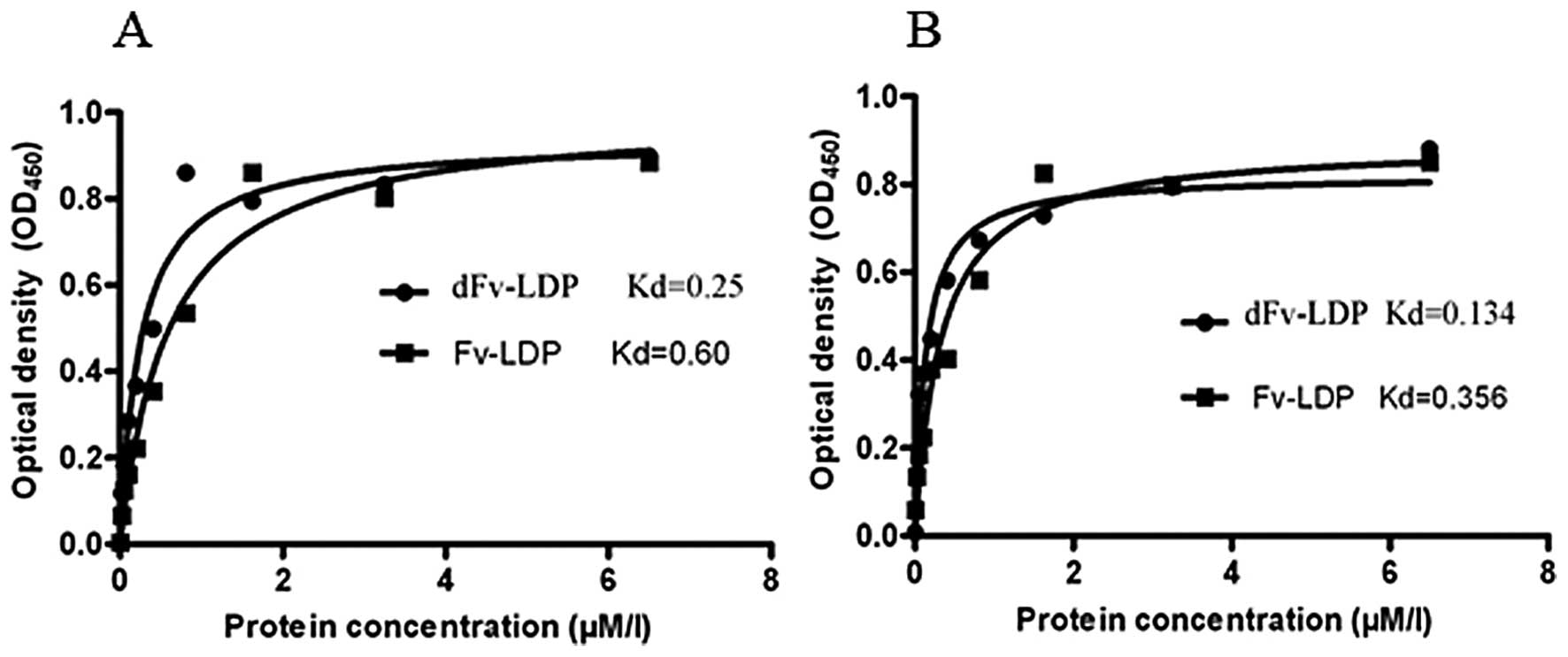
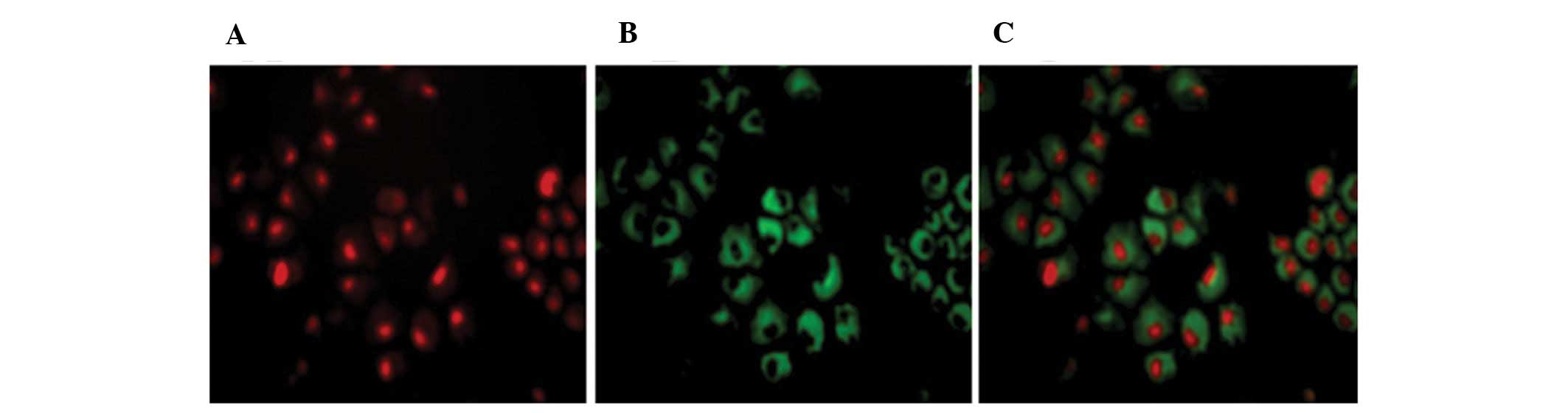
Binding capability with tumor cells
To investigate the binding of fusion protein dFv-LDP
with HCC, enzyme-linked immunosorbent assay (ELISA) was used to
determine the affinity of dFv-LDP with Bel-7402 and HepG 2 cell
lines. As shown in Fig. 2, fusion
protein dFv-LDP could bind with tumor cells in a dose-dependent and
saturable manner (Fig. 2), and the
affinity constant is about 2- or 3-fold increased compared to the
Fv-LDP, which contained just a single scFv. The binding of dFv-LDP
with tumor cells was also displayed by immunofluorescence, as shown
in Fig. 3, which indicated that the
gelatinases are abundantly expressed around the tumor cells, and
the fusion protein dFv-LDP could efficiently bind them.
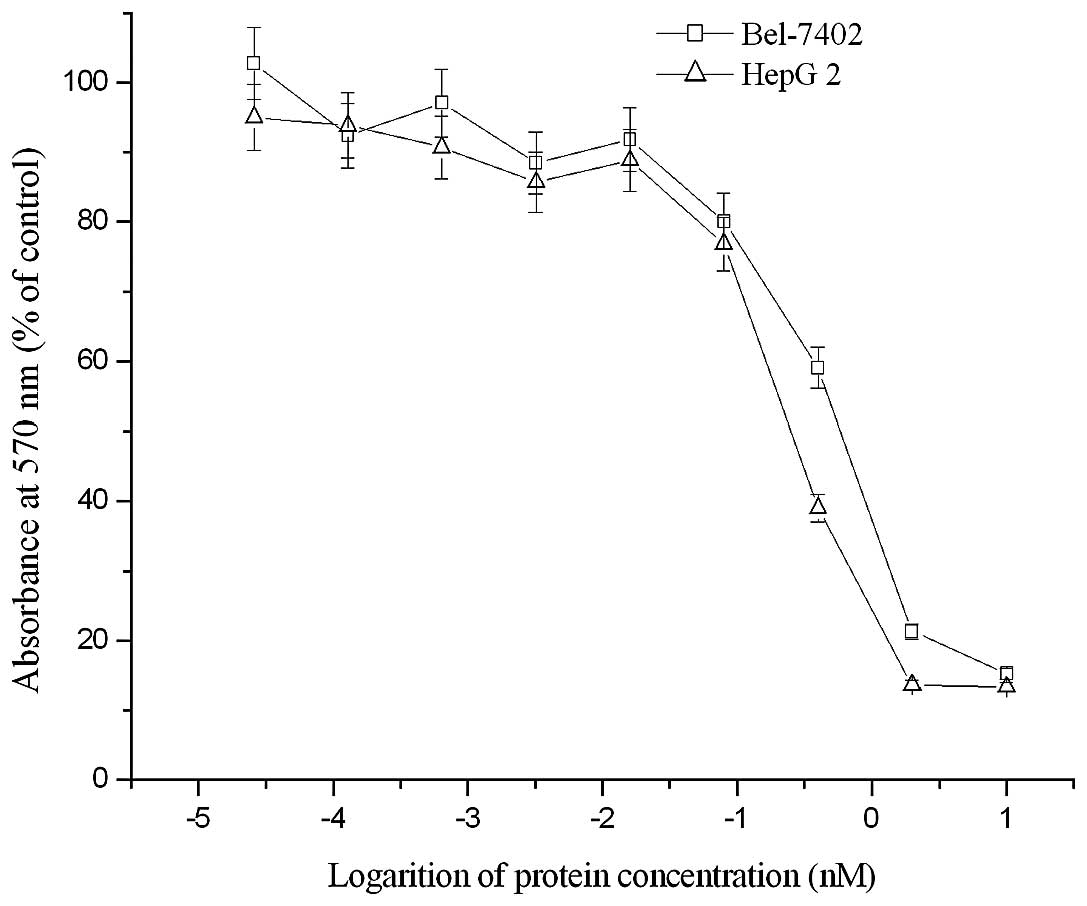
Ctytotoxicity of dFv-LDP-AE in vitro
As examined by MTT assay, the enediyne-energized
fusion protein dFv-LDP-AE displayed potent cytotoxicity to two HCC
cell lines. As shown in Fig. 4, the
IC50 of dFv-LDP-AE to Bel-7402 and HepG 2 tumor cells
were ~4.5×10−10 M and 7.7×10−10 M,
respectively.
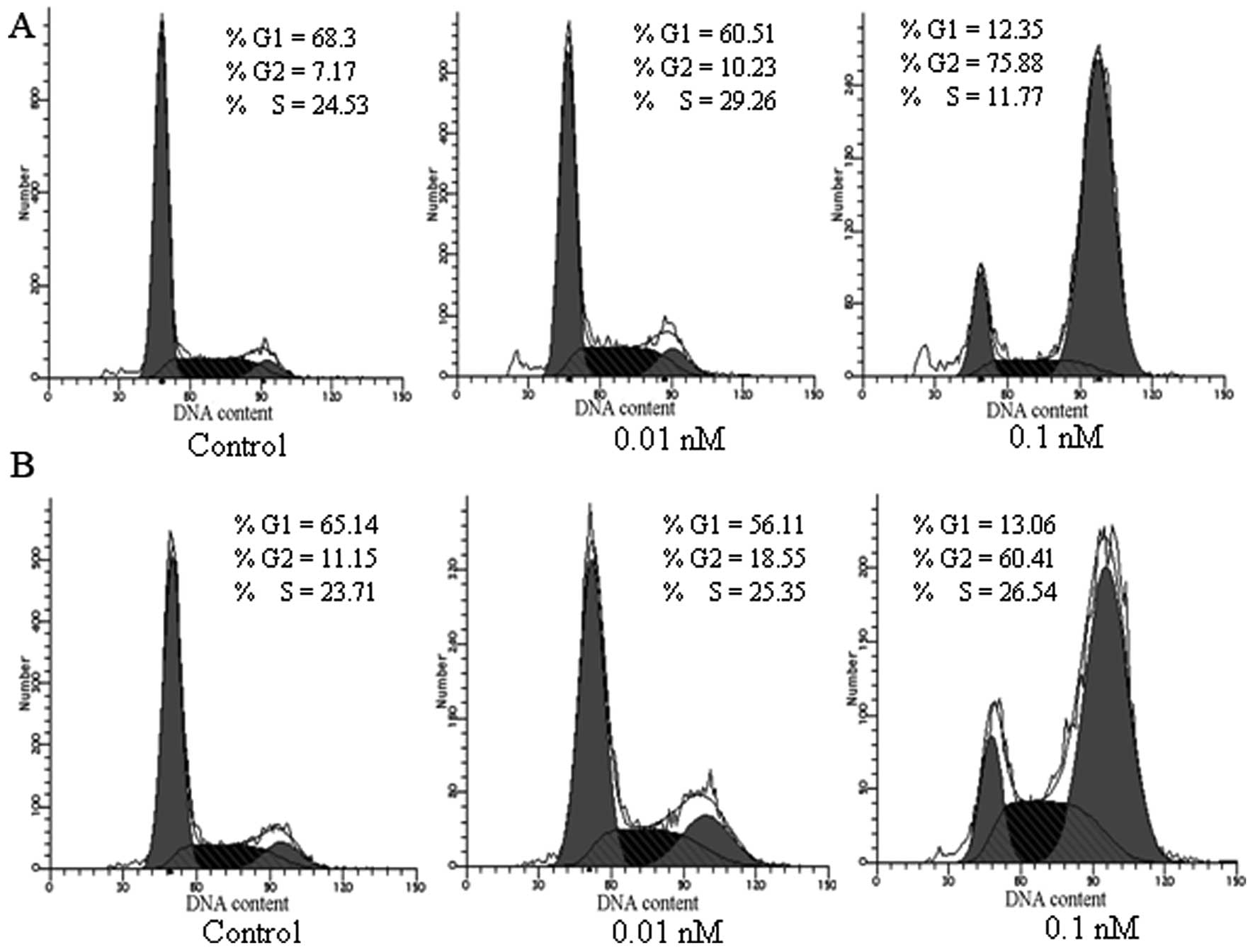
Cell cycle analysis
HepG 2 and Bel-7402 tumor cells were PI stained and
the cell cycle progression was evaluated using flow cytometry. As
shown in Fig. 5, an apparent
increase of cells in the G2 phase by 3.06–68.71% with a
corresponding decrease of cells in the G1 phases, was observed in
Bel-7402 cells upon treatment with 0.01 nM and 0.1 nM dFv-LDP-AE.
Similarly, an increase of cells arrest in G2 phase by 7.4–49.26%
with a corresponding decrease of cells in the G1 phase was detected
in HepG 2 cells after exposure to dFv-LDP-AE at indicated doses,
thus, indicating that dFv-LDP-AE had potent inhibition on the
progression of HCC tumor cells.
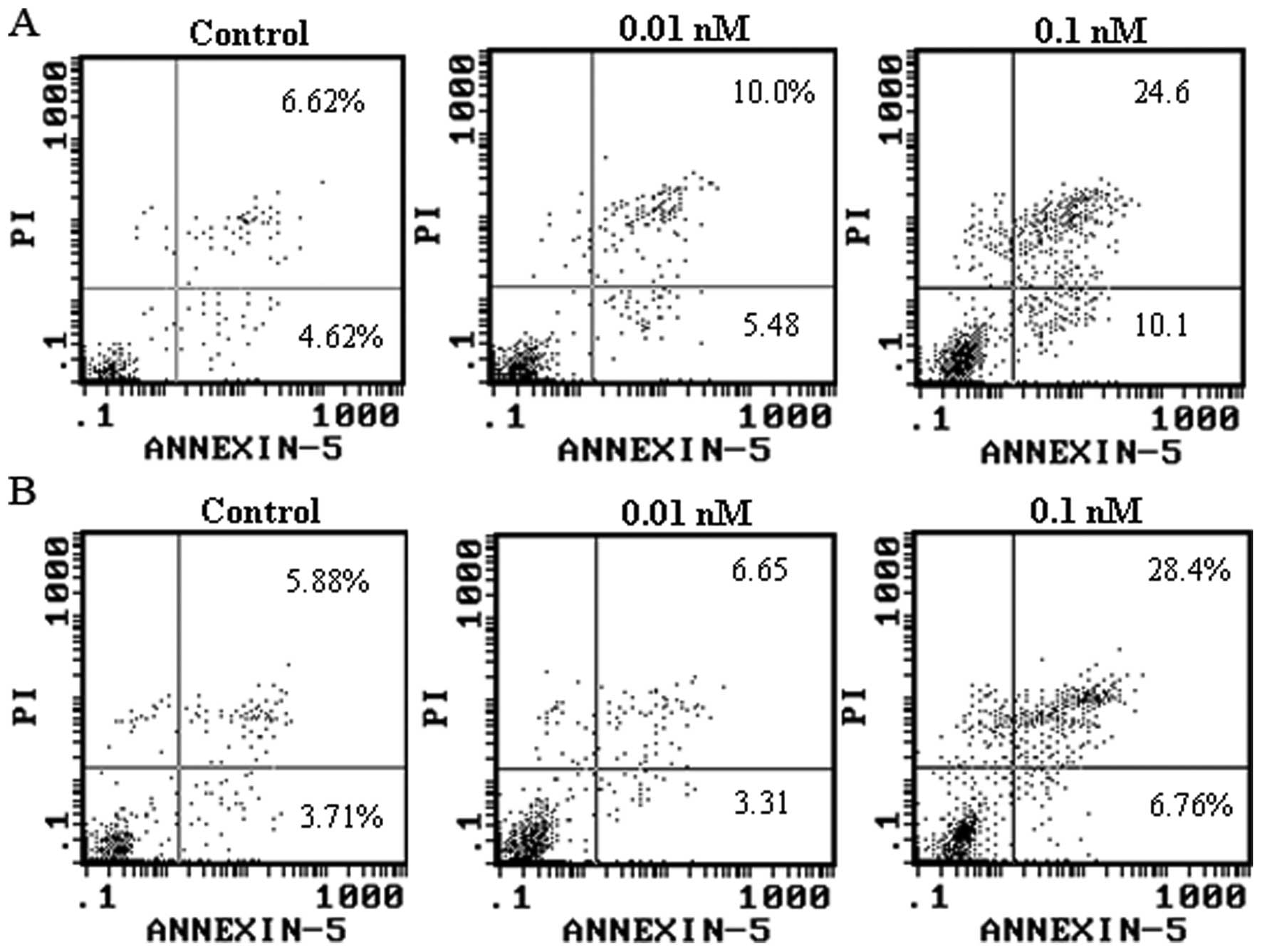
Apoptosis analysis
FITC-Annexin V/PI analysis showed that the energized
fusion protein dFv-LDP-AE induced apoptosis in Bel-7402 and HepG 2
cells. As shown in Fig. 6, 0.01 and
0.1 μM dFv-LDP-AE induced apoptosis in 15.48 and 34.7% of Bel-7402
cells, respectively. Similarly, 0.01 and 0.1 μM dFv-LDP-AE resulted
in apoptosis in 9.9 and 35.16% of HepG 2 cells, respectively.
The antitumor effect of dFv-LDP-AE in
vivo
The in vivo antitumor effects of dFv-LDP-AE
were investigated on the inhibition of subcutaneously transplanted
murine H22 in Kunming mice. The tumor-bearing mice were treated by
tail vein injection once at 48 h after transplantation. The results
showed that dFv-LDP-AE significantly inhibited the growth of
hepatoma 22 tumors (Table I). As
evaluated on Day 12, dFv-LDP-AE at 1.25, 1.0 and 0.75 mg/kg
suppressed the tumor growth by 89.5% (P<0.05 vs. LDM), 85.2%
(P<0.01 vs. control) and 77.4% (P<0.01 vs. control),
respectively, whereas the free LDM at tolerated dose of 0.06 mg/kg
showed an inhibition rate of 73.6% and the ‘naked’ fusion protein
dFv-LDP at 1.25 mg/kg also showed a moderate inhibition rate
(37.6%) on tumor growth. During the whole experiment, all treated
mice survived at the final time, no severe side-effects were
observed during the treatment and the body weight of the mice had
increased a little.
 | Table ITumor growth inhibition effects of
dFv-LDP-AE on hepatoma 22 in mice. |
Table I
Tumor growth inhibition effects of
dFv-LDP-AE on hepatoma 22 in mice.
| Groups | Dose (mg/kg) | Mouse no.
(begin/end) | BWC (mean ± SD,
g) | Tumor weight (mean ±
SD, g) | Inhibition ratio
(%) |
|---|
| Control | | 10/10 | 11.632±0.95 | 3.1±0.36 | - |
| LDM | 0.06 | 10/10 | 3.57±2.48 | 0.82±0.61 | 73.6a |
| dFv-LDP-AE | 0.75 | 10/10 | 3.28±1.46 | 0.7±0.44 | 77.4a |
| 1.0 | 10/10 | 3.36±1.24 | 0.46±0.32 | 85.2a |
| 1.25 | 10/10 | 2.79±2.47 | 0.33±0.21 | 89.5a,b |
| dFv-LDP | 1.25 | 10/10 | 10.56±2.17 | 1.93±0.70 | 37.6 |
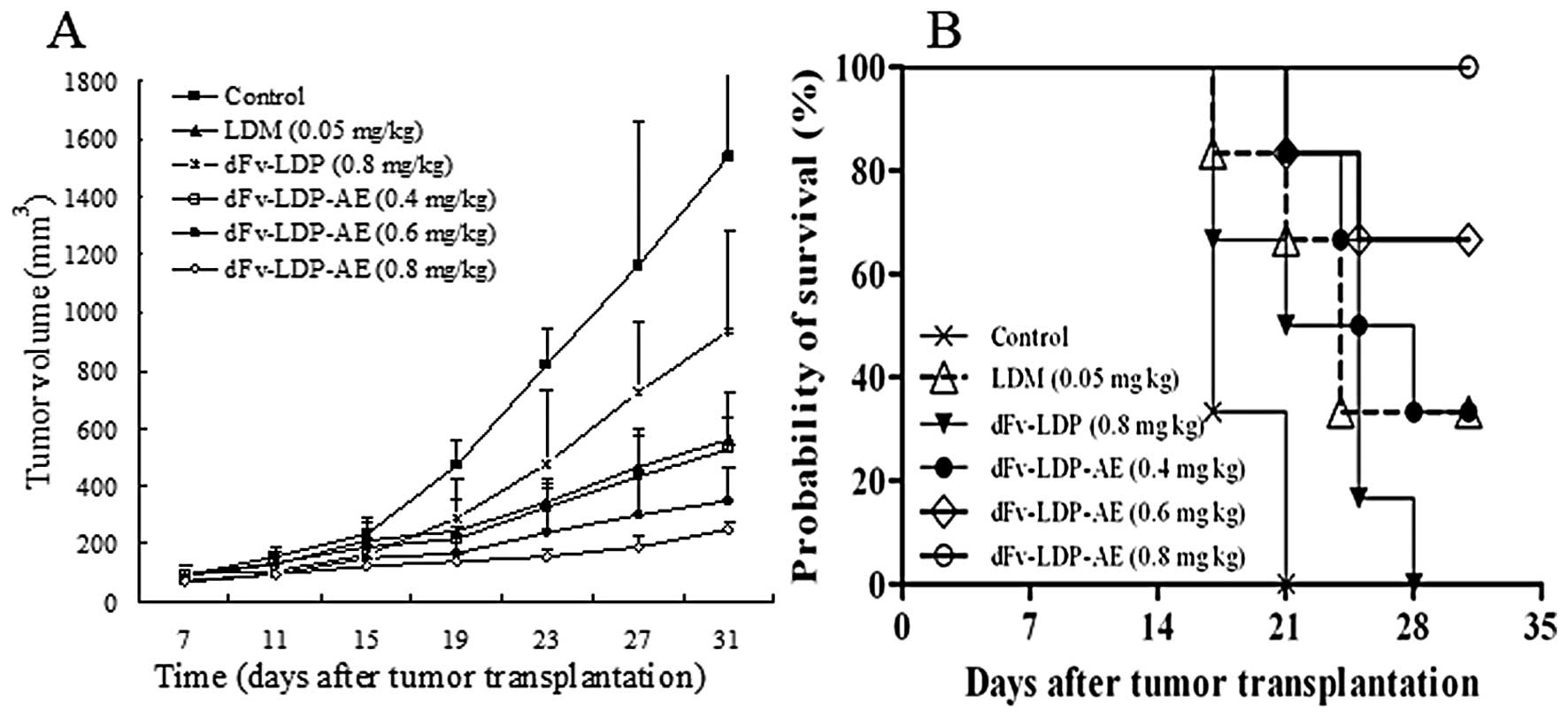
The antitumor activities of dFv-LDP-AE were also
evaluated in nude mice with xenograft of Bel-7402 human hepatoma.
When the tumors reached about 100 mm3 in size, the mice
were randomized into 6 treatment groups (n=6 per group). The
treatments were started by intravenously injection twice
approximately on Day 7 and 14 after tumor implantation. As shown in
Fig. 7A, Bel-7402 xenografts grew
rapidly in nude mice, and the tumor volume in the control group
increased from 22 to 1522 mm3 (around 70-fold increase)
over 31 days duration of the experiment. Mice treated with LDM at
the tolerated dose of 0.05 mg/kg showed an inhibition rate of
63.4%. dFv-LDP-AE at 0.8 mg/kg suppressed the tumor growth by
87.3%, which demonstrated statistically significant differences
(P<0.01) compared with that of LDM at 0.05 mg/kg. The
therapeutic efficacy in groups of dFv-LDP-AE was dose-dependent.
The fusion protein dFv-LDP (0.8 mg/kg) also showed certain
therapeutic efficacy (around 38.8% inhibition). These results
suggested that dFv-LDP-AE could inhibit the growth of Bel-7402
xenografts significantly in nude mice; and the therapeutic efficacy
of dFv-LDP-AE was apparently stronger than that of LDM (P<0.01),
as compared under the tolerable dosage. i.e. the targeting delivery
of LDM by an anti-gelatinase antibody fragment enhanced the
antitumor effects significantly. It was also noteworthy that the
enediyne-energized fusion protein dFv-LDP-AE was shown to be
significantly more effective than dFv-LDP or LDM used alone in
prolonging the survival of mice (P<0.05, Fig. 7B).
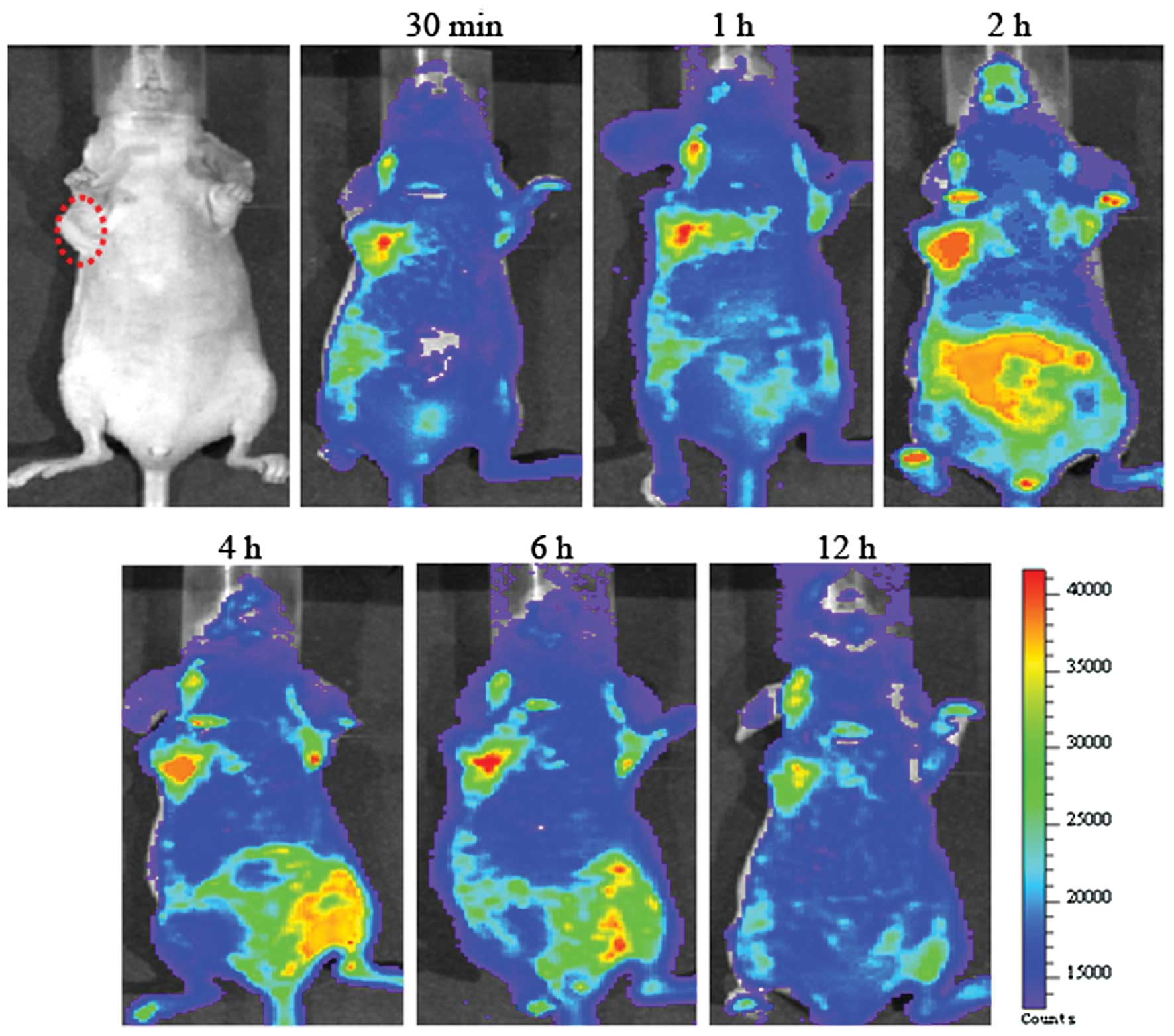
The small animal optical imaging using
FITC-labeled fusion protein
We previously reported the tumor targeting
capability of dFv-LDP to certain tumor cells and the injection dose
was 20 mg/kg. As the FITC is not a very good fluorescence in
imaging in vivo (17), its
wavelength is relatively short and it could not penetrate thick
tissue, in order to make the in vivo imaging much more
clear, the higher dose of 40 mg/kg FITC-labeled fusion protein
dFv-LDP was injected via tail vein. As shown in Fig. 8, we observed significant tumor
retention of FITC-labeled dFv-LDP in the tumor foci, the
FITC-labeled dFv-LDP saturated the tumor and its surrounding sites
within 30 min after injection. The signal of fluorescence was still
visible at the tumor site and maintaining the signal at 12 h after
administration. As we expected, the dFv-LDP demonstrated very good
tumor targeting potential, which was mainly located at tumor sites.
However, the question regarding the dFv-LDP with high liver and
kidney uptake remained unsolved, therefore causing a problem in the
systemic toxicity to normal organs.
Discussion
In this study, we demonstrate that the tandem
anti-gelatinase scFv format improved the biodistribution and
therefore enhanced the antitumor effects of LDM. By covalent
association of a tandem anti-gelatinase scFv to the N′ terminal of
LDM, the accumulation of LDM in the peritumor tissue could be
increased, and the tumor killing capability, the G2/M phase arrest
or the induction of apoptosis would be greatly enhanced. Therefore,
the inhibition rate of LDM and dFv-LDP-AE on tumor growth is
apparently improved from 73.6 to 89.5% in hepatoma 22 xenograft
tumor model and from 63.4 to 87.3% in hepatoma Bel-7402 xenograft
tumor model. The explanation for this difference is the tumor
targeting ability of the tandem scFv format. From the results of
the optical imaging, the degree and specificity of tumor
localization were due to dFv-LDP-AE, which was presumably as a
result of its affinity with gelatinase overexpression in tumor
sites.
Gelatinases are abundantly overexpressed in HCC and
its stromal cells, and play an important role in tumor
microenvironment (18,19). In this study, it found that the
‘naked’ fusion protein dFv-LDP had an inhibition rate of 38.8% at a
dosage of 0.8 mg/kg in Bel-7402 xenograft, which indicated that the
fusion protein dFv-LDP not only played as a carrier of AE, but also
possessed certain antitumor efficacy in vivo. This might be
attributed to the dual effects of dFv-LDP on tumor growth
inhibition and the disturbed balance of tumor microenvironment.
In the small animal optical imaging experiment, the
fast tumor location of dFv-LDP at 30 min after administration was
observed. When accumulation of dFv-LDP-AE in the target tissue is
achieved, therapeutic efficacy is displayed by a potent
tumor-killing power of AE. The rapid accumulation of dFv-LDP-AE in
tumor site indicated that the dFv-LDP-AE might not decompose or
decay before being delivered to the target site. Thus, the
dFv-LDP-AE effects could be internalization or display of a
‘bystander’ effect, killing nearby tumor cells in addition to the
directly targeted cells.
In summary, in this study we demonstrated that the
tandem format dFv-LDP-AE showed improved antitumor effect and
biodistribution compared to the LDM by increasing the targeting
delivery of LDM indicating that the gelatinases (MMP-2 and MMP-9)
are a valid target for antibody-directed therapy. Although, more
studies are warranted to further explore targeting ability and
decreasing the immunogenicity for the murine origin of the scFv
used, as well as lowering the kidney and liver adsorption.
Acknowledgements
This research was supported by grants from
‘Significant new drug development’ Science and Technology Major
Projects of China (2009ZX09301-003; 2009ZX09401-005;
2010ZX09401-407).
References
|
1
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edeline J, Raoul JL, Vauleon E,
Guillygomac'h A, Boudjema K and Boucher E: Systemic chemotherapy
for hepatocellular carcinoma in non-cirrhotic liver: a
retrospective study. World J Gastroenterol. 15:713–716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohr L, Yeung A, Aloman C, Wittrup D and
Wands JR: Antibody-directed therapy for human hepatocellular
carcinoma. Gastroenterology. 127:S225–S231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith LM, Nesterova A, Ryan MC, et al:
CD133/prominin-1 is a potential therapeutic target for
antibody-drug conjugates in hepatocellular and gastric cancers. Br
J Cancer. 99:100–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bjorklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
6
|
Svagzdys S, Lesauskaite V, Pangonyte D,
Saladzinskas Z, Tamelis A and Pavalkis D: Matrix
metalloproteinase-9 is prognostic marker to predict survival of
patients who underwent surgery due to rectal carcinoma. Tohoku J
Exp Med. 223:67–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KR, Bae JS, Choi HN, et al: The role
of serum response factor in hepatocellular carcinoma: an
association with matrix metalloproteinase. Oncol Rep. 26:1567–1572.
2011.PubMed/NCBI
|
|
8
|
Chen R, Cui J, Xu C, et al: The
significane of MMP-9 over MMP-2 in HCC invasiveness and recurrence
of hepatocellular carcinoma after curative resection. Ann Surg
Oncol. Jun 17–2011.(Epub ahead of print).
|
|
9
|
Alley SC, Okeley NM and Senter PD:
Antibody-drug conjugates: targeted drug delivery for cancer. Curr
Opin Chem Biol. 14:529–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao RG: Pharmacology and therapeutic
applications of enediyne antitumor antibiotics. Curr Mol Pharmacol.
1:50–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao RG and Zhen YS: Enediyne anticancer
antibiotic lidamycin: chemistry, biology and pharmacology.
Anticancer Agents Med Chem. 8:121–131. 2008.
|
|
12
|
Guo XF, Zhu XF, Shang Y, Zhang SH and Zhen
YS: A bispecific enediyne-energized fusion protein containing
ligand-based and antibody-based oligopeptides against epidermal
growth factor receptor and human epidermal growth factor receptor 2
shows potent antitumor activity. Clin Cancer Res. 16:2085–2094.
2010. View Article : Google Scholar
|
|
13
|
Xin C, Ye S, Ming Y, et al: Efficient
inhibition of B-cell lymphoma xenografts with a novel recombinant
fusion protein: anti-CD20Fab-LDM. Gene Ther. 17:1234–1243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Liu XJ, Hu L, et al: Factor VII
light chain-targeted lidamycin targets tissue factor-overexpressing
tumor cells for cancer therapy. Int J Mol Med. 29:409–415.
2012.
|
|
15
|
Zhong G, Zhang S, Li Y, Liu X, Gao R, Miao
Q and Zhen Y: A tandem scFv-based fusion protein and its
enediyne-energized analogue show intensified therapeutic efficacy
against lung carcinoma xenograft in athymic mice. Cancer Lett.
295:124–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang SH, Cheng X, Zhong GS, Xiong DS and
Shao RG: In vivo imaging analysis of biodistribution of
FITC-labeled Rituximab in lymphoma-bearing nude mice. Zhonghua Yi
Xue Za Zhi. 90:2367–2370. 2010.(In Chinese).
|
|
17
|
Hama Y, Urano Y, Koyama Y, et al: In vivo
spectral fluorescence imaging of submillimeter peritoneal cancer
implants using a lectin-targeted optical agent. Neoplasia.
8:607–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerg M, Kopitz C, Schaten S, et al:
Distinct functionality of tumor cell-derived gelatinases during
formation of liver metastases. Mol Cancer Res. 6:341–351. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Määttä M, Soini Y, Liakka A and
Autio-Harmainen H: Differential expression of matrix
metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in
hepatocellular and pancreatic adenocarcinoma: implications for
tumor progression and clinical prognosis. Clin Cancer Res.
6:2726–2734. 2000.
|