Introduction
Colorectal cancer (CRC) represents a significant
global health problem. It was estimated that 50% of people in the
western world will develop a colorectal adenoma by the age of 50,
with about one in ten patients progressing to malignancy (1). If be diagnosed at early stage,
non-metastatic CRC can be surgically resected with a favorable
prognosis. However, CRC metastases are associated with a poor
prognosis and are often refractory to chemotherapy. Since CRC has a
defined precursor cell population and exhibits slow progression to
metastasis, a successful cancer treatment strategy will have to be
based on the elimination of cancer stem cell (CSC) population.
Thus, identifying CSCs is the crucial first step in the treatment
of CRC.
CSCs were firstly found in acute myeloid leukemia
(AML) and later in other tumor types. They have been identified
through their expression of specific cell surface markers, such as
CD34+CD38− was associated with AML(2); CD44+CD24− was
for breast tumors(3); and
CD44+CD24−ESA+ was for pancreatic
tumors(4). One of the well
documented CSC markers is CD133. CD133+ population is
enriched in many tumor tissues including CRCs (5,6). On
the other hand, since a single CD44+ CRC cell can form a
sphere in vitro with stem cell features, and generate a
xenograft tumor in vivo with the properties of the original
tumor, CD44 was proposed as a robust marker for colon CSCs
(7,8). In addition, CD44 was also reported as
the marker for gastric cancer CSCs (9).
Another potential colon CSC marker is ALDH1, a
detoxifying enzyme that oxidizes intracellular aldehydes and
converts retinol to retinoic acid. Selection of CD133+,
CD44+ cells with ALDH activity enriched somewhat the CSC
population (10). However, either
CD133 and CD44 or their combination can be used effectively as a
marker for the identification of CSCs is still disputable (11). To assess whether CD44, CD133, or a
combination of CD44 and CD133 can represent CSCs of CRC, we studied
the expression pattern of popular markers on six CRC cell lines.
Among them, SW620 cells were classified into four subpopulations
based on the CD44 and CD133 expression. The capability of colony
formation, proliferation, apoptosis, drug resistance, as well as
the migratory and invasion potential of each subpopulation were
subsequently analized. Our data suggested that CD44 and CD133 or
their combination cannot universally be used to establish the
identity of the CSCs for all CRCs, but
CD44+CD133− seems likely to represents the
CSCs in SW620 cells.
Materials and methods
Cell lines and culture
Colon cancer cell lines, Colo205, DLD1, HCT116,
HT29, SW480 and SW620 originated from the American Type Culture
Collection (ATCC, Manassas, VA, USA), and cultured in DMEM
containing 10% FBS supplemented with 100 IU/ml penicillin and 100
μg/ml streptomycin. All culture reagents were from Invitrogen
(Carlsbad, CA, USA).
Western blot analysis
Cells were lysed on ice by mammalian protein
extraction reagent (ThermoFisher Scientific, Waltham, MA, USA) plus
protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA). After
removing insoluble debris by centrifugation at 16,000 × g for 30
min at 4°C, the supernatant was designated as whole cell lysate.
Protein concentrations were determined with Bradford method
(Bio-Rad, Hercules, CA, USA). Protein (40 μg) for each cell lysate
was separated by SDS-PAGE and transferred onto PVDF membranes
(Bio-Rad). Membranes were blocked with 5% dry milk in TBST and
immunoblotted with primary antibodies as follows: CD44, ESA
(eBioscience, San Diego, CA, USA), CD133 (Miltenyi Biotec, Auburn,
CA, USA) and ALDH1A1 (LifeSpan Biosciences, Seattle, WA, USA).
β-tubulin antibody was used for loading control. HRP conjugated
secondary antibodies (Jackson ImmunoResearch Laboratories, West
Grove, PA, USA) and enhanced chemiluminescence (Pierce, Rockford,
IL, USA) were used to detect the protein bands. Digital images of
luminescence were taken by IVIS system (Caliper Life Sciences,
Hopkinton, MA, USA).
Immunofluorescence assay
Cells (1−103) were planted onto 8-well
glass chamber slides (Fisher Scientific, Hampton, NH, USA) and
cultured for 24 h. After briefly rinsed with PBS twice, the cells
were fixed with 4% paraformaldehyde for 30 min and washed with PBS
three times. Then, the fixed cells were blocked with 10% normal
goat serum plus 1% BSA (Sigma-Aldrich) for 30 min, and incubated
with PE-conjugated CD133 (Miltenyi Biotec), FITC-conjugated CD44
and eFluor 660-conjugated ESA (eBioscience) for 1 h at 4°C in the
dark. Subsequently, the slides were cover slipped with mounting
medium (Dako) containing DAPI to counter stain the nuclei.
Flow cytometry analysis and isolation of
cell subpopulation
The expression profiles of CD133 and CD44 in
cultured cells were analyzed by flow cytometry. Briefly,
1−106 cells were incubated with 100 μl of 1% BSA in PBS
containing 1 μg of CD16/CD32 (eBioscience) for 30 min on ice to
block unspecific Fc interaction, then labeled with PE-conjugated
anti-CD133, FITC-conjugated anti-CD44 and eFluor 660-conjugated
anti-ESA antibodies for 1 h. Labeled cells were resuspended in PBS
with 1% FBS, and analyzed by flow cytometer (BD Biotechnology).
Isotypic IgG and unstained cells served as negative controls. By
using the same setup, CD44 and CD133 co-labeled SW620 cells were
sorted by FACS to obtain CD133+CD44+,
CD133+CD44−,
CD133−CD44+ and
CD133−CD44− subpopulations. Propidium iodide
(1 μg/ml) (PI, Invitrogen) was added into the suspension of cells
to exclude the dead cells during sorting.
Colony-formation and cell proliferation
assay
SW620, SW480 and their sorted cells were seeded to
96-well plates with an estimated single cell per well by limited
dilution. Fourteen days later, the colonies were counted under
phase contrast microscope. Cell proliferation rates were determined
with CyQuant cell proliferation assay kit (Invitrogen). Cells
(2,500) were seeded in 96-well plates with 200 μl growth medium per
well. Cells were cultured for up to 5 days. At each selected time
point started at 24 h, one plate was retrieved from incubator and
stored at −70°C after the removal of culture medium. Once all the
plates were collected, the cell numbers (total DNA) were
quantitated by following the manufacturer’s protocol.
Apoptosis assay based on caspase 3/7
activity
Aliquots of 1.5−104 sorted cells were
seeded on 96-well plates. After 24 h, 2 μM of camptothecin (CPT,
Sigma-Aldrich) was added as treatment groups, medium with vehicle
DMSO alone (Sigma) was also setup as the control group. After an
additional 48 h of incubation, cellular apoptotic activity was
assessed with caspase-Glo kit (Promega, Madison, WI) following the
recommended procedures of the manufacturer. The fold increase in
activity was calculated based on the normalized activity of
untreated cells. The basal level activity of different
subpopulation was also plotted. Each sample was measured three
times.
Tumor invasion and migration assay
These assays were performed by using BD BioCoat
Tumor Invasion System. FluoroBlok 24-Multiwell Insert Plate with an
8 μm pore size PET membrane was used for migration assay and the
same insert plate uniformly coated with BD Matrigel Matrix (BD
Biosciences) was used for the invasion assay. Sorted SW620 cell
subpopulations (1−106) in 500 μl serum-free medium was
seeded to the apical chamber, then 750 μl of chemoattractant (10%
FBS in DMEM) was added to each of the basal chambers. After 48
hours incubation, cells migrated to the lower chamber were stained
with 4 μg/ml calcein-AM in HBSS for 1 h at 37°C, 5% CO2.
Fluorescence intensity was quantified by a bottom-reading plate
reader. Insert membranes were also examined and fluorescent
pictures were taken under an inverted fluorescence microscope.
Statistical analysis
All of the data were analyzed with the GraphPad
Prism (GraphPad Software, Inc., La Jolla, CA). Results are
expressed as mean ± SD. Comparisons between two groups were
assessed by two-tailed Student’s t-test. Differences between groups
were considered significant with P<0.05. All experiments were
performed at least twice to confirm reproducibility.
Results
Expression profiles of CD133, CD44, ALDH1
and ESA in selected colon cancer cell lines
CD133, CD44, ESA and ALDH1 are widely considered as
markers of cancer stem/progenitor-like cells. To test this
hypothesis, western blotting was performed to analyze the
expression profiles of these proteins in six colon cancer cell
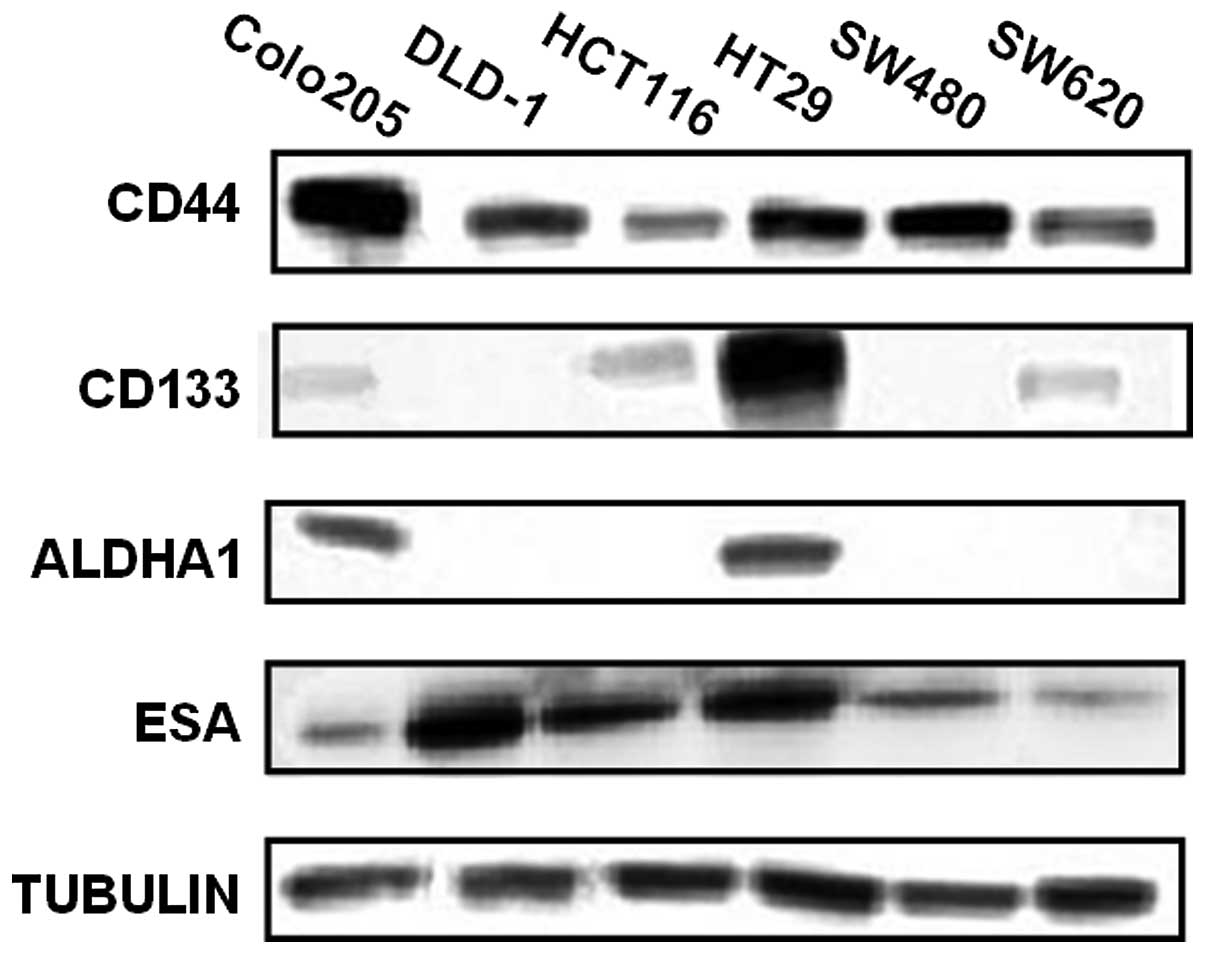
lines. As shown in Fig. 1, CD44 and
ESA were detected in all tested cell lines with different
expression levels; but CD133 was only detectable in Colo205,
HCT116, HT29 and SW620; and ALDH1A1 only in Colo205 and HT29.
Expression profiles of CD133 and CD44 by
flow cytometric analysis
The relative percentages of cells expressing each
single surface marker (CD133, CD44 and ESA) or multiple markers
were determined by flow cytometric analysis. All the cells in the
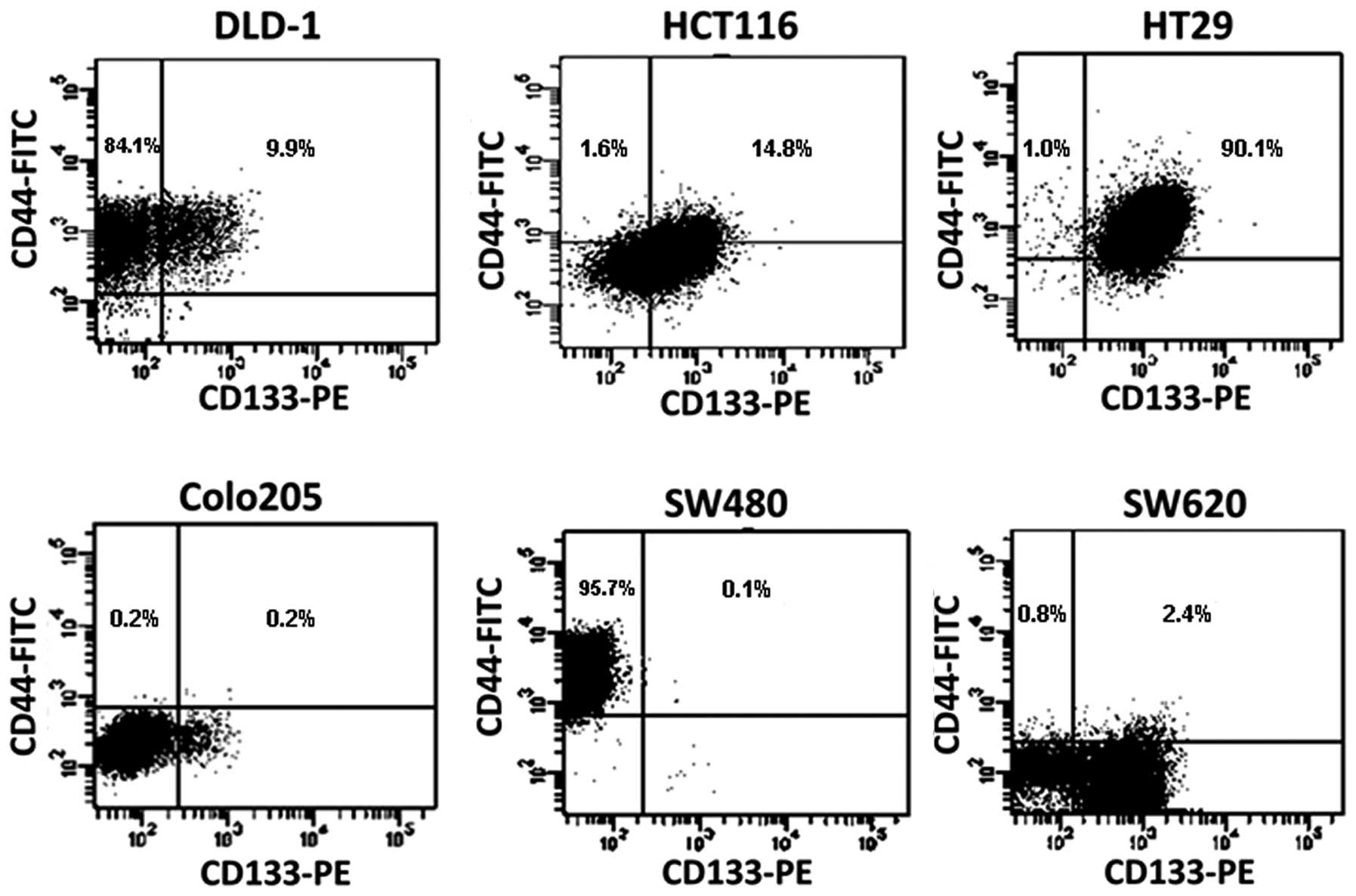
six cell lines were ESA positive (not shown). As shown in Table I, for single marker, ~94% of DLD1
cells were CD44 positive but only 12% were CD133 positive. By
comparison, 69.6% of HCT116 cells were CD133 positive, but only
16.4% were CD44 positive. In contrast, almost all HT29 cells
expressed these two surface markers. An interesting observation was
from SW480 and SW620 lines which derived from the same patient, as
the early stage cells, SW480 were almost all CD44 positive (95.8%),
while only a very small percentage (0.2%) of cells were CD133
positive. In the later stage invasive SW620 cells, CD133 positive
cells were around 60%, but only a small percentage (3.2%) was CD44
positive. These data suggest that ESA maybe a surface marker for
colon tumor cells, but cannot be a CSC marker. Since the majority
of cells express either CD133 or CD44 in many types of colon tumor
cells, it is unlikely that a single marker of CD133 or CD44 can be
used to distinguish CSCs.
 | Table IExpression of CD133+,
CD44+ and ESA+ in colon cancer cells. |
Table I
Expression of CD133+,
CD44+ and ESA+ in colon cancer cells.
| Colo205 | DLD1 | HT29 | HCT116 | SW480 | SW620 |
|---|
| CD133+
cells (%) | 6.90± 0.11 | 12.0±0.38 | 98.30±0.20 | 69.62±1.51 | 0.23±0.06 | 59.63±1.40 |
| CD44+
cells (%) | 1.63±0.23 | 94.0±0.21 | 91.1±0.20 | 16.4±0.69 | 95.8±0.17 | 3.22±0.63 |
| ESA+
cells (%) | 99.93±0.12 | 99.97±0.06 | 99.97±0.06 | 99.87±0.05 | 99.97±0.06 | 99.97±0.06 |
In contrast to high percentage of cells expressing
for single marker of CD44 or CD133, the percentage of cells with
co-expression of CD44 and CD133 markers on the same cell is lower
for most of cell lines, such as only 0.1% of SW480 cells, 0.2% of
Colo205 cells, 2.4% of SW620 cells expressed CD133 and CD44
simultaneously. However, they are still high in DLD1 (9.9%), HCT116
(14.8%) and especially for HT29 (90.1%) cell lines (Fig. 2). Of note, the percentage of
CD44+CD133− cells is also very small in four
of six cell lines, such as Colo205 (0.2%), HCT (1.6%), HT (1.0%)
and SW620 (0.8%). These data suggested that the minority of the CSC
subpopulation can be represented by the combination of CD133 and
CD44 markers, not necessary the positive co-expression of both
markers.
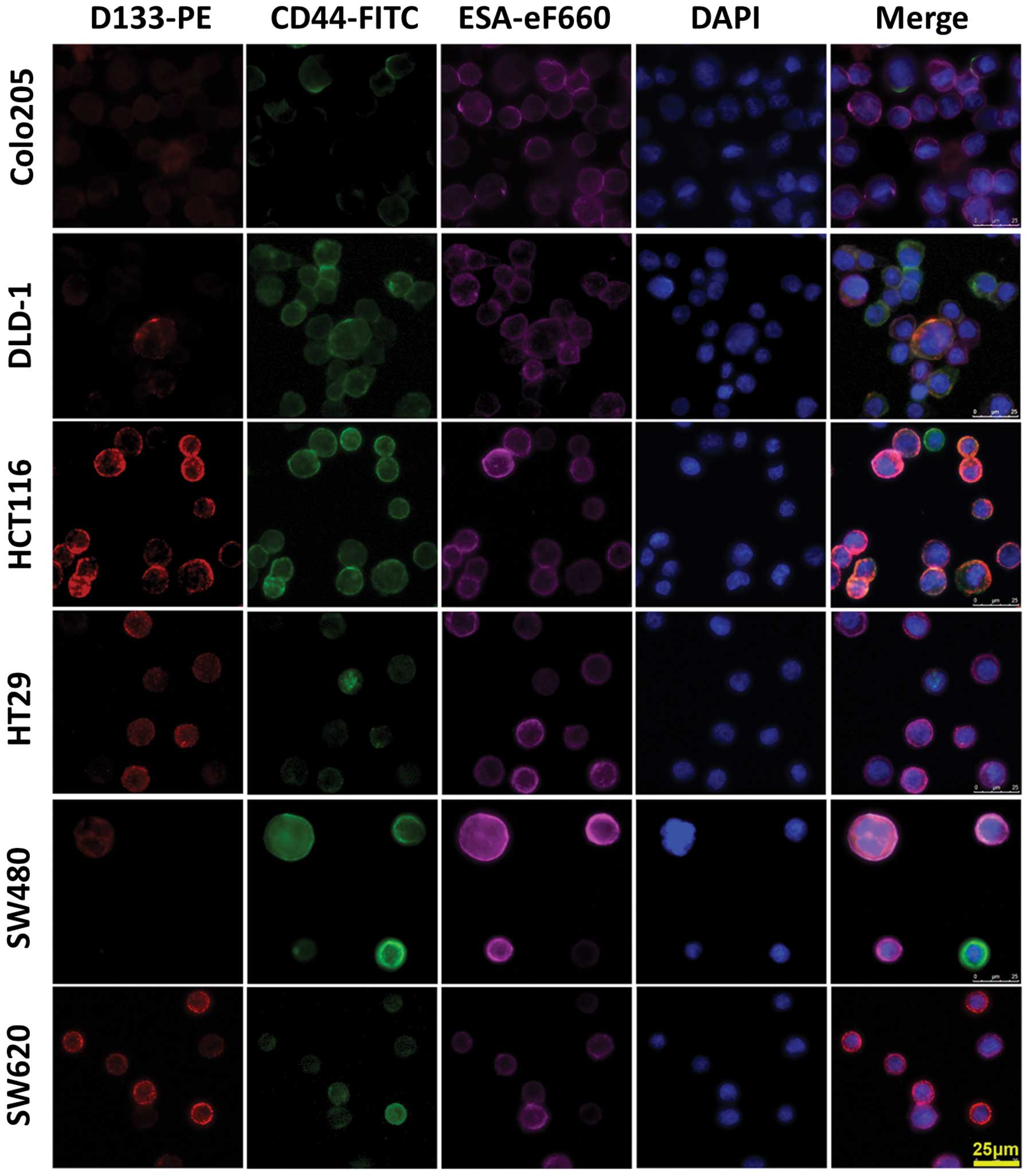
Immunofluorescence assay
To confirm the western blotting and flow cytometry
data, cells labeled with PE-conjugated CD133 antibody (red) and
FITC-conjugated CD44 antibody (green) were fixed, and the nuclei
were counter-stained with DAPI (blue). These cell samples were
examined under a fluorescence microscope. The fluorescent
distribution pattern in individual cell line from the
immunostaining with single antigen was consistent with the western
blotting data. Overlapped multicolor images of
triple-immunostaining comfirmed the existence of
CD44+CD133− and
CD44+CD133+ cells (Fig. 3).
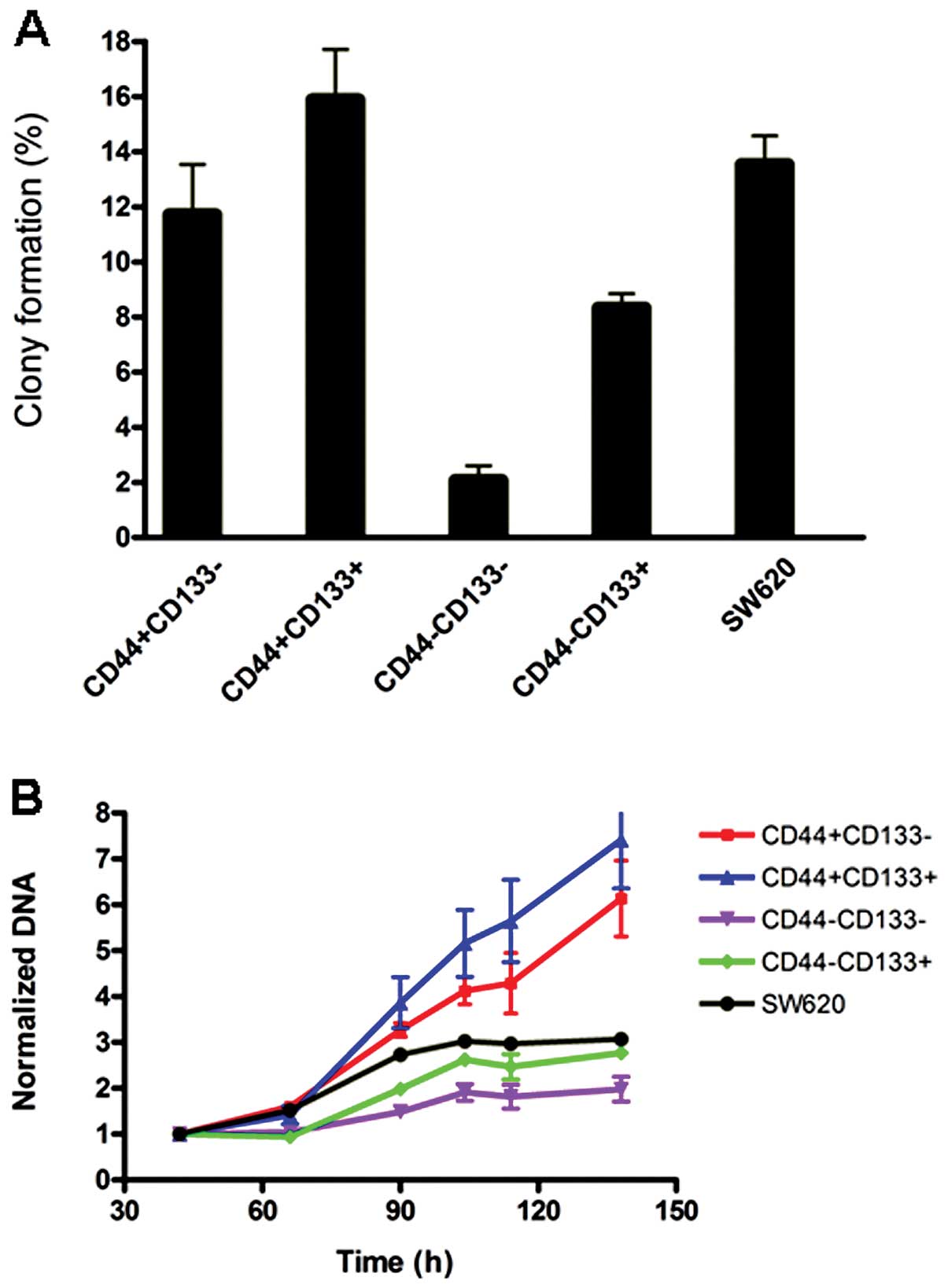
Evaluation of colony formation and cell
proliferation in different subpopulations
To exam the properties of cell subpopulations
expressing different surface markers, and explore whether the
combination of CD44 and CD133 can be used to identify CSCs from
certain colon cancer cells, SW620 cells were selected for further
characterization. SW620 cells were sorted into
CD44+CD133+,
CD44+CD133−,
CD44−CD133+ and
CD44−CD133− subpopulations by FACS. Then 200
μl of the suspension containing one single cell was planted into
each well of 96-well plates. The number of colonies formed were
counted after two weeks of culture. The results showed that
CD44+CD133+ cells have the strongest clone
forming capability among all subpopulations, and the
CD44−CD133− subpopulation produced the least
clones (Fig. 4A). The data for
unsorted control SW620 mixture were likely overestimated as
compared to sorted subpopulations, since sorting would damage
slightly the cells resulting in lower colony formation rate.
The relative proliferation rates of sorted cell
subpopulations from the SW620 line were analyzed by measuring the
total DNA content. As shown in Fig.
4B, all subpopulations only displayed slight differences in
growth rate, CD133+ CD44+ cells had the
fastest, and CD133− CD44− the slowest growth
rate. CD44 expression seems correlated with growth rate by the
comparison of the CD44+ and CD44− cells.
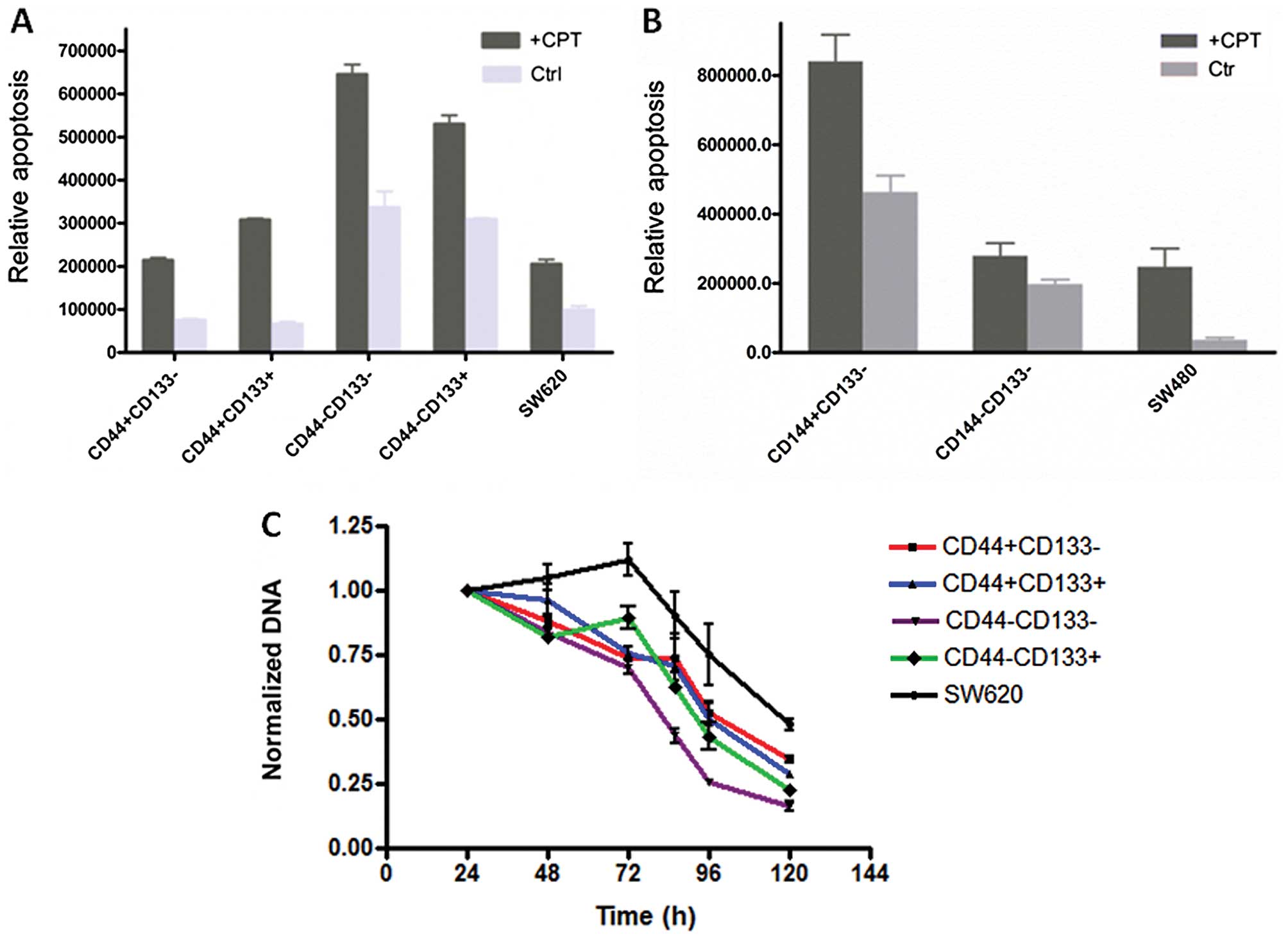
Spontaneous apoptosis and drug
resistance
CD44+ cells from SW620 had the least
spontaneous apoptosis, and were more resistant to the drug CPT
(Fig. 5A). SW480 cells seemed to
behave differently, a small portion of CD44 negative cells
undergoing a less spontaneous apoptosis, even though they were more
resistant to CPT, similar to the corresponding subpopulations of
the SW620 line (Fig. 5B).
Considering that the SW620 line is derived from the SW480 line,
these data suggested either CD44 or CD133 alone has no direct
correlation to their capability of drug resistance or to survive.
It is also worth to note that unsorted cells of SW620 and SW480
lines showed a relatively low apoptosis rate, suggesting that cells
with different markers support each other with respect to growth.
To further test this, the proliferation profiles of sorted SW620
subpopulation cells were analyzed under the stress of drug
treatment. As shown in Fig. 5C, the
drug has the greatest impact on the growth of
CD44−CD133− cells, and the least on
CD44+CD133− and unsorted SW620 cells. These
data are consistent with the results of the apoptotic assay.
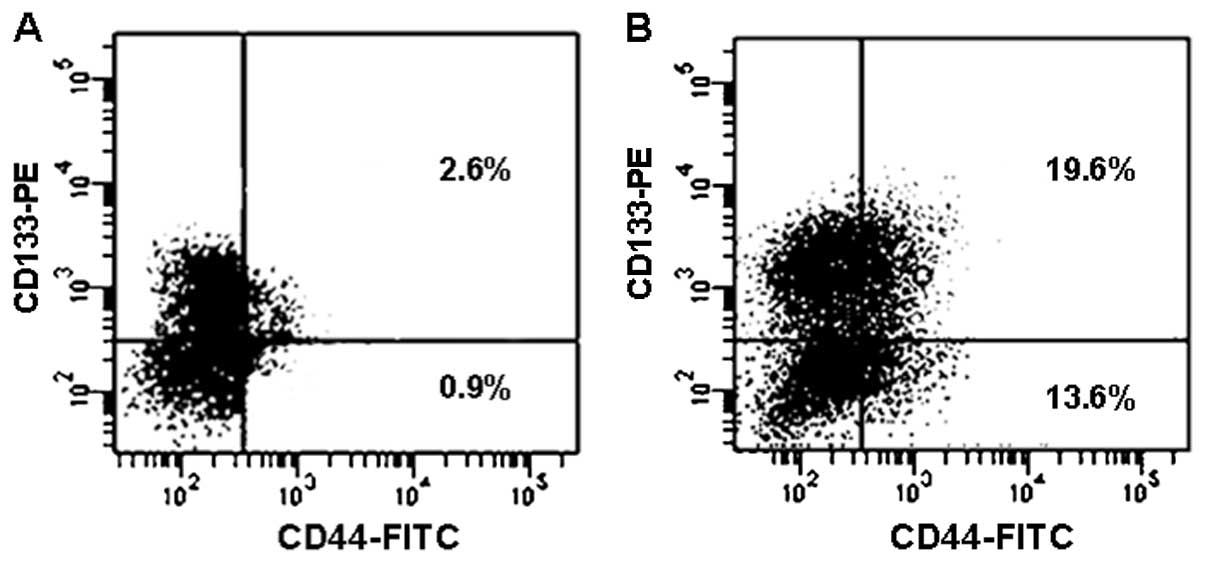
Next the changes of surface markers were examined
for the viable cells after CPT treatment. As shown in Fig. 6, the percentage of CD44+
cells in the unsorted SW620 cells were increased by 24 h of
treatment with 2 μM CPT; CD44+CD133+ cells
showed increases from 2.6 to 19.6%. However, percentagewise,
CD44+CD133− increased the most from 0.9 to
13.6%.
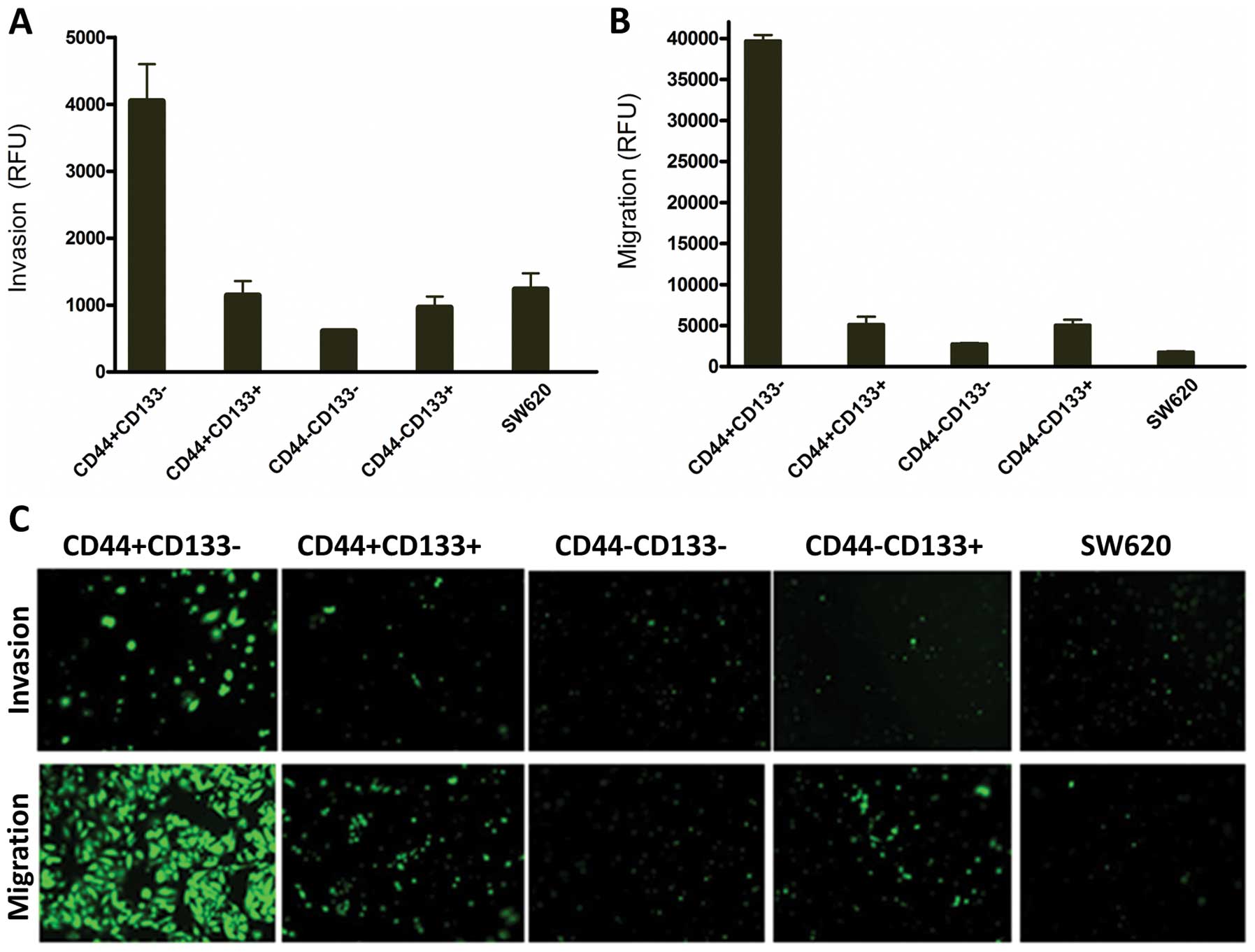
CD44+CD133−
subpopulation has the strongest invasion and migration
capability
The sorted SW620 cells with
CD44+CD133−,
CD44+CD133+,
CD44−CD133− and
CD44−CD133+ phenotypes and unsorted SW620
cells were allowed to invade for 40 h using the BD Matrigel
Invasion Chamber assay. As shown in Fig. 7A, CD44+CD133−
cells were 6-fold more invasive than
CD44−CD133− and 3-fold more than the unsorted
cells. The CD44−CD133− and
CD44−CD133+ were weakly invasive and only
scare spots of fluorescence were observed under the inverted
fluorescent microscope (Fig. 7C).
These data suggest CD44+CD133− cells were the
most invasive ones. Migration study conducted by BD Matrigel
Chamber without coated membranes showed that the CD44-CD133- cells
and unsorted cells were weakly migratory, while the
CD44+CD133− cells had the strongest migration
capability; more precisely, CD44+CD133− cells
had a 14-fold higher migration rate than those of
CD44−CD133− cells as measured by fluorescence
intensity (Fig. 7B). These data
were confirmed by visual examination under an inverted fluorescent
microscope (Fig. 7C).
Discussion
CRC is the second leading cause of cancer-related
mortality in developed countries. It is believed that cancers are
developed from, and are maintained by CSCs arising from a resident
normal stem/progenitor cell within the tissue bearing the
characteristics of malignancy. A number of studies have
demonstrated the existence of CSCs in CRC tissues (5,6). These
CSCs have been characterized by their expression of specific cell
surface biomarkers, such as CD133, CD44. CD44 is a transmembrane
glycoprotein involved in cell adhesion, migration and drug
resistance.
CD133 is a membrane protein originally classified as
a marker of primitive hematopoietic and neural stem cells. CD133
was suggested to play a role in tumor angiogenesis as
CD133+ glioma cells produce proangiogenic factors that
can directly modify endothelial cell behavior (12). CD133 is recognized as a CSC marker
in brain, colon, melanoma, bone sarcomas, non-small cell lung
cancer, and other solid tumors (5,6,13–16),
but this notion was challenged by studies from other groups. CD133
was found widely distributed in many epithelial tissues, and CD133
expression does not correlate with the ability of colon tumors to
metastasize, as 40% human CRCs that metastasized to the liver are
CD133 negative (11). CD133
expression can also be modulated by oxygen levels (17). Therefore, CD133 as CSC marker need
to be re-evaluated and additional CSC surface markers maybe
involved in maintaining CSC properties (18). To evaluate whether CD44, CD133 or
combination of both can represent CSCs of CRC, we analyzed their
protein expression levels in several CRC lines by western
blotting.
As shown in Table I,
CD44 and ESA are relatively highly expressed in all cell lines,
suggesting that they are a common denominator but cannot be the
sole CSC markers in CRCs. CD133 had the highest protein expression
in HT29 cells; the percentage of cells expressing each marker is
loosely related to total protein level. Such as HT29 had a higher
CD133 protein level than HCT116 cells, the percentage of CD133
positive cells was also higher in HT29 than in HCT116 cells (98%
vs. 70%). The total protein level of CD133 is similar in Colo205
and SW620, but the percentage of CD133 positive cells in SW620 is
much higher than those in Colo205 (60% vs. 7%). These data suggest
that it is unlikely that CD133 alone can be a useful marker for
CRCs, at least not for all types of colon cancer cells.
Subsequently, the CD44/CD133 co-expression profiles
of these CRCs were analyzed by flow cytometry. The co-expression of
CD44+CD133+ cells represented a smaller
percentage, especially in SW480 (0.1%) and SW620 (2.4%) cell lines
(Fig. 2); but it is still very high
in DLD1, HCT116 and HT29 cell lines. Thus, these data indicate that
the co-expression of CD44 and CD133 was not a minority of CSC
subpopulation for half of cells we tested. However,
CD44+CD133− cells represent a minority in
four of six cell lines. To examine the possibility that the
combination of CD44 and CD133 can still represent the marker for
some colon cancer stem cells, the SW620 cell line, which showed
four defined subpopulations for CD44 and CD133 expression, was
selected for further characterization. The SW620 line is a
metastatic counterpart of the non-metastatic SW480 line, and both
cell lines were derived from a colon carcinoma of the same patient
(19). SW620 cells were sorted into
four subpopulations (CD44+CD133+,
CD44+CD133−,
CD44−CD133+ and
CD44−CD133−). Their capability of colony
formation, proliferation rate, spontaneous apoptosis, drug
resistance, as well as their migratory and invasion rates were
analyzed and compared.
For colony formation, as shown in Fig. 4, the double positive lines had the
highest and double negative the lowest capability while the pair of
CD44 positive subpopulations had the greater colony capability
compared to the double negative pair. The similar pattern was also
observed in the proliferation assay (Fig. 4B). These results suggest CD44
expression plays a major role in the CRC colony formation and
proliferation. The data are supported by a previous study involving
60 patients, in which it was shown that CD44 and CD133 positive
cells did not co-localize within colorectal cancer.
CD44+ cells can effectively form a sphere in
vitro and initiate a tumor in vivo. Knockdown of CD44,
but not CD133, prevented colony formation and inhibited
tumorigenicity in a xenograft model (8).
In addition to robust cell growth, CD44+
cells had less spontaneous apoptosis and were more resistant to
drug induced cell death (Fig. 5A).
This is consistent with results from breast cancer cells, in which
CD44 positive cells were shown to express higher levels of the
anti-apoptotic protein Bcl-2 (20).
It was reported that CD44 is involved in drug resistance and
metastasis in prostate cancer (21). Overall, the mixed cells have less
spontaneous apoptosis and SW620 is more resistant to CPT induced
apoptosis than SW480 (compare last column in Fig. 5A and B). This is consistent with the
data produced using cisplatin (22). In vitro, SW620 cells were
also more irradiation and chemotherapy resistant (23). The mechanism of CD133+
cell resistance to apoptosis is believed to be due to the
production of interleukin-4 (IL-4) (24).
Surviving cells from drug treatment show only minor
change on CD44 expression, but the CD133 expression fluctuates
dependent on the CD44 expression on the same cell (data not shown).
Overall, the CD44+ cells (both
CD44+CD133+ and
CD44+CD133− cells) were enriched after drug
treatment (Fig. 6). This behavior
is different for the colon cancer cell lines HT29 and Caco2, which
were reported in CD44 and CD133 reduced after treatment with sodium
butyrate (25). It is conceivable
that these changes may well be cell line and drug dependent. To
date, either CD44+CD133+ or
CD44+CD133− SW620 cells show characteristics
of CSCs. However, the data showed that
CD44+CD133− are more migratory and invasive
in the Matrigel-based assay. It appears that when CD44 is positive,
CD133 plays a negative role, and when CD44 is negative; CD133 plays
a positive role (Fig. 7). This
finding is consistent with the general role of CD44. The CD44
receptor is well documented to interact with the P-glycoprotein to
promote cell migration and invasion in cancer (26) and claimed as the invasive marker of
glioma (27). A clinicopathological
analysis in 189 consecutive CRC patients showed that CD133 was only
detected in 29 tumors (15.3%). There was no difference in the
distribution of CD133 expressing cells between the invasive and
surface area. It was concluded that CD133 expression does not play
a dominant role in CRC migration and invasion (28).
In two other clinical studies, CD44s expression
reflected more the aggressiveness of the primary tumor (29) and the depth of invasion (30). In addition, the positive correlation
of CD44 with metastasis was also demonstrated in prostate CSCs,
which showed that cells with enhanced clonogenic, tumor-initiating
and metastatic capacities were enriched in the CD44+
cell population. CD44 knockdown inhibited prostate cancer
regeneration and metastasis (31).
Based on above reports and our present data, CD44 is a relatively
more robust marker for colorectal CSC, and
CD44+CD133− is more likely the CSC maker for
SW620 cells. However, since the more invasive SW620 has less
CD44+ cells than SW480, this suggests that CD44 cannot
be the sole determinant of cell migration and invasion.
CD44 was reported as a metastasis suppressor for
some prostate cancers, and the expression of the standard form of
CD44 decreased during the progression of prostate cancer to a
metastatic state (32). CD44 also
plays a similar negative role in pancreatic cancer as its
progression was accompanied by an almost complete loss of CD44
expression, due to alternative splicing of the CD44 pre-RNA. The
extracellular matrix can influence the expression of CD44 isoforms
and thereby may facilitate tumor invasion (33). This may also be the case for some
CRC, as the transition from SW480 to SW620 preceded with a decrease
in standard form of CD44 expression, an isoform of CD44 could be
expressed on SW620 cells or SW620 has a CD44 with different
post-translational modification (such as the glycosylation).
Overall, there is a wide range of expression of both
CD44 and CD133 in CRC cells. The data from expression profiling
suggest that CD44 or CD133 alone is likely not enough to establish
the identity of the CSCs for CRCs. The combination of CD44 and
CD133 correlated with more features of CSCs, but they cannot be
generalized and applied for all colon cancer cells. CSC surface
markers vary depending on the individual source and the stage of
colon cancers. For SW620 cells, CD44+CD133−
correlated with most of features proposed for CSCs, such as
minority, drug resistance and invasion capability. Coincidentally,
the majority of SW480 cells, the non-metastatic precursor cells of
SW620, have the CD44+CD133− surface marker,
therefore, CD44+ and CD133− are not the only
determinants of CSCs for these cancer cells. It is possible that
there are additional CSC surface markers involved in maintaining
CSC properties, or alternatively, the CSC hypothesis may not be a
universal model that can be applied to all cancers or all patients
with the same disease.
Acknowledgements
This study was supported by a funding from the
National Cancer Institute (1P50CA128323, U24CA126588).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CSCs
|
cancer stem cells
|
|
ESA
|
epithelial specific antigen
|
|
ALDH1
|
aldehyde dehydrogenase 1
|
|
FACS
|
fluorescence activated cell
sorting
|
|
CPT
|
camptothecin. SDS-PAGE, sodium dodecyl
sulfate polyacrylamide gel electrophoresis
|
|
PVDF
|
polyvinylidene difluoride
membranes
|
|
HRP
|
horse- radish peroxidase
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
DMSO
|
dimethyl sulfoxide
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium
|
|
BSA
|
bovine serum albumin
|
References
|
1
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
6
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalerba P, Dylla SJ, Park IK, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du L, Wang H, He L, et al: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang EH, Hynes MJ, Zhang T, et al:
Aldehyde dehydrogenase 1 is a marker for normal and malignant human
colonic stem cells (SC) and tracks SC overpopulation during colon
tumorigenesis. Cancer Res. 69:3382–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both
CD133+ and CD133− metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|
|
12
|
Bao S, Wu Q, Sathornsumetee S, et al: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al Dhaybi R, Sartelet H, Powell J and
Kokta V: Expression of CD133+ cancer stem cells in
childhood malignant melanoma and its correlation with metastasis.
Mod Pathol. 23:376–380. 2010.
|
|
14
|
Artells R, Moreno I, Diaz T, et al: Tumour
CD133 mRNA expression and clinical outcome in surgically resected
colorectal cancer patients. Eur J Cancer. 46:642–649. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baba T, Convery PA, Matsumura N, et al:
Epigenetic regulation of CD133 and tumorigenicity of
CD133+ ovarian cancer cells. Oncogene. 28:209–218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tirino V, Desiderio V, Paino F, et al:
Human primary bone sarcomas contain CD133+ cancer stem
cells displaying high tumorigenicity in vivo. FASEB J.
25:2022–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griguer CE, Oliva CR, Gobin E, et al:
CD133 is a marker of bioenergetic stress in human glioma. PLoS ONE.
3:e36552008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bidlingmaier S, Zhu X and Liu B: The
utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J Mol Med. 86:1025–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Futschik M, Jeffs A, Pattison S, et al:
Gene expression profiling of metastatic and nonmetastatic
colorectal cancer cell lines. Genome Lett. 1:26–34. 2002.
View Article : Google Scholar
|
|
20
|
Madjd Z, Mehrjerdi AZ, Sharifi AM,
Molanaei S, Shahzadi SZ and Asadi-Lari M: CD44+ cancer
cells express higher levels of the anti-apoptotic protein Bcl-2 in
breast tumours. Cancer Immun. 9:42009.
|
|
21
|
Hao JL, Cozzi PJ, Khatri A, Power CA and
Li Y: CD147/EMMPRIN and CD44 are potential therapeutic targets for
metastatic prostate cancer. Curr Cancer Drug Targets. 10:287–306.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huerta S, Harris DM, Jazirehi A, et al:
Gene expression profile of metastatic colon cancer cells resistant
to cisplatin-induced apoptosis. Int J Oncol. 22:663–670.
2003.PubMed/NCBI
|
|
23
|
Kawamoto H, Yuasa T, Kubota Y, et al:
Characteristics of CD133(+) human colon cancer SW620 cells. Cell
Transplant. 19:857–864. 2010.
|
|
24
|
Todaro M, Alea MP, Di Stefano AB, et al:
Colon cancer stem cells dictate tumor growth and resist cell death
by production of interleukin-4. Cell Stem Cell. 1:389–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haraguchi N, Ohkuma M, Sakashita H, et al:
CD133+CD44+ population efficiently enriches
colon cancer initiating cells. Ann Surgical Oncol. 15:2927–2933.
2008.
|
|
26
|
Miletti-Gonzalez KE, Chen S, Muthukumaran
N, et al: The CD44 receptor interacts with P-glycoprotein to
promote cell migration and invasion in cancer. Cancer Res.
65:6660–6667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiranowska M, Ladd S, Smith SR and
Gottschall PE: CD44 adhesion molecule and neuro-glial proteoglycan
NG2 as invasive markers of glioma. Brain Cell Biol. 35:159–172.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: a clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bendardaf R, Algars A, Elzagheid A, et al:
Comparison of CD44 expression in primary tumours and metastases of
colorectal cancer. Oncol Rep. 16:741–746. 2006.PubMed/NCBI
|
|
30
|
Huh JW, Kim HR, Kim YJ, et al: Expression
of standard CD44 in human colorectal carcinoma: association with
prognosis. Pathol Int. 59:241–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu C, Kelnar K, Liu B, et al: The
microRNA miR-34a inhibits prostate cancer stem cells and metastasis
by directly repressing CD44. Nat Med. 17:211–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao AC, Lou W, Dong JT and Isaacs JT: CD44
is a metastasis suppressor gene for prostatic cancer located on
human chromosome 11p13. Cancer Res. 57:846–849. 1997.PubMed/NCBI
|
|
33
|
Abetamann V, Kern HF and Elsasser HP:
Differential expression of the hyaluronan receptors CD44 and RHAMM
in human pancreatic cancer cells. Clin Cancer Res. 2:1607–1618.
1996.PubMed/NCBI
|