Introduction
Dopaminergic neurons play a crucial role in a
variety of brain functions such as voluntary movement which is
severely affected in Parkinson’s disease (PD), a common
neurodegenerative movement disorder. Metabolic stress has been
identified as an important trigger factor for the neurodegenerative
process of PD. In particular, it has been consistently found that
the activity of mitochondrial respiratory chain complex I (CXI) is
reduced 40% in PD patients. In this context of metabolic
dysfunction in PD, ATP-sensitive potassium (KATP)
channels are of special interest, because their open probability
directly depends on the metabolic state of a cell (1). KATP channels are closed at
high ATP-to-ADP ratios and open in response to decreased ATP and
increased ADP levels. By this mechanism, KATP channel
activity exerts a powerful control mechanism of cellular
excitability and affect the cell’s physiologic activity. However,
for the common form of sporadic PD, it remain unclear if the
metabolic dysfunction could contributed to its development through
KATP channel.
Rotenone, pesticide and toxin that inhibit complex
I, results in selective DA degeneration (1–3).
Studies in vivo have shown that chronic, systemic
administration of rotenone produces dopaminergic degeneration and
Lewy body-like cytoplasmic inclusions, which closely mimic the
pathology of PD (1). Rotenone
treatment also functions as an effective PD model in vitro,
resulting in toxicity to dopaminergic cells (4). Partial inhibition of complex I by
rotenone has been shown to increase mitochondrial production of ROS
(5,6), which may be the precipatory event in
toxicity models. However, the basis for rotenone-induced selective
toxicity to dopaminergic neurons remains ambiguous.
Recent evidence suggests that the increased
oxidative stress within dopaminergic neurons, due to dopamine (DA)
metabolism and oxidation, combined with a complex I
inhibition-induced ROS production may lead to cell death by
overloading the oxidative capacity of dopaminergic cells. Liss
et al suggest that the KATP channels is related
with the selective death of dopaminergic cells in the midbrain, but
the mechanism remained unclear (7).
Consequently, we wanted to know what is the relationship between DA
metabolism and the KATP channels. We supposed that
rotenone, as complex I inhibition in PD model, affect DA metabolism
and oxidation, which associate with KATP channel
activity and then contribute to the dysfunction of the dopaminergic
cells. Therefore, in this study we sought to investigate whether
tyrosine hydroxylase (TH), the rate-limiting enzyme (8) of DA synthesis, was involved in open
probability of KATP channel in PC12 cells induced by
rotenone. We found that in PC12 cells rotenone treatment enhanced
KATP channel opening and decreased the expression of TH
which could be inversed by the KATP channel inhibitor
glibenclamide. Thus, the present study delivers important new
insights into the molecular pathways that may contribute to one of
pathological mechanism on dopaminergic degeneration in dopaminergic
neurons.
Materials and methods
Materials
PC12 cells were obtained from the Cell Bank in the
Chinese Academy of Sciences (Shanghai, China). DMEM/F12 culture
medium and fetal bovine serum (FBS) were purchased from Gibco-BRL
(Rockville, MD, USA); RNA extraction reagent (TRIzol), RNA reverse
transcription reagent and PCR expansion reagent kit were from
Takara Biotechnology Co., Ltd. (Dalian, China). Protein lysis
buffer, rabbit anti-β-actin were from CST Co. (Danvers, MA, USA).
Rabbit anti-TH was from Millipore Co. (Billerica, MA, USA). Goat
anti-Kir6.2 was from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). ATP Assay kit was from Beyotime Institute of Biotechnology
(Jiangsu, China). Mn (III) TBAP was from Biosense Laboratories AS
(Bergen, Norway). All the other chemicals, including rotenone,
3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT),
lipophilic cationic dye JC-1, PI and fluorescent
Ca2+-indicator dye were obtained from Sigma (St. Louis,
MO, USA).
Cell culture and treatment
PC12 cell line were cultured in a DMEM/F12 medium
containing 10% inactivated FBS, 100 U/ml penicillium and 100 mg/l
streptomycin at the conditions of 37°C and 5% CO2.
Pancreatin (0.125%) was used to digest for passage. Logarithmic
phase cells were used for all the experiments. For the drug
treatment, the cells were inoculated in a culture plate or a
culture flask for 12 h, and then added with the corresponding
drugs, while the blank control group was only added with the
culture solution of the same volume.
Cell viability measurement
Cell viability was assessed by the MTT assay
(9). Briefly, PC12 cells were
seeded in 96-well plates (2×103 cells/well) and then the
rotenone treatment was administered. After treatment, cells were
washed with PBS and incubated with MTT (5 mg/ml) in culture medium
at 37°C for another 3 h. Then formazan blue, which formed in cells,
was solubilized in 100 μl of DMSO. The absorption values were
measured at a wavelength of 490 nm using a Sunrise Remote
Microplate Reader (Grodlg, Austria). The viability of PC12 cells in
each well was presented as the percentage of control cells.
Confocal fluorescence microscopy on
Kir6.2 co-localization
After cells were cultured in culture dishes for 24
h, they were washed with ice-cold PBS and fixed in PBS-buffered 4%
paraformaldehyde at room temperature for 20 min. Then, cells were
washed with PBS and blocked with 10% horse serum for 10 min and
incubated overnight with the goat anti-Kir6.2 antibodies (1:100) at
4°C. Thereafter, the cells were washed with PBS and incubated with
fluorescein isothiocyanate (FITC)-labeled rabbit anti-goat IgG at
37°C for 1 h, PI for 1 min. Finally, cells were observed under a
confocal fluorescence-microscope system (TCS SP-2, Leica).
Detection of the KATP opening
by patch clamp technique
The treated PC12 cells were digested by 0.125%
pancreatin until deformed. Then the cells were washed and resuspend
with the extracellular solution, and dripped on the slides. Within
20 min, the cells sunk and stuck to the slides and were prepared
for patch clamp experiments. The whole-cell recording mode was used
to detect the KATP opening state with patch clamp
amplifier EPC-10 (HEKA, Germany) and the micropipette puller
PUL-100 (WPI, Worcester, MA, USA). Cells of middle size and with
very smooth outline were used. The intracellular solution
(potassium gluconate 140 mM, KCl 10 mM, MgCl2 5 mM, EGTA
0.5 mM, ATP 0.5 mM, HEPES 10 mM, pH 7.2 with Tris-OH) was used.
While the extracellular solution included NaCl 150 mM, KCl 5 mM,
MgCl2 1 mM, glucose 10 mM, HEPES 10 mM, CaCl2
2 mM, pH 7.4 (with Tris-OH) (10).
All experiments were performed at room temperature. The pClamp 6.01
procedure was used to collect and analyze the data.
Intracellular calcium ion, ROS, ATP
detection
When detecting the alteration of intracellular
Ca2+ concentration in PC12 cells induced by rotenone,
the cells were treated in the same way as described above and added
with the Fluo-3-AM probe (75 mM) for 1 h at 37°C, then the cells
were collected and the fluorescence intensity detection was
conducted by a FACSVantage SE flow cytometer at an excitation
wavelength of 355 nm and an emission wavelength of 485 nm.
When intracellular ROS production in PC12 cells
induced by rotenone was detected, the cells were washed by PBS and
fluorescent probe DCFH-DA diluted with serum-free culture medium
(1:5,000) and added in culture medium. After being cultured for 1
h, the cells were collected and the fluorescence intensity
detection was conducted by flow cytometry.
The alteration of intracellular ATP concentration in
PC12 cells treated with rotenone was detected by the method of
luciferase bioluminescent according to the kit instruction of the
manufacturer and tested by the Lmax II Luminometer (Molecular
Devices, Sunnyvale, CA, USA).
Cell culture medium pH detection
The pH of cell culture medium was measured at room
temperature by pH meter (pHS-3D, Shanghai, China).
Mitochondrial transmembrane potential
detection by flow cytometry
The changes of mitochondrial transmembrane potential
in PC12 cells were detected by flow cytometry. Briefly, the cells
were stained with JC-1 (20 μg/ml) for 20 min at 37°C. The JC-1
fluorescence intensity on cells was measured by a FACSVantage SE
flow cytometer with an excitation wavelength of 490 nm and an
emission wavelength of 527 nm.
Preparations of RNA extraction and
reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from 1×106 treated
PC12 cells using cold TRIzol reagent and RNA (500 ng) was used to
RT-PCR with RT reagent Kit according to the manufacturer’s
protocol. The primers of Kir6.2 used for PCR were: sense,
5′-CCGCCAGCTTGATGA GGAC-3′, and antisense, 5′-GGACCGCAACTCAGGACA
AG-3′, with product of 146 bp. The PCR amplification method to
detect Kir6.2 among the samples was set as follows: pre-denatured
at 94°C for 4 min, 35 cycles × 30 sec at 94°C, 30 sec at 55°C and
30 sec at 72°C. PCR products were detected by 2% agarose gel
electrophoresis. TH and β-actin mRNA were detected by qRT-PCR with
the following primers: for TH: sense, 5′-AGGGCTGCTGTCTTCCTAC-3′ and
antisense, 5′-GCTGTGTCTGGGTCAAAGG-3′; for β-actin: sense,
5′-AGGCCAACCGTGAAAAGATG-3′ and antisense, 5′-AC
CAGAGGCATACAGGGACAA-3′; with the product of 81 and 88 bp,
respectively. The reaction system contains 5 μl SYBR-Green Mix,
sense and antisense primers each 30 μM, cDNA 1 μl, and RNA-free
H2O supplied to 10 μl. Real-time PCR parameters were: 3
min at 95°C, 35 cycles × 10 sec at 95°C, 20 sec at 55°C, then
65–95°C for dissolved curve. The amount of TH mRNA was normalized
by β-actin mRNA levels as the endogenous reference and relative to
the control is then given by 2−ΔΔCt.
TH protein expression by western blot
analysis
The treated cells were collected and broken down by
CST Lysis Buffer (including 10% Ser/Thr inhibitor, 10% Tyr
inhibitor and 10% PMSF), then centrifuged in 12,000 × g for 15 min
at 4°C, the supernatant was collected, 5X protein loading buffer
was added and boiled for 10 min. After SDS-PAGE, the proteins were
transferred to the PVDF membrane. Membranes were washed in water 2
times, and blocked with 5% BSA which contained 0.05% Tween-20 for 1
h, then incubated overnight in primary antibody (diluted with 5%
BSA of 1:2,000) at 4°C. Membranes were washed with TBST 3 times,
and then incubated in the corresponding horseradish peroxidase
(HRP)-conjugated secondary antibody (diluted with 5% BSA of
1:3,000) for 1 h at ambient temperature. The ECL chemiluminescent
method was used to observe the results and then analyzed in the
ChemDoc imager (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Each batch of experiments were repeated for at least
3–5 times. Statistical analyses were performed using the SPSS 10.0
package (SPSS Inc., Chicago, IL, USA). Data were expressed as the
mean ± SD of 3–5 independent experiments. ANOVA and Student’s
t-test were performed to determine the statistical significance.
Differences between groups were considered to be significant at
P<0.05.
Results
Functional KATP channels exist
on the outer membrane of the PC12 cells
The results on functional KATP
localization on PC12 is contradictory, some researchers suggest
functional KATP exist on the outer membrane of the PC12,
but others showed contrary results (11–13).
Therefore, we first ascertained whether functional KATP
channels exist in PC12 cells. In neural and dopaminergic cells the
KATP channel usually is transcribed by SUR1/Kir6.2 gene
which is important for the physiology of dopaminergic cells
(14,15), we detected Kir6.2 expressed at mRNA
levels on PC12 cells by RT-PCR methods.
While we detected the expression of Kir6.2 mRNA in
PC12 cells, as a positive or negative control, we also tested the
expression of Kir6.2 in rat cardiac muscle and small intestine
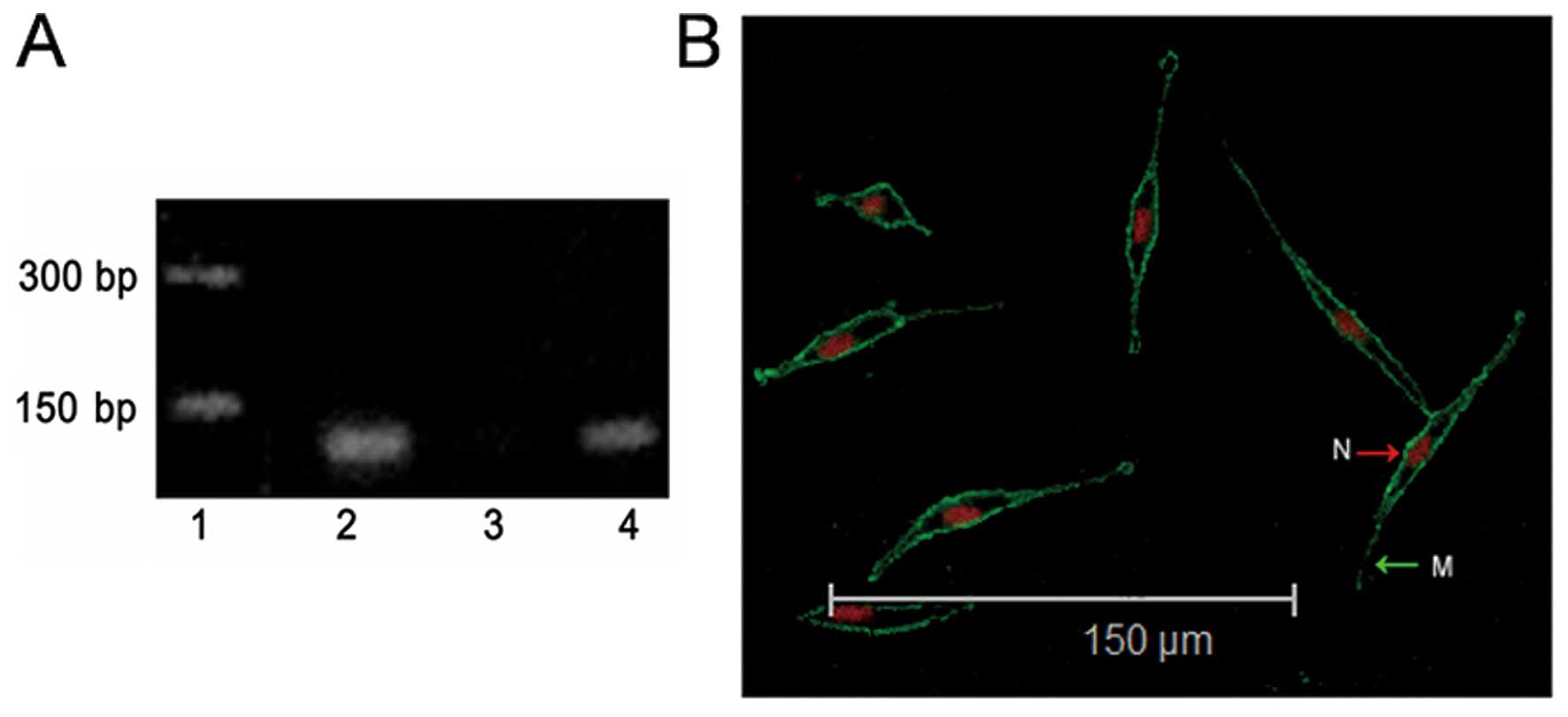
(16). Results in Fig. 1A show that Kir6.2 was expressed in
PC12 cells and rat cardiac tissues. To confirm this result, we
assessed KATP channel colocalization on PC12 cells with
Kir6.2 antibody by laser confocal microscopy. The expression of
Kir6.2 protein on the outer membrane of the PC12 cells were
observed (Fig. 1B). Both results
indicated that KATP channels exist in PC12 cells.
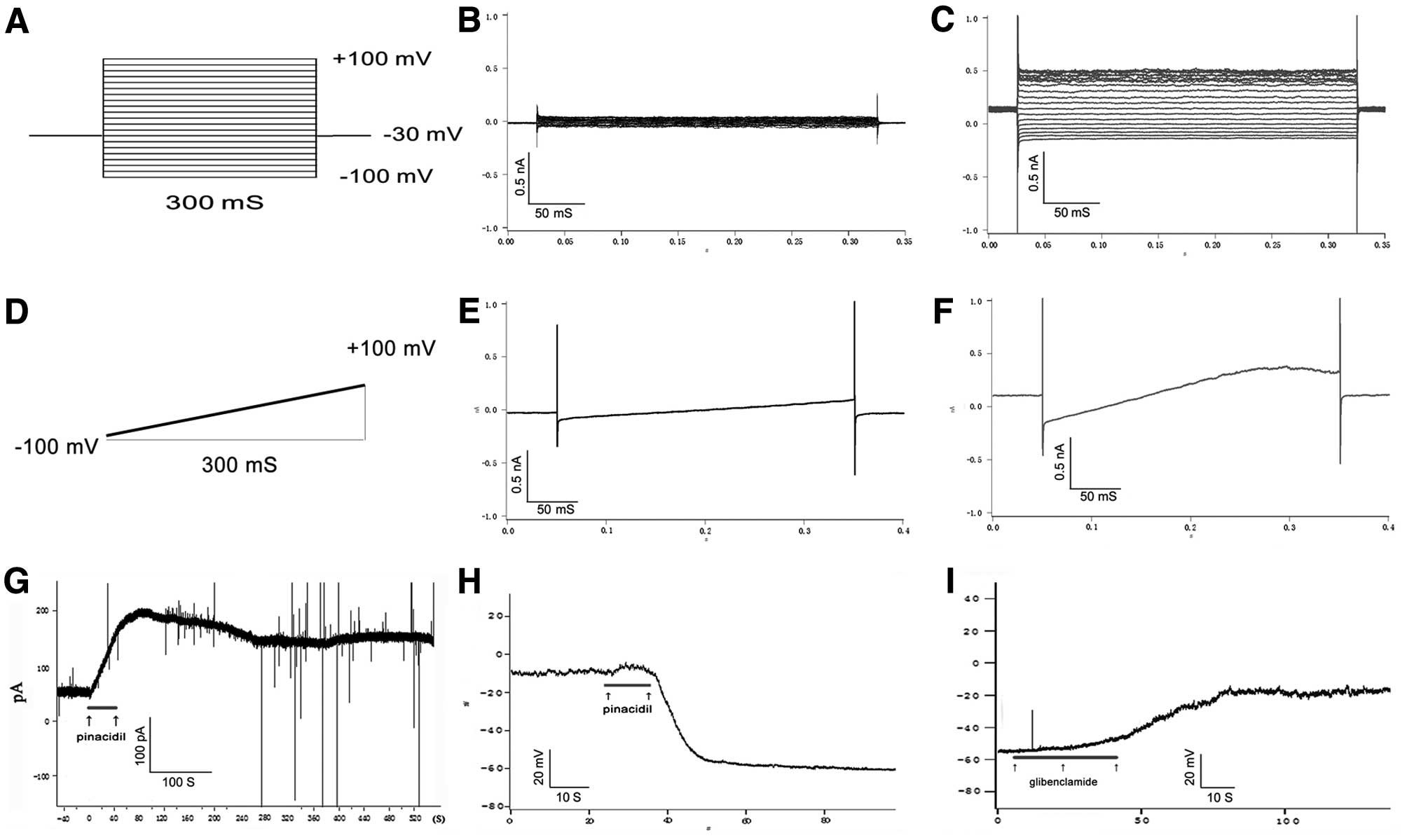
To determine whether these KATP channels
worked on PC12 cells, whole cell patch clamp technology was
implemented to detect opening state of KATP channels
with KATP channel selective opener, pinacidil, and the
selective inhibitor, glibenclamide. Results showed that the outward
current induced by pinacidil is non-voltage dependent and can be
inhibited by glibenclamide (Fig.
2). This is a typical characteristic of functional
KATP channel (10,17).
Effect of acute and chronic rotenone
treatment on the cell vitality of PC12
There are various states of energic stress such as
acute and chronic energic disturbance in compensated or
decompensated manner in dopaminergic neurons. To understand whether
various energic states influence activity of KATP
channels, the treatment with different doses of rotenone on PC12
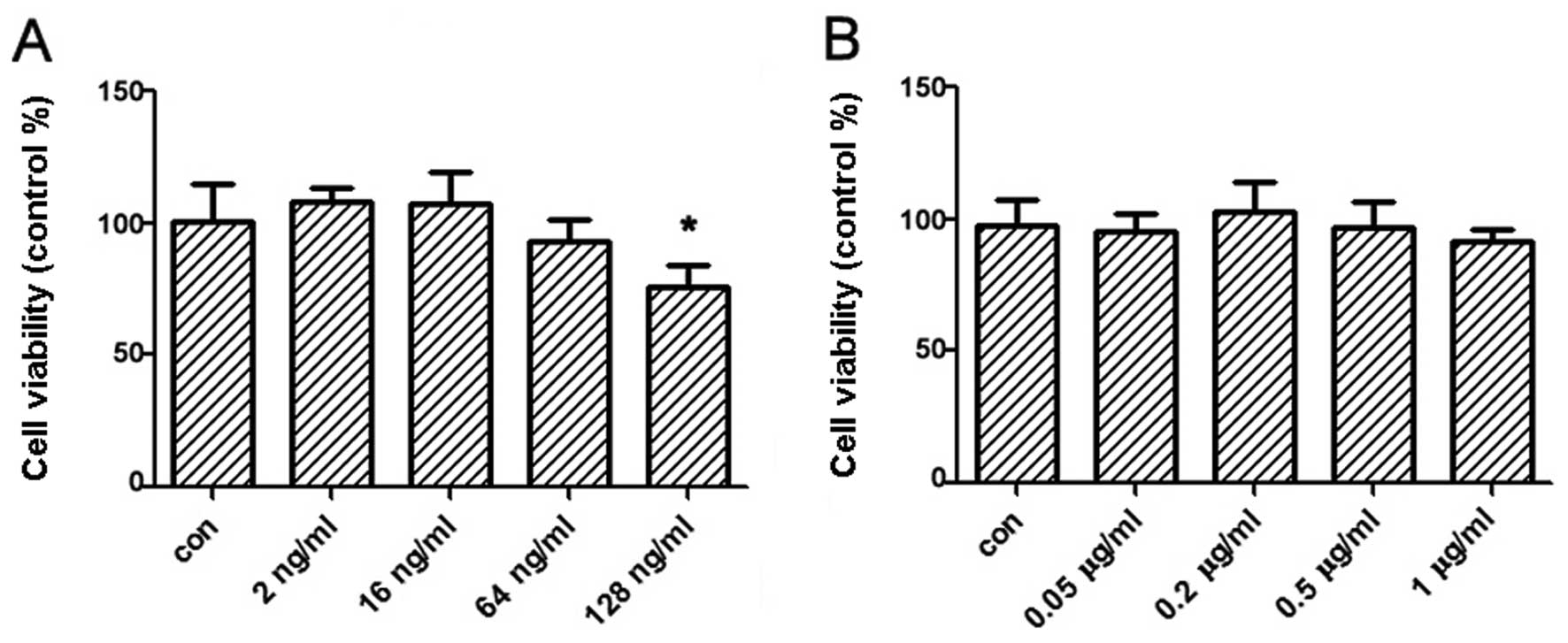
cells was implemented. Therefore, we firstly determined the
concentration of rotenone treated on PC12 cells by MTT assay so
that the direct toxicity on cells by rotenone was excluded. Results
showed that the concentration of rotenone ≤1 μg/ml for 1-h
treatment and 64 ng/ml for 24-h treatment had no effect on the cell
vitality. Therefore, the suitable dose of rotenone treatment on
PC12 cells was selected in further experiments (Fig. 3).
KATP channel opening state and
cell energy state affected by acute rotenone treatment for 15
min
The ROS increase and ATP decrease occurred and
aggravated the time of rotenone treatment (18) were the important factors to affect
the states of the KATP channel, whole cell patch clamp
was used to record the opening state affected by the energic state
induced by rotenone and the recorded on the opening state of
KATP channels were completed within 15 min after the
adding of the rotenone in this parts of the study. Results showed
that treatment with various doses of rotenone (0.05–1 μg/ml) on
PC12 cells elicited outward current in a dose-dependent manner
(Fig. 4A). This outward current was
inhibited by glibenclamide, the specific KATP inhibitor,
which are commonly used to identify the current of KATP
opening (Fig. 4B and C).
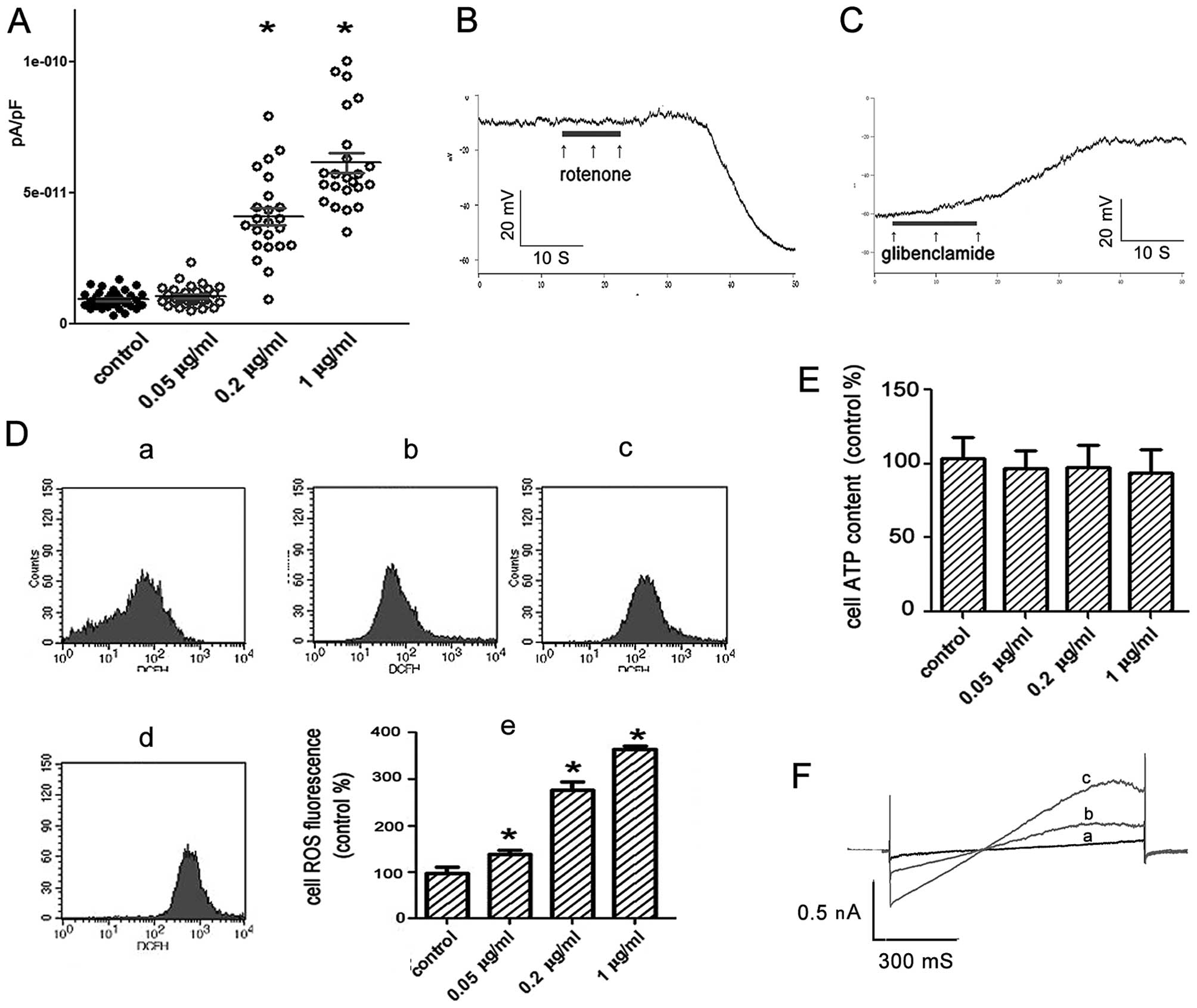 | Figure 4KATP channel opening
states and physiologic states of PC12 cells treated by rotenone for
15 min. (A) Patch-clamp whole cell recording of the currents by
voltage-clamp steps at depolarization of +60 mV under the rotenone
treatment (n>20). Control, 9.39E-12±3.39E-12 pA/pF; 0.05 μg/ml,
1.06E-11±3.97E-12 pA/pF; 0.2 μg/ml, 4.11E-11±1.60E-11 pA/pF; 1
μg/ml, 6.15E-11±1.82E-11 pA/pF. (B and C) Continuous current clamp
recording of the membrane potential. (B) Treated with rotenone 1
μg/ml, (C) with hyperpolarization induced by rotenone,
glibenclamide 20 μM was added. (D) ROS level of the cells treated
by rotenone and analyzed by flow cytometry. a, control; b, 0.05
μg/ml; c, 0.2 μg/ml; d, 1 μg/ml; e, general trend (control,
100±12%; 0.05 μg/ml, 137±9%; 0.2 μg/ml, 275±18%; 1 μg/ml, 363±8%).
(E) ATP level of the cells treated by rotenone and analyzed by
luciferase bioluminescent method. (F) Effects of Mn (III) TBAP on
the voltage-clamp ramps responded currents induced by rotenone,
n=3; (a, rotenone 1 μg/ml + Mn (III) TBAP 30 μM; b, rotenone 1
μg/ml + Mn (III) TBAP 10 μM; c, rotenone 1 μg/ml).
*P<0.05 vs. the control group. |
To understand what factors did affect the opening of
KATP channel under this circumstance, we investigated
the intracellular ATP and ROS concentration after treatment with
rotenone on PC12 cells for 15 min. Results showed that treatment
with rotenone markedly increased intracellular ROS production in a
dose-dependent manner (Fig. 4D).
The concentration of intracellular ATP was not obviously decreased
(Fig. 4E). Consequently, one of the
reasons for the opening of the KATP was the increased
ROS production in PC12 cells induced by rotenone.
To confirm this assumption, PC12 cells were treated
with both rotenone (1 μg/ml) and a superoxide dismutase mimetic Mn
(III) TBAP (30 μM). We found that Mn (III) TBAP (30 μM) markedly
prevented rotenone-induced currents on PC12 cells (Fig. 4F). Therefore, results suggest that
the rotenone-induced currents was mainly caused by the increased
ROS production on PC12 cells treated with rotenone for 15 min.
KATP channel opening state and
cell energy state affected by chronic rotenone treatment for 24
h
To explore KATP channel opening states
induced by rotenone for 24 h, PC12 cells were measured by whole
cell patch clamp after treatment with various doses of rotenone
(2–64 ng/ml) for 24 h. Results showed treatment with rotenone in
low concentration (2 and 16 ng/ml) on PC12 cells elicited outward
current. Since the outward current induced by the 2 and 16 ng/ml
treatment can be inhibited by glibenclimade, it was considered as
the current of KATP channel opening. However, treatment
with rotenone in high concentration (64 ng/ml) did not alter
outward current on PC12 cells (Fig.
5A). Then, we further added the KATP opener
pinacidil (100 nM) or another high dose of rotenone (1 μg/ml) in
the PC12 cells treated with rotenone (64 ng/ml), opening of
KATP channels was not observed (Fig. 5B). It was demonstrated that the
inhibition of KATP channels by rotenone for long-term
treatment (24 h) was not reversed by pinacidil.
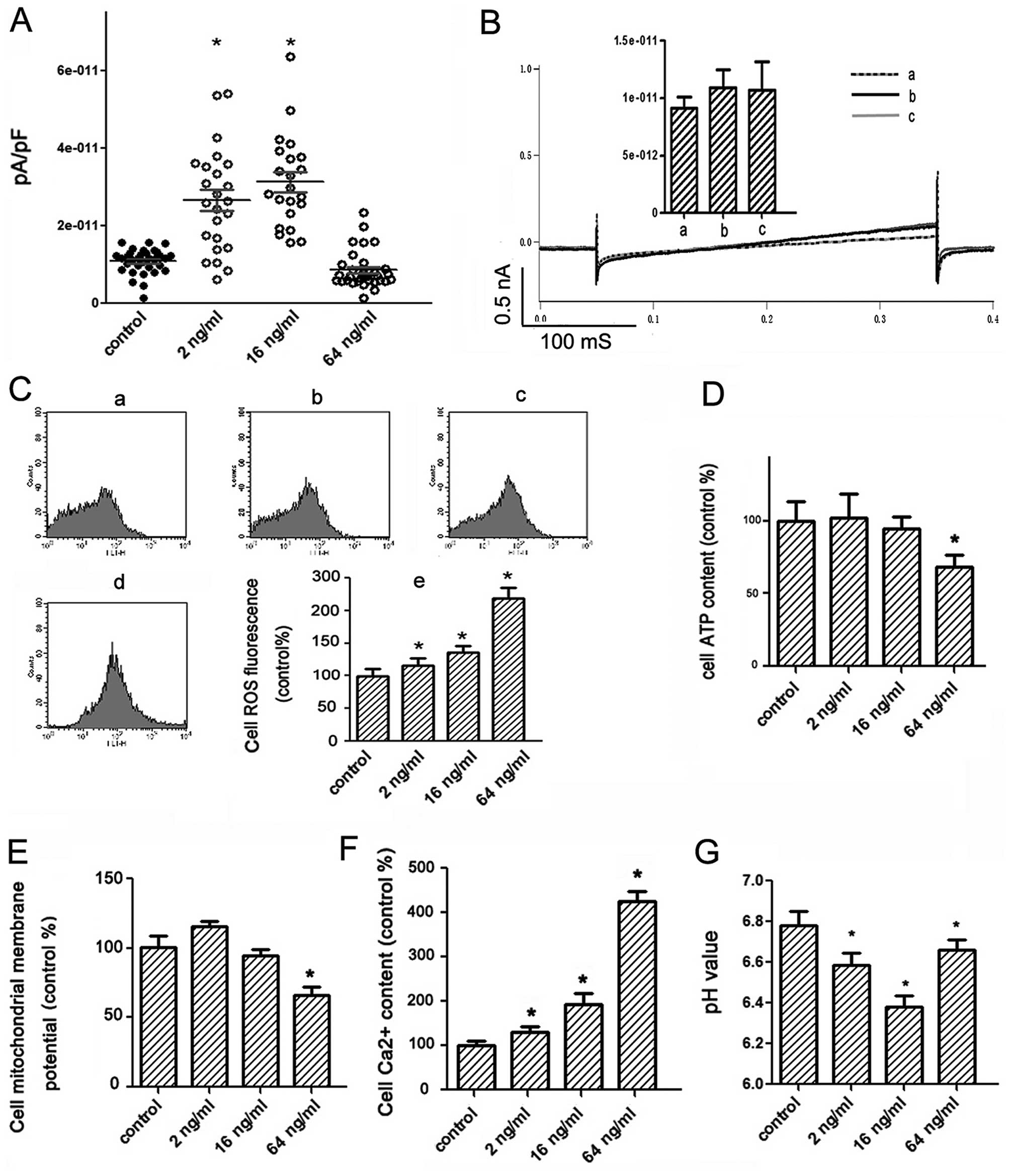 | Figure 5KATP channel opening
states and physiologic states of PC12 cells treated by rotenone
(2–64 ng/ml) for 24 h. (A) Patch-clamp whole cell recording of the
currents by voltage-clamp steps at depolarization of +60 mV
(n>20). Control, 1.09E-11±3.19E-12 pA/pF; 2 ng/ml,
2.65E-11±1.32E-11 pA/pF; 16 ng/ml, 3.12E-11±1.17E-11 pA/pF; 64
ng/ml, 8.54E-12±4.80E-12 pA/pF. (B) Patch clamp testing the
KATP channel function of the cells which had been
treated by rotenone (64 ng/ml) for 24 h with the channel opener
(currents responded to voltage-clamp ramps were recorded and the
data for the bars were the currents (pA/pF) recorded at 250 ms),
n=3 (a, without channel opener; b, with rotenone 1 μg/ml; c, with
pinacidil 100 nM). (C) ROS levels analyzed by flow cytometry. a,
control; b, rotenone 2 ng/ml; c, rotenone 16 ng/ml; d, rotenone 64
ng/ml; e, general trend. (D) ATP levels analyzed by luciferase
bioluminescent method. (E) Mitochondrial transmembrane potential
analyzed by flow cytometry. (F) Ca2+ level analyzed by
flow cytometry. (G) pH value of the culture medium measured by pH
meter. *P<0.05 vs. the control group. |
To investigate which is the main reason for
KATP channel opening states, we then measured the ROS
and the ATP levels on PC12 cells induced by rotenone. Results
showed that treatment with various dose rotenone (2–64 ng/ml) led
to increase in ROS production on PC12 cells in a
concentration-dependent manner (Fig.
5C). The alteration of ATP concentration on PC12 cells induced
by rotenone in low dose was not clearly observed. Treatment with
rotenone in high dose (64 ng/ml) notably reduced ATP production on
the cells (Fig. 5D).
The cellular calcium ion level and the mitochondrial
membrane potentials could reflect the physiological states of
cells. So, we tested them by flow cytometry. Results showed the
mitochondrial membrane potentials were decreased and calcium ion
levels were increased by treatment with rotenone in a
dose-dependent manner, respectively (Fig. 5E and F).
When cells are under energic stress, glycolysis is
promoted to produce more acidic metabolites. We measured the pH
value of culture medium on PC12 cells treated with various dose
rotenone. The pH value of culture medium on PC12 cells treated with
low dose rotenone (2 and 16 ng/ml) was 6.59±0.06 and 6.38±0.05,
respectively, which was lower than the control (6.78±0.07).
However, pH value in culture medium on cells treated with high dose
(64 ng/ml) rotenone was 6.66±0.05, which recovered near to the
control level (Fig. 5G). Results
shown that treatment with rotenone in high dose might cause more
injury to PC12 cells which may have lost their metabolism
compensation function and induce irreversible physiologic
dysfunction. It is suggested that pH might be one of the important
causes to affect open state of KATP channels.
Inhibition of KATP channel
increased expression of TH
To determine if the opening states of
KATP could affect the TH expression on PC12 cells, which
could help us to understand the function of this channel in DA
synthesis, KATP inhibitor glibenclamide and opener
pinacidil were used to treat cells for 24 h to manipulate the
opening state of this channel, then the TH expression was assayed.
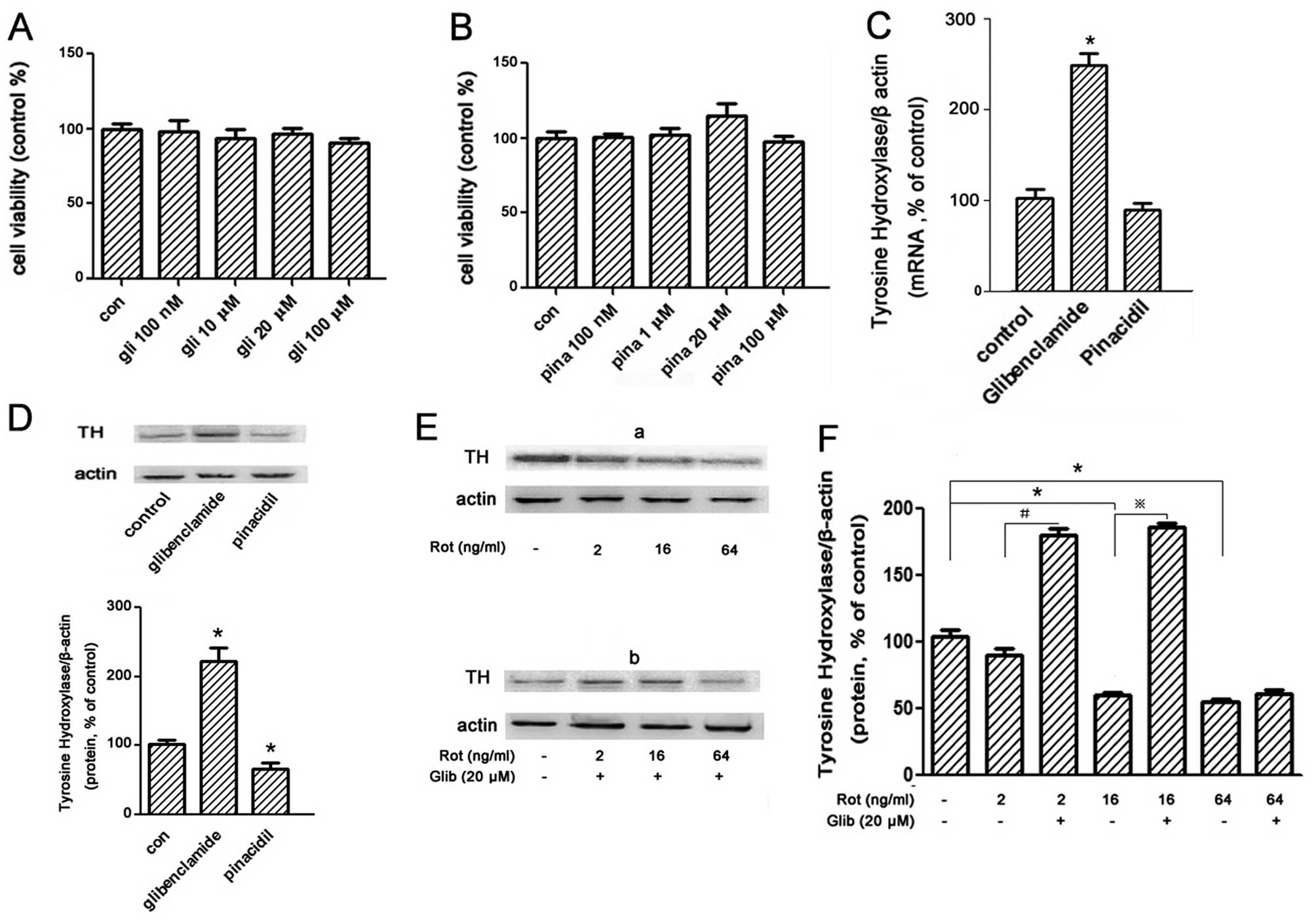
First, we tested the effect of these chemicals to the viability of
PC12 cells by MTT assay to find the suitable concentration to be
used in the follow experiment which would have no adverse effect.
Results showed that it was best for both at ≤100 μM (Fig. 6A and B).
We then measured TH expression on PC12 cells treated
with pinacidil 100 nM and glibenclamide 20 μM for 24 h,
respectively. Results showed that TH expression at mRNA level
increased notably (2.47-fold vs. control) by glibenclamide, whilst
no alteration of TH expression was observed after treatment with
pinacidil (Fig. 6C). But for TH
expression at protein level measured by western blot analysis,
treatment with glibenclamide increased expression 2.2-fold more
than control and decreased 0.35-fold by pinacidil less than control
(Fig. 6D). This suggests that
treatment with glibenclamide (20 μM) for 24 h markedly promote the
translation and expression of TH on PC12 cells.
Since rotenone leads to the opening of
KATP channel on PC12 in our experiment, we next
determined whether it can affect the TH expression. Results showed
that treatment with various rotenone (2–64 ng/ml) 24 h decreased
the TH expression in a dose-dependent manner on PC12 cells
(Fig. 6Ea and F). But glibenclamide
(20 μM) inhibited the decrease of TH expression by rotenone
(Fig. 6Eb and F). This suggess that
the TH expression reduced by rotenone related with the opening of
KATP channel because it was notably inversed by the
KATP inhibitor glibenclamide.
Discussion
In this study we tested the KATP channel
on PC12 cells to explore how the different energic states caused by
various doses of rotenone to affect the KATP opening
state and whether the KATP opening state influences the
expression of TH which is related with DA synthesis.
The PC12 cell line derived from the rat
pheochromocytoma is considered close to the dopamine terminal
neurons compared with other cell lines. It is often used as an
in vitro model to study the physiology of central dopamine
neurons. Recent evidence indicates that activation of
KATP channels in PC12 cells confers protection against
mitochondrial complex-I inhibition-induced cell death (19) and could have potential beneficial
effects in Parkinson’s disease. Further understanding of the
mechanisms that underlie this interesting phenomenon may lead to
the new insight for the treatment of neurodegenerative diseases.
KATP channels are reportedly present in both plasma and
mitochondrial membranes. In this study we sought to determine if
the KATP channel in plasma membranes contributes to
neuroprotection. Our data showed that PC12 cells distributed
functional KATP channels on the plasma membranes which
was discrepant in different research (11–13).
The reason is that the cells in the different states and culture
conditions may lead to different energic and redox states which
affect expression and function of the KATP channel.
Our results suggest that treatment with rotenone in
different doses could affect the KATP opening states
differently. The treatment with various dose of rotenone (0.05–1
μg/ml) on PC12 cells within 15 min elicited outward current in
dose-dependent manner which was inhibited by glibenclamide, the
specific KATP inhibitor. In this circumstance,
intracellular ROS increased by rotenone for 15 min was also
observed and could be the main factor to cause the opening of this
channel. In the rotenone treatment (2–64 ng/ml) for 24 h, cells
with 2–16 ng/ml treatment with mild intracellular ROS increase
could elicited outward current, while cells with 64-ng/ml treatment
with more serious intracellular ROS increase could not. There is
evidence that rotenone treatment could cause serious oxidative
environment and exhaust the glutathione of dopaminergic cells
(20,21), and this may cause the oxidation of
the channel hydrosulfide. When the hydrosulfide of the channel was
oxidized, it becomes closed (22).
Therefore, we supposed that increasing lactic acid, ROS, might
contribute to promote the opening of KATP channel
(23). However, treatment with
rotenone (64 ng/ml) for 24 h upregulated ROS production until
overload, so that it led to inactivation of the KATP
channel.
In PD patient, there is not only a serious decrease
of the number of the dopaminergic cells, but also notably reduced
dopamine synthesis in the remaining cells (8,24). We
sought to determine the relationship between KATP
channel and TH expression which is dopamine synthesis rate-limiting
enzyme. We then correlated with detection of KATP
channel subunits using specific antibodies and valuation of the
presence of functional KATP channels in the plasma
membrane of PC12 cells induced by rotenone using the patch-clamp
technique. TH expression in PC12 cells induced by rotenone were
valuated by means of western blot analysis. The overall results
suggest that opening state of the KATP was related with
the TH expression in PC12 cells, which would affect the synthesis
of dopamine. Treatment with KATP inhibitor,
glibenclamide, notably enhanced the TH expression in PC12 cells,
but KATP channel opener, pinacidil, did not reduce TH
expression markedly in cells. The reasons probably is that TH
expression is low in the normal cells, and a part of the
KATP channel of PC12 is usually quite open (about 40% of
the cells). In addition, a previous study indicated that the
inhibition of KATP may enhance the TH expression in
vivo. They found that the use of glibenclimade after infarction
promoted the myocardial TH expression and the sympathetic
reinnervation, which is harmful under this circumstance (25), but may be a potential target for the
PD therapy. The mechanism of the inhibition of the KATP
that promoted the TH expression remains unclear. Because the
inhibition of the KATP conduced to membrane
depolarization, a consideration is the expression of the
transcription factor Nurr1 might be enhanced by prolonged membrane
depolarization, TH is a downstream gene of this transcription
factor (26). TH expression can
also be induced by the outer membrane depolarization (27). This may suggest the intimacy between
the state of KATP and the occupation of dopamine
turnover in these cells.
In conclusion, we have demonstrated for the first
time that activation of plasma membrane KATP channels
induced by rotenone inhibits TH expression which influence the DA
synthesis in PC12 cells. Further elucidation of the elements up-
and downstream of KATP channels may open novel
therapeutic strategies for the treatment of various
neurodegenerative diseases.
Acknowledgements
We are grateful for the financial support from the
National Nature Science Foundation of China (Project no. 81070222)
and the Nature Science Foundation of Chongqing (Project no. CSTC,
2009BA5083).
Abbreviations:
|
PC12
|
pheochromocytoma cell line
|
|
PI
|
propidium iodide
|
|
TH
|
tyrosine hydroxylase
|
|
KATP channel
|
ATP sensitive potassium channel
|
|
PD
|
Parkinson’s disease
|
|
DA
|
dopamine
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)
2,5-diphenyltetrazolium bromide
|
|
Mn (III) TBAP
|
Mn (III) tetrakis (4-benzoic acid)
porphrin chloride
|
References
|
1
|
Betarbet R, Sherer TB, MacKenzie G,
Garcia-Osuna M, Panov AV and Greenamyre JT: Chronic systemic
pesticide exposure reproduces features of Parkinson’s disease. Nat
Neurosci. 3:1301–1306. 2000.
|
|
2
|
Dawson TM and Dawson VL: Molecular
pathways of neurodegeneration in Parkinson’s disease. Science.
302:819–822. 2003.
|
|
3
|
Di Monte DA: The environment and
Parkinson’s disease: is the nigrostriatal system preferentially
targeted by neurotoxins? Lancet Neurol. 2:531–538. 2003.
|
|
4
|
Hartley A, Stone JM, Heron C, Cooper JM
and Schapira AH: Complex I inhibitors induce dose-dependent
apoptosis in PC12 cells: relevance to Parkinson’s disease. J
Neurochem. 63:1987–1990. 1994.PubMed/NCBI
|
|
5
|
Pitkanen S and Robinson BH: Mitochondrial
complex I deficiency leads to increased production of superoxide
radicals and induction of superoxide dismutase. J Clin Invest.
98:345–351. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Votyakova TV and Reynolds IJ:
ΔΨm-dependent and -independent production of reactive oxygen
species by rat brain mitochondria. J Neurochem. 79:266–277.
2001.
|
|
7
|
Liss B, Haeckel O, Wildmann J, Miki T,
Seino S and Roeper J: K-ATP channels promote the differential
degeneration of dopaminergic midbrain neurons. Nat Neurosci.
8:1742–1751. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Javoy-Agid F, Hirsch EC, Dumas S,
Duyckaerts C, Mallet J and Agid Y: Decreased tyrosine hydroxylase
messenger RNA in the surviving dopamine neurons of the substantia
nigra in parkinson’s disease: an in situ hybridization study.
Neuroscience. 38:245–253. 1990.
|
|
9
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu C, Yang K, Liu Q, Wakui M, Jin GZ, Zhen
XC and Wu J: Tetrahydroberberine blocks ATP-sensitive potassium
channels in dopamine neurons acutely-dissociated from rat
substantia nigra pars compacta. Neuropharmacology. 59:567–572.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koshimura K, Tanaka J, Murakami Y and Kato
Y: Effect of high concentration of glucose on dopamine release from
pheochromocytoma-12 cells. Metabolism. 52:922–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai KK, McCrossan ZA and Abbott GW:
Activation of mitochondrial ATP-sensitive potassium channels
increases cell viability against rotenone-induced cell death. J
Neurochem. 84:1193–1200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamensdorf I, He LP, Nechushtan A,
Harvey-White J, Eisenhofer G, Milan R, Rojas E and Kopin IJ: Effect
of glipizide on dopamine synthesis, release and metabolism in PC12
cells. Eur J Pharmacol. 388:147–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liss B and Roeper J: ATP-sensitive
potassium channels in dopaminergic neurons: transducers of
mitochondrial dysfunction. News Physiol Sci. 16:214–217.
2001.PubMed/NCBI
|
|
15
|
Zingman LV, Hodgson DM, Bast PH, Kane GC,
Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki
T, et al: Kir6.2 is required for adaptation to stress. Proc Natl
Acad Sci USA. 99:13278–13283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olson TM and Terzic A: Human
KATP channelopathies: diseases of metabolic homeostasis.
Pflugers Arch. 460:295–306. 2010.PubMed/NCBI
|
|
17
|
Van den Top M, Lyons DJ, Lee K, Coderre E,
Renaud LP and Spanswick D: Pharmacological and molecular
characterization of ATP-sensitive K+ conductances in
CART and NPY/AgRP expressing neurons of the hypothalamic arcuate
nucleus. Neuroscience. 144:815–824. 2007.PubMed/NCBI
|
|
18
|
Sherer TB, Betarbet R, Testa CM, Seo BB,
Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A and
Greenamyre JT: Mechanism of toxicity in rotenone models of
Parkinson’s disease. J Neurosci. 23:10756–10764. 2003.
|
|
19
|
Tai KK and Truong DD: Activation of
ATP-sensitive potassium channel confers protection against
rotenone-induced cell death: therapeutic implications for
Parkinson’s disease. J Neurosci Res. 69:559–566. 2002.PubMed/NCBI
|
|
20
|
Saravanan KS, Sindhu KM and Mohanakumar
KP: Acute intranigral infusion of rotenone in rats causes
progressive biochemical lesions in the striatum similar to
Parkinson’s disease. Brain Res. 1049:147–155. 2005.PubMed/NCBI
|
|
21
|
Liu YM, Jiang B, Bao YM and An LJ:
Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction
in rotenone-induced PC12 cells. Toxicol In Vitro. 22:430–437. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Islam MS, Berggren PO and Larsson O:
Sulfhydryl oxidation induces rapid and reversible closure of the
ATP-regulated K+ channel in the pancreatic β-cell. FEBS
Lett. 319:128–132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alekseev AE, Hodgson DM, Karger AB, Park
S, Zingman LV and Terzic A: ATP-sensitive K+ channel
channel/enzyme multimer: metabolic gating in the heart. J Mol Cell
Cardiol. 38:895–905. 2005.
|
|
24
|
Kastner A, Hirsch E, Agid Y and Javoy-Agid
F: Tyrosine hydroxylase protein and messenger RNA in the
dopaminergic nigral neurons of patients with Parkinson’s disease.
Brain Res. 606:341–345. 1993.PubMed/NCBI
|
|
25
|
Lee TM, Lin MS and Chang NC: Effect of
pravastatin on sympathetic reinnervation in postinfarcted rats. Am
J Physiol Heart Circ Physiol. 293:H3617–H3626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He XB, Yi SH, Rhee YH, Kim H, Han YM, Lee
SH, Lee H, Park CH, Lee YS, Richardson E, et al: Prolonged membrane
depolarization enhances midbrain dopamine neuron differentiation
via epigenetic histone modifications. Stem Cells. 29:1861–1873.
2011. View Article : Google Scholar
|
|
27
|
Kilbourne EJ, McMahon A and Sabban EL:
Membrane depolarization by isotonic or hypertonic KCl: differential
effects on mRNA levels of tyrosine hydroxylase and dopamine
β-hydroxylase mRNA in PC12 cells. J Neurosci Methods. 40:193–202.
1991.
|