Introduction
Integrins comprise a large family of αβ
heterodimeric cell-surface receptors that are expressed in a wide
variety of cells. They mediate diverse processes and are involved
in cell-cell and cell-matrix interactions such as cell adhesion and
migration, cell survival and differentiation. It is now well
documented that integrins play a crucial role in cancer
progression, metastasis and neoangiogenesis.
There are two members of integrin β3 family: αvβ3
and αIIbβ3. αvβ3 integrin is strongly expressed on the surface of
the smooth muscle cells, endothelial cells, monocytes and
platelets. Dysregulation of β3 integrin expression is associated
with the pathogenesis of several diseases, including cancer. Many
invasive tumour cells, including melanoma show an overexpression of
this integrin. There are also reports indicating the correlation
between αvβ3 integrin expression and the stage of tumour
progression (1–5). β3 integrins are also strongly involved
in tumour-induced angiogenesis and have been described as
pro-angiogenic factors (6,7). The role of αvβ3 integrin in tumour
angiogenesis is related not to its expression by neoplastic cells,
but rather to its expression by host endothelial cells (8). Moreover, it was proven that
antagonists of αvβ3 inhibit angiogenic processes, including
endothelial cell adhesion and migration, whereas factors, which
increase αvβ3 integrin expression, induce angiogenesis (9,10).
αIIbβ3 integrin expression is limited mainly to
platelets, megakaryocytes, human blood monocytes, granulocytes, and
large granular lymphocytes (11).
However, there is increasing evidence that αIIbβ3 integrin is also
present in the tumour cells (3).
Its expression is connected with tumour thickness, invasion
abilities and metastatic potential of human and mouse melanomas
(3,8). Various studies showed that αIIbβ3 is
constitutively expressed at a high-affinity state and is highly
involved in tumour cell adhesion and invasion (12).
αIIbβ3 integrin is also involved in tumour-induced
platelet aggregation, which has been described as an important step
of metastasis pathway. Tumour cells during migration in blood
vessels can form complexes with platelets. This process, resulting
from direct binding of platelets to tumour cells, is essential for
metastasis (8,13).
The β3 integrins appear to have an important
stimulatory role in tumour progression and metastasis and that is
why β3 integrins have often been proposed as potential targets for
cancer diagnostic and therapeutic approaches. Application of
anti-integrin antibodies and RGD (Arg-Gly-Asp) related peptides
have revealed promising effects in anticancer therapy (14–17).
One of the most interesting integrin-targeting tools are short
interfering RNAs (siRNAs).
In this study, the in vitro and in
vivo properties of B16 mouse melanoma cells with lower
expression of integrin β3 were evaluated. Proliferation rate,
adhesive properties and the ability to migrate and metastasize were
studied. In order to achieve cells with low expression of integrin
β3, transfection with siRNA was employed. B16 cells that fail to
express integrin β3 show impaired motility and ability to bind to
extracellular matrix (ECM) proteins, and are unable to colonize
lungs. These results provide supplementary data for a direct role
of integrin β3 in the adhesion, migration and metastasis processes
of mouse melanoma cells and prove that the silencing of integrin
expression can be efficiently and selectively obtained using
siRNAs.
Materials and methods
Cell culture
The mouse melanoma B16 cells were obtained from the
American Type Culture Collection (Rockville, MD, USA) and
maintained in the Cell Culture Collection of the Institute of
Immunology and Experimental Therapy Polish Academy of Sciences
(IIET, PASc), Wroclaw, Poland. Cells were cultured in RPMI medium
supplemented with 4 mM L-glutamine, 4.5 g/l glucose, 1.5 g/l
NaHCO3 (both from Sigma-Aldrich Chemie GmbH, Steinheim,
Germany), 100 U/ml penicillin, 100 μg/ml streptomycin (both from
Polfa Tarchomin S.A., Warsaw, Poland) and 10% FBS (Sigma-Aldrich
Chemie GmbH).
siRNA
The siRNAs (sense and antisense strands) were
purchased from Qiagen (Qiagen Inc., Valencia, USA) and were diluted
according to manufacturer’s instructions and then stored at −20°C.
The following sequences were tested for their effectiveness in
silencing integrin β3 expression: Sequence M1: sense
r(GCCGUGAAUUGUACCUACA)dTdT, antisense r(UGUAGGUACAAUUCACGGC)dGdT;
Sequence M2: sense r(CGGUGAGCUUUAGUAUCGA)dTdT, antisense
r(UCGAUACUAAAGCUCACCG)dTdG. As a control, a negative siRNA, with no
homology to mRNA databases was used (Silencer® Negative
Control #1 siRNA, Ambion).
In vitro transfections were performed using
HiPerFect reagent (Qiagen Inc.) as recommended by the manufacturer.
Cells were plated on a 24-well plate in 0.5 ml of medium RPMI-O-MEM
without antibiotics and FBS (4×104 cells per well).
Shortly after plating, cells were transfected with 100 μl of the
transfection mixture containing 5 or 25 nM of siRNA. Cells were
washed 6 h after transfection and the procedure was repeated 48 h
later.
Integrin quantification
The expression of integrin β3 (CD61) (Becton
Dickinson, San Jose, USA) was determined by flow cytometry. B16
cells (1×105) were mixed with an appropriate volume of
McAb solution (pre-chilled to 4°C). Cells were incubated for 30 min
on an ice bath, and subsequently washed twice with PBS
(supplemented with 2% fetal bovine serum). Cell surface
fluorescence was measured using a FACS Calibur flow cytometer
(Becton Dickinson). Damaged cells were labeled with propidium
iodide solution to each test tube just before data acquisition.
Data for damaged cells were not analyzed. Data analysis was
performed using WinMDI 2.8 software.
Semi-quantitative PCR
Total RNA extraction, DNA digestion and cDNA
synthesis was performed with RNAlater RNA Stabilization Reagent™
(Qiagen Inc.) according to the manufacture’s procedure. PCR
reaction was performed using the following primers: integrin β3:
forward 5′TCAGATGCGCAAGCTTACTAGC3′, reverse
5′TCAGCACGTGTTTGTAGCCAA3′; GAPDH: forward:
5′ATGACATCAAGAAGGTGGTG3′, reverse: 5′CATACCAGGAAATGAGCTTG3′. PCR
cycling conditions were 94°C for 30 sec, 55°C for 30 sec, and 72°C
for 1 min, 35 cycles for integrin β3 expression and 25 cycles for
GAPDH. PCR products were dissolved in 1.7% agarose gel with
ethidium bromide.
Antiproliferative assays
Cells were plated in 96-well plates (Sarstedt, Inc.
Newton, NC, USA) at the density of 8×103 cells per well
in 100 μl of culture medium without FBS and antibiotics. After 24 h
of incubation at standard conditions (37°C in humid atmosphere with
5% CO2), cells were treated with siRNA suspended in 100
μl of medium FBS and antibiotics-free. The cytotoxic assays were
performed after 24, 48 and 72 h exposure of the cultured cells to
varying concentrations siRNA, e.g. 1, 5 and 25 nM. The amount of
HiPerFect was stable (3 μl per well). The SRB method was used as
described by Skehan and coworkers (18). The optical densities of the samples
were measured on a Multiskan RC photometer (Labsystems, Helsinki,
Finland) at λ=540 nm.
Adhesion assay
Flat-bottomed, 96-well plates were coated with
fibrinogen (10 μg/ml suspended in 7.5% NaHCO3, Merck,
Darmstadt, Germany) and blocked with 1% BSA (Sigma-Aldrich Chemie
GmbH) in TSM buffer (20 mM Tris-HCl pH 8.0, 150 nM NaCl, 1 mM
CaCl2, 2 mM MgCl2). Cells were suspended in
0.5% solution of BSA, added into plates in the amount of
2.5×104 and incubated for 1 h at 37°C. Unbound cells
were washed out twice with TSM buffer and dyed with 0.2% solution
of crystalline violet in methanol. After 30 min of incubation at
4°C, cells were washed with PBS-Ca2+Mg2+, dried and
suspended in 20% methanol. The absorbance was measured at λ=570 nm
in a computer-interfaced, 96-well microtiter plate reader Multiskan
RC photometer.
Migration assay
Migration chamber preparation
Fibronectin assay: 8-μm insert membranes (Falcon BD
Biosciences, USA) were sterilely covered with fibronectin (100
μg/ml, Falcon BD Biosciences). Both sides of the membrane were
covered with 20 μl of the fibronectin suspension and incubated for
30 min at 37°C. Fibronectin was removed and the inserts were washed
three times with sterile water. Subsequently, both sides of the
membrane were immersed in a 0.1% albumin solution and incubated for
15 min. The inserts were washed three times with sterile water and
dried. The prepared inserts were not stored, but used immediately
after preparation.
Migration assay
The siRNA M2-transfected, negative siRNA-transfected
and non-treated B16 cells were suspended in DMEM with no FBS, and
applied to the upper section of the migration chamber, with
2.9×105 cells/insert. Culture medium supplemented with
10% FBS applied to the lower section served as chemoattractant.
The migration was carried out at 37°C with 5%
CO2. The time of migration was initially optimised and
for B16 cells was 2 h. Thereafter (following the manufacturer’s
instructions), the cells from the upper side of the membrane were
removed with a cotton swab. The cells on the bottom side of the
membrane were fixed and stained with a Diff-Quick set (Medion
Diagnostics, Düdingen, Switzerland) and counted by light
microscopy. The number of cells per membrane was determined,
accumulated into groups, and the average was presented.
Metastasis assay
Eight- to twelve-week-old C57BL/6/IiW female mice
were purchased from Maria Skłodowska-Curie Memorial Cancer Center
and Institute of Oncology, Warsaw (Poland) and kept under specific
pathogen-free (SPF) conditions. All experiments were performed
under standard laboratory conditions according to Interdisciplinary
Principles and Guidelines for the Use of Animals in Research,
Marketing and Education issued by the New York Academy of Science
Ad Hoc Committee on Animal Research and were approved by the 1st
Local Committee for Experiments with the Use of Laboratory Animals,
Wroclaw, Poland.
Mice were inoculated intravenously (i.v.) with
3×105 B16 cells (collected from in vitro culture)
in 0.2 ml of Hank’s medium into the lateral tail vein. Mice were
sacrificed by cervical dislocation (21 days after cells
inoculation). Lungs were excited and weighed immediately, and lung
metastatic foci were counted.
Results
Inhibition of integrin β3 synthesis by
RNA interference in vitro
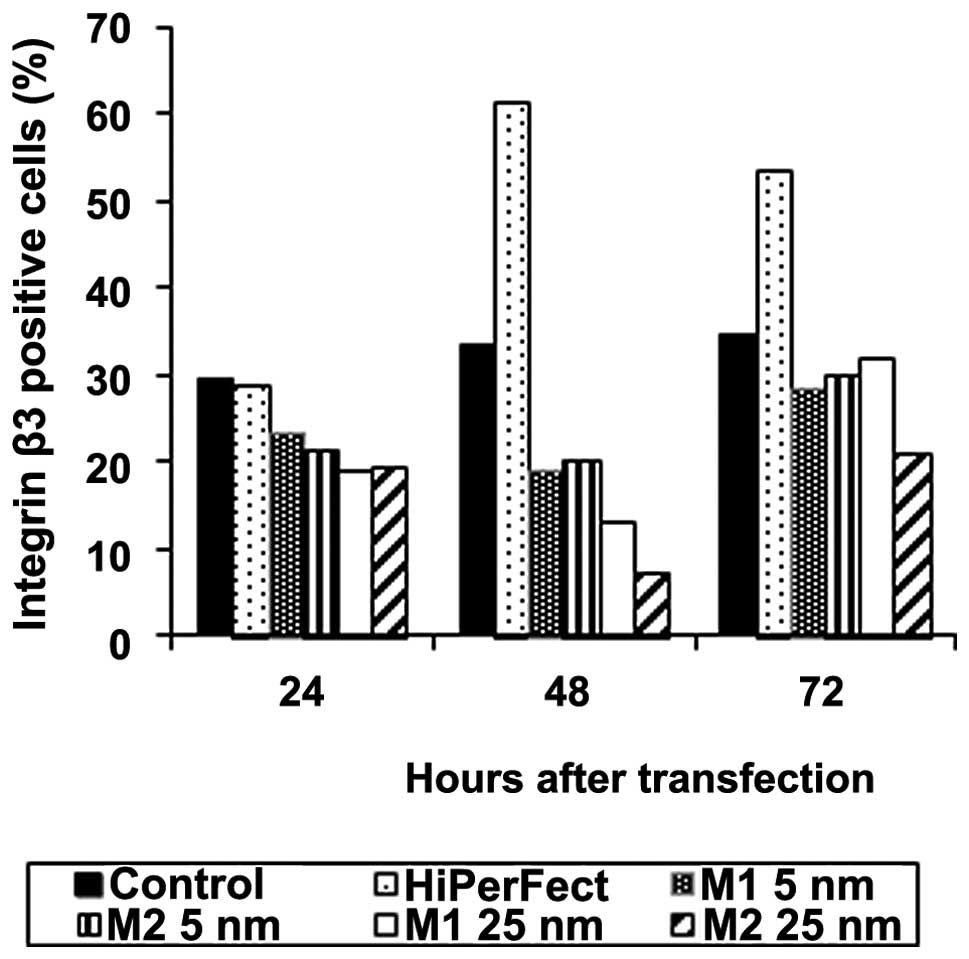
B16 cells were transfected with 5 or 25 nM of M1 and
M2 siRNAs. The expression of integrin β3 was measured by
cytofluorometry after 24, 48 and 72 h after transfection. Both
siRNA sequences led to the reduction of integrin β3 expression as
compared to control, non-transfected cells; however, the sequence
M2 appeared to be more potent. In both cases, the silencing effect
increased with siRNA concentration. However, we also showed that
the most effective concentration of siRNA was 25 nM and further
increase in siRNA amount did not enhance the effect (data not
shown). Moreover, our experiments confirmed that siRNA-mediated
silencing of integrin β3 expression is transitory, with a highest
inhibition of protein expression after 48 h after transfection. We
observed almost 80% reduction of integrin β3 expression on B16
cells 48 h after transfection with M2 siRNA compared to untreated
cells (Fig. 1).
None of the tested sequences showed cytotoxicity.
The inhibition of B16 cells proliferation reached only 5%, 24 h
after transfection as compared to the control, non-transfected
cells, irrespective of siRNA sequence and concentration applied.
B16 cells treated with siRNAs achieved a control proliferation rate
72 h after transfection.
Taking the above-mentioned results into account, we
chose M2 sequence for further studies. Comparing the efficacy of
integrin β3 silencing by a single and a double transfection, we
found that it is possible to obtain a significant increase in the
inhibition of integrin β3 expression due to a transfection repeated
after additional 48 h. In that case, the inhibition of integrin β3
expression on B16 cells could reach even 98% (mean inhibition was
87±8%, which corresponded to 48±11% drop in the mean fluorescence
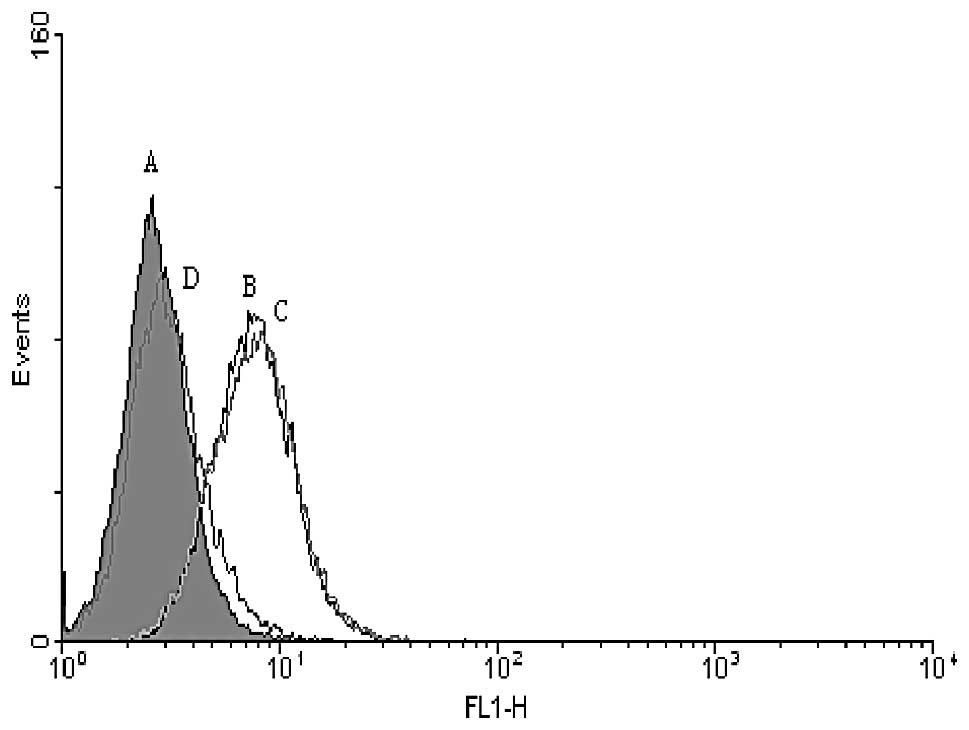
canal values). Cells restored integrin β3 expression after 96 h
after the first transfection. For negative siRNA, with no homology
to any known mRNA, we showed a slight (5%) and insignificant
increase in the integrin β3 expression. The representative
histogram of transfected B16 cells is shown in Fig. 2.
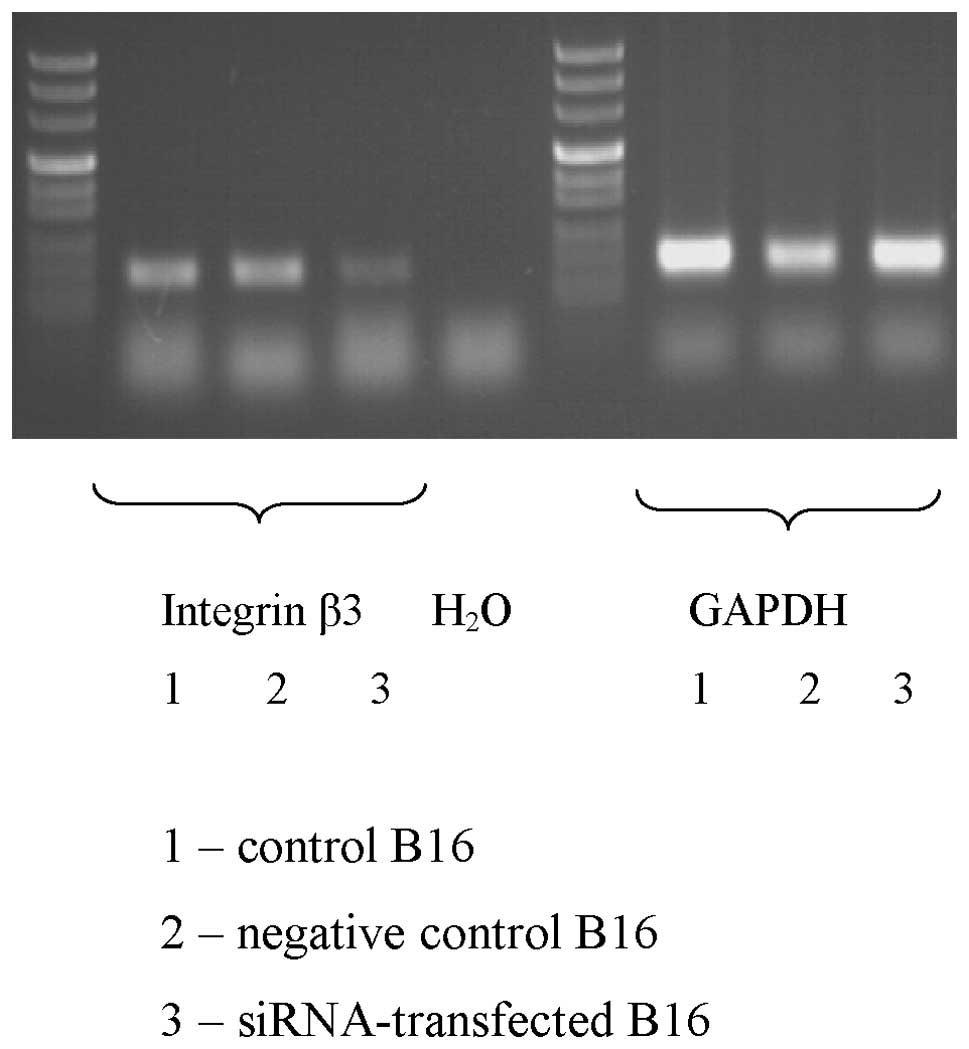
These changes were confirmed on mRNA level.
Semi-quantitative PCR revealed a marked decrease in the expression
of mRNA for integrin β3 as a result of the siRNA transfection. No
significant differences were observed in the expression of integrin
β3 mRNA between control, untreated cells and cells transfected with
negative siRNA (Fig. 3).
Inhibition of cell adhesion to matrix
proteins by RNA interference in vitro
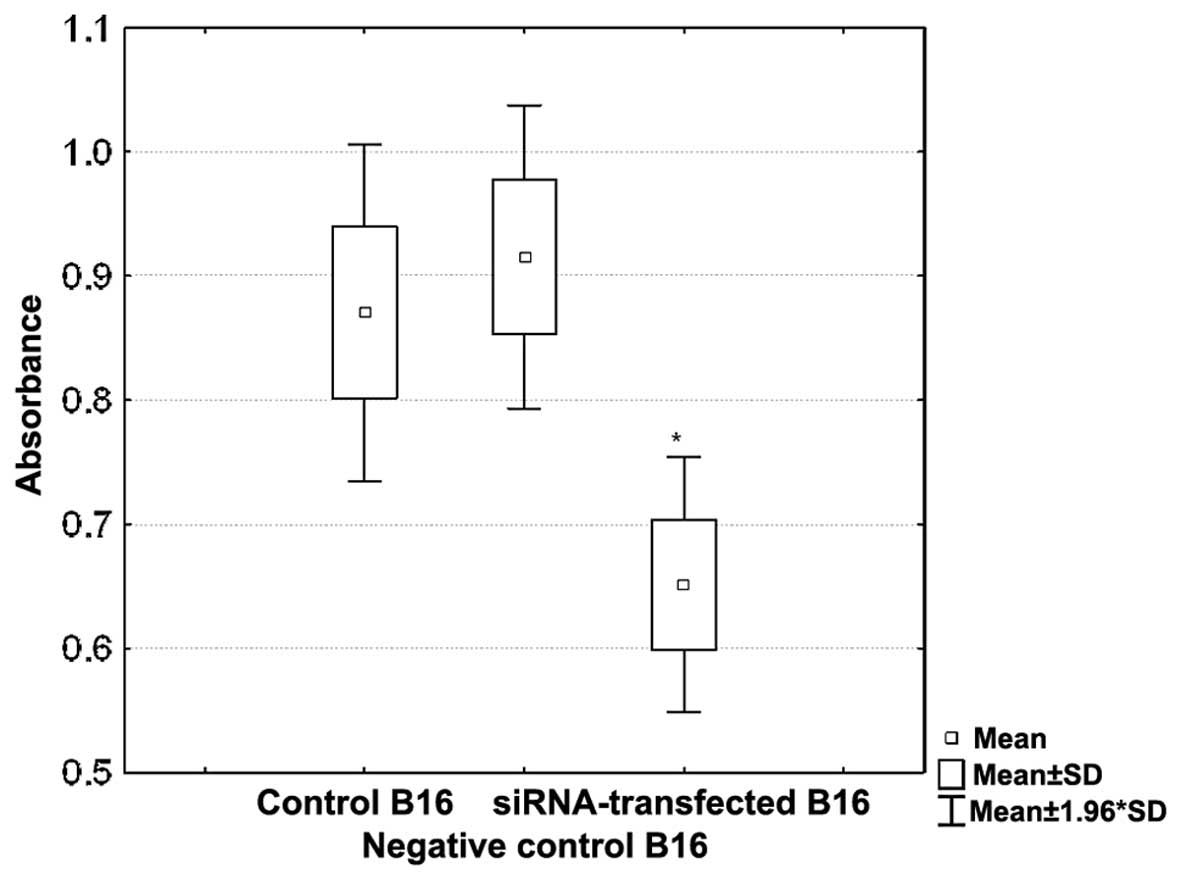
To estimate the possible effects of integrin β3
silencing on the cell-ECM interactions, we studied adhesive
properties of B16 cells on fibrinogen-coated plates. B16 cells were
transfected with 25 nM of siRNA and the transfection was repeated
after 48 h. The expression of integrin β3 was measured by
cytofluorometry after additional 24 h. The experiment was repeated
twice and in each attempt almost 90% silencing of integrin β3 was
obtained. It corresponded to a statistically significant impairment
of the adhesion to fibrinogen. siRNA-transfected B16 cells bound to
fibrinogen-coated wells were 31% weaker in comparison to the
control, non-transfected cells (Fig.
4).
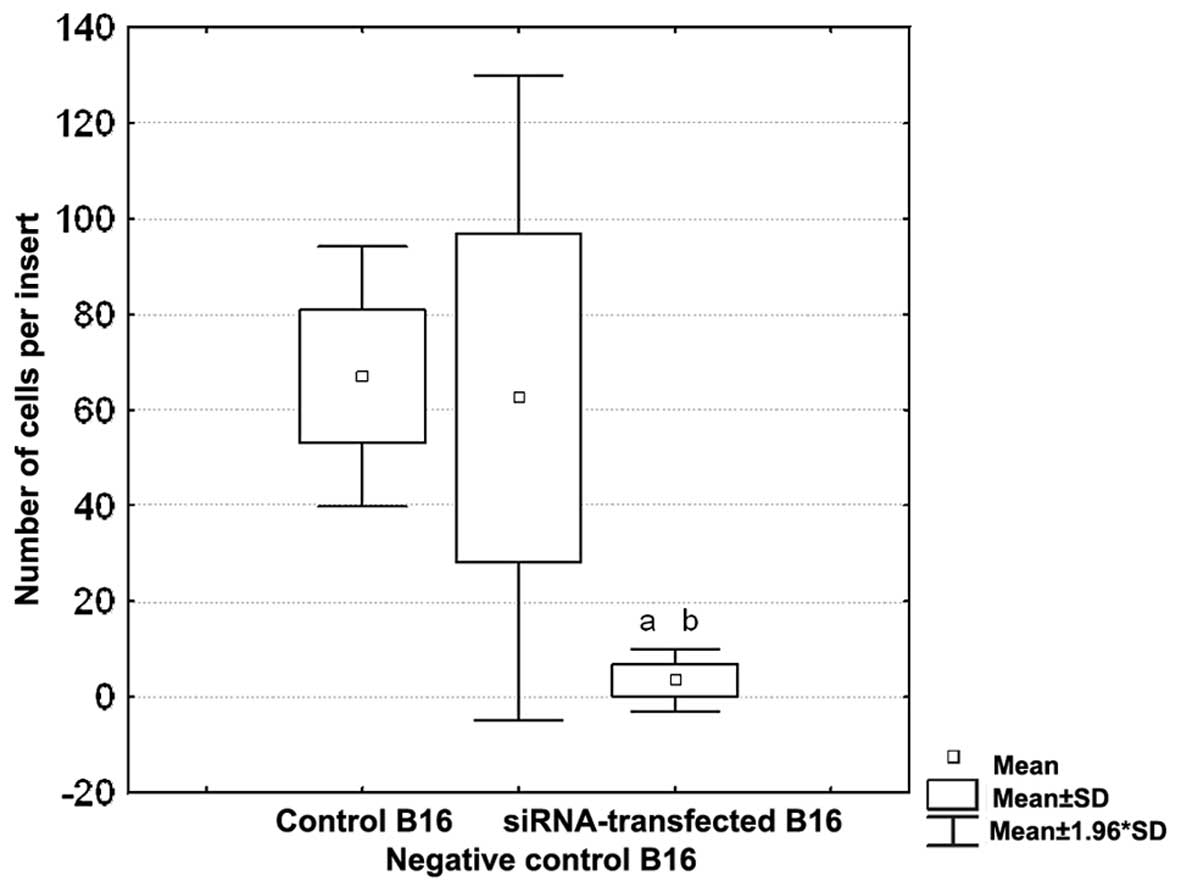
Inhibition of cell migration by RNA
interference in vitro
To verify the influence of integrin β3 on the
motility of B16 melanoma cells, the migration assay was performed.
B16 cells with silenced expression of integrin β3 (80% lower than
the control, untreated cells) were applied. Inhibition of integrin
β3 expression caused almost complete impairment of the ability of
B16 cells to migrate through the fibronectin-coated inserts
(Fig. 5). Mean number of B16
siRNA-transfected cells detected on the bottom side of the membrane
was 4±3, whereas this value for the control, untreated cells was
67±14 (p<0.01). No influence of the transfection with negative
siRNA on the cell motility was observed (63±34).
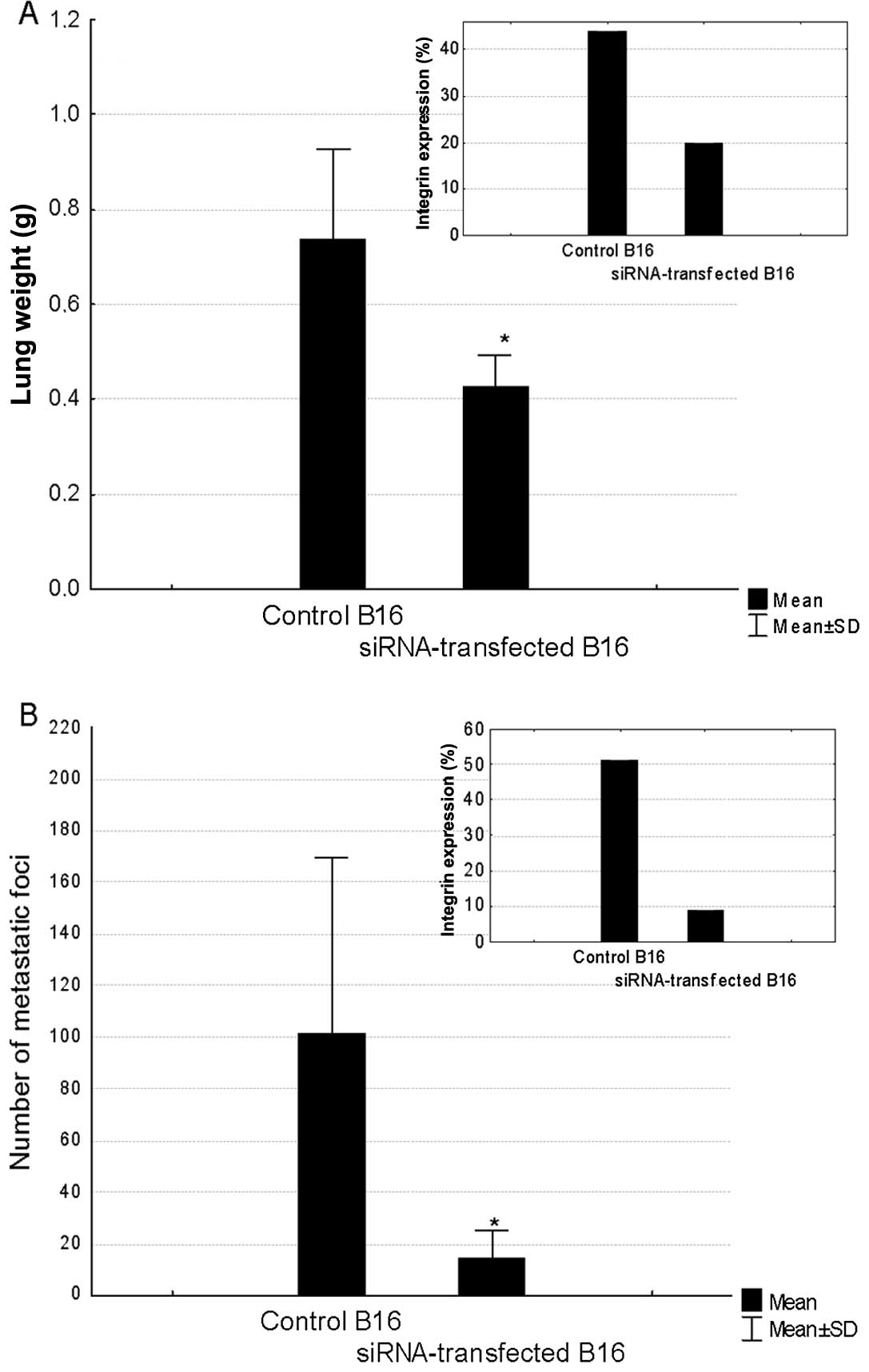
Inhibition of metastatic potential by RNA
interference
C57/BL6 mice were inoculated intravenously (i.v.)
with B16 cells transfected with anti-integrin β3 siRNA, negative
siRNA or non-transfected, control ones. A correlation between the
level of silencing of integrin β3 expression and the inhibition of
metastatic potential of B16 cells was observed. In the first
experiment, the expression of integrin β3 on siRNA-treated cells
was inhibited by 55%. At the end of the experiment, the lungs were
excised and weighed. The mean lung weight in the control mice was
0.74 g. It was significantly decreased in the group of mice
inoculated with B16 cells transfected with siRNA against integrin
β3 (Fig. 6A). The 42% drop in the
lung weight in these mice corresponded to 55% decrease in the
expression of CD61 in the transfected cells measured by
cytofluorymetry prior to the melanoma cells inoculation. However,
83% silencing of integrin β3 expression led to 86% drop in the
number of lung metastatic foci as compared to the control values
(Fig. 6B). No significant
inhibition of metastatic potential of B16 cells treated with
negative siRNA was observed.
Discussion
Many studies have shown that the expression of
integrins alters frequently during malignant transformation. These
changes comprise both alterations in the number and identity of
integrin receptors on cancer cells (8). Special attention is focused on the
role of both αvβ3 and αIIbβ3 in tumour growth, invasion and
metastasis. Tumour cells expressing αvβ3 and/or αIIbβ3 display
increased survival and growth in vivo(3), and increased metastatic potential
(19). Upregulation of integrin
expression results in alteration of the ability of malignant cells
to interact with the extracellular matrix, and promotes migration
as well as facilitates survival outside the tumour
microenvironment.
The importance of both αvβ3 and αIIbβ3 has been
extensively studied in melanoma. Presence of β3 subunit is a
characteristic of melanoma, and is strongly associated with the
disease progression and poor prognosis (1–2,20).
Integrins have been shown to be potential targets
for drug development for therapeutic applications including
anticancer treatment (21).
Biological methods targeting integrins include monoclonal
antibodies (16,22,23),
peptides containing RGD or KGD motifs (24,25),
RGD analogues (26), and more
recently, siRNAs (27,28). RNA interference (RNAi) is a
sequence-specific post-transcriptional gene silencing by
double-stranded RNA. This mechanism, first discovered by Mello and
Fire in Caenorhabditis elegans is present and conserved in a
range of organisms (29). Despite
the endogenous origin, siRNA can be introduced efficiently into the
cells. For over a decade now, siRNAs have been successfully used
for targeting and knockdown of sequence-specific mRNAs and has
become a key experimental tool for the analysis of gene function.
SiRNA have also moved into the clinic; several siRNA-based
therapeutic strategies have entered clinical trials (30).
Herein, we report for the first time that siRNA can
selectively and efficiently silence the expression of integrin β3
subunit in B16 melanoma cells. The effect is manifested 48 h after
transfection and can be significantly enhanced by double
transfection (first, shortly after seeding of the cells and second,
48 h later). Integrin β3-silencing does not affect the
proliferation rate of B16 cells.
Clinically, metastatic phenotype of melanoma tumours
depends on peculiar adhesive, invasive and migratory properties of
tumour cells. This is mostly correlated with the expression of the
adhesion receptor integrin αvβ3 and αIIbβ3.
In order to metastasise, tumour cells need to detach
from the primary tumour, gain access to blood vessels, survive in
blood stream, then attach to vascular endothelial cells,
extravasate from blood vessels and finally, colonize distant
tissues and organs. These steps are strongly dependent on the
cross-talk between tumour and endothelial cells as well as on
cell-ECM interactions. Among ECM ligands for β3 integrins,
fibrinogen, fibronectin and vitronectin are of special importance
(31–34). It has been shown that in
fibrinogen-deficient mice, a significant reduction in the number of
lung metastases formed by B16 melanoma and LLC (Lewis Lung
Carcinoma) cells was observed (35). Proteolytic fragments or recombinant
peptides containing certain domains of fibronectin can inhibit
integrin-mediated adhesion, angiogenesis and metastasis in various
experimental tumour models (36–39;
reviewed in refs. 21,41). In our studies, the transfection of
B16 melanoma cells with siRNA for integrin β3, resulting in 90%
silencing of protein expression, corresponding to a statistically
significant impairment of the adhesion to fibrinogen.
siRNA-transfected B16 cells bound to fibrinogen-coated plates were
31% weaker than the control, integrin-positive cells. These
observations probably point toward the involvement of other
adhesive proteins in the interactions between B16 melanoma cell and
fibrinogen. These may include α4β1 or α5β1 integrins (40,41).
In these studies, we also show that siRNA-mediated
silencing of integrin β3 expression significantly affects the
metastatic potential of B16 cells. B16 cells that express lower
levels of integrin β3 form less metastatic foci in lungs when
injected into tail vein in comparison to the control
non-transfected cells. This may result from the impairment of
several steps which are crucial for the colonization of distant
organs, i.e. i) survival in bloodstream, ii) attachment to vascular
endothelial cells, iii) basal membrane disintegration, iv)
extravasation from vessel lumen, and v) establishment of secondary
tumours. Integrin β3 expressed on the surface of B16 cells is
involved in all these steps. Since the integrin β3-knockdown is
transitory, it seems that the impairment of early steps of this
‘metastatic cascade’ is crucial for long-term effects observed in
our studies. It has been shown that the survival rate of tumour
cells in bloodstream may be connected with the interactions between
tumour cells and platelets, which, in turn, seem to be fibrinogen
related. Recent studies have demonstrated that platelets and
fibrinogen facilitate each other in protecting tumour cells from
natural killer cytotoxicity (42).
It has also been suggested that the formation of
platelet-fibrin-tumour cell aggregates may be causally related to
endothelial adhesion and metastatic potential (43–45).
Since the adhesion to fibrinogen is inhibited in β3-deficient
cells, this may explain the low metastatic potential of
siRNA-transfected B16 cells.
β3-silenced cells are probably unable to adhere to
vessel walls. It may be suggested that the production and/or
activation of matrix metalloproteinases (MMPs) essential for
basement membrane disruption is inhibited (46,47).
This may clearly affect the migration of B16 cells through the
vessel walls. We also show that silencing of β3 expression in B16
cells leads to a dramatic loss of migratory properties. This could
be explained both by the inhibition of B16 cells-ECM interactions
as well as by the abrogation of signal transduction pathways
promoting cell motility (48–51).
In summary, our experiments have proved that siRNA
transfection is an effective tool for the silencing of integrin β3
expression in B16 melanoma cells. The inhibition of integrin β3
expression on cell surface is correlated with impaired motility,
ability to bind to ECM proteins and significantly lower metastatic
potential. Furthermore, our studies suggest that the impairment of
early steps of this ‘metastatic cascade’ is crucial for long-term
effects.
References
|
1
|
Albelda SM, Mette SA, Elder DE, Stewart R,
Damjanovich L, Herlyn M and Buck CA: Integrin distribution in
malignant melanoma: association of the β3 subunit with tumor
progression. Cancer Res. 50:6757–6764. 1990.
|
|
2
|
Hieken TJ, Farolan M, Ronan SG, Shilkaitis
A, Wild L and Das Gupta TK: β3 integrin expression in melanoma
predicts subsequent metastasis. J Surg Res. 63:169–173. 1996.
|
|
3
|
Trikha M, Timar J, Zacharek A, Nemeth JA,
Cai Y, Dome B, Somlai B, Raso E, Ladanyi A and Honn KV: Role for β3
integrins in human melanoma growth and survival. Int J Cancer.
101:156–167. 2002.
|
|
4
|
Cooper CR, Chay CH and Pienta KJ: The Role
of alpha(v)beta(3) in prostate cancer progression. Neolplasia.
4:191–194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rezaeipoor R, Chaney EJ, Oldenburg AL and
Boopart S: Expression order of alpha-v and beta-3 integrin subunits
in the N-methyl-N-nitrosourea-induced rat mammary tumor model.
Cancer Invest. 27:496–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leu SJ, Lam SC and Lau LF: Pro-angiogenic
activities of CYR61 (CCN1) mediated through integrins avb3 and a6b1
in human umbilical vein endothelial cells. J Biol Chem.
277:46248–46255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nam JO, Kim JE, Jeong HW, Lee SJ, Lee BH,
Choi JY, Park RW, Park JY and Kim IS: Identification of the αvβ3
integrin-interacting motif of βig-h3 and its anti-angiogenic
effect. J Biol Chem. 278:25902–25909. 2003.
|
|
8
|
Mizejewski GJ: Role of integrins in
cancer: survey of expression patterns. Proc Soc Exp Biol Med.
222:124–138. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Minamiguchi K, Kumagai H, Masuda T, Kawada
M, Ishizuka M and Takeuchi T: Thiolutin, an inhibitor of HUVEC
adhesion to vitronectin, reduces paxillin in HUVECs and suppresses
tumour cell-induced angiogenesis. Int J Cancer. 93:307–316. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nikos E, Tsopanoglou NE, Andriopoulou P
and Maragoudakis ME: On the mechanism of thrombin-induced
angiogenesis: involvement of αvβ3-integrin. Am J Physiol Cell
Physiol. 283:1501–1510. 2002.PubMed/NCBI
|
|
11
|
Burns GF, Cosgrove L, Triglia T, Beall JA,
Lopez AF, Werkmeister JA, Begley CG, Haddad AP, d’Apice AJ, Vadas
MA, et al: The IIb-IIIa glycoprotein complex that mediates platelet
aggregation is directly implicated in leukocyte adhesion. Cell.
45:269–280. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Timar J, Trikha M, Szekeres K, Bazaz R,
Tovari J, Silletti S, Raz A and Honn KV: Autocrine motility factor
signals integrin-mediated metastatic melanoma cell adhesion and
invasion. Cancer Res. 56:1902–1908. 1996.PubMed/NCBI
|
|
13
|
Oleksowicz L, Mrowiec Z, Schwartz E,
Khorshidi M, Dutcher J and Puszkin E: Characterization of
tumour-induced platelet aggregation: the role of immunorelated GPIb
and GPIIb/IIIa expression by MCF-7 breast cancer cells. Thromb Res.
79:261–274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheu JR, Lin CH, Peng HC and Huang TF:
Triflavin, an Arg-Gly-Asp-containing peptide, inhibits human
cervical carcinoma (HeLa) cell-substratum adhesion through an
RGD-dependent mechanism. Peptides. 15:1391–1398. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yun Z, Menter DG and Nicolson GL:
Involvement of integrin alphavbeta3 in cell adhesion, motility, and
liver metastasis of murine RAW117 large cell lymphoma. Cancer Res.
56:3103–3111. 1996.PubMed/NCBI
|
|
16
|
Cohen SA, Trikha M and Mascelli MA:
Potential future clinical applications for the GPIIb/IIIa
antagonist, abciximab in thrombosis, vascular and oncological
indications. Pathol Oncol Res. 6:163–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Auzzas L, Zanardi F, Battistini L,
Burreddu P, Carta P, Rassu G, Curti C and Casiraghi G: Targeting
alphavbeta3 integrin: design and applications of mono- and
multifunctional RGD-based peptides and semipeptides. Curr Med Chem.
17:1255–1299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skehan P, Storeng R, Sudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang YS, Chen YQ, Timar J, Nelson KK,
Grossi IM, Fitzgerald LA, Diglio CA and Honn KV: Increased
expression of alpha IIb beta 3 integrin in subpopulations of murine
melanoma cells with high lung-colonizing ability. Int J Cancer.
51:445–451. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Belle PA, Elenitsas R, Satyamoorthy K,
Wolfe JT, Guerry DI, Schuchter L, Van Belle TJ, Albelda S, Tahin P,
Herlyn M and Elder DE: Progression-related expression of beta3
integrin in melanomas and nevi. Hum Pathol. 33:562–567.
1999.PubMed/NCBI
|
|
21
|
Perdih A and Dolenc MS: Small molecule
antagonists of integrin receptors. Curr Med Chem. 17:2371–2392.
2010. View Article : Google Scholar
|
|
22
|
Brooks PC, Stromblad S, Klemke R, Visscher
D, Sarkar FH and Cheresh DA: Antiintegrin alpha v beta 3 blocks
human breast cancer growth and angiogenesis in human skin. J Clin
Invest. 96:1815–1822. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitjans F, Meyer T, Fittschen C, Goodman
S, Jonczyk A, Marshall JF, Reyes G and Piulats J: In vivo therapy
of malignant melanoma by means of antagonists of αv integrins. Int
J Cancer. 87:716–723. 2000.
|
|
24
|
Isoai A, Ueno Y, Giga-Hama Y, Goto H and
Kumagai HA: A novel Arg-Gly-Asp containing peptide specific for
platelet aggregation and its effect on tumor metastasis: a possible
mechanism of RGD peptide-mediated inhibition of tumor metastasis.
Cancer Lett. 65:259–264. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buerkle MA, Pahernik SA, Sutter A, Jonczyk
AKM and Dellian M: Inhibition of the alpha-nu integrins with a
cyclic RGD peptide impairs angiogenesis, growth and metastasis of
solid tumours in vivo. Br J Cancer. 86:788–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perkins JJ, Duong LT, Fernandez-Metzler C,
Hartman GD, Kimmel DB, Leu C-T, Lynch JJ, Prueksaritanont T, Rodan
GA, Rodan SB, et al: Non-peptide alpha(v)beta(3) antagonists:
identification of potent, chain-shortened RGD mimetics that
incorporate a central pyrrolidinone constraint. Bioorg Med Chem
Lett. 13:4285–4288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han HD, Mangala LS, Lee JW, Shahzad MM,
Kim HS, Shen D, Nam EJ, Mora EM, Stone RL, Lu C, et al: Targeted
gene silencing using RGD-labeled chitosan nanoparticles. Clin
Cancer Res. 16:3910–3922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roman J, Ritzenthaler JD, Roser-Page S,
Sunx and Han S: Alpha5beta1-integrin expression is essential for
tumor progression in experimental lung cancer. Am J Respir Cell Mol
Biol. 43:684–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature.
391:806–811. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hart IR, Birch M and Marshall JF: Cell
adhesion receptor expression during melanoma progression and
metastasis. Cancer Metastasis Rev. 10:115–128. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seftor RE: Role of the beta3 integrin
subunit in human primary melanoma progression: multifunctional
activities associated with alpha(v)beta3 integrin expression. Am J
Pathol. 153:1347–1351. 1998. View Article : Google Scholar
|
|
33
|
Switala-Jelen K, Dabrowska K, Opolski A,
Lipinska L, Nowaczyk M and Gorski A: The biological functions of
beta3 integrins. Folia Biol. 50:143–152. 2004.PubMed/NCBI
|
|
34
|
Akiyama SK, Olden K and Yamada KM:
Fibronectin and integrins in invasion and metastsis. Cancer
Metastasis Rev. 14:173–189. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000.PubMed/NCBI
|
|
36
|
Yi M and Ruoslahti E: A fibronectin
fragment inhibits tumor growth, angiogenesis and metastasis. Proc
Natl Acad Sci USA. 98:620–624. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong W, Liu Y, Huang B, Lei Z, Wu F-H, Li
D, Feng Z-H and Zhang G-M: Recombinant CBD-HepII polypeptide of
fibronectin inhibits αvβ3 signaling and hematogenous metastasis of
tumor. Biochem Biophys Res Commun. 367:144–149. 2008.
|
|
38
|
Ramos OH, Kauskot A, Cominetti MR, Bechyne
I, Salla Pontes CL, Chareyre F, Manent J, Vassy R, Giovannini M,
Legrand C, et al: A novel avb3-blocking disintegrin containing the
RGD motive DisBa-01, inhibits bFGF-induced angiogenesis and
melanoma metastasis. Clin Exp Metastasis. 25:53–64. 2008.
View Article : Google Scholar
|
|
39
|
Sheldrake HM and Patterson LH: Function
and antagonism of beta3 integrins in the development of cancer
therapy. Curr Cancer Drug Targets. 9:519–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gehlsen KR, Davis GE and Sriramarao P:
Integrin expression in human melanoma cells with differing invasive
and metastatic properties. Clin Exp Metastasis. 10:111–120. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rebhun RB, Cheng H, Gershenwald JE, Fan D,
Fidler IJ and Langley RR: Constitutive expression of the alpha4
integrin correlates with tumorigenicity and lymph node metastasis
of the B16 murine melanoma. Neoplasia. 12:173–182. 2010.PubMed/NCBI
|
|
42
|
Zheng S, Shen J, Jiao Y, Zhang C, Wei M,
Hao S and Zeng X: Platelets and fibrinogen facilitate each other in
protecting tumor cells from natural killer cytotoxicity. Cancer
Sci. 100:859–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cavanaugh PG, Sloane BF and Honn KV: Role
of the coagulation system in tumor-cell-induced platelet
aggregation and metastasis. Haemostasis. 18:37–46. 1988.PubMed/NCBI
|
|
44
|
Crissman JD, Hatfield JS, Menter DG,
Sloane B and Honn KV: Morphological study of the interaction of
intravascular tumor cells with endothelial cells and subendothelial
matrix. Cancer Res. 48:4065–4072. 1988.PubMed/NCBI
|
|
45
|
Liu Y, Zhao F, Gu W, Yang H, Meng Q, Zhang
Y, Yang H and Duan Q: The roles of platelet GPIIb/IIIa and
alphavbeta3 integrins during HeLa cells adhesion, migration, and
invasion to monolayer endothelium under static and dynamic shear
flow. J Biomed Biotechnol. 2009:8292432009.PubMed/NCBI
|
|
46
|
Ria R, Vacca A, Ribatti D, Di Raimondo F,
Merchionne F and Dammacco F: Alpha(v)beta(3) integrin engagement
enhances cell invasiveness in human multiple myeloma.
Haematologica. 87:836–845. 2002.PubMed/NCBI
|
|
47
|
Sato T, Sakai T, Noguchi Y, Takita M,
Hirakawa S and Ito A: Tumor-stromal cell contact promotes invasion
of human uterine cervical carcinoma cells by augmenting the
expression and activation of stromal matrix metalloproteinases.
Gynecol Oncol. 92:47–56. 2004. View Article : Google Scholar
|
|
48
|
Cary LA and Guan JL: Focal adhesion kinase
in integrin-mediated signaling. Front Biosci. 4:D102–D113. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hao H, Naomoto Y, Bao X, Watanabe N,
Sakurama K, Noma K, Motoki T, Tomono Y, Fukazawa T, Shirakawa Y, et
al: Focal adhesion kinase as potential target for cancer therapy
(Review). Oncol Rep. 22:973–979. 2009.PubMed/NCBI
|
|
50
|
Yang J, Price MA, Li GY, Bar-Eli M, Salgia
R, Jagedeeswaran R, Carlson JH, Ferrone S, Turley EA and McCarthy
JB: Melanoma proteoglycan modifies gene expression to stimulate
tumor cell motility, growth, and epithelial-to-mesenchymal
transition. Cancer Res. 69:7538–7547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hou CH, Yang RS, Hou SM and Tang CH: TNF-α
increases αvβ3 integrin expression and migration in human
chondrosarcoma cells. J Cell Physiol. 226:792–799. 2011.
|