Introduction
Gastric cancer is one of the most common types of
malignant tumor and is the second leading cause of cancer-related
mortality worldwide, with an overall survival of approximately 10
months (1). Despite advances in the
treatment of gastric cancer, the prognosis of gastric cancer
remains poor. It has been acknowledged that gastric carcinogenesis
is a multistep process, involving numerous genetic and epigenetic
alterations, such as abnormalities in growth factors/receptors,
angiogenic factors and cell cycle regulators. These abnormalities
also define the biological characteristics of gastric cancer cells,
which may serve as therapeutic targets for gastric cancer (2). Thus, additional novel targets for
therapeutic development have been identified and are being
explored.
A previous study demonstrated that vascular
endothelial growth factor (VEGF) is a potent pro-angiogenic factor
which not only stimulates endothelial cell proliferation, migration
and survival, but also increases vascular permeability. The
promotion of angiogenesis is a well-known prerequisite for tumor
growth, invasion and metastasis (3). It has been reported that tumor cells
secrete VEGF, and this process is necessary for tumor growth
(4). Evidence has shown that solid
tumors do not grow beyond the volume of 2–3 mm3 in the
absence of neo-angiogenesis due to the insufficient diffusion of
oxygen and nutrients from the blood vessels. VEGF directly
activates the VEGF receptor expressed in tumor cells, leading to an
autocrine activation of primary cancer growth and acts as a
survival factor for the VEGF receptor-expressing tumor cells
(5,6). The inhibition of VEGF has shown
promising results in reducing tumor metastasis and/or primary tumor
growth in a number of models (7).
Blocking of VEGF-D by a mouse monoclonal anti-human-VEGF-D antibody
has been shown to be effective in halting primary tumor growth and
suppressing local tumor metastasis in a mouse xenograft tumor
model. Similarly, neutralizing antibodies against VEGF-receptor
(R)3 have been shown to inhibit lymph node metastasis and soluble
VEGF-R3, trapping both VEGF-C and VEGF-D, and thus blocking
lymphangiogenesis and lymph node metastasis in several models
(7). In addition, VEGF-Trap
(8), antisense oligonucleotides
(9) and RNA interference (RNAi)
have also been used to inhibit VEGF in tumor therapy studies. As a
result, VEGF may be an important target for tumor therapy.
Small interfering RNA (siRNA) has emerged as a
powerful strategy for the investigation of gene expression and
function compared with antisense oligonucleotides and neutralizing
antibodies. siRNA leads to greater specificity and efficiency when
targetting genes, and it may be designed in diverse ways due to its
versatility. In addition, siRNA is becoming an important tool for
the study of biological processes and has the potential for
therapeutic applications in human cancer diseases.
In our previous studies (10), we constructed lentivirus-mediated
VEGF siRNA, and proved that it can suppress gastric cancer cell
growth and induce apoptosis in SGC7901 human gastric cancer cells.
The aim of this study was to evaluate whether VEGF siRNA can
inhibit gastric cancer growth in vivo and elucidate the
mechanism of apoptosis induced by VEGF siRNA in SGC7901 cells.
Materials and methods
Cell culture
The SGC7901 human gastric cancer cell line was
obtained from the Chinese Type Culture Collection (Shanghai,
China). Cells were cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100
μg/ml). Cells were incubated at 37°C in a humidified incubator with
5% CO2.
Construction of lentiviral vectors and
transfection
Lentiviral vectors for human VEGF siRNA encoding a
green fluorescent protein (GFP) sequence were constructed by
GeneChem Co. (Shanghai, China). The target siRNA sequence was
CAGGAGT ACCCTGATGAGATC (GenBank accession no. NM003376). The
lentiviral vectors containing VEGF-C siRNA were constructed by
ligating the HpaI/XhoI digests of pGCL-GFP and the
VEGF siRNA PCR products were confirmed by DNA sequencing. The
negative control siRNA was provided by GeneChem. The
lentivirus-encoded siRNA targeting VEGF and the control were
prepared and titered to 2×109 (TU/ml) as previously
described (10).
The SGC7901 cells (2×105) were seeded in
6-well plates overnight before transfection. The virus
[multiplicity of infection (MOI)=10] was added to each well
containing an enhanced infection solution (EIS; Genechem) and
incubated for 8–12 h at 37°C, followed by incubation for 96 h in
complete RPMI-1640 medium. The cells were then harvested for
subsequent studies.
Human gastric tumor xenograft mouse
model
Four-week-old male Balb/c nude mice were purchased
from the Nanjing Peng Sheng Biotechnology Development Company,
Nanjing, China (certification no. 2007–004). The mice were housed
in a pathogen-free animal facility and were randomly divided into
the following 3 groups with 6 mice/group: the blank control,
negative control lentivirus (Nc-Lv) and VEGF-RNAi-Lv group. Cells
(2×106/0.2 ml) were subcutaneously injected into the
right axillary fossa of each mouse. Once the tumors had emerged,
tumor growth was monitored every 3 days and measured in 2
dimensions. Tumor volume was calculated using the formula V =
W2 × L/2, where ‘L’ and ‘W’ are the longest and shortest
diameters, respectively, and the tumor growth curve was drawn.
After a 2-week treatment, the mice were sacrificed, the tumor
weight and inhibition rate were evaluated and the tumors were
obtained for RT-PCR, western blot analysis and pathological
examination. All experimental procedures were carried out according
to the National Guidelines for the Care and Use of Laboratory
Animals and were approved by the Animal Care and Use Committee.
Histological sections and staining
For the histological analysis, the tumor tissues
were immediately fixed in 4% neutral-buffered formalin and embedded
in paraffin after the nude mice were sacrificed and the slides were
prepared for hematoxylin and eosin (H&E) staining. After
deparaffinization and redehydration the slides were stained with
H&E.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA from tumor cells and tissues was isolated
with a total RNA extraction kit (Sangon, Shanghai, China) according
to the manufacturer’s instructions. Total RNA (1 μg) was
reverse-transcribed into cDNA with the PrimeScript RT-PCR kit
(Takara Bio, Inc., Dalian, China). Subsequently, 2 μl of cDNA
product were subjected to PCR amplification with TaqDNA polymerase
(Bioer Technology, Beijing, China) on a thermal cycler. The PCR
primers used in this study are shown in Table I. Human GAPDH (hGAPDH) was used as
the internal control. The PCR conditions were as follows: 1 cycle
of denaturation at 94°C for 10 min, followed by 30 cycles at 94°C
for 1 min, 55°C for 1 min and 72°C for 1 min, before a final
extension at 72°C for 10 min. The PCR products were loaded onto 2%
agarose gels and visualized with ethidium bromide under UV light.
This experiment was performed 3 times and representative data are
shown.
 | Table IhGAPDH and VEGF primers used in this
study. |
Table I
hGAPDH and VEGF primers used in this
study.
| Gene | Primer sequence | PCR product length
(bp) |
|---|
| hGAPDH | Sense:
5′-GGCTCTCCAGAACATCAT-3′ | 240 |
| Antisense:
5′-CACCTGGTGCTCAGTGTA-3′ | |
| VEGF | Sense:
5′-CTACCTCCACCATGCCAAGT-3′ | 411 |
| Antisense:
5′-AAATGCTTTCTCCGCTCTGA-3′ | |
Western blot analysis
Whole-cell protein extracts from SGC7901 cells and
the tumor xenografts were prepared with the Tissue and Cell Lysis
Solution (BIOS, Beijing, China), according to the manufacturer’s
instructions. Protein concentrations were determined using a BCA
assay kit (BIOS). Samples were adjusted to equal protein
concentrations and volume and subjected to SDS-PAGE and transferred
onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA).
Following blocking, the membranes were incubated with primary
antibodies against SIRT1, p53, p21, Bcl-2 and survivin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and then incubated with
HRP-conjugated secondary antibodies. The specific protein was
detected using a SuperSignal protein detection kit (Pierce,
Rockford, IL, USA).
ELISA
Ninty-six hours post lentivirus infection, the
SGC7901 cell supernatants were collected to detect the VEGF levels.
The absorbance at 450 nm was measured using a human VEGF ELISA kit
(Jingmei Biotech Co., Ltd., Beijing, China) according to the
manufacturer’s instructions and the concentration of VEGF in the
supernatants was calculated.
Statistical analysis
The data are expressed as the means ± SD. The
significance of the data was determined by one-way ANOVA analysis.
A P-value <0.05 was considered to indicate a statistically
significant difference. All the statistical analyses were performed
with SPSS 13.0 software.
Results
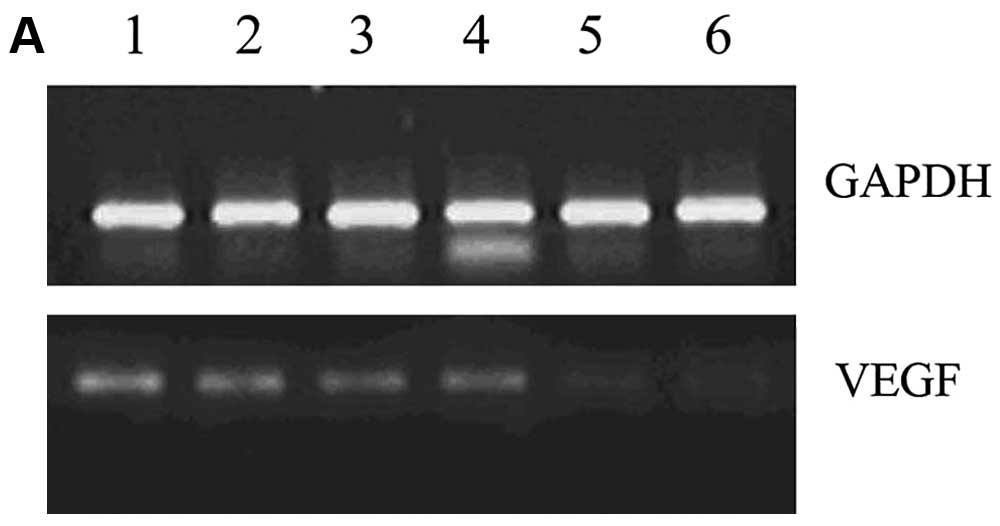
Effect of VEGF siRNA on the expression of
VEGF in SGC7901 cells
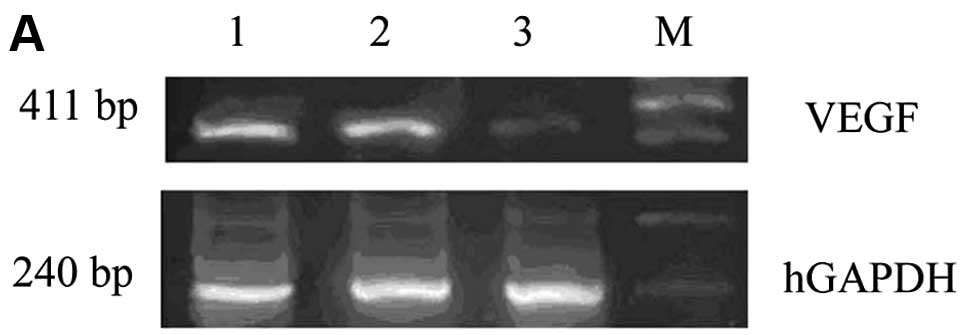
The silencing effects of VEGF siRNA in SGC7901 cells
were evaluated by RT-PCR and ELISA. The RT-PCR results showed that
the VEGF mRNA expression in the VEGF siRNA group was significantly
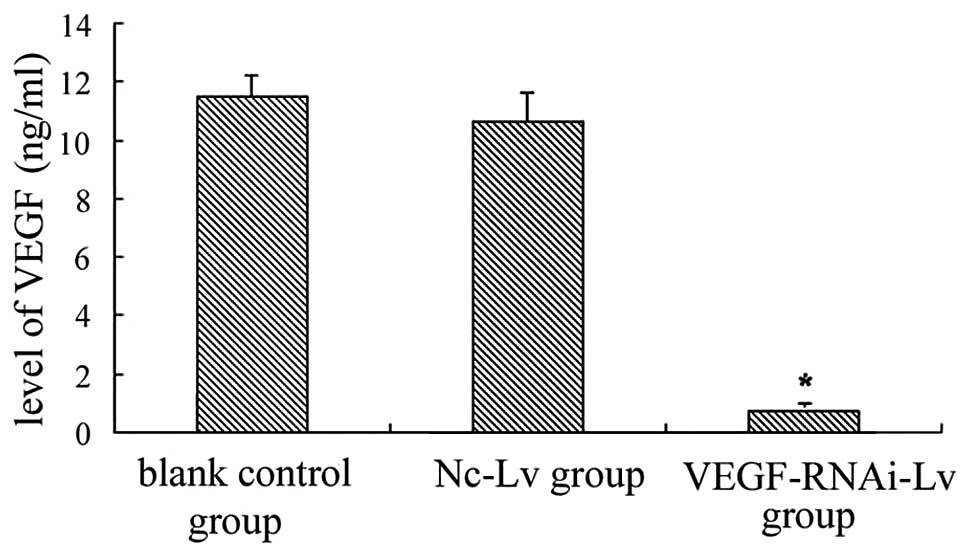
suppressed compared to the control groups (Fig. 1). In accordance with this, ELISA
indicated that the VEGF protein level in the VEGF siRNA group was
decreased by 93.6% (0.699±0.054 ng/ml) compared to the blank
control group (11.459±0.782 ng/ml) and the negative control group
(10.642±0.981 ng/ml) (Fig. 2).
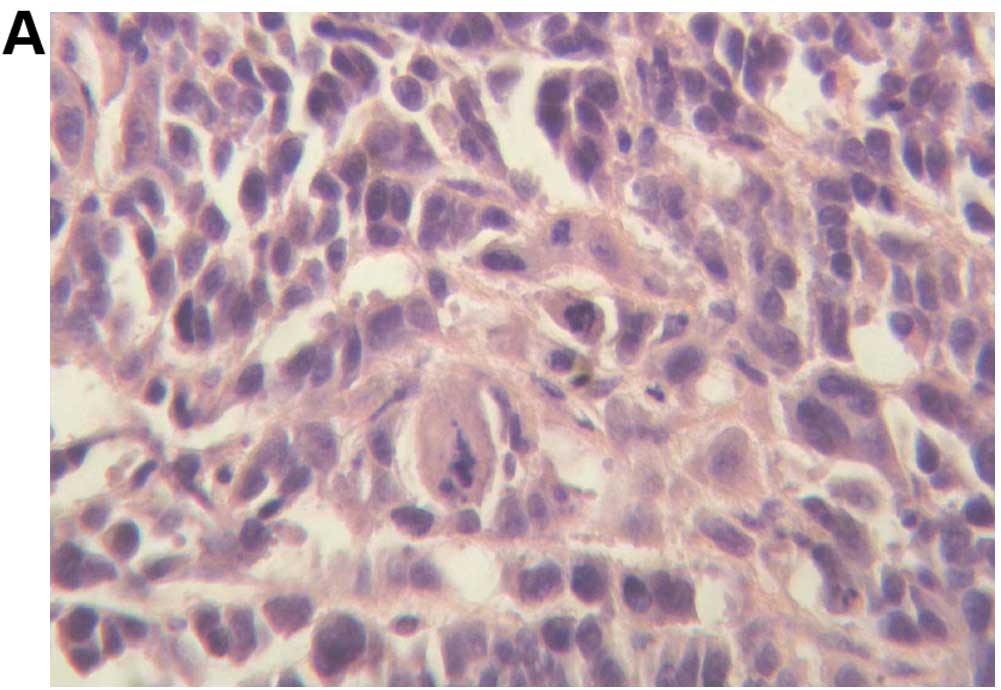
Histological staining results
To confirm the biological characteristics of the
xenograft tumors, H&E staining was performed. The results
indicated that the xenograft tumors in the 3 groups were poorly
differentiated carcinoma, with large areas of necrosis in the blank
control and negative control groups. However, there was no necrosis
in the siRNA-treated group (Fig.
3).
Influence of VEGF siRNA on gastric tumor
growth in xenografts
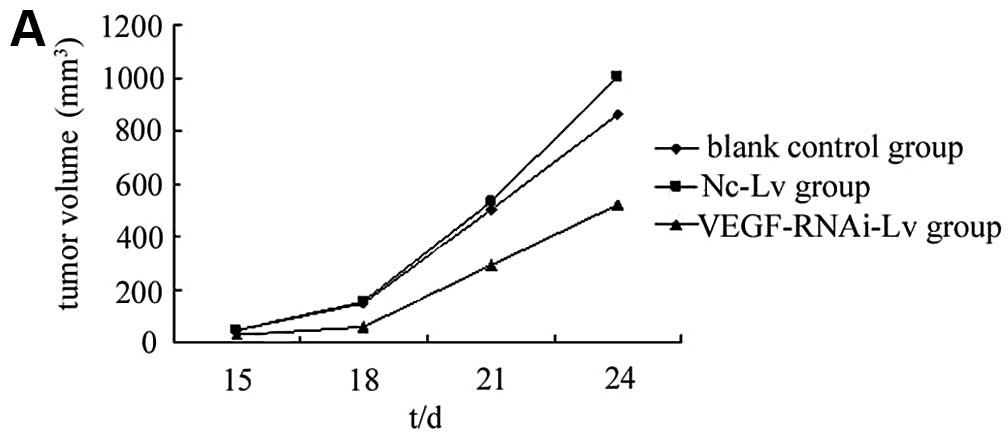
We then investigated the possibility of using VEGF
as a target gene for gastric tumor therapy in the nude mouse tumor
xenograft model. The data showed that on day 10, all mice formed a
palpable tumor at the sites of injection. Animals were sacrificed
on day 24. The mean tumor size of the blank control group was
856.84±89.39 mm3; the mean tumor size of the Nc-Lv
negative control group was 1002.01±142.07 mm3 and that
of the VEGF-RNAi-Lv group was 518.01±67.98 mm3. There
were no significant differences between the blank control group and
the Nc-Lv negative control group (P>0.05) (Fig. 4). However, the VEGF-RNAi-Lv group
showed significant tumor growth suppression compared to the blank
control group (P<0.05). No toxicity was observed in the mice, as
assessed by changes in behavior, appearance or weight.
VEGF siRNA suppresses VEGF expression in
tumor xenografts
We then examined the effect of VEGF siRNA on VEGF
mRNA in vivo by RT-PCR. The VEGF mRNA expression was
significantly decreased in the VEGF-RNAi-Lv group compared to the
blank control group (Fig. 5A and
B). However, the difference between the Nc-Lv group and the
blank control group was not statistically significant (P>0.05).
We also detected the VEGF protein level of these 3 groups. The
results showed that the expression of the VEGF protein in the
VEGF-RNAi-Lv group was suppressed significantly. Similar resutls
were obsereved with the VEGF mRNA expression (Fig. 5C and D).
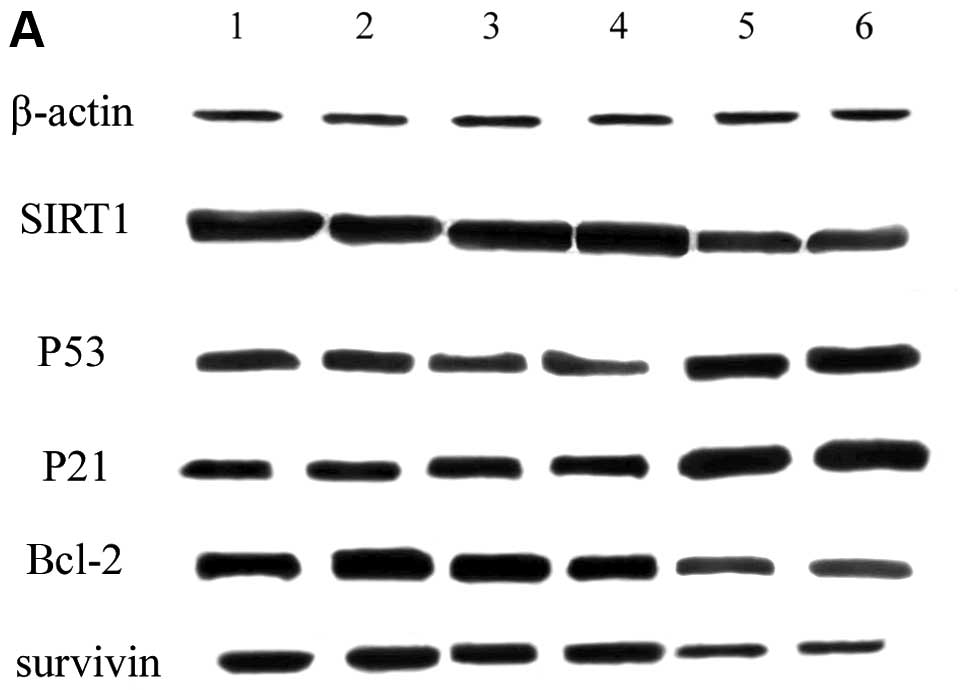
VEGF siRNA affects the expression of
apoptosis-associated proteins
We show that the downregulation of VEGF by RNAi
induces apoptosis in SGC7901 cells. It is known that SIRT1, p53,
Bcl-2, survivin and p21 are important proteins associated with
apoptosis. Therefore, we detected the levels of these
apoptosis-associated proteins by western blot analysis. The results
indicated that the expression of SIRT1, Bcl-2 and survivin was
downregulated (P<0.05) in the VEGF-RNAi-Lv group; however, the
expression of p53 and p21 was upregulated (P<0.05) in the
VEGF-RNAi-Lv group. There was no significant difference between the
Nc-Lv control group and the blank control group (Fig. 6).
Discussion
The VEGF system is essential for angiogenesis, and
VEGF overexpression frequently correlates with increased
microvascularity and metastasis and decreased spontaneous apoptosis
(11). VEGF is expressed in a
variety of cells, including smooth muscle, endothelial, epithelial
and a number of cancer cells (12).
It has been reported that VEGF is overexpressed in many types of
human cancer and cell lines, such as ovarian, breast, lung and
gastric cancer. Evidence has shown that VEGF is associated with
migration and proliferation and prolongs cell survival in gastric
cancer. The effects of VEGF on gastric cancer cell proliferation,
differentiation, migration and survival predicates VEGF for
potential therapeutic antitumor strategies.
RNA interference is a naturally occurring endogenous
regulatory process where short double-stranded RNA (dsRNA) causes
sequence-specific post-transcriptional gene silencing. In the
cytoplasm, long dsRNAs are cleaved by the endoribonuclease Dicer
into siRNA and loaded onto a RNA-induced silencing complex (RISC),
causing mRNA degradation and gene silencing (13). Compared with the traditional gene
silencing techniques, RNAi is a highly efficient and specific
regulatory process. At present, RNAi is widely used to inhibit
genes involved in signaling transduction, angiogenesis, drug
resistance and regulating apoptosis and cell cycle changes in
cancer studies (14).
It has been reported that the downregulation of
survivin expression by siRNA may induce apoptosis in pancreatic
cancer cells (PC-2 cell line) and enhance its sensitivity to
radiotherapy (15). Yao et
al (16) transfected COX-2
siRNA into the human gastric cancer cell line SGC7901, and showed
that the downregulation of COX-2 can significantly inhibit the
growth of gastric cancer cells in vitro and in vivo
and suppress the migration and tube formation of human umbilical
vein endothelial cells.
Recent studies (17–20)
have demonstrated that siRNA targeting VEGF may specifically
suppress VEGF expression in breast, prostate, colorectal cancer and
a number of other cancer cells. By contrast, data on cancer
treatments with lentivirus-mediated VEGF siRNA are rare.
In this study, we applied lentivius-mediated siRNA
to inhibit VEGF expression in SGC7901 cells and in a nude mouse
model of subcutaneous xenografts. We demonstrate that the silencing
of VEGF may suppress the growth of xenograft tumors. Compared to
the controls, the VEGF siRNA-treated mice showed a significant
suppression of tumor growth. This antitumor efficacy may be
attributed to the inhibition of tumor angiogenesis as suggested by
a reduced VEGF expression, demonstrating that siRNA targeting VEGF
may be an effective pathway for inhibiting gastric tumor growth,
and may represent a novel treatment for VEGF overexpression in
gastric cancer. This strategy may also have great potential for use
in clinical trials for the treatment of gastric tumors.
In a previous study (21), we demonstrated that VEGF silencing
induced apoptosis in SGC7901 cells. However, the precise mechanism
involved remains unclear. Since p53 is important in cell apoptosis,
it is interesting to investigate whether VEGF siRNA has an effect
on p53, Therefore, we studied the correlation between p53 and
apoptosis induced by VEGF silencing. Our results showed that the
expression of the p53 protein increased with siRNA targeting VEGF,
which indicated that p53 was involved in the process of SGC7901
cell apoptosis induced by VEGF silencing. It has been acknowledged
that p53 translocates into the nucleus to activate p21 while
suppressing Bcl-2 and survivin gene expression, leading to cell
apoptosis (22,23). Therefore, we detected the expression
levels of p21, Bcl-2 and survivin, and the results showed that in
the VEGF-RNAi-Lv-treated group, p21 expression was upregulated,
while the expression of Bcl-2 and survivin was downregulated,
suggesting that in principle, VEGF siRNA efficiently inhibited
intracellular signal transduction.
SIRT1, a proto member of the sirtuin family, not
only modifies histones through deacetylation but also deacetylates
many non-histone proteins that are involved in cell growth,
apoptosis, neuronal protection, cell senescence and tumorigenesis
(24). p53 has been shown to be a
downstream target of SIRT1. SIRT1 physically interacts with p53 and
deacetylates the p53 protein with a specificity at its C-terminal
Lys382 residue, decreases p53-mediated transcriptional activation
and reduces the downstream protein (p21) level (25). It has also been reported that the
inhibition of SIRT1 causes p53 hyperacetylation and increases
p53-dependent transcriptional activity (23), causing a decrease in cell growth,
cell viability and the colony-forming ability of prostate cancer
cells (26). Therefore, we further
analyzed the expression pattern of the p53 and SIRT1 proteins. Our
data showed that SIRT1 was downregulated significantly, while p53
was upregulated significantly after siRNA silencing. The reason may
be that SIRT1 blocks the nuclear translocation of p53 via its
deacetylation, resulting in the inhibition of p53 function as a
transcriptional regulator (27).
p53 transcriptional activity increases when SIRT1 is inhibited. The
mechanism of VEGF siRNA-induced apoptosis in gastric cancer cells
is as follows: siRNA decreases VEGF expression, which inhibits
SIRT1 expression, leading to the p53 transcriptional upregulation,
the activation of downstream p21 and the suppression of Bcl-2 and
survivin. Although VEGF silencing did not reach 100% knockdown in
the SGC7901 cells, signal transduction was efficiently inhibited,
supporting the effective antitumor response in vivo.
Taken together, the results from our study
demonstrated that lentivirus-mediated VEGF siRNA inhibited tumor
growth in SGC7901 cell xenografts. These data indicate that
lentivirus-mediated siRNA targeting VEGF offers a future option
against gastric cancer.
Acknowledgements
This study was supported by the Foundation of
Science and Technology Development Project of Shandong Province
(grant no. 2008GG10002018).
References
|
1
|
Claerhout S, Lim JY, Choi W, et al: Gene
expression signature analysis identifies vorinostat as a candidate
therapy for gastric cancer. PLOS One. 6:e246622011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong Y, Guo MZ, Ye ZJ, Zhang XL, Zhao YL
and Yang YS: Silence of HIN-1 expression through methylation of its
gene promoter in gastric cancer. World J Gastroenterol. 17:526–533.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le XF, Mao W, Lu C, Thornton A, Heymach
JV, Sood AK and Bast RC Jr: Specific blockade of VEGF and HER2
pathways results in greater growth inhibition of breast cancer
xenografts that overexpress HER2. Cell Cycle. 7:3747–3758. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanson J, Gorman J, Reese J and Fraizer G:
Regulation of vascular endothelial growth factor, VEGF gene
promoter by the tumor suppressor, WT1. Front Biosci. 12:2279–2290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bachelder RE, Crago A, Chung J, Wendt MA,
Shaw LM, Robinson G and Mercurio AM: Vascular endothelial growth
factor is an autocrine survival factor for neuropilin-expressing
breast carcinoma cells. Cancer Res. 61:5736–5740. 2001.PubMed/NCBI
|
|
6
|
Matsuura M, Onimaru M, Yonemitsu Y, et al:
Autocrine loop between vascular endothelial growth factor (VEGF)-C
and VEGF receptor-3 positively regulates tumor-associated
lymphangiogenesis in oral squamoid cancer cells. Am J Pathol.
175:1709–1721. 2009. View Article : Google Scholar
|
|
7
|
Rinderknecht M, Villa A, Ballmer-Hofer K,
Neri D and Detmar M: Phage-derived fully human monoclonal antibody
fragments to human vascular endothelial growth factor-c block its
interaction with VEGF receptor-2 and 3. PLoS One. 5:e119412010.
View Article : Google Scholar
|
|
8
|
Lassoued W, Murphy D, Tsai J, Oueslati R,
Thurston G and Lee WM: Effect of VEGF and VEGF trap on vascular
endothelial cell signaling in tumors. Cancer Biol Ther.
10:1326–1333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng LF, Li YJ, Wang H, Zhao JL, Wang XF
and Hu YS: Combination of vascular endothelial growth factor
antisense oligonucleotied therapy and radiotherapy increases the
curative effects against maxillofacial VX2 tumors in rabbits. Eur J
Radio. 78:272–276. 2011. View Article : Google Scholar
|
|
10
|
Yin YM, Yu H, Zhou YB, Zhang WQ and Lv R:
Effects of lentivirus-mediated RNA interference on VEGF expression
in human gastric cancer cells. Shandong Med J. 49:51–53. 2009.(In
Chinese).
|
|
11
|
Menendez D, Krysiak O, Inga A, Krysiak B,
Resnick MA and Schonfelder G: A SNP in the flt-1 promoter
integrates the VEGF system into the p53 transcriptional network.
Proc Natl Acad Sci USA. 103:1406–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Oosterom AT and Bruijn EA: Vascular endothelial growth factor
and angiogenesis. Pharmacol Rev. 56:549–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Lu Z, Wientjes MG and Au JL:
Delivery of siRNA therapeutics: barriers and carriers. AAPS J.
12:492–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo P, Coban O, Snead N, Trebley J,
Hoeprich S, Guo S and Shu Y: Engineering RNA for targeted siRNA
delivery and medical application. Adv Drug Deliv Rev. 62:650–666.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A and
Qin ZY: Down-regulation of survivin expression by small interfering
RNA induces pancreatic cancer cell apoptosis and enhances its
radiosensitivity. World J Gastroenterol. 12:2901–2907.
2006.PubMed/NCBI
|
|
16
|
Yao L, Liu F, Hong L, Sun L, Liang S, Wu K
and Fan DM: The function and mechanism of COX-2 in angiogenesis of
gastric cancer cells. J Exp Clin Cancer Res. 30:132011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun P, Gao J, Liu YL, Wei LW, Wu LP and
Liu ZY: RNA interference (RNAi)-mediated vascular endothelial
growth factor-C (VEGF-C) reduction interferes with
lymphangiogenesis and enhances epirubicin sensitivity of breast
cancer cells. Mol Cell Biochem. 308:161–168. 2008. View Article : Google Scholar
|
|
18
|
Kim SH, Lee SH, Tian H, Chen X and Park
TG: Prostate cancer cell-specific VEGF siRNA delivery system using
cell targeting peptide conjugated polyplexes. J Drug Target.
17:311–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasan MR, Ho SH, Owen DA and Tai IT:
Inhibition of VEGF induces cellular senescence in colorectal cancer
cells. Int J Cancer. 129:2115–2123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raskopf E, Vogt A, Sauerbruch T and
Schmitz V: siRNA targeting VEGF inhibits hepatocellular carcinoma
growth and tumor angiogenesis in vivo. J Hepatol. 49:977–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun P, Yu H, Zhang WQ, Liu Y and Lv R:
Mechanism of the apoptosis of human gastric cancer cell line SUN
SGC7901 induced by vascular endothelial growth factor siRNA. J Med
Postgrad. 24:350–353. 2011.(In Chinese).
|
|
22
|
Bredow S, Juri DE, Cardon K and Tesfaigzi
Y: Identification of a novel Bcl-2 promoter region that counteracts
in a p53-dependent manner the inhibitory p2 region. Gene.
404:110–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guha M and Altieri DC: Survivin as a
global target of intrinsic tumor suppression networks. Cell Cycle.
8:2708–2710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng CX: SIRT1, is it a tumor promoter or
tumor suppressor? Int J Biol Sci. 5:147–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamakuchi M and Lowenstein CJ: MiR-34,
SIRT1 and p53: the feedback loop. Cell Cycle. 8:712–715. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung HB and Ahmad N: Role of p53 in the
anti-proliferative effects of Sirt1 inhibition in prostate cancer
cells. Cell Cycle. 8:1478–1483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han MK, Song EK, Gou Y, Ou X, Mantel C and
Broxmeyer HE: SIRT1 regulates apoptosis and Nanog expression in
mouse embryonic stem cells by controlling p53 subcellular
localization. Cell Stem Cell. 2:241–251. 2008. View Article : Google Scholar : PubMed/NCBI
|