Introduction
The development of ligand-targeted therapeutics in
anticancer therapy has gained momentum in recent years (1). Systemic cytotoxic chemotherapy shows
little selectivity and side effects. One strategy to improve the
lack of selectivity is to couple therapeutics to antibodies or
smaller molecule peptides (2).
Among the most relevant peptide receptors, the bombesin receptors
are of major interest, because they were found to be overexpressed
in various cancers such as prostate (3,4),
breast (5,6), and small cell lung cancer (7). The human counterparts of bombesin,
namely gastrin-releasing peptide (8,9) and
neuromedin B (8), have been found
in mammalian tissue. Gastrin-releasing peptide receptors (GRPR) are
overexpressed on a variety of human tumors such as prostate,
breast, and lung cancer. Bombesin (BBN) is a 14 amino acid peptide
with high affinity for these GRPRs. Bombesin and gastrin-releasing
peptide (GRP) are potent neuropeptides expressed by prostate cancer
neuroendocrine cells and are related to the progression of this
malignancy.
Nanoliposomes are double-membrane lipid vesicles
capable of packaging drugs for various delivery applications
(10). Nano-pegylated liposomes can
evade the reticuloendothelial system and remain in the circulation
for prolonged periods, improving tumor targeting and efficacy in
animal models (11,12). Nano-pegylated liposomes provide
passive targeting because nanoliposome accumulation in tumors is by
means of the enhanced permeability and retention (EPR) effect
through leaky tumor vasculature (12). Preclinical studies have shown that
cytotoxic agents entrapped in pegylated liposomes tend to
accumulate in tumors (13,14).
Preclinical studies of tumor therapy with
radionuclide-liposome conjugates or liposome-mediated
radiotherapeutics have been reported (15–18).
Rhenium-188 is a radionuclide used for imaging and
therapeutic dual applications due to its short physical half-life
of 16.9 h with 155 keV gamma emissions for imaging, and its 2.12
MeV β emission with maximum tissue penetration range of 11 mm for
tumor therapeutics (19). In
addition, 188Re can be obtained from a commercial
nuclear generator, which makes it convenient for routine research
and clinical use.
Chemoradiotherapy is a standard treatment for
patients with locally advanced rectal cancer. Direc targeted
therapy approaches target tumor antigens to change signalling by
monoclonal antibodies, peptide or small molecule drugs. Drugs can
actively target tumors using tumor-specific antibody or peptide
ligands binding to receptors that are present on tumor cells. In
this study, a new combination of peptide targeted
radiochemo-therapeutics was designed and studied for treating solid
tumors of the pancreas by intravenous administration. The in
vivo nuclear images of tumor, prolonged survival time and
therapeutic efficacy of radiochemo-therapeutics of
188Re-(DXR)-liposome-BBN were evaluated in AR42J
malignant pancreas solid tumor-bearing mice.
Materials and methods
Materials
The 188W/188Re generator was
purchased from Oak Ridge National Laboratory (Oak Ridge, USA).
Elution of the 188W/188Re generator with
normal saline provided solutions of carrier-free 188Re
as sodium perrhenate (NaReO4). The pegylated liposome
(Nano-X) was provided by Taiwan Liposome Co. (Taipei, Taiwan).
N,N-bis(2-mercaptoethyl)-N′,N′-diethylethylenediamine (BMEDA) were
purchased from ABX (Radeberg, Germany). Stannous chloride
(SnCl2) was purchased from Merck (Darmstadt, Germany).
Glucoheptonate powder and doxorubicin was purchased from
Sigma-Aldrich Corp.(Bangalore, India). Sepharose CL-6B column were
purchased from GE Healthcare (Uppsala, Sweden). All other chemicals
were purchased from Merck. Dulbecco's modified Eagle's medium
(DMEM) cell culture medium and fetal bovine serum (FBS) was
purchased from Gibco (USA).
Cell cultures and animal model
The AR42J human pancreas carcinoma cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). It was grown in Dulbecco's modified Eagle's medium (DMEM)
medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 2
mM L-glutamine at 37°C in 5% CO2. Cells were detached
with 0.05% trypsin/0.53 mM EDTA in Hanks' balanced salt solution
(HBSS). Four-week-old male nude mice were obtained from the
National Animal Center of Taiwan (Taipei, Taiwan), with food and
water being provided ad libitum in the animal house of the
INER. Animal protocols were approved by the Institutional Animal
Care and Use Committee (IACUC) at the Institute of Nuclear Energy
Research. Mice were subcutaneously inoculated with 2×106
tumor cells in the right hind flank. Ten days after inoculation,
the animals developed tumors of ~50–100 mm3 in size.
Preparation of
188Re-(DXR)-liposome-BBN
The method for radiolabeling BMEDA with
188Re was as previously described (15,16).
The labeling efficiency of the 188Re-BMEDA complexes was
checked by paper chromatography with normal saline as the eluent.
188Re-BMEDA was encapsulated in the liposomes using the
ammonium sulfate gradient loading procedure, the labeling processes
of 188Re-(DXR)-liposome-BBN were as follows. The
pegylated nanoliposomes (0.5 ml), DSPE-PEG-BBN (5 μl; 40 mg/ml)with
or without DXR (80 μl; 140 mg/ml) had added high specific activity
188Re-BMEDA (450–650 MBq per 0.5 ml) solution and
incubation at 60°C for 30 min. The 188Re-liposome-BBN or
188Re-DXR-liposome-BBN was separated from free
188Re-BMEDA using sepharose CL-6B column (GE Healthcare)
eluted with normal saline. The labeling efficiency of the pegylated
nanoliposomes was determined using the activity in pegylated
nanoliposomes or liposome-DXR-BBN after separation divided by the
total activity before separation. The amount of doxorubicin trapped
inside the liposome was analyzed.
Calculation of the amount of bombesin
molecules on the surface of liposomes
The amount of bombesin on liposome solution was
determined by BCA assay (bombesin molecules/ml liposome). The
particle size and the number of phospholipid molecules per liposome
can be measured by particle analyzer and phosphate assay
separately. The amount of particle per ml liposome solution can be
calculated through the concentration of phospholipid and particle
size of liposome (vesicles/ml liposome). Finally, the amount of
bombesin molecules per vesicle through the amount of bombesin on
liposome solution (bombesin molecules/ml liposome) and the amount
of particle per ml liposome solution were calculated. In this
experiment, the average amount of bombesin molecules on the surface
of liposome is 511.
MicroSPECT imaging and
semi-quantification analysis of targeted
188Re-liposome-BBN
Imaging was acquired using low-energy,
high-resolution collimators at 1, 24, 48 and 72 h after intravenous
injection of 188Re-liposome-BBN with or without cold BBN
(2 mg/mouse). For imaging acquisition, the mice were anesthetized
with 1–2% isoflurane in 100% O2. The energy window was
set at 155 keV ± 10–15%, the FOV (field of view) was 12.5 cm. SPECT
imaging was followed by CT image acquisition (X-ray source: 50 kV,
0.4 mA; 256 projections) with the animal in exactly the same
position. Images were calibrated to standardized uptake values
(SUV) (15,20). For calculating standardised tumor
uptake value (SUV), known radio activity Re-188 was
performenced as reference. The SUV was determined from the regions
of interest (ROI) on the tumor with uptake. The SUV was calculated
according to the following standard formula: measured activity
concentration (μCi/g)/[injected dose (μCi)/body weight (g)]. The
images revealed a high uptake in tumors at 1 and 24 h after
intravenous injection.
Therapeutic efficacy studies
Nude mice were used and each was subcutaneously
inoculated with AR42J cells (2×106) in the right hind
flank. Approximately 10 days after inoculation, tumor-bearing mice
were divided randomly into groups, 8–10 mice per group. The study
was divided in two experiments: (A) combinational
radio-chemotherapeutic efficacy of
188Re-(DXR)-liposome-BBN in AR42J tumor-bearing mouse
model, (B) dose-dependent effect of radio-chemotherapeutic efficacy
of 188Re-(DXR)-liposome-BBN in AR42J tumor-bearing mouse
model. In every experiment, one group was randomly selected as the
control. In study (A), 4 groups of mice were treated with
188Re-DXR-liposome-BBN (17.76 MBq/100 μl of
188Re, 2 mg/kg DXR and 0.4 μmol phospholipids),
188Re-liposome-BBN (17.76 MBq/100 μl of 188Re
and 0.4 μmol phospholipids), Lipo-Dox-BBN (2 mg/kg DXR and 0.4 μmol
phospholipids) and normal saline by single i.v. injection,
respectively. In study (B), 6 groups of mice were treated with
188Re-DXR-liposome-BBN (17.76 MBq/100 μl of
188Re, 2 mg/kg DXR and average 3.7 μmol phospholipids),
188Re-DXR-liposome-BBN (11.84 MBq/100 μl of
188Re, 2 mg/kg DXR and average 3.7 μmol phospholipids),
188Re- liposome-BBN (11.84 MBq/100 μl of
188Re and average 3.7 μmol phospholipids),
188Re-liposome-BBN (17.76 MBq/100 μl of 188Re
and average 3.7 μmol phospholipids), Lipo-Dox-BBN (2 mg/kg DXR and
average 3.7 μmol phospholipids) and normal saline by single i.v.
injection, respectively. Treatments were initiated when the volume
of tumors was ~50–100 mm3. The treatments were performed
on day 0 as a single dose. Tumor was measured twice weekly by a
digital calipers to document tumor growth. Tumor measurements were
converted into tumor volume (V) using the formula (21,22): V
= (Y × W2)/2; where Y and W are the larger and smaller
perpendicular diameters, respectively. All data are expressed as
mean ± standard deviation. The mean tumor growth inhibition rate
(MGI) was calculated according to the volume of the tumor (23): growth rate of the treated
group/growth rate of untreated group. Following standard animal-use
protocols, termination was mandated on reaching one or both of the
following criteria: a tumor weight of >2 g (2 ml volume) or
total body weight loss of >20% (24). The combination therapeutic
enhancement results were evaluated by the combination index (CI)
(23,25). The CI expected growth inhibition
rate/observed growth inhibition rate. The expected growth rate of
combination treatment = tumor growth inhibition rate of drug A only
× tumor growth inhibition rate of drug B only. In this study, drug
A is 188Re-DXR-liposome-BBN, and drug B is lipo-Dox-BBN.
An index >1 points to the synergistic effect, while that of
<1 indicates less than an additive effect.
Results
Labeling efficiency of
188Re-(DXR)-liposome-BBN
The encapsulation efficiency of
188Re-BMEDA in pegylated liposome-BBN and liposome-BBN
contain DXR was 54 and 76%, respectively. The radiochemical purity
of 188Re-(DXR)-liposome-BBN was >95%. The average
particle size of 188Re-(DXR)-liposome (~90 nm) was
similar to the particle sizes before 188Re-BMEDA
encapsulation.
MicroSPECT/CT imaging of
188Re-liposome-BBN
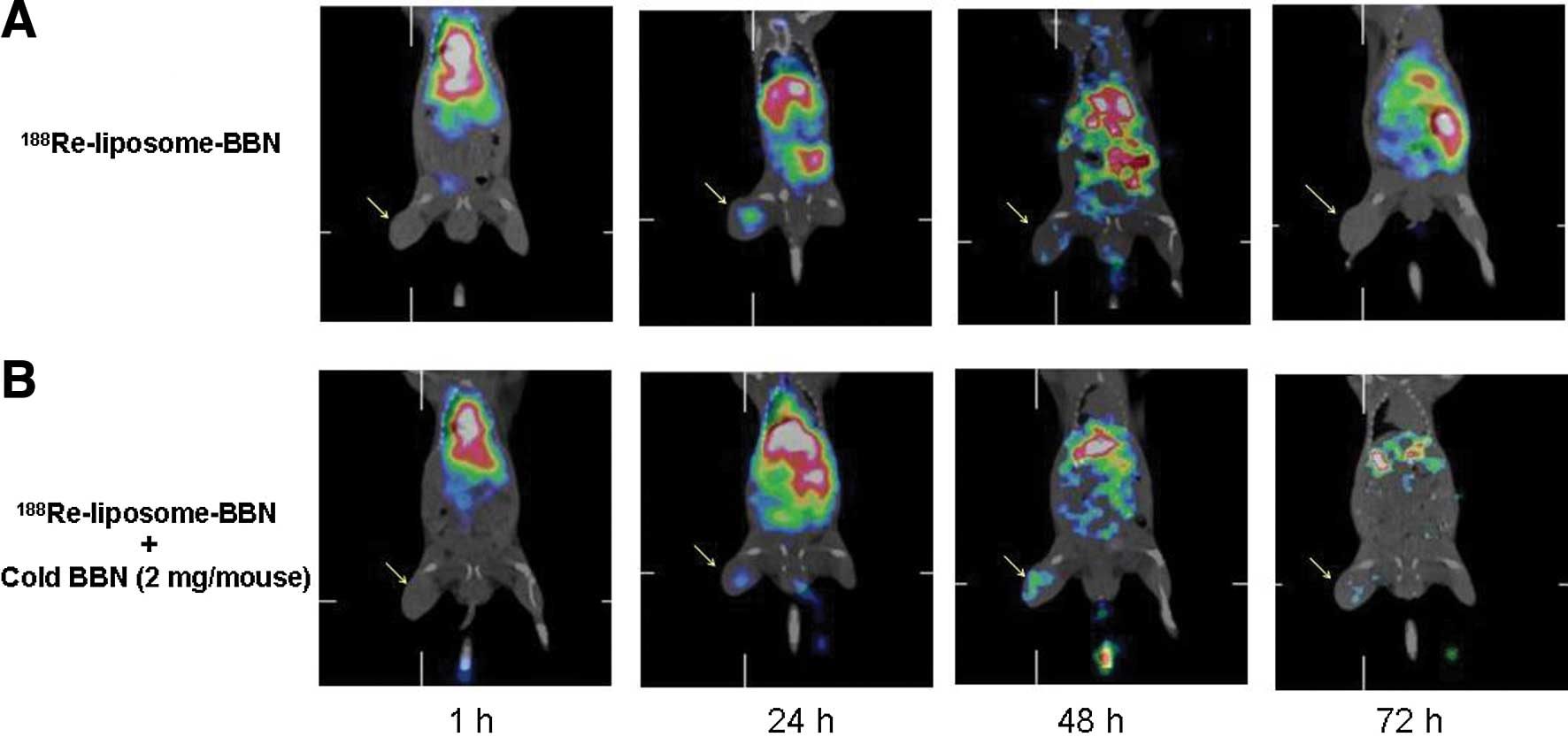
To confirm the specific targeting of the tumor sites
of passive 188Re-liposome-BBN, microSPECT/CT imaging was
performed. The microSPECT/CT imaging showed accumulated
188Re-liposome-BBN in the liver, spleen and tumor
(Fig. 1A). The microSPECT/CT
imaging of 188Re-liposome-BBN pointed to significant
target and uptake in the tumors until 48 h after intravenous
injection. The SUV of 188Re-liposome-BBN in tumor reach
the peak at 24 h after injection (2.13±0.98) (Table I). Contrary to microSPECT/CT imaging
of 188Re-liposome-BBN with cold BBN (2 mg/mouse), the
microSPECT/CT imaging showed significant decrease of
188Re-liposome-BBN uptake in the tumors at 24 h after
injection (Fig. 1B).
 | Table IThe standardized uptake value (SUV)
analysis of microSPECT/CT imaging of 188Re-liposome-BBN
with or without cold BBN (2 mg/mouse) in AR42J tumor-bearing mouse
model (n=3). |
Table I
The standardized uptake value (SUV)
analysis of microSPECT/CT imaging of 188Re-liposome-BBN
with or without cold BBN (2 mg/mouse) in AR42J tumor-bearing mouse
model (n=3).
| Time (h) |
188Re-liposome-BBN |
188Re-liposome-BBN + cold BBN
(2 mg/mouse) |
|---|
| 1 | 1.84±0.38 | 1.70±0.30 |
| 24 | 2.13±0.98 | 1.82±0.31 |
| 48 | 1.29±0.76 | 1.50±0.63 |
| 72 | 0.87±0.51 | 0.84±0.42 |
Therapeutic efficacy of
188Re-(DXR)-liposome-BBN
In experiment (A) combinational
radio-chemotherapeutic efficacy of
188Re-(DXR)-liposome-BBN in AR42J tumor-bearing mouse
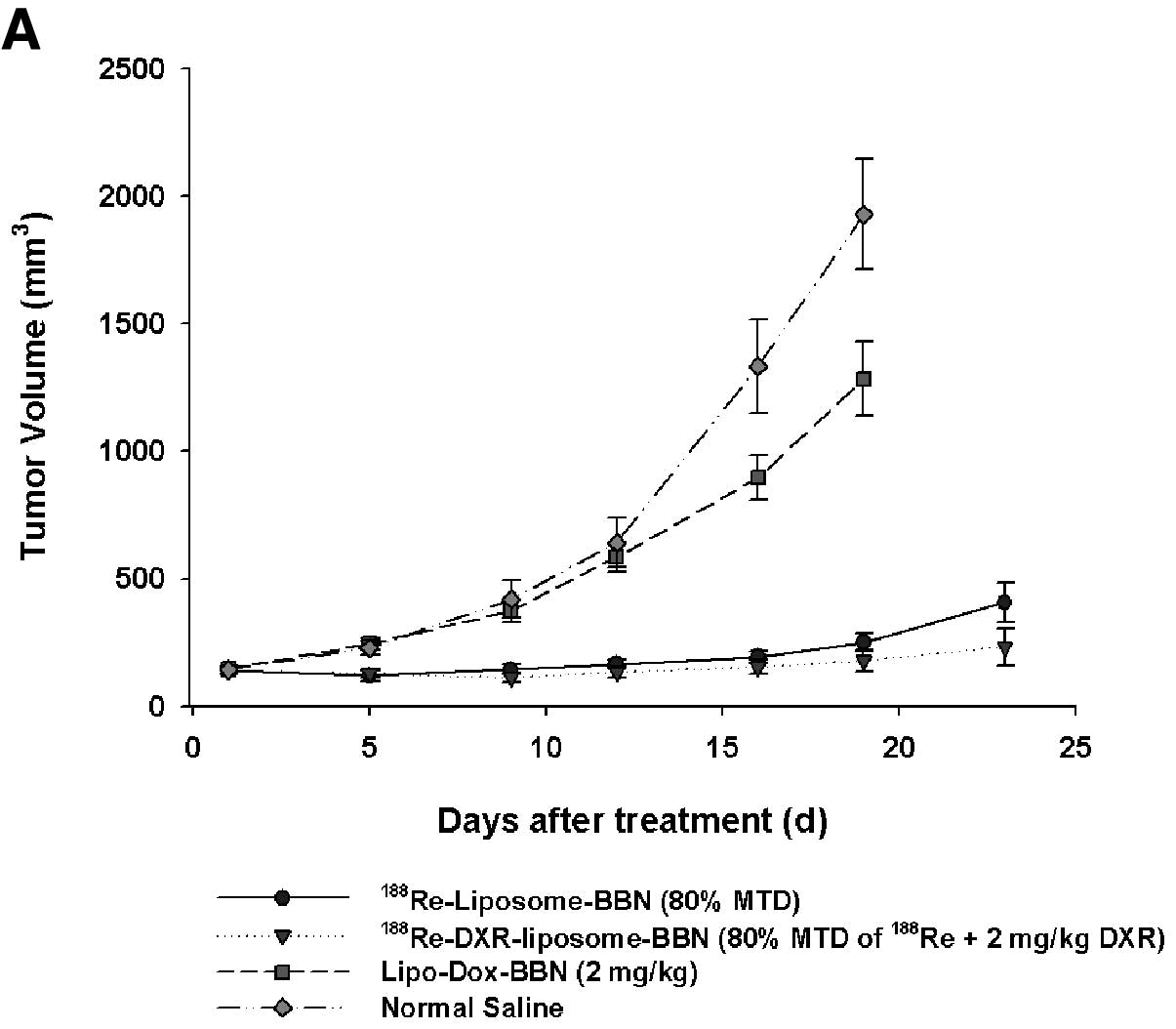
model, tumor volume growth and inhibition after various treatments
from 0 to 23 days are plotted in Fig.
2A. In contrast to the mean tumor volume of 1929±218
mm3 in the untreated normal saline group at 19 d, the
mean tumor volume of the treated groups at 19 d with
188Re-DXR-liposome-BBN (17.76 MBq, 2 mg/kg DXR),
188Re-liposome-BBN (17.76 MBq) and Lipo-Dox-BBN (2 mg/kg
DXR) were 178±42, 251±37 and 1284±144 mm3, respectively.
As shown in Fig. 1A, the mean
growth inhibition rates achieved by
188Re-DXR-liposome-BBN (17.76 MBq, 2 mg/kg DXR),
188Re-liposome-BBN (17.76 MBq) and Lipo-Dox-BBN (2 mg/kg
DXR) were 0.092, 0.130 and 0.666, respectively. Significant
additive tumor growth inhibition effect was demonstrated by the
radiochemo-combination treatment with 188Re-DXR-liposome
(CI 0.946; Table II).
 | Table IIThe combinational radio-chemo
therapeutic efficacy of 188Re-(DXR)-liposome-BBN in
AR42J tumor-bearing mouse model. |
Table II
The combinational radio-chemo
therapeutic efficacy of 188Re-(DXR)-liposome-BBN in
AR42J tumor-bearing mouse model.
| Tumor growth
inhibition | Survival |
|---|
|
|
|
|---|
| Treatment
modality | MGIa | Expectedb | CIc | Median survival
time (d) | P-valued | Life span
(%)e |
|---|
|
188Re-liposomes-BBN | 0.130 | | | 40.25 | 0.0002 | +75.00 |
|
188Re-DXR-liposomes-BBN | 0.092 | 0.087 | 0.946 | 43.00 | 0.0000 | +86.96 |
| Lipo-Dox-BBN (2
mg/kg) | 0.666 | | | 23.83 | 0.0169 | +3.61 |
| Normal saline | | | | 23.00 | | |
In experiment (B) dose-dependent effect of
radio-chemotherapeutic efficacy of
188Re-(DXR)-liposome-BBN in AR42J tumor-bearing mouse
model, tumor volume growth and inhibition after various treatments
from 0 to 26 days are plotted in Fig.
3A. In contrast to the mean tumor volume of 2339±329
mm3 in the untreated normal saline group at 18 d, the
mean tumor volume of the treated groups at 18 d with
188Re-DXR-liposome-BBN (17.76 MBq, 2 mg/kg DXR),
188Re-DXR-liposome-BBN (11.84 MBq, 2 mg/kg DXR),
188Re-liposome-BBN (11.84 MBq),
188Re-liposome-BBN (17.76 MBq) and Lipo-Dox-BBN (2 mg/kg
DXR) were 328±77, 457±52, 509±72, 414±77 and 1274±150
mm3, respectively. As shown in Fig. 2A, the mean growth inhibition rates
achieved by 188Re-DXR-liposome-BBN (17.76 MBq, 2 mg/kg
DXR), 188Re-DXR-liposome-BBN (11.84 MBq, 2 mg/kg DXR),
188Re-liposome-BBN (11.84 MBq),
188Re-liposome-BBN (17.76 MBq) and Lipo-Dox-BBN (2 mg/kg
DXR) were 0.140, 0.198, 0.218, 0.177 and 0.545, respectively.
Significant additive tumor growth inhibition effect was
demonstrated by the radiochemo-combination treatment with
188Re-DXR-liposome (11.84 MBq, 2 mg/kg DXR) and
188Re-DXR-liposome (17.76 MBq, 2 mg/kg DXR) (CI 0.610
and 0.689, respectively; Table
III).
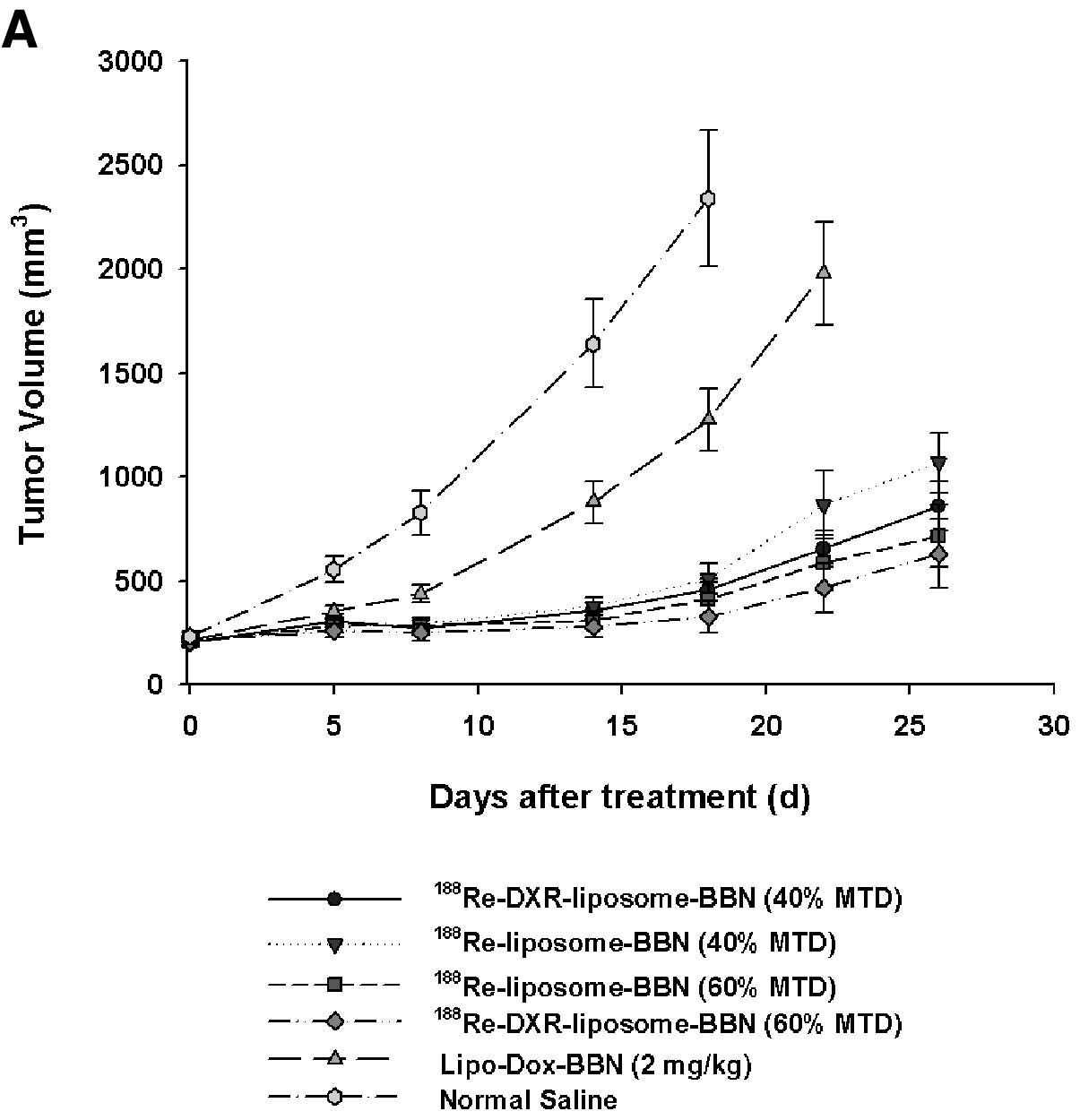 | Figure 3Tumor growth and survival curve. (A)
Tumor growth volume (mm3) versus time (days) for nude
mice implanted with AR42J tumors after administering
188Re-DXR-liposome-BBN (17.76 MBq of 188Re, 2
mg/kg DXR), 188Re-DXR-liposome-BBN (11.84 MBq of
188Re, 2 mg/kg DXR), 188Re-liposome-BBN
(11.84 MBq of 188Re), 188Re-liposome-BBN
(17.76 MBq of 188Re), Lipo-Dox-BBN (2 mg/kg DXR) and
normal saline by single i.v. injection on day 0. Tumor growth was
significantly inhibited by the highest dose radiochemo-combination
treatment of 188Re-DXR-liposome-BBN. (B) Survival curve
for nude mice bearing AR42J tumors after i.v. injection of
188Re-DXR-liposome-BBN (17.76 MBq of 188Re, 2
mg/kg DXR), 188Re-DXR-liposome-BBN (11.84 MBq of
188Re, 2 mg/kg DXR), 188Re-liposome-BBN
(11.84 MBq of 188Re), 188Re- liposome-BBN
(17.76 MBq of 188Re), Lipo-Dox-BBN (2 mg/kg DXR) and
normal saline by single i.v. injection on day 0. Prolonged survival
time and better survival rate of mice were observed after
administration of highest dose radiochemo-combination treatment of
188Re-DXR-liposome-BBN. P-value for comparisons of
survival curves of various treatment groups are listed in Table III. |
 | Table IIIThe therapeutic efficacy of
dose-dependent effect of radio-chemo therapeutic efficacy of
188Re-(DXR)-liposome-BBN in AR42J tumor-bearing mouse
model. |
Table III
The therapeutic efficacy of
dose-dependent effect of radio-chemo therapeutic efficacy of
188Re-(DXR)-liposome-BBN in AR42J tumor-bearing mouse
model.
| Tumor growth
inhibition | Survival |
|---|
|
|
|
|---|
| Treatment
modality | MGIa | Expectedb | CIc | Median survival
time (d) | P-valued | Life span
(%)e |
|---|
|
188Re-DXR-liposome-BBN (40%
MTD) | 0.195 | 0.119 | 0.610 | 43.50 | 0.0000 | +81.25 |
|
188Re-DXR-liposome-BBN (60%
MTD) | 0.140 | 0.096 | 0.689 | 45.50 | 0.0000 | +89.58 |
|
188Re-liposome-BBN (40%
MTD) | 0.218 | | | 30.33 | 0.0000 | +26.37 |
|
188Re-liposome-BBN (60%
MTD) | 0.177 | | | 45.33 | 0.0000 | +88.88 |
| Lipo-Dox-BBN (2
mg/kg) | 0.545 | | | 28.33 | 0.0578 | +18.04 |
| Normal saline | | | | 24.00 | 0.0000 | |
In study (A), the survival curves for the different
treatment groups are compared in Fig.
2B. The median survival time for the normal saline control mice
was 23 d. The median survival times for the mice treated with
188Re-DXR-liposome-BBN (17.76 MBq, 2 mg/kg DXR),
188Re-liposome-BBN (17.76 MBq) and Lipo-Dox-BBN (2 mg/kg
DXR) were 43.00 d (P<0.05), 40.25 d (P<0.05) and 23.83 d,
respectively. The P-values for the differences among the survival
curves of the various treatment groups are shown in Table II.
In study (B), the survival curves for the different
treatment groups are compared in Fig.
3B. The median survival time for the normal saline control mice
was 24 d. The median survival times for the mice treated with
188Re-DXR-liposome-BBN (17.76 MBq, 2 mg/kg DXR),
188Re-DXR-liposome-BBN (11.84 MBq, 2 mg/kg DXR),
188Re-liposome-BBN (11.84 MBq),
188Re-liposome-BBN (17.76 MBq) and Lipo-Dox-BBN (2 mg/kg
DXR) were 45.50 d (P<0.05), 43.50 d (P<0.05), 30.33 d
(P<0.05), 45.33 d (P<0.05) and 23.83 d, respectively. The
P-values for the differences among the survival curves of the
various treatment groups are shown in Table III.
Discussion
The nuclear molecular imaging such as single photon
emission computer tomography (SPECT) are valuable tools for new
anticancer drug discovery and development in preclinical animal
models of human disease. In our previous studies, the results of
biodistribution, pharmacokinetics and micro-SPECT/CT imaging
demonstrated the benefits of passive radio-therapeutics of
188Re-liposome in C26 colon carcinoma ascities, C26
colon solid tumor and HT-29 colon solid tumor animal models
(15,16,20,26).
In this study, the tumor targeting and therapeutic efficacy of
bimodality radiochemo-combination treatment of
188Re-(DXR)-liposome-BBN was investigated. The in
vivo microSPECT/CT imaging result of Fig. 1 displays AR42J tumor targeted by
188Re-liposomes-BBN, the decrease uptake of
188Re-liposome-BBN was shown after administration of
cold BBN. These results indicated 188Re-liposome with
BBN peptide targets the gastrin-releasing peptide receptors in the
tumor.
The high energy β emitters of 188Re (2.12
MeV) have a mean tissue penetration range of 3.5 mm and maximum
tissue penetration range of 10.15 mm (27,28),
which enable 188Re to kill tumor cells through a
cross-fire or non-specific cell killing effect. Li et al
have studied the therapeutic efficacy of radioimmunotherapy (RIT)
of 188Re-labeled herceptin, the tumor inhibition rate
(IR) was 48.8±4.9 after the 4th week of 188Re-herceptin
(11.1 MBq) administration by intravenous injection (29). Huang et al and
Papahadjopoulos et al studied therapies of
doxorubicin-encapsulating liposome in mice bearing C-26 colon
carcinoma, the life span (%) of treatment with 3 and 10 mg/kg
doxorubicin encapsulating liposome by triple injection was 1.3 and
5.1%, respectively (30,31). In this study, we use
radiochemo-therapeutic 188Re-DXR-liposome-BBN for drug
delivery to improve therapeutic efficacy and to reduce toxicity. In
therapeutic experiment (A) and (B), the results (Table II) show dose-dependent anti-tumor
activity of 188Re. The results (Table III) of comparisons of the
therapeutic efficacy treatmentments with irradiation only (such as
188Re-liposome-BBN) or anti-tumor chemical drug only
(such as Lipo-Dox-BBN) or dual radio-chemotherapy (such as
188Re-DXR-liposome-BBN) revealed that
188Re-DXR-liposome-BBN showed a better tumor growth
inhibition rate, a higher survival ratio and life span of AR42J
tumor-bearing mice treated with single doses (0.140, 45.50 d and
89.58%, respectively). The additive tumor regression effect of
radiochemo-therapeutics of 188Re-DXR-liposome-BBN was
also demonstrated in Table
III.
Many effects have been demonstrated by conjugating
various targeting ligands to the liposome surface to increase
specificity of interaction of liposomal drugs with targeted cells
and to enhance the amount of drugs delivered into tumor cells.
Antibodies, peptides, growth factors and folate can selectively
bind to target antigens or receptors overexpressed on the tumor
cell. RGD-modified liposomes (32–34),
integrin-targeted paclitaxel nanoparticles (35–38),
and folate-conjugated liposomes (39) have been demonstrated to increase the
intracellular delivery and therapeutic efficacy of chemotherapeutic
agents in vivo. In our study, BBN targeted peptide
conjugated with radio-chemotherapeutics
188Re-(DXR)-liposome was designed for treating solid
pancreatic tumors by intravenous administration.
Apoptosis occurs spontaneously and is enhanced by
irradiation. Radiation-induced apoptosis has been observed in
vivo (40,41). Radiation-induced apoptosis is
considered to be one of the main cell death mechanisms following
exposure to irradiation. Apoptosis plays a modest role in the
treatment response of most solid tumors, which constitute the main
human malignancies. This is observed in vivo such as
intestinal crypt, salivary and lacrimal glands with non-dividing
cells, lymphocytes that are non-dividing, and is frequently seen
early within 4–6 h after irradiation (40,42,43).
188Re kills tumor cells through a cross-fire or
non-specific cell killing effect. Li et al have studied the
therapeutic efficacy of radioimmunotherapy (RIT) of
188Re-labeled herceptin, the tumor inhibition rate (IR)
was 48.8±4.9 after the 4th week of 188Re-herceptin (11.1
MBq) administration by intravenous injection (29). Radiochemotherapeutics of
188Re-DXR-liposome attained significant survival time,
ascites inhibition (decreased by 49 and 91% at 4 days after
treatment; P<0.05) and tumor inhibition in mice (15,44).
Radiotherapeutics with 188Re-liposomes provided better
survival time (increased by 34.6% of life span; P<0.05), tumor
and ascites inhibition (decreased by 63.4 and 83.3% at 7 days after
treatment; P<0.05) in mice compared with chemotherapeutics of
5-fluorouracil (5-FU) (45). In
conclusion, we used peptide targeted radiochemo-therapeutic
multifunctional nanoliposome as carrier for drug delivery to
improve therapeutic efficacy. The inhibition of tumor growth in
mice treated with 188Re-DXR-liposome-BBN was precisely
controlled and had longer survival time than those treated with
anti-cancer drug, 188Re-liposome-BBN (MGI = 0.130; 75%),
Lipo-Dox-BBN (MGI = 0.666; 3.61%) and untreated control mice. The
additive tumor regression effect (Table II) was observed (CI 0.946) for
co-delivery radiochemo-therapeutics of
188Re-DXR-liposome-BBN.
Acknowledgements
The authors would like to thank Ching-Jun Liou for
his help with the preparation of 188Re, and Cheng-Hui
Chuang for her technical assistance in microSPECT/CT.
References
|
1
|
Allen TM: Ligand-targeted therapeutics in
anticancer therapy. Nat Rev Cancer. 2:750–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maison W and Frangioni JV: Improved
chemical strategies for the targeted therapy of cancer. Angew Chem
Int Ed Engl. 42:4726–4728. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markwalder R and Reubi JC:
Gastrin-releasing peptide receptors in the human prostate: relation
to neoplastic transformation. Cancer Res. 59:1152–1159.
1999.PubMed/NCBI
|
|
4
|
Sun B, Halmos G, Schally AV, Wang X and
Martinez M: Presence of receptors for bombesin/gastrin-releasing
peptide and mRNA for three receptor subtypes in human prostate
cancers. Prostate. 42:295–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gugger M and Reubi JC: Gastrin-releasing
peptide receptors in non-neoplastic and neoplastic human breast. Am
J Pathol. 155:2067–2076. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Halmos G, Wittliff JL and Schally AV:
Characterization of bombesin/gastrin-releasing peptide receptors in
human breast cancer and their relationship to steroid receptor
expression. Cancer Res. 55:280–287. 1995.PubMed/NCBI
|
|
7
|
Toi-Scott M, Jones CL and Kane MA:
Clinical correlates of bombesin-like peptide receptor subtype
expression in human lung cancer cells. Lung Cancer. 15:341–354.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minamino N, Kangawa K and Matsuo H:
Neuromedin B: a novel bombesin-like peptide identified in porcine
spinal cord. Biochem Biophys Res Commun. 114:541–548. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McDonald TJ, Jornvall H, Tatemoto K and
Mutt V: Identification and characterization of variant forms of the
gastrin-releasing peptide (GRP). FEBS Lett. 156:349–356. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brannon-Peppas L and Blanchette JO:
Nanoparticle and targeted systems for cancer therapy. Adv Drug
Deliv Rev. 56:1649–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen TM and Cullis PR: Drug delivery
systems: entering the mainstream. Science. 303:1818–1822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Torchilin VP: Recent advances with
liposomes as pharmaceutical carriers. Nat Rev Drug Discov.
4:145–160. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Newman MS, Colbern GT, Working PK, Engbers
C and Amantea MA: Comparative pharmacokinetics, tissue
distribution, and therapeutic effectiveness of cisplatin
encapsulated in long-circulating, pegylated liposomes (SPI-077) in
tumor-bearing mice. Cancer Chemother Pharmacol. 43:1–7. 1999.
View Article : Google Scholar
|
|
14
|
Vaage J, Donovan D, Wipff E, et al:
Therapy of a xenografted human colonic carcinoma using cisplatin or
doxorubicin encapsulated in long-circulating pegylated stealth
liposomes. Int J Cancer. 80:134–137. 1999. View Article : Google Scholar
|
|
15
|
Chang YJ, Chang CH, Yu CY, et al:
Therapeutic efficacy and microSPECT/CT imaging of
188Re-DXR-liposome in a C26 murine colon carcinoma solid
tumor model. Nucl Med Biol. 37:95–104. 2010.PubMed/NCBI
|
|
16
|
Chen MH, Chang CH, Chang YJ, et al:
MicroSPECT/CT imaging and pharmacokinetics of
188Re-(DXR)-liposome in human colorectal
adenocarcinoma-bearing mice. Anticancer Res. 30:65–72.
2010.PubMed/NCBI
|
|
17
|
Emfietzoglou D, Kostarelos K and Sgouros
G: An analytic dosimetry study for the use of radionuclide-liposome
conjugates in internal radiotherapy. J Nucl Med. 42:499–504.
2001.PubMed/NCBI
|
|
18
|
Ting G, Chang CH and Wang HE: Cancer
nanotargeted radiopharmaceuticals for tumor imaging and therapy.
Anticancer Res. 29:4107–4118. 2009.PubMed/NCBI
|
|
19
|
Ercan MT and Caglar M: Therapeutic
radiopharmaceuticals. Curr Pharm Des. 6:1085–1121. 2000.PubMed/NCBI
|
|
20
|
Chang YJ, Chang CH, Chang TJ, et al:
Biodistribution, pharmacokinetics and microSPECT/CT imaging of
188Re-BMEDA-liposome in a C26 murine colon carcinoma
solid tumor animal model. Anticancer Res. 27:2217–2225.
2007.PubMed/NCBI
|
|
21
|
Carlsson G, Gullberg B and Hafstrom L:
Estimation of liver tumor volume using different formulas - an
experimental study in rats. J Cancer Res Clin Oncol. 105:20–23.
1983.PubMed/NCBI
|
|
22
|
Fang F, Wang AP and Yang SF: Antitumor
activity of a novel recombinant mutant human tumor necrosis
factor-related apoptosis-inducing ligand. Acta Pharmacol Sin.
26:1373–1381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morgillo F, Kim WY, Kim ES, Ciardiello F,
Hong WK and Lee HY: Implication of the insulin-like growth
factor-IR pathway in the resistance of non-small cell lung cancer
cells to treatment with gefitinib. Clin Cancer Res. 13:2795–2803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maddalena ME, Fox J, Chen J, et al:
177Lu-AMBA biodistribution, radiotherapeutic efficacy, imaging, and
autoradiography in prostate cancer models with low GRP-R
expression. J Nucl Med. 50:2017–2024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan Y, Sun X, Xu M, et al: Efficacy of
recombinant methioninase in combination with cisplatin on human
colon tumors in nude mice. Clin Cancer Res. 5:2157–2163.
1999.PubMed/NCBI
|
|
26
|
Chen LC, Chang CH, Yu CY, et al:
Biodistribution, pharmacokinetics and imaging of
188Re-BMEDA-labeled pegylated liposomes after
intraperitoneal injection in a C26 colon carcinoma ascites mouse
model. Nucl Med Biol. 34:415–423. 2007.PubMed/NCBI
|
|
27
|
Iznaga-Escobar N: 188Re-direct
labeling of monoclonal antibodies for radioimmunotherapy of solid
tumors: biodistribution, normal organ dosimetry, and toxicology.
Nucl Med Biol. 25:441–447. 1998. View Article : Google Scholar
|
|
28
|
O'Donoghue JA, Bardies M and Wheldon TE:
Relationships between tumor size and curability for uniformly
targeted therapy with beta-emitting radionuclides. J Nucl Med.
36:1902–1909. 1995.PubMed/NCBI
|
|
29
|
Li G, Wang Y, Huang K, Zhang H, Peng W and
Zhang C: The experimental study on the radioimmunotherapy of the
nasopharyngeal carcinoma overexpressing HER2/neu in nude mice model
with intratumoral injection of 188Re-herceptin. Nucl Med
Biol. 32:59–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang SK, Mayhew E, Gilani S, Lasic DD,
Martin FJ and Papahadjopoulos D: Pharmacokinetics and therapeutics
of sterically stabilized liposomes in mice bearing C-26 colon
carcinoma. Cancer Res. 52:6774–6781. 1992.PubMed/NCBI
|
|
31
|
Papahadjopoulos D, Allen TM, Gabizon A, et
al: Sterically stabilized liposomes: improvements in
pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad
Sci USA. 88:11460–11464. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao H, Wang JC, Sun QS, Luo CL and Zhang
Q: RGD-based strategies for improving antitumor activity of
paclitaxel-loaded liposomes in nude mice xenografted with human
ovarian cancer. J Drug Target. 17:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong XB, Huang Y, Lu WL, et al: Enhanced
intracellular delivery and improved antitumor efficacy of
doxorubicin by sterically stabilized liposomes modified with a
synthetic RGD mimetic. J Control Release. 107:262–275. 2005.
View Article : Google Scholar
|
|
34
|
Xiong XB, Huang Y, Lu WL, et al:
Intracellular delivery of doxorubicin with RGD-modified sterically
stabilized liposomes for an improved antitumor efficacy: in vitro
and in vivo. J Pharm Sci. 94:1782–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng S, Su B, Li W, et al:
Integrin-targeted paclitaxel nanoliposomes for tumor therapy. Med
Oncol. 28:1180–1187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wartchow CA, Alters SE, Garzone PD, et al:
Enhancement of the efficacy of an antagonist of an extracellular
receptor by attachment to the surface of a biocompatible carrier.
Pharm Res. 21:1880–1885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Wartchow CA, Danthi SN, et al: A
novel antiangiogenesis therapy using an integrin antagonist or
anti-Flk-1 antibody coated 90Y-labeled nanoparticles.
Int J Radiat Oncol Biol Phys. 58:1215–1227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du H, Cui C, Wang L, Liu H and Cui G:
Novel tetrapeptide, RGDF, mediated tumor specific liposomal
doxorubicin (DOX) preparations. Mol Pharm. 8:1224–1232
|
|
39
|
Turk MJ, Waters DJ and Low PS:
Folate-conjugated liposomes preferentially target macrophages
associated with ovarian carcinoma. Cancer Lett. 213:165–172. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Milas L, Stephens LC and Meyn RE: Relation
of apoptosis to cancer therapy. In Vivo. 8:665–673. 1994.PubMed/NCBI
|
|
41
|
Meyn RE, Stephens LC, Hunter NR and Milas
L: Apoptosis in murine tumors treated with chemotherapy agents.
Anticancer Drugs. 6:443–450. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cunningham D: Current status of colorectal
cancer: CPT-11 (irinotecan), a therapeutic innovation. Eur J
Cancer. 32A(Suppl 3): S1–S8. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chung KY and Saltz LB: Adjuvant therapy of
colon cancer: current status and future directions. Cancer J.
13:192–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen LC, Chang CH, Yu CY, et al:
Pharmacokinetics, micro-SPECT/CT imaging and therapeutic efficacy
of 188Re-DXR-liposome in C26 colon carcinoma ascites
mice model. Nucl Med Biol. 35:883–893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsai CC, Chang CH, Chen LC, et al:
Biodistribution and pharmacokinetics of 188Re-liposomes
and their comparative therapeutic efficacy with 5-fluorouracil in
C26 colonic peritoneal carcinomatosis mice. Int J Nanomed.
6:2607–2619. 2011.
|