Introduction
Charged particle therapy research and its clinical
application has been expanding since its introduction in the early
1960’s. Today, proton therapy is the prevailing form of charged
particle therapy with 37 facilities around the world treating
patients with various types of cancers including uveal melanoma,
unresectable sarcomas, and basal skull or paraspinal tumors that
require a significantly higher dose of ionizing radiation (1–4).
Proton therapy is also considered advantageous relative to
conventional forms of photon radiotherapy in cases where precise
localization of the radiologic effects to a tumor is imperative
during treatment. Quintessential examples of the types of tumors
where proton therapy is the most advantageous in treatment include
prostate cancer and pediatric neoplasms (5–7).
In the 1990’s, the advantages of proton therapy
eventually led to an expansion of the field of charged particle
therapy to include carbon ion radiotherapy. Today, carbon ion
radiotherapy centers at a limited number of locations worldwide are
currently treating patients for the same types of cancers commonly
treated elsewhere with protons (8–14).
Carbon ions are typically accelerated at energies between 140 and
400 MeV for applications in a clinical setting whereas energies
between 65 and 200 MeV are most commonly utilized when accelerating
protons (11,15). Proton and carbon radiotherapy are
both effective for precisely treating and delineating a localized
tumor during treatment with ionizing radiation. This is due to the
beam of accelerated particles gradually depositing increasing
amounts of energy along a path in the biological tissue (16,17).
At a certain depth in living tissue and organs, the majority of a
particle beam’s energy (and therefore dose) is deposited along a
relatively short traversal of the beam path termed the ‘Bragg
peak’. This narrow region is known as an area of high linear energy
transfer, or LET, and is where a significant amount of energy from
a particle beam is deposited into the tissue. This property enables
the majority of a significant dose of ionizing radiation to be
controllably localized to a relatively small tumor volume when
treating oncology patients with these charged particle beams in
clinical settings.
The therapeutic value of these charged particle
therapies is in part, defined through their relative biological
effectiveness, or ‘RBE’, which is defined as the ratio of a given
dose of charged particles at a specific depth passing through air,
water, or a biological tissue relative to the dose of X-rays
required (or known) to produce an equal biological effect of a
given dose of charged particles at a specific depth within air,
water, or biological tissue. The RBE incident along particle beam
paths, and most notably, at the Bragg peak for protons and carbon
ions in radiotherapy has been a key factor when comparing these two
types of charged particle therapies. Bragg peak RBE values of
proton beams determined experimentally range between 1.0 and 2.1,
and therapeutic values are estimated to be of 1.0 or 1.1 depending
on the treatment center (15,18–20).
Reported RBE values for carbon ions in radiotherapy have, in
contrast, varied considerably, in part due to the limited number of
facilities where these charged particle beams are available to
patients worldwide. While empirical values for carbon ion RBEs at
the Bragg range between 2.3 and 5 in a basic research setting, a
consensus has yet to be reached on a definitive value to apply
clinically for this particular type of radiotherapy (21–25).
In both carbon and proton radiotherapy, the Bragg peak of a
particle beam can be manipulated throughout the entirety of a tumor
in order to deliver a maximum dose of radiation to all malignant
cells. When this technique is applied to a tumor, a spread out
Bragg peak, or ‘SOBP’, can be delivered to the malignancy in its
entirety.
Due to the large overlap in applications for these
two similar types of therapies, we investigated the cellular
lethality of protons accelerated at 70 MeV/n and carbon ions
accelerated at 290 MeV/n preceding, beyond, and located at the
Bragg peak using Chinese hamster ovary (CHO) cells as a mammalian
cell model utilizing particle accelerators at the National
Institute of Radiological Sciences (NIRS) in Japan. Wild-type,
homologous recombination mutant 51D1 (RAD51D mutant), and
non-homologous end joining mutant xrs5 (Ku80 deficient) cell lines
were used to evaluate cell lethality per dose at discrete points
along a continuous path of ionizing radiation of X-rays, γ-rays,
protons, or carbon ions tracking through an Opticell™ stacked
culture cell system. We found that all forms of radiation were
dependent on dose as evaluated by cellular lethality, however, only
carbon ions produced cellular lethality that was dependent on LET
at the Bragg peak. Among the various cell lines used, xrs5 cells
alone displayed cellular lethality that was completely dependent on
dose regardless of the type of radiation exposure. Conversely,
wild-type cells, and to a lesser extent, 51D1 cells were most
sensitive to carbon ion exposure, least sensitive to γ-ray
exposure, and showed intermediate sensitivity to protons.
Collectively, our findings suggest that carbon ion therapy is
advantageous over proton therapy in light of carbon ion irradiation
characteristics. Most noteworthy are the higher LET values at the
Bragg peak and the fact that LET levels themselves in combination
with dose are primary determinants of cellular lethality when
treating a tumor. Ultimately, the specific genetics of any given
malignancy with respect to its DNA damage repair proficiency
affects the final extent of therapeutic advantage gained through
the use of carbon ion beams in patients treated at charged particle
therapy centers.
Materials and methods
Radiation conditions
Particle-based irradiation experiments were carried
out at the NIRS in Chiba, Japan. Carbon ions were accelerated to
290 MeV/n using the Heavy Ion Medical Accelerator (HIMAC)
synchrotron and protons were accelerated at 70 MeV/n using the
NIRS-930 cyclotron delivery port in C-8. Dose rates for carbon ions
and protons were set at 1 Gy/min. γ-ray irradiation experiments
were carried out at a dose rate of ~2.5 Gy/min at Colorado State
University (Fort Collins, CO) using a Model Mark I-68A (SS0056)
6,000Ci 137Cesium sealed source model (J.L. Shepherd,
Carlsbad, CA). X-ray irradiation experiments were carried out at
the NIRS using a Titan X-ray generator (Shimadzu, Japan) with a
peak tube/voltage potential of 200 kVp, a tube intensity of 20 mA,
0.5 mm of aluminum and copper filters, at a dose rate of 1 Gy/min.
Irradiations were carried out at room temperature.
Cell culture
Original Chinese hamster ovary epithelial wild-type
cells (CHO 10B2) were kindly supplied by Dr Joel Bedford (Colorado
State University, Fort Collins, CO). DNA repair-deficient CHO
mutant cell lines for the: i) homologous recombination pathway
(51D1 cells; CHO AA8 RAD51D mutant cell lines) and ii) the
non-homologous end-joining pathway (xrs5 cells; Ku80 gene
deficient) were kindly supplied by Dr Larry Thompson (Lawrence
Livermore National Laboratory, Livermore, CA)(26,27).
All cells were grown and maintained in α-MEM (Invitrogen, Carlsbad,
CA) supplemented with 10% fetal bovine serum (FBS, Sigma, St.
Louis, MO), 1X antibiotics and antimycotics (anti-anti,
Invitrogen), at 37°C in CO2 incubators at 5%
CO2 and 100% humidity. Doubling times were ~12 h for all
cell lines.
Irradiation procedure and cell survival
assays
Cultured cells were trypsinized and re-suspended
into growth medium containing α-MEM with 10% FBS and antibiotics
(anti-anti, Invitrogen). Once re-suspended, 10 ml of medium
containing between 500 and 700 cells were placed into each
individual Opticell™ (Thermo Scientific, Rochester, NY) cell
culture container ~1 h prior to irradiation. All samples were then
appropriately organized and irradiated. Radiation physics
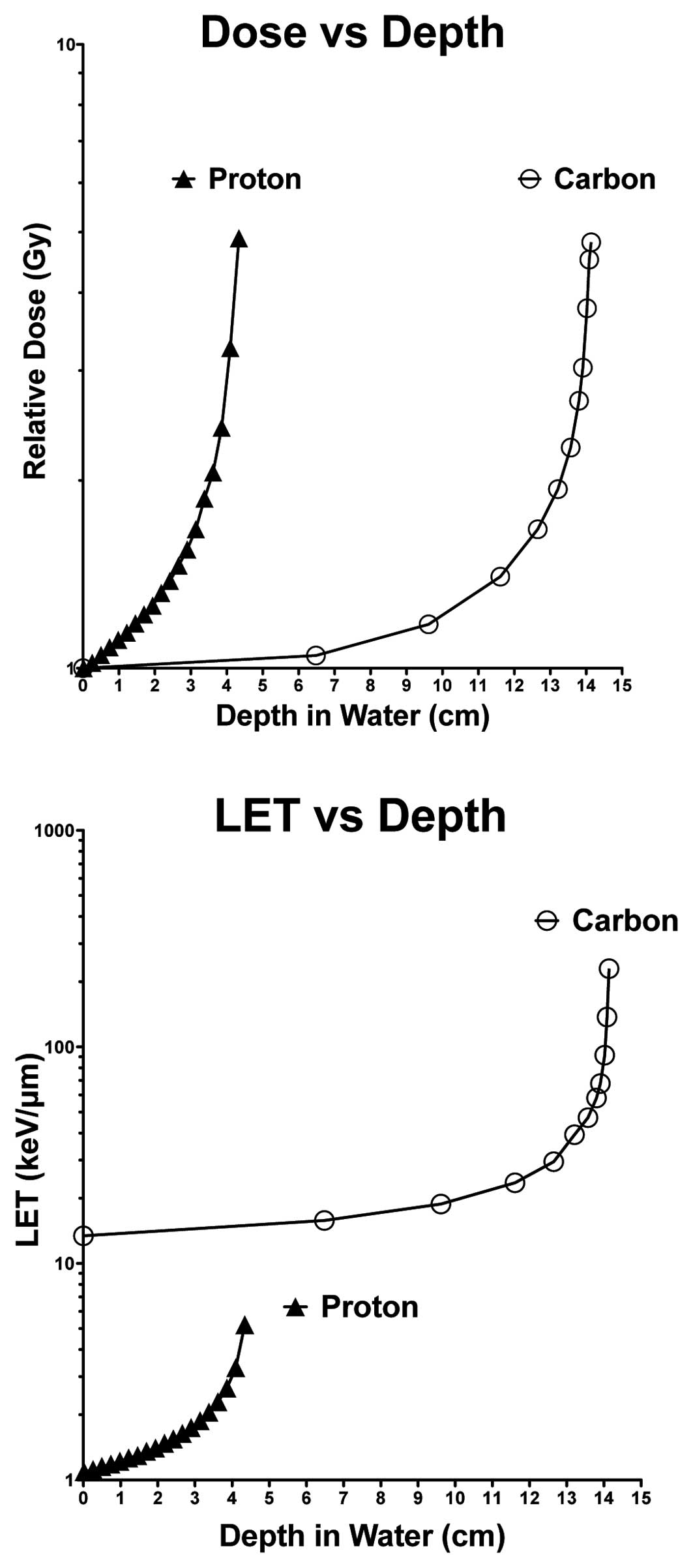
quantitative values including dose distribution and LET
distribution for both of the proton and carbon beams used is
summarized in Fig. 1. Immediately
following radiation, all cells were incubated at 37°C with 5%
CO2 humidity for 7–10 days. After this culturing period,
tissue culture vessels were then washed with 0.9% NaCl, fixed in
100% ethanol, and stained with 0.1% crystal violet. Colonies
containing >50 cells were scored as a surviving colony. A
minimum of three independent experiments was carried out for each
type of radiation studied. Survival curves were drawn for
individual data points plotting a given Opticell container’s
‘depth’ from doses calculated according to values presented in
Fig. 1.
Data treatment and statistical
analysis
All experimental data were analyzed using the Prism
5™ software. Standard errors of the means for all data points were
calculated for all experimental data points and are depicted in
each figure.
Results
Radiation physics parameters
Depth distribution values for the dose and LET were
calculated and plotted against corresponding depths in water for
the 70 MeV/n accelerated protons and 290 MeV/n accelerated carbon
ions (Fig. 1). These maximum doses
were delivered at depths of ~4 and 14 cm (in water), for protons
and carbon ions, respectively. In regard to LET distribution
values, carbon ions displayed higher LET values at all depths (in
water) relative to the protons, and delivered a peak LET at a depth
of ~14 cm (in water). Proton LET values were significantly lower
relative to those for carbon ions and a peak LET at a depth of ~4
cm (in water).
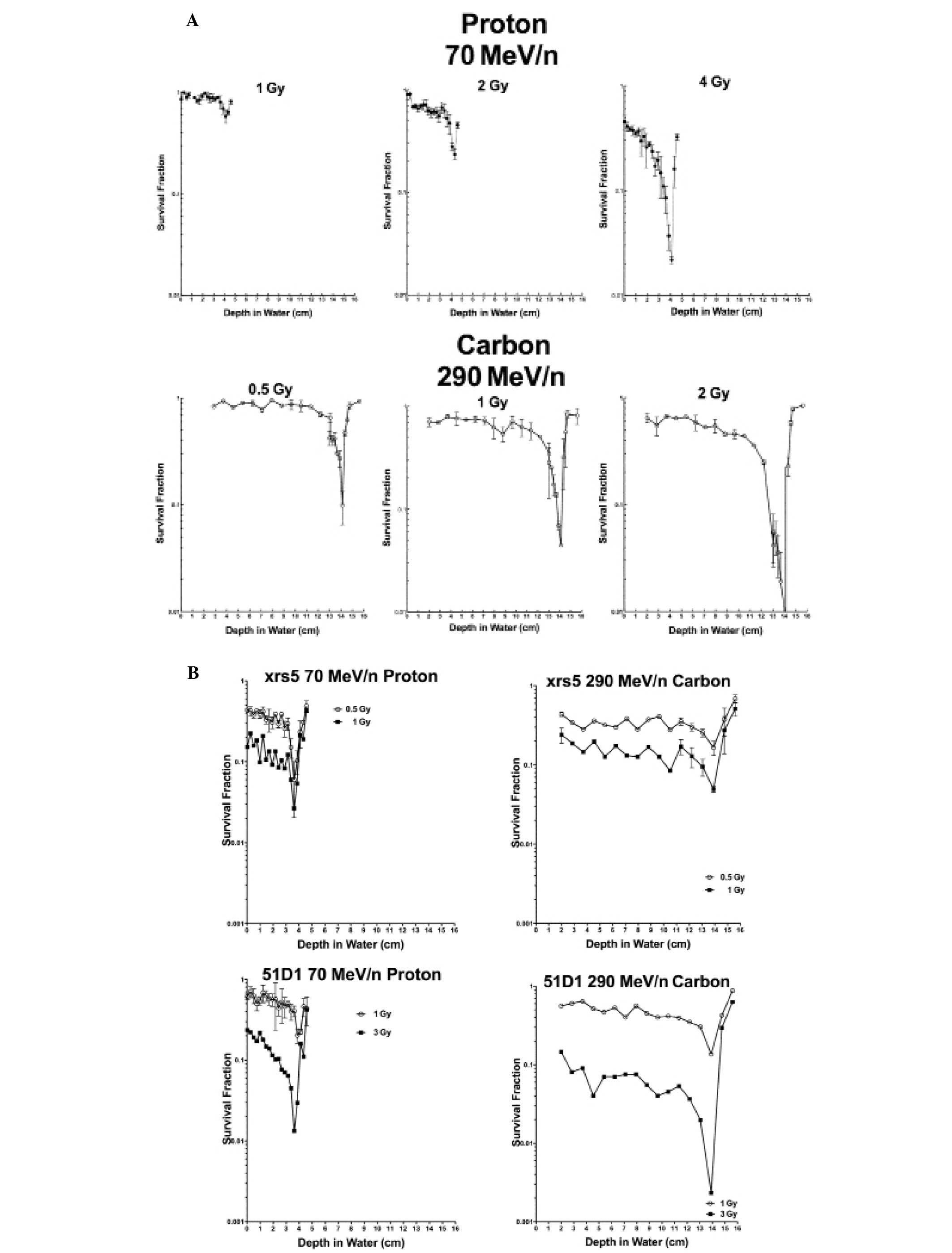
Dose-depth distribution effect of proton
and carbon beam cellular lethality
Stacked Opticell culture system cell survival assays
were carried out using CHO wild-type xrs5 and 51D1 cells exposed to
70 MeV/n accelerated protons and doses of 290 MeV/n accelerated
carbon ions. Carbon ions yielded lower survival fractions relative
to protons at their respective Bragg peaks for wild-type and 51D1
cells at equal and lower doses (Fig.
2). xrs5 cells had comparable survival values for proton and
carbon ions at doses of 0.5 and 1 Gy (Fig. 2).
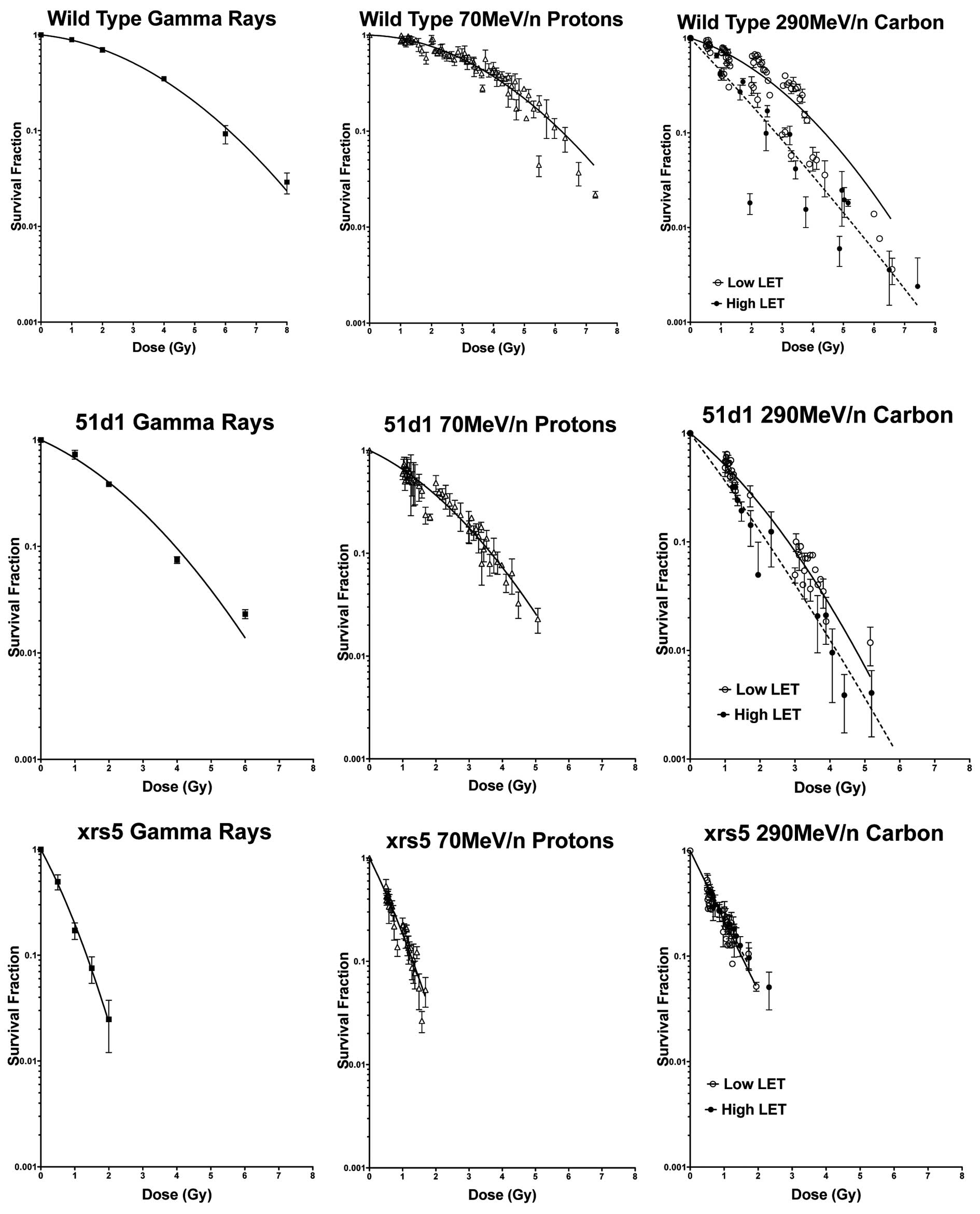
Determination of dose-dependent cell
survival
Cell survival was evaluated in response to the dose
for all three cell lines using γ-rays, 70 MeV/n accelerated
protons, and 290 MeV/n accelerated carbon ions. Both wild-type and
51D1 cells were most sensitive to carbon ions, and displayed
responses in cell survival that were dependent on both the dose and
LET for this particular form of radiation. In contrast, xrs5 cells
displayed relatively comparable cell survival responses to dose for
all types of radiation exposure, including exposure to high LET
(defined as LET values >30 keV/μm) carbon ions (Fig. 3).
RBE values for wild-type cells
RBE values were calculated based on the average D10
values representative of a particular form of radiation. High LET
290 MeV/n carbon ions had the highest average RBE of 2.03, followed
by low LET 290 MeV/n carbon ions with an RBE of 1.29 and 70 MeV/n
protons with an RBE of approximately 1.
Discussion
Our results show that the degree of cell killing
assessed via cell survival assays was dependent on the dose for all
types of radiation used in our study (Fig. 3). Of the various types of radiation
used, carbon ions produced cell survival levels that were dependent
on both the dose and amount of LET exposure (Fig. 3). The LET values of carbon ions near
Bragg peak are about one hundred times higher than protons and
other low LET radiation values (Fig.
1). In regards to notable differences observed in survival
responses between the different cell lines used, wild-type and
homologous recombination mutant 51D1 cells tended to be the most
sensitive to carbon ion radiation, especially at regions of high
LET (Figs. 2 and 3). On the other hand, xrs5 cells displayed
essentially the same sensitivity to the three types and LET
radiation used in our study (Figs.
2B and 3). At lower doses (of 1
Gy) in particular, non-homologous end joining deficient cells
trended towards a higher sensitivity to either carbon ions or
protons than did homologous recombination mutants, which is
consistent with the relative roles of these two repair pathways in
double-stranded DNA damage (Figs.
2B and 3).
When comparing the effectiveness between carbon ion
radiotherapy and proton radiotherapy, our study suggests that
carbon ions are advantageous to protons in the sense that cellular
lethality is dependent on both dose and LET values for carbon ions
(for cells proficient in non homologous end joining DNA repair),
whereas cell survival for protons is dependent only on dose.
Additionally, carbon ions in our study displayed LET values that
were significantly higher than protons at the Bragg peak regions,
which is also advantageous, especially when considering that an
SOBP of high LET radiation is actually administered to a tumor as a
whole.
A previous study reported findings similar to ours
when evaluating the cellular lethality for xrs5 cells in response
to increasing LET exposure of carbon ions (28). Concurrently, these two studies
suggest that loss of non-homologous end joining DNA repair capacity
undermines the carbon ion cellular lethality dependence on both the
quality of radiation and quantity of LET exposure, and that only
patients diagnosed with NHEJ DNA repair-competent tumors will
maximally benefit from carbon ion therapy. To the best of our
knowledge, our present study is one of the first to investigate
whether cells deficient in the other major cellular DNA repair
pathway and incapable of homologous recombination also display this
phenomenon. Results from the present study demonstrate that
homologous recombination-deficient cells demonstrate a dependence
on the quality of radiation and quantity of LET when cell survival
is measured. However, investigative efforts are still required to
definitively reiterate or reinforce this finding.
Another finding from our study that is worth
mentioning are the RBE values derived from D10 values representing
our monoenergetic proton and carbon beams. We discovered average
RBE values calculated from D10 doses to be 2.03 at high LET Bragg
peak regions and 1.29 at low LET regions outside the Bragg peak for
wild-type cells exposed to carbon ions. The average calculated RBE
for protons was approximately 1.0 for wild-type cells. From the
perspective of RBE in our study, carbon ions may be considered
advantageous to protons when using this wild-type in vitro
model. RBE values for wild-type cells in our study are comparable
to those depicted in previous studies (15,29,30).
In light of our findings, it would be significant to
determine if carbon ions remain advantageous over protons in terms
of a LET deposition and cell survival dependence on both dose and
LET deposition at lower energy levels where a Bragg peak for
accelerated carbon ions is located at the same depth in water as a
comparative proton beam. Additionally, a future investigative
effort evaluating the degree of synergy between inhibitors of
various DNA repair pathways (i.e. homologous recombination and
non-homologous end joining repair) combined with either proton or
carbon radiation exposure could shed light on our findings. Further
research involving proton and carbon in vitro experiments
and more effective particle therapy treatment modalities are
required for types of localized cancers where these types of
radiation are applicable.
Acknowledgements
This work was a part of Research Project with Heavy
Ions at NIRS-HIMAC and with NIRS-Cyclotron. This research is
partially supported by International Open Laboratory at NIRS.
References
|
1
|
Ares C, Hug EB, Lomax AJ, et al:
Effectiveness and safety of spot scanning proton radiation therapy
for chordomas and chondrosarcomas of the skull base: first
long-term report. Int J Radiat Oncol Biol Phys. 75:1111–1118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hug EB and Slater JD: Proton radiation
therapy for chordomas and chondrosarcomas of the skull base.
Neurosurg Clin N Am. 11:627–638. 2000.PubMed/NCBI
|
|
3
|
Pontvert D: Value of proton therapy in
tumors other than melanomas of the eye and sarcomas of the base of
the skull. Pathol Biol. 41:1181993.(In French).
|
|
4
|
Munzenrider JE and Liebsch NJ: Proton
therapy for tumors of the skull base. Strahlenther Onkol. 175(Suppl
2): 57–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
St Clair WH, Adams JA, Bues M, et al:
Advantage of protons compared to conventional X-ray or IMRT in the
treatment of a pediatric patient with medulloblastoma. Int J Radiat
Oncol Biol Phys. 58:727–734. 2004.PubMed/NCBI
|
|
6
|
Timmermann B: Proton beam therapy for
childhood malignancies: status report. Klin Padiatr. 222:127–133.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hattangadi JA, Rombi B, Yock TI, et al:
Proton radiotherapy for high-risk pediatric neuroblastoma: early
outcomes and dose comparison. Int J Radiat Oncol Biol Phys. Dec
2–2011.(Epub ahead of print).
|
|
8
|
Mizoe JE, Hasegawa A, Jingu K, et al:
Results of carbon ion radiotherapy for head and neck cancer.
Radiother Oncol. 103:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishikawa H, Tsuji H, Kamada T, et al:
Carbon ion radiation therapy for prostate cancer: results of a
prospective phase II study. Radiother Oncol. 81:57–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Combs SE, Kalbe A, Nikoghosyan A, et al:
Carbon ion radiotherapy performed as re-irradiation using active
beam delivery in patients with tumors of the brain, skull base and
sacral region. Radiother Oncol. 98:63–67. 2011. View Article : Google Scholar
|
|
11
|
Silari M: Applications of particle
accelerators in medicine. Radiat Prot Dosimetry. 146:440–450. 2011.
View Article : Google Scholar
|
|
12
|
Schulz-Ertner D, Nikoghosyan A, Thilmann
C, et al: Results of carbon ion radiotherapy in 152 patients. Int J
Radiat Oncol Biol Phys. 58:631–640. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akutsu Y, Yasuda S, Nagata M, et al: A
phase I/II clinical trial of preoperative short-course carbon-ion
radiotherapy for patients with squamous cell carcinoma of the
esophagus. J Surg Oncol. 105:750–755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Li S, Wang XH, et al: Results of
carbon ion radiotherapy for skin carcinomas in 45 patients. Br J
Dermatol. 166:1100–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paganetti H, Niemierko A, Ancukiewicz M,
et al: Relative biological effectiveness (RBE) values for proton
beam therapy. Int J Radiat Oncol Biol Phys. 53:407–421. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez-Lafrasse C and Balosso J: From
the carbon track to therapeutic efficiency of hadrontherapy. Cancer
Radiother. 16:16–24. 2012.PubMed/NCBI
|
|
17
|
Terasawa T, Dvorak T, Ip S, Raman G, Lau J
and Trikalinos TA: Systematic review: charged-particle radiation
therapy for cancer. Ann Intern Med. 151:556–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carabe A, Moteabbed M, Depauw N, Schuemann
J and Paganetti H: Range uncertainty in proton therapy due to
variable biological effectiveness. Phys Med Biol. 57:1159–1172.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsunemoto H, Morita S, Ishikawa T, et al:
Proton therapy in Japan. Radiat Res (Suppl). 8:S235–S243. 1985.
View Article : Google Scholar
|
|
20
|
Wouters BG, Lam GK, Oelfke U, Gardey K,
Durand RE and Skarsgard LD: Measurements of relative biological
effectiveness of the 70 MeV proton beam at TRIUMF using Chinese
hamster V79 cells and the high-precision cell sorter assay. Radiat
Res. 146:159–170. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weyrather WK and Kraft G: RBE of carbon
ions: experimental data and the strategy of RBE calculation for
treatment planning. Radiother Oncol. 73(Suppl 2): S161–S169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peschke P, Karger CP, Scholz M, Debus J
and Huber PE: Relative biological effectiveness of carbon ions for
local tumor control of a radioresistant prostate carcinoma in the
rat. Int J Radiat Oncol Biol Phys. 79:239–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui X, Oonishi K, Tsujii H, et al: Effects
of carbon ion beam on putative colon cancer stem cells and its
comparison with X-rays. Cancer Res. 71:3676–3687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frese MC, Yu VK, Stewart RD and Carlson
DJ: A mechanism-based approach to predict the relative biological
effectiveness of protons and carbon ions in radiation therapy. Int
J Radiat Oncol Biol Phys. 83:442–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ando K and Kase Y: Biological
characteristics of carbon-ion therapy. Int J Radiat Biol.
85:715–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeggo PA and Kemp LM: X-ray-sensitive
mutants of Chinese hamster ovary cell line. Isolation and
cross-sensitivity to other DNA-damaging agents. Mutat Res.
112:313–327. 1983.PubMed/NCBI
|
|
27
|
Hinz JM, Tebbs RS, Wilson PF, et al:
Repression of mutagenesis by Rad51D-mediated homologous
recombination. Nucleic Acids Res. 34:1358–1368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weyrather WK, Ritter S, Scholz M and Kraft
G: RBE for carbon track-segment irradiation in cell lines of
differing repair capacity. Int J Radiat Biol. 75:1357–1364. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elsasser T, Weyrather WK, Friedrich T, et
al: Quantification of the relative biological effectiveness for ion
beam radiotherapy: direct experimental comparison of proton and
carbon ion beams and a novel approach for treatment planning. Int J
Radiat Oncol Biol Phys. 78:1177–1183. 2010. View Article : Google Scholar
|
|
30
|
Belli M, Cera F, Cherubini R, et al:
RBE-LET relationships for cell inactivation and mutation induced by
low energy protons in V79 cells: further results at the LNL
facility. Int J Radiat Biol. 74:501–509. 1998. View Article : Google Scholar : PubMed/NCBI
|