Introduction
Approximately four per 100,000 people succumb to
leukemia each year in Taiwan, according to reports from the
Department of Health, Executive Yuan of Taiwan (1,2). The
current primary treatment for leukemia is chemotherapy, however the
survival rates remain unsatisfactory. Acute myeloid leukemia (AML)
increases the number of myeloid cells in the bone marrow and
interrupts their maturation, resulting in hematopoietic
insufficiency (3–5). Differentiation induction, as a
therapeutic strategy, can have a powerful impact on hematopoietic
malignancies, in particular on myeloid leukemia (6,7).
Therefore, discovering a new antileukemia agent that is more
effective and less toxic for leukemia patients is necessary.
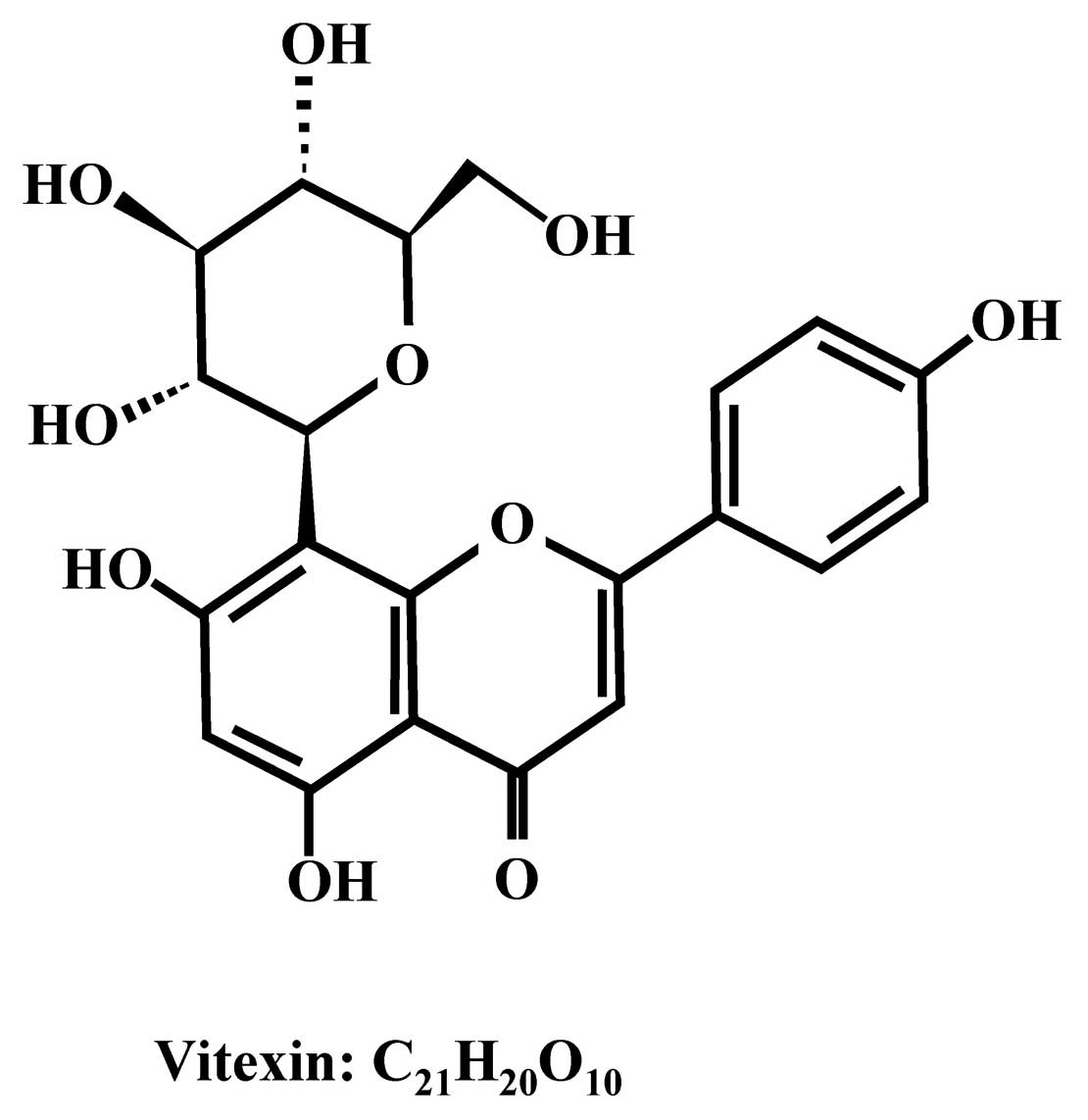
Vitexin is a natural apigenin flavone glucoside,
found in the Desmodium species (8,9). It
has been reported to exhibit biological activities, including
antioxidant and anti-inflammatory effects (8). Vitexin is now known to also possess
antitumor activities by targeting apoptotic cell death in human
breast cancer cell lines and potent inhibition on tumor necrosis
factor α (TNF-α)-induced cell death (10). Therefore, our study investigated the
effects of vitexin on the induction of apoptosis in U937 human
leukemia cells.
Apoptosis assures the homeostasis of tissues during
development, host defense and aging (11,12).
Divergent cell survival due to insufficient apoptosis has been
linked to the development and/or progression of human malignancies
(13). Nevertheless, cancer cells
with mutations or abnormalities in the expression of other genes
that regulate apoptosis can display intrinsic resistance to
chemotherapy-induced apoptosis (11,14).
This suggests that acquired defects in the apoptotic process play
an important role in the development of drug resistance. In
addition, several transcription factors have been shown to be
targets of vitexin action, which may mediate vitexin-induced
programmed cell death (15). This
study investigated whether vitexin could induce cell apoptosis in
U937 human leukemia cells, as there is currently no available
information regarding its cytotoxic effects on human leukemia
cells.
Materials and methods
Chemicals and reagents
Vitexin (Fig. 1),
dimethylsulfoxide (DMSO), propidium iodide (PI),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
RNase A, Triton X-100 and cyclosporine A were purchased from
Sigma-Aldrich Corp. (St. Louis, MO, USA). All primary and secondary
antibodies were obtained from Santa Cruz Biotechnology Inc., (Santa
Cruz, CA, USA). TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.6),
potassium phosphates and z-VAD were purchased from Merck Co.
(Darmstadt, Germany). RPMI-1640 medium, fetal bovine serum (FBS),
penicillin-streptomycin and L-glutamine were obtained from
Gibco/Life Technologies (Grand Island, NY, USA). All of the
chemicals used were of reagent grade.
Cell cultures
The human lymphoma U937 cell line was purchased from
the Food Industry Research and Development Institute (Hsinchu,
Taiwan, R.O.C.). The cells were grown in RPMI-1640 medium
supplemented with 10% heat-inactivated FBS, 100 μg/ml
penicillin-100 U/ml streptomycin and 2 mM L-glutamine, and grown in
a humidified 5% CO2 atmosphere at 37˚C. The cells were
subcultured every third day (16).
Cell viability and morphological
changes
The U937 human leukemia cells were placed in 96-well
cell culture plates at an initial concentration of 1×104
cells/ml and incubated with various concentrations of vitexin (0,
50, 100, 200 and 400 μM). After a 24-h incubation period, MTT
solution (0.5 mg/ml) was added into the wells for 4 h. The growth
medium was removed, and the formazan crystals formed by oxidation
of the MTT dye were dissolved with 100 μl 0.04 N HCl in
isopropanol. The absorbance was measured at 570 nm by ELISA reader
and the cell survival ratio was expressed as a percentage of the
control as previously described (17,18).
For morphological changes, cells were cultured in 12-well plates at
a density of 2×105 cells/well and treated with or
without 200 μM of vitexin for 24 h. Morphological changes in
vitexin-treated cells were examined and photographed using
phase-contrast light microscopy (19). All results were obtained from three
independent experiments.
DNA laddering fragmentation assay
Approximately 2×105 cells/well of U937
cells were grown in 12-well plates and treated with vitexin or
vitexin plus z-VAD (a pan-caspase inhibitor) for 24 h. DNA was
extracted from vitexin-treated and untreated cells with the Tissue
and Cell Genomic DNA purification kit (Genemark Technology Co.,
Ltd. Tainan, Taiwan). DNA fragmentation was visualized by 1.5%
agarose gel electrophoresis as previously described (20,21).
Flow cytometric analysis for apoptosis by
TUNEL assay
TUNEL staining was performed according to the
manufacturer’s protocols (in situ cell death detection kit;
Roche Diagnostics, Boehringer Mannheim, Germany). Cells
(1×106/well) were individually plated into six-well
plates and exposed to 1 μM vitexin and vitexin plus z-VAD or
cyclosporine A (a mitochondrial membrane potential inhibitor) for
24 h. After treatment, cells were collected and fixed in 4%
formaldehyde overnight, placed in 0.1% Triton X-100/PBS, washed
with 0.1% PBS twice, then stained with 100 μl of terminal
deoxynucleotidyl transferase-containing solution and incubated in
the dark for 30 min at 37˚C. Following TUNEL staining, all samples
were washed three times and resuspended in 0.5 ml of PBS containing
PI (10 μg/ml) and DNase free-RNase A (200 μg/ml). TUNEL-positive
cells were analyzed by flow cytometry. The median fluorescence
intensity was quantified with CellQuest software (BD Biosciences)
(21,22). TUNEL assays were performed in
triplicate for three independent experiments.
Caspase activity determinations
Caspase activity in cell lysates was measured using
the manufacturer’s protocols (caspase-3, -7 and -9 colorimetric
assay kits; R&D Systems Inc., Minneapolis, MN, USA). Cells were
re-suspended in medium at an initial concentration of
5×106 cells and pelleted and re-suspended in 0, 50, 100,
200 and 400 μM of vitexin for 24 h. Cells were lysed in lysis
buffer [50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 10 mM EGTA, 10 mM
digitonin and 2 mM DTT]. The cell lysates (50 μg proteins) were
incubated with caspase-3, -7 and -9 specific substrates
(Ac-DEVD-pNA and Ac-LEHD-pNA) at 37˚C for 1 h. Caspase activity and
absorbance were measured with an enzyme-linked immunosorbent assay
reader at OD405 (16,23).
All results are from three independent experiments.
Measurement of mitochondrial membrane
potential
Cells were seeded in 24-well cell culture plates at
an initial concentration of 2×105 cells/ml and were
maintained with 0, 50, 100, 200 and 400 μM of vitexin for 24 h.
Flow cytometry was then used to determine the level of ΔΨm.
Following incubation, cells were harvested, washed twice by PBS,
and then stained with 500 μl of 100 nM of DiOC6 that was
stored at -20˚C as a 1 μmol/l stock in DMSO for the level of ΔΨm.
Subsequently, cells were maintained in a dark room for 30 min at
37˚C and all samples were analyzed immediately by flow cytometry,
as previously described (19,24).
Western blot analysis
Western blot analysis to determine the levels of
various proteins was performed as previously described (18,19).
Cells were washed with PBS and lysed into the PRO-PREP™ protein
extraction solution (iNtRON Biotechnology, Gyeonggi-do, Korea)
before being placed in a 10-cm dish at an initial concentration of
1×107 cells and incubated with 200 μM of vitexin for
6,12,18 and 24 h. An equal amount of cell lysate was separated by
10% gel using sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE). Proteins were electro-transferred to a
nitrocellulose membrane using iBot Dry Blotting system
(Invitrogen/Life Technologies). The membrane was then blocked in 5%
powdered non-fat milk in PBST solution (0.1% Tween-20 in PBS) for 1
h. The primary antibodies caspase-9, caspase-3 and Bcl-2 were
diluted in blocking solution and then incubated with the membrane
overnight. The membrane was then covered with an alkaline HRP
conjugated secondary IgG antibody (goat anti-rabbit and goat
anti-mouse) for 1 h. After incubating with the second antibody, the
membranes were reacted with enhanced chemiluminescence (ECL)
solution (Western blotting detection kit, Immobilon Western HRP
substrate, Millipore, Billerica, MA, USA). Signals were detected by
X-ray film (GE Healthcare, Piscataway, NJ, USA). β-actin was
included as a loading control. The radiograms were scanned and the
band density was quantified using NIH ImageJ program (Bethesda, MD,
USA) (18).
Statistical analysis
Data were expressed as the mean ± SD and differences
between control and vitexin-treated groups were analyzed by
Student’s t-test. *p<0.05 was considered to indicate
statistically significant differences.
Results
Effects of vitexin on morphological
changes and cell viability of U937 human leukemia cells
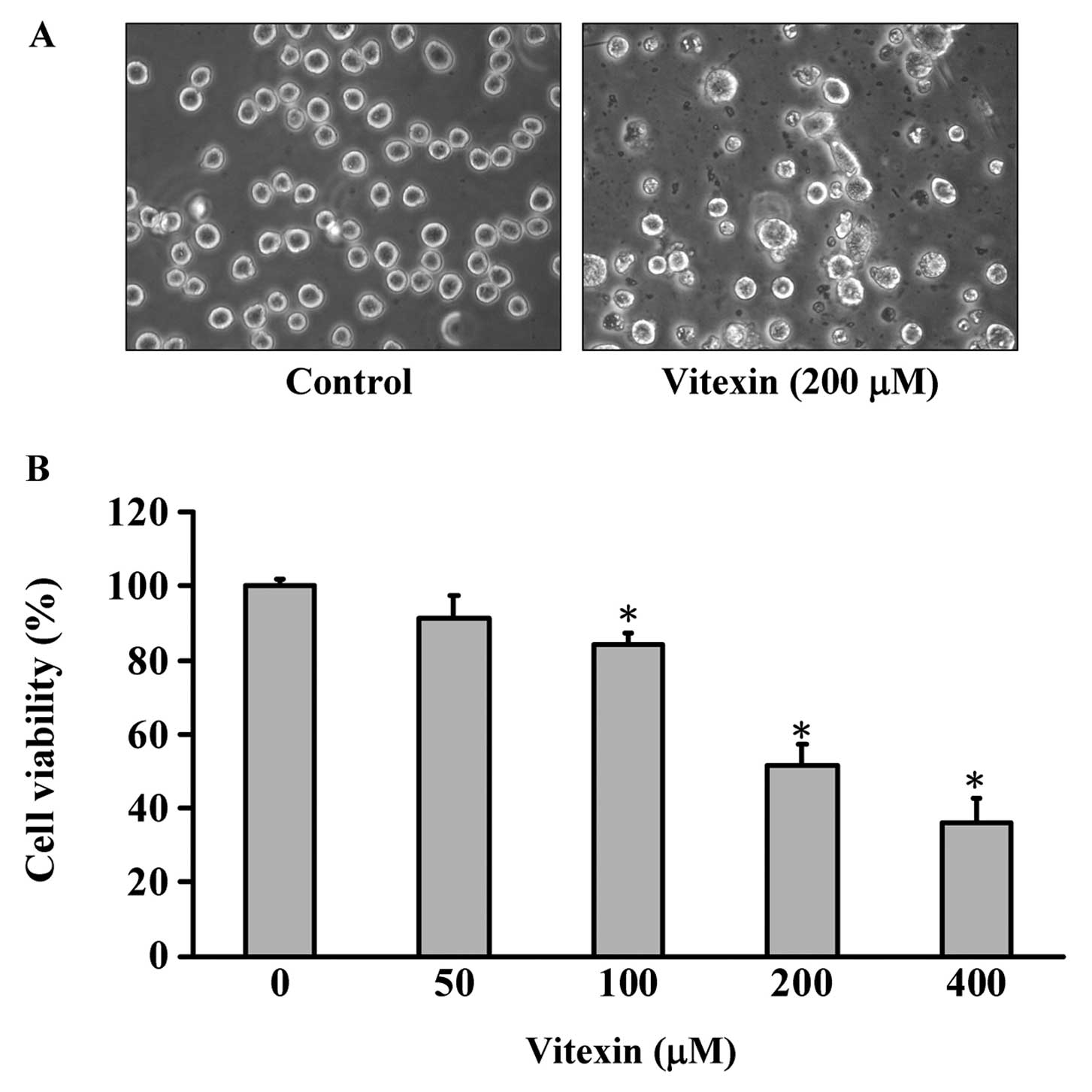
The morphological changes shown in Fig. 2A indicate that the cells in the
vitexin-treated and the control group differ significantly. Some of
the cells detached from the surface and debris were also observed
in the plate of the vitexin-treated group, but the control cells
were well spread with flattened morphology (Fig. 2A). To determine the growth
inhibition effects of vitexin, cells were treated with different
concentrations (50, 100, 200 and 400 μM) of vitexin for 24 h. Cell
viability was determined by the MTT assay. Concentration- and
time-dependent effects of vitexin are shown in Fig. 2B, and the viable cells were
significantly reduced in the vitexin-treated U937 human leukemia
cells. The concentration required to inhibit growth by 50%
(IC50) for U937 human leukemia cells was ~200.34 μM at
24 h. We suggest that vitexin reduced the proportion of viable U937
human leukemia cells in a concentration- and time-dependent
manner.
Effects of DNA laddering fragmentation in
U937 cells
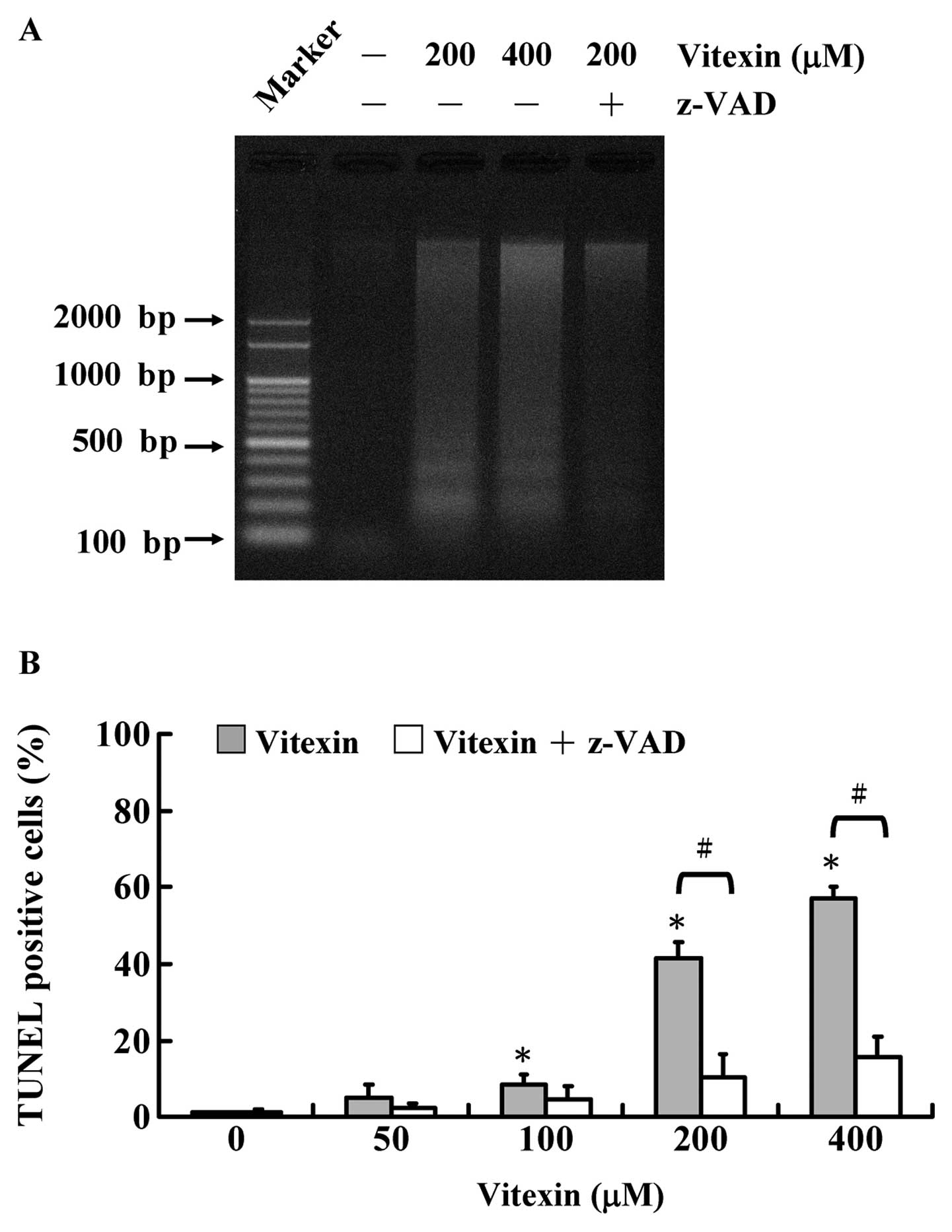
DNA laddering provides evidence for a
vitexin-induced apoptotic process characterized by DNA
fragmentation. The agarose gel electrophoresis of DNA extracted
from U937 cells treated with vitexin at various concentrations (200
and 400 μM) for 48 h was carried out and the results revealed that
DNA fragmentation occurred in vitexin-treated U937 cells (Fig. 3A). We also noted that pretreatment
with z-VAD (a pan-caspase inhibitor) is likely to protect a
vitexin-provoked DNA ladder of U937 cells. Similarly, TUNEL
positive cells were also observed in U937 cells after exposure to
100, 200 and 400 μM of vitexin, and z-VAD attenuated
vitexin-induced TUNEL positive cells in comparison to untreated
control cells, as shown in Fig. 3B.
Thus, vitexin concentration-dependently induced apoptosis by DNA
fragmentation. Based on this finding, we suggest that
vitexin-induced apoptotic death of U937 cells is correlated with
caspase-dependent effects.
Effects of vitexin on the caspase-3, -7,
and -9 activities in U937 cells
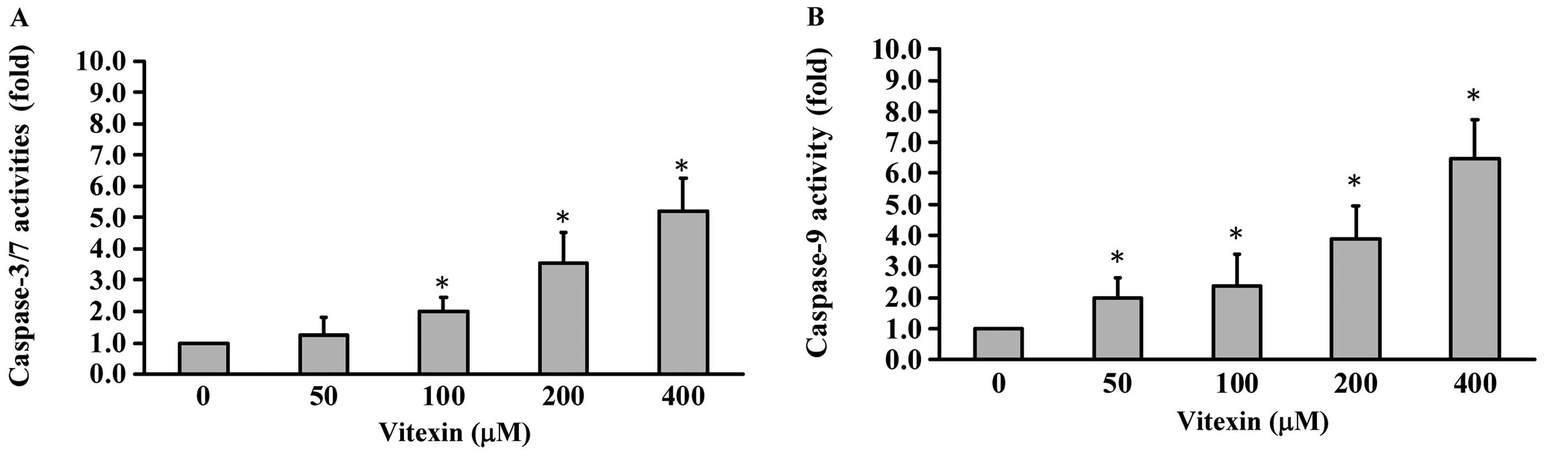
We determined the roles of individual caspases in
vitexin-induced apoptosis. In order to evaluate the effects of
vitexin on the activities of caspase-3, -7 and -9 in U937 cells,
caspase activity assays were applied to investigate related caspase
activities. The results in Fig. 4A
and B show that various concentrations (0, 50, 100, 200 and 400 μM)
of vitexin promoted caspase-3, -7 (Fig.
4A) and caspase-9 (Fig. 4B)
activities in a concentration-dependent manner. We confirm that
vitexin-induced apoptosis is mediated by activations of caspase-3,
-7 and -9 signaling. Thus, we suggest that intrinsic apoptotic
signaling contributed to vitexin-triggered apoptosis of U937 cells
in vitro.
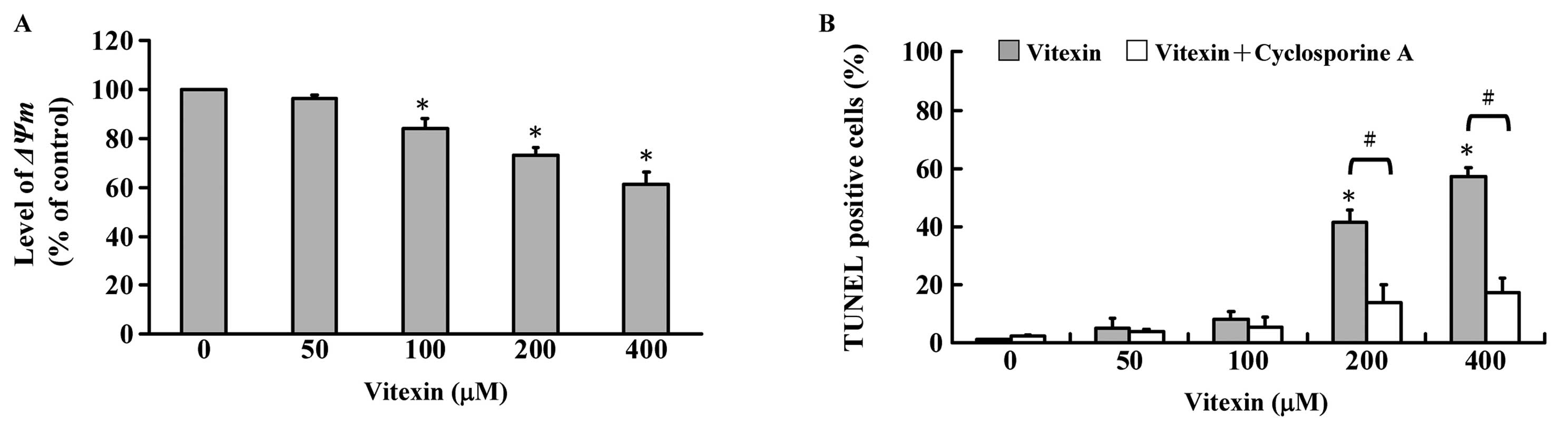
Effects of vitexin on mitochondrial
membrane potential and its inhibitor (cyclosporine A) in U937
cells
Cells were treated with 50, 100, 200 and 400 μM of
vitexin for 24 h. The alterations of ΔΨm were determined by
staining with DiOC6 and then analyzed by flow cytometry,
and representative data are shown in Fig. 5A demonstrating that vitexin
decreased the level of ΔΨm in U937 cells and this effect is a
concentration-dependent response. To explore whether
vitexin-induced apoptosis is mediated through mitochondrial
depolarization, cyclosporine A (a ΔΨm inhibitor) was used for
measuring TUNEL positive cells. We found that cyclosporine A is
able to decrease vitexin-stimulated apoptosis in U937 cells as seen
in Fig. 5B. We suggest that
vitexin-induced apoptosis is involved in the mitochondrial
signaling pathway.
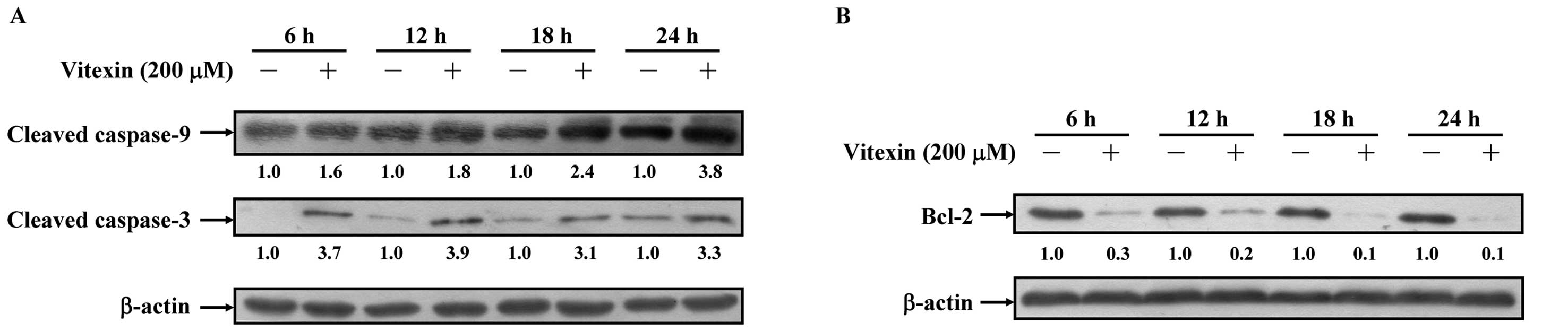
Effects of vitexin on the levels of
apoptosis-associated protein expression in U937 cells
We confirmed that vitexin-induced apoptosis is
associated with mitochondria-dependent protein expressions. Our
data indicate that vitexin promoted the expressions of cleaved
caspase-3 and cleaved caspase-9 in U937 cells (Fig. 6A). The Bcl-2 family proteins located
on the mitochondrial membrane are important for suppression of
mitochondrial manifestations of apoptosis (12,16).
Fig. 6B shows that a decrease of
Bcl-2 expression occurred in vitexin-treated U937 cells.
Consequently, the induction of the mitochondrial pathway plays a
central role in vitexin-induced apoptosis of U937 cells in
vitro.
Discussion
Vitexin, a natural apigenin flavone glucoside, has
been reported to exhibit antioxidative and anti-inflammatory
properties, and to have growth inhibitory effects in human breast
cancer cell lines (9,25), but few reports demonstrate its
inhibitory effects on U937 human leukemia cells. In this study, we
found that different concentrations (50, 100, 200, 400 μM) of
vitexin significantly inhibited the growth of U937 human leukemia
cells (Fig. 2B). These data
indicate that vitexin inhibited cell proliferation and induced
apoptosis (Fig. 3) in the U937
human leukemia cells, and vitexin induced morphological changes
(Fig. 2A) and reduced the
percentage of viable cells in the human U937 leukemia cell in a
dose- and time-dependent manner.
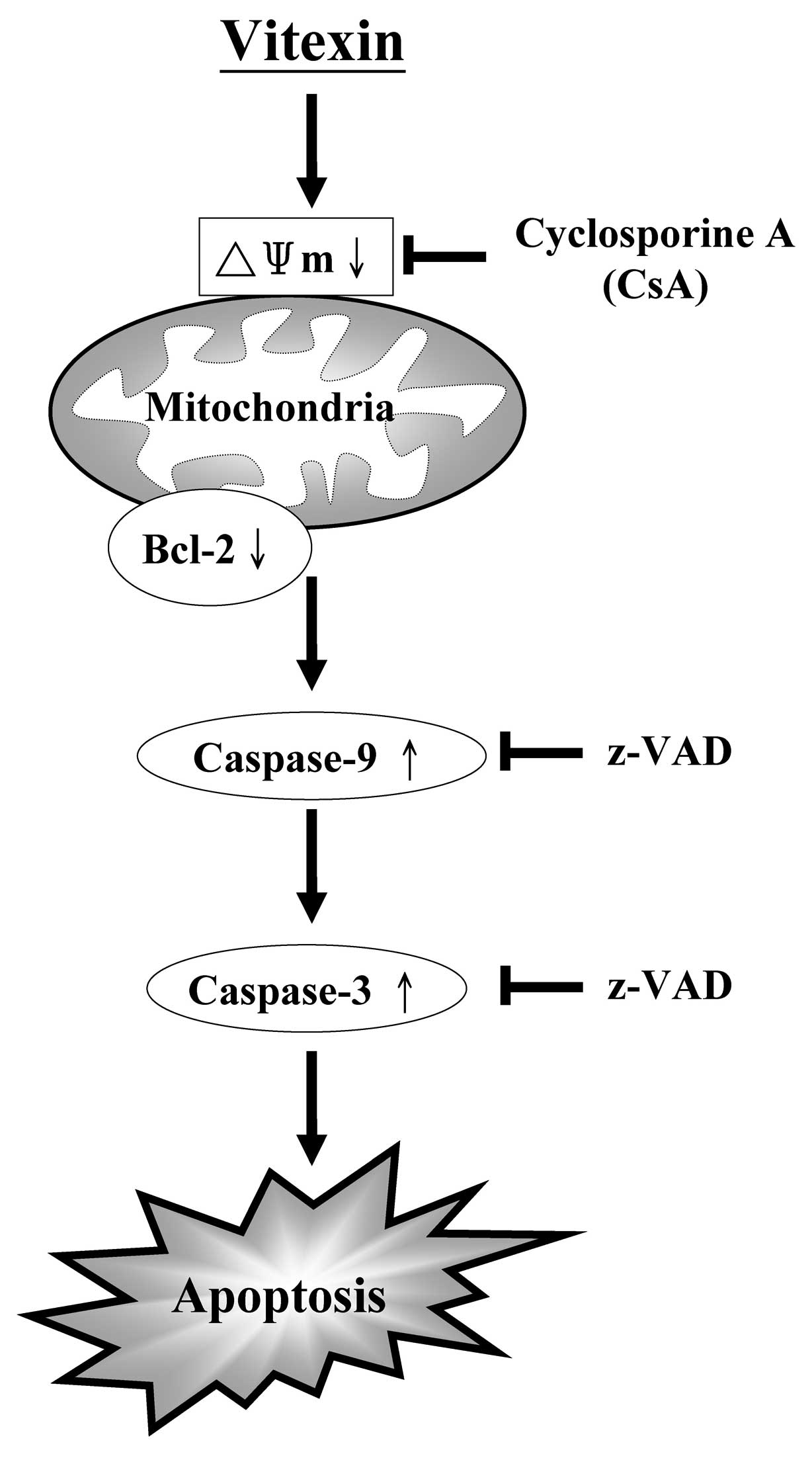
It is well known that mitochondria are implicated as
being a center mechanism and one of the apoptotic targets (26,27),
and Bcl-2 family protein expression is likely to influence
mitochondrial depolarization (28).
Consequently, the activations of caspase-9 and -3 are the key
mediators of cell apoptosis (12,28).
In the current study, we demonstrated that vitexin inhibited the
levels of Bcl-2 (Fig. 6B) which led
to the disruption of ΔΨm (Fig. 5A)
in U937 cells. Importantly, vitexin stimulated caspase-9 and -3
activities (Fig. 4) and protein
expressions (Fig. 6) in U937 cells.
Pretreatment with z-VAD (a pan-caspase inhibitor) (Fig. 3A) and cyclosporine A (Fig. 5B) led to a decrease in
vitexin-induced TUNEL positive cells, compared with cells treated
alone. Hence, vitexin-provoked apoptosis could be inhibited via
suppressing the mitochondrial and caspase-dependent pathways. Taken
together, these results suggest the potential use of the
anti-leukemia activity of vitexin and confirmed that vitexin may be
used as a treatment for diseases such as leukemia. Our study is
also in agreement with a previous study by Zhou et al
addressing the biological activity and anticancer actions of
vitexin in tumor cell lines (9). In
conclusion, the induction of apoptotic cell death by vitexin in
U937 human leukemia cells was detected as an activation of
caspase-3, -7 and -9 (Fig. 7).
Based on these experiments, we suggest that vitexin enhances the
cytotoxicity and induces an apoptotic cell death in U937 human
leukemia cells.
Acknowledgements
This study was partly supported by the grant-in-aid
from the National Science Council, Republic of China (Taiwan)
(NSC-101-2313-B-039-008).
References
|
1
|
Lin JP, Yang JS, Lin JJ, et al: Rutin
inhibits human leukemia tumor growth in a murine xenograft model in
vivo. Environ Toxicol. 27:480–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang YL, Hung CC, Chen JS, et al: IKZF1
deletions predict a poor prognosis in children with B-cell
progenitor acute lymphoblastic leukemia: a multicenter analysis in
Taiwan. Cancer Sci. 102:1874–1881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lowenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SJ, Kim KH, Park JS, et al:
Comparative analysis of cell surface proteins in chronic and acute
leukemia cell lines. Biochem Biophys Res Commun. 357:620–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stahnke K, Eckhoff S, Mohr A, Meyer LH and
Debatin KM: Apoptosis induction in peripheral leukemia cells by
remission induction treatment in vivo: Selective depletion and
apoptosis in a CD34+ subpopulation of leukemia cells.
Leukemia. 17:2130–2139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olsson I, Bergh G, Ehinger M and Gullberg
U: Cell differentiation in acute myeloid leukemia. Eur J Haematol.
57:1–16. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai JC, Huang GJ, Chiu TH, et al:
Antioxidant activities of phenolic components from various plants
of Desmodium species. Afr J Pharm Pharmacol. 5:468–476.
2011. View Article : Google Scholar
|
|
9
|
Zhou Y, Liu YE, Cao J, et al: Vitexins,
nature-derived lignan compounds, induce apoptosis and suppress
tumor growth. Clin Cancer Res. 15:5161–5169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banskota AH, Tezuka Y, Adnyana IK, et al:
Hepatoprotective effect of combretum quadrangulare and its
constituents. Biol Pharm Bull. 23:456–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelloff GJ, Crowell JA, Steele VE, et al:
Progress in cancer chemoprevention: Development of diet-derived
chemopreventive agents. J Nutr. 130:467S–471S. 2000.PubMed/NCBI
|
|
12
|
Lavrik IN, Golks A and Krammer PH:
Caspases: pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klampfer L, Cammenga J, Wisniewski HG and
Nimer SD: Sodium salicylate activates caspases and induces
apoptosis of myeloid leukemia cell lines. Blood. 93:2386–2394.
1999.PubMed/NCBI
|
|
15
|
Aggarwal BB, Van Kuiken ME, Iyer LH,
Harikumar KB and Sung B: Molecular targets of nutraceuticals
derived from dietary spices: Potential role in suppression of
inflammation and tumorigenesis. Exp Biol Med. 234:825–849. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
17
|
Chang YH, Yang JS, Kuo SC and Chung JG:
Induction of mitotic arrest and apoptosis by a novel synthetic
quinolone analogue, CWC-8, via intrinsic and extrinsic apoptotic
pathways in human osteogenic sarcoma U-2 OS cells. Anticancer Res.
29:3139–3148. 2009.
|
|
18
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
19
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo CL, Wu SY, Ip SW, et al: Apoptotic
death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma
cells is mediated through the ROS, mitochondrial depolarization and
caspase-3-dependent signaling responses. Int J Oncol. 39:319–328.
2011.
|
|
21
|
Chung JG, Yang JS, Huang LJ, et al:
Proteomic approach to studying the cytotoxicity of YC-1 on U937
leukemia cells and antileukemia activity in orthotopic model of
leukemia mice. Proteomics. 7:3305–3317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu PP, Liu KC, Huang WW, et al: Triptolide
induces apoptosis in human adrenal cancer NCI-H295 cells through a
mitochondrial-dependent pathway. Oncol Rep. 25:551–557.
2011.PubMed/NCBI
|
|
23
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee H, Park MT, Choi BH, et al:
Endoplasmic reticulum stress-induced JNK activation is a critical
event leading to mitochondria-mediated cell death caused by
β-lapachone treatment. PLoS One. 6:e215332011.PubMed/NCBI
|
|
25
|
Dong L, Fan Y, Shaox and Chen Z: Vitexin
protects against myocardial ischemia/reperfusion injury in
Langendorff-perfused rat hearts by attenuating inflammatory
response and apoptosis. Food Chem Toxicol. 49:3211–3216. 2011.
View Article : Google Scholar
|
|
26
|
Kim R, Emi M, Tanabe K and Murakami S:
Role of the unfolded protein response in cell death. Apoptosis.
11:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Breckenridge DG, Germain M, Mathai JP,
Nguyen M and Shore GC: Regulation of apoptosis by endoplasmic
reticulum pathways. Oncogene. 22:8608–8618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orrenius S: Reactive oxygen species in
mitochondria-mediated cell death. Drug Metab Rev. 39:443–455. 2007.
View Article : Google Scholar : PubMed/NCBI
|