Introduction
Lung cancer is the leading cause of cancer-related
mortality not only in China, but worldwide. Non-small cell lung
cancer (NSCLC) accounts for 80% of lung cancer cases with a 5-year
survival rate of 16% (1). Surgical
resection of primary NSCLC is frequently followed by tumor
recurrence at distant sites as a result of occult micrometastasis
(2). Despite improvements in
diagnosis and treatment, the overall survival for lung cancer
patients is disappointing and the majority of deaths are associated
with metastasis to the brain, lung and bones (3). Thus, there is an urgent need to
further understand the molecular mechanisms of NSCLC progression
and to discover new molecular targets for treatment.
Proteolytic ectodomain release, a process known as
‘shedding’, has been recognized as a key mechanism for regulating
the function of a diversity of cell surface proteins. A disintegrin
and metalloproteases (ADAMs) have emerged as the major proteinase
family that mediates ectodomain shedding. ADAM-mediated shedding is
essential for a number of biological processes, such as cell fate
determination, cell migration, wound healing, cell proliferation
and angiogenesis (4). Moreover,
numerous members of the ADAM family such as ADAM9, 10 and 17 have
been proven to be associated with cancer cell proliferation,
migration and invasion (5),
implying that ADAMs play pivotal roles in cancer formation and
progression. A recent study has also highlighted the potential of
targeting ADAM family members as a new approach for anticancer
therapy (6). For cancer metastasis,
it has been demonstrated that the overexpression of ADAM9 in NSCLC
correlates with brain metastasis (7). ADAM17 plays an important role in
hepatocellular carcinoma metastasis (8). ADAM10 silencing or rhADAM10 have been
shown to have no effect on cell viability but markedly reduce the
invasiveness and migration of pancreatic cancer cells (9). ADAM10 has been demonstrated as one of
the key molecules in several of the shedding events characterized
to date and has also been implicated in the shedding of a number of
substrates that drive cancer progression, including the Notch
receptor, EGF, ErbB2, E-cadherin, L1-CAM and inflammatory cytokines
(10). Previous studies have shown
that ADAM10 is overexpressed in a variety of human cancers, such as
pancreatic, oral squamous cell carcinoma, ovarian and uterine
cancers (9,11,12).
In addition, certain in vitro studies have revealed that the
downregulation of ADAM10 with specific small interfering RNA
(siRNA) results in the reduced migration and invasion of cancer
cells via the inhibition of the cleavage of substrates, such as
CLCX-16 and L1-CAM (9,13). With respect to lung cancer, a
previous report indicated that the expression of the active form of
ADAM10 was increased in NSCLC tissues and it has been hypothesized
that ADAM10 may be involved in NSCLC tumor angiogenesis and/or
metastasis (14). Despite the
abovementioned reports, the involvement of ADAM10 in human lung
cancer remains unclear.
It has previously been described that several
prominent substrates of ADAM10 involving different signaling
pathways, such as Notch receptor, ErbB2/ERK and
E-cadherin/β-catenin play important roles in the initiation and/or
progression of lung cancer (15–17).
Notch proteins, the transmembrane receptors, are highly conserved
in the development and the determination of cell fate. To date, 4
mammalian transmembrane receptors (Notch1–4) and 5 transmembrane
ligands (Jagged1, 2, δ1, 3 and 4) have been identified (18). Recently it has been increasingly
recognized that the Notch signaling pathway plays an important role
in the initiation and/or progression of lung cancer (15,19,20).
Furthermore, growing evidence suggests that Notch1 promotes the
proliferation, invasion and migration of lung cancer cells through
the activation of the signaling pathway (21,22).
However, the underlined mechanisms need to be further investigated.
Clinical data have demonstrated that 30% of NSCLC cases have
increased Notch1 activity and 10% of NSCLC cases have
gain-of-function mutations on the Notch1 gene (23). In NSCLC adenocarcinoma, tumor cell
Notch1 and VEGF expression have been shown to be independently
associated with poor prognosis (24). In mammals, both ADAM10 and ADAM17
cleave Notch under certain situations; however, the embryos of
ADAM10 knockout mice have shown a reduced or disorganized
expression of Notch target genes and enhanced levels of Notch
protein, indicating a lack of cleavage/activation (25). To date, there is no evidence
regarding the lack of Notch signaling or processing in ADAM17
knockout mice. As a result, we hypothesized that ADAM10 promotes
NSCLC progression via the cleavage of its substrate, the Notch1
receptor, and that the specific targeting of ADAM10 represents a
more effective strategy for NSCLC cancer therapy compared with the
traditional treatment. The aim of this study was to investigate the
involvement of ADAM10 in NSCLC progression and to further explore
the potential mechanisms of the biological behavioral changes
affected by ADAM10 in NSCLC cells.
In the present study, we examined the protein
expression of ADAM10 and Notch1 in NSCLC tissues and corresponding
normal tissues. The results showed that the expression of ADAM10
was upregulated in NSCLC tissues and that the elevated expression
of ADAM10 significantly correlated with NSCLC metastasis. In
addition, there was positive correlation between the expression of
ADAM10 and Notch1 in NSCLC tissues. We provide evidence that the
knockdown endogenous expression of ADAM10 by RNA interference may
inhibit A549 NSCLC cell migration and invasion, and that this
anticancer effect of silencing ADAM10 expression may target the
Notch1 signaling pathway.
Materials and methods
Reagents and antibodies
G418 and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). The γ-secretase
inhibitor (DAPT) was purchased from Calbiochem (San Diego, CA, USA)
and used at a concentration of 10 μmol/l. The antibody against
human ADAM10 was purchased from eBioscience (San Diego, CA, USA).
Antibodies to the C terminal of human Notch1/NICD and β-actin were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Anti-human ERK1/2 and p-ERK1/2 were from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Anti-human β-catenin antibody was
purchased from R&D Systems (Minneapolis, MN, USA).
Clinical tumor specimens
Clinical NSCLC specimens were collected between
December 2009 and October 2010 at the Tongji Hospital (Wuhan,
China). For each patient, a sample of adjacent and apparently
non-affected tissue was also obtained and used as the normal
control. All specimens from patients who underwent surgery were
formalin-fixed and paraffin-embedded for histopathological
diagnosis and immunohistochemical analysis. The histological
diagnosis of each tumor was confirmed on hematoxylin and
eosin-stained sections. Histological classification was assessed in
all patients according to the World Health Organization
International Histological Classification of Tumors (18). The clinical features of the patients
are presented in Table I. All
patients provided informed consent for the excess pathological
specimens obtained for research purposes. Research was carried out
in compliance with the Helsinki Declaration and the approval of the
Ethics Committee of Tongji Medical College.
 | Table ICorrelation between ADAM10 expression
and clinicopathological factors in tumor tissues from 56 patients
with NSCLC. |
Table I
Correlation between ADAM10 expression
and clinicopathological factors in tumor tissues from 56 patients
with NSCLC.
| | ADAM10
expression | |
|---|
| |
| |
|---|
| Factors | n | Low histoscore
(0–4) | High histoscore
(6–9) | P-value |
|---|
| Age (years) |
| <60 | 34 | 16 | 18 | NS (0.65) |
| ≥60 | 22 | 9 | 13 | |
| Gender |
| Male | 41 | 18 | 23 | NS (0.85) |
| Female | 15 | 7 | 8 | |
| Tumor size
(cm) |
| <5 | 30 | 16 | 14 | NS (0.16) |
| ≥5 | 26 | 9 | 17 | |
| Histological
type |
| Squamous cell
carcinoma | 27 | 13 | 14 | NS (0.68) |
|
Adenocarcinoma | 25 | 11 | 14 | |
| Adenosquamous cell
carcinoma | 4 | 1 | 3 | |
| Histological
differentiation |
| Poor | 13 | 3 | 10 | 0.045a |
| Moderate | 25 | 10 | 15 | |
| Well | 18 | 12 | 6 | |
| Lymph node
metastasis |
| Negative | 18 | 13 | 5 | 0.004a |
| Positive | 38 | 12 | 26 | |
| Distant
metastasis |
| Negative | 37 | 21 | 16 | 0.011a |
| Positive | 19 | 4 | 15 | |
Immunohistochemical (IHC) analysis and
semi-quantitative evaluation
The antibody staining against ADAM10 or Notch1 was
performed on 4-μm histological sections of formalin-fixed,
paraffin-embedded tumor and adjacent normal samples as previously
described (19). For each spot,
areas of most intense and/or predominant staining pattern were
scored by eye. The immunostaining pattern for each case was
independently evaluated by 3 investigators (P.W., Y.L. and Y.W.)
using a two-headed microscope. The cells with brown staining were
considered positively stained. Immunostaining for markers was
classified by a semiquantitative method based on a scale that takes
into account the intensity and distribution of the staining:
staining intensity was categorized as 0 (negative), 1 (weak), 2
(moderate) or 3 (strong). Staining areas were scored as 0 (no
positive cells), 1 (≤25% positive cells), 2 (>25% and ≤50%
positive cells) and 3 (>50% positive cells). To gauge both the
staining intensity and distribution simultaneously, the average
values of intensity for each tissue were multiplied by the average
values for percentage area stained in each tissue to derive a
composite histoscore (histoscore = percentage area stained ×
staining intensity). For example, a tissue with intense, uniform
staining was assigned the maximum histoscore of 9. For statistical
analysis, the histoscore of each section no <6 was classified as
high expression (histoscore 6–9) and low expression cases included
both negative (histoscore 0) and weakly positive cases (histoscore
1–4). Assigning a histoscore is now a commonly used method for
evaluating both staining uniformity and intensity in tissues in
order to better correlate results between multiple samples from
immunohistochemical analysis (19).
Cell culture and stable ADAM10
knockdown
The human NSCLC cell line, A549, purchased from the
China Center for Type Culture Collection (CCTCC; Wuhan, China), was
cultured according to the guidelines provided and passaged in our
laboratory for <6 months after receipt.
Three short hairpin (sh)RNA oligonucleotides were
synthesized to target 3 different regions in human ADAM10 cDNA: 1,
GGGTCTGTTATTGATGGAAGA; 2, GCTGATGAGAA GGACCCTACA; and 3,
GCATACACAAGTGTGCATTAA. They were cloned respectively into shRNA
expression vector, pGenesil-4, with U6 promoter (Genesil Corp.,
Wuhan, China), containing selectable marker GFP to facilitate the
selection of stably transfected cells. The recombinant shADAM10 and
control shCon plasmids were transfected into A549 cells using
Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA). The cells were
then screened with G418 (800 μg/ml), and the stably transfected
cell clones were picked after 3 weeks and the silencing effect was
detected by western blot analysis.
Scratch wound assay
The selected A549 cells were plated in 6-well plates
at a concentration of 3×105 cells/well. Approximately 48
h later, the confluent cell layers were carefully scratched using
sterile pipette tips. Non-adherent cells and cellular debris were
removed by washing with phosphate-buffered saline (PBS). Cells were
observed under a microscope and digitally photographed at different
times. Inhibition of cell migration was assessed when the wound in
the control was closed and quantified by using a measuring tool in
Photoshop CS2 (Adobe Systems, Inc., USA).
Cell invasion assay
Cell invasion was examined using Transwell chambers
(8.0 μm) according to the manufacturer’s instructions (Costar, USA)
and the top chambers were coated with Matrigel (BD Biosiences,
USA). The cells were transferred to the top chamber of each
prepared Transwell chamber at a density of 4×105
cells/ml (100 μl). The bottom chamber contained DMEM supplemented
with 10% FBS. The cells were allowed to migrate for 24 h at 37°C.
Non-invading cells were removed from the top surfaces with a cotton
swab. The membranes were fixed in 95% ethanol and stained with 0.1%
crystal violet. The cells that had penetrated to the bottom surface
of each membrane were counted with 10 random fields on each
microscope slide.
Western blot analysis
The cell pellet was lysed in buffer containing 10
mmol/l Tris-HCl (pH 8.0), 1 mmol/l MgCl2, 1% NP-40, 0.5%
sodium deoxycholate, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mg/l
aprotinin and 0.02 mg/l leupeptin. Following cell protein
quantification, 60 μg of protein were subjected to 7.5% SDS-PAGE.
The proteins separated on the PAGE gel were transferred onto
nitrocellulose membranes, which were blocked for 2 h at room
temperature. The membranes were then incubated overnight at 4°C
with primary antibodies, depending on the assays, which were
anti-human antibodies against ADAM10, Notch1/NICD, ERK1/2,
p-ERK1/2, β-catenin and β-actin. After being washed 3 times with
TBST (50 mmol/l Tris-HCl pH 7.6, 150 mmol/l NaCl, 0.1% Tween-20),
the membranes were incubated with secondary antibodies conjugated
with horseradish peroxidase, and then washed 3 times with TBST
again. Immunoreactive bands were visualized by an enhanced
chemiluminescence (ECL) system and the level of protein expression
was determined using Image Quant TL software (Amersham Pharmacia
Biotech, USA).
Immunofluorescence
Cells were plated on glass coverslips in 6-well
culture plates. After 24 h, the cells were fixed with 4%
paraformaldehyde for 15 min. Subsequently, the cells were
permeabilized with 0.1% Triton X-100 for 15 min at room
temperature, washed with PBS and blocked with PBS containing 0.5%
(w/v) bovine serum albumin (BSA) and 0.15% (w/v) glycine (BSA
buffer) for 1 h at room temperature. Cells were treated with
anti-Notch1/NICD antibody (1:50 dilution in BSA buffer) for 1 h at
room temperature. Cells were then washed with BSA buffer and
incubated with secondary antibody conjugated with Rhodamine for 1 h
at room temperature. DAPI (200 ng/ml) was used for staining the
nuclei. Images were acquired by a Nikon fluorescence microscope,
with NIS-Elements 3.1 software.
Statistical analysis
The direction and strength of the association
between ADAM10 and Notch1 were evaluated and compared with the
Spearman correlation test. Experimental differences were examined
for statistical significance using the χ2 or Student’s
t-test. Data are reported as the means ± SD and a P-value <0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed with the Superior
Performance Software System (SPSS) 15.0 for Windows (SPSS, Inc.,
Chicago, IL, USA).
Results
Expression of ADAM10 protein in NSCLC
clinical specimens
In order to investigate the potential role of ADAM10
expression in NSCLC progression, immunohistochemical analysis was
carried out to evaluate the endogenous protein expression in the
paraffin-embedded tumor and adjacent normal tissues. Fig. 1A illustrates the ADAM10 protein
expression in 2 representative patients. Through statistical
analysis of ADAM10 protein expression in NSCLC tissues from 56
patients, we found that the histoscore of ADAM10 was statistically
increased in tumor tissues compared to normal tissues (P<0.01,
Fig. 1B). Furthermore, the strong
immunoreactivity of ADAM10 expression was displayed in both lymph
node and distant metastatic NSCLC samples (Fig. 1C). Compared with non-metastatic
tumor tissues (Fig. 2A), the
statistical data revealed that the ADAM10 expression levels were
significantly elevated in the metastatic tissues (P<0.05;
Fig. 1D). These findings suggest a
possible link between ADAM10 overexpression and metastasis in
NSCLC.
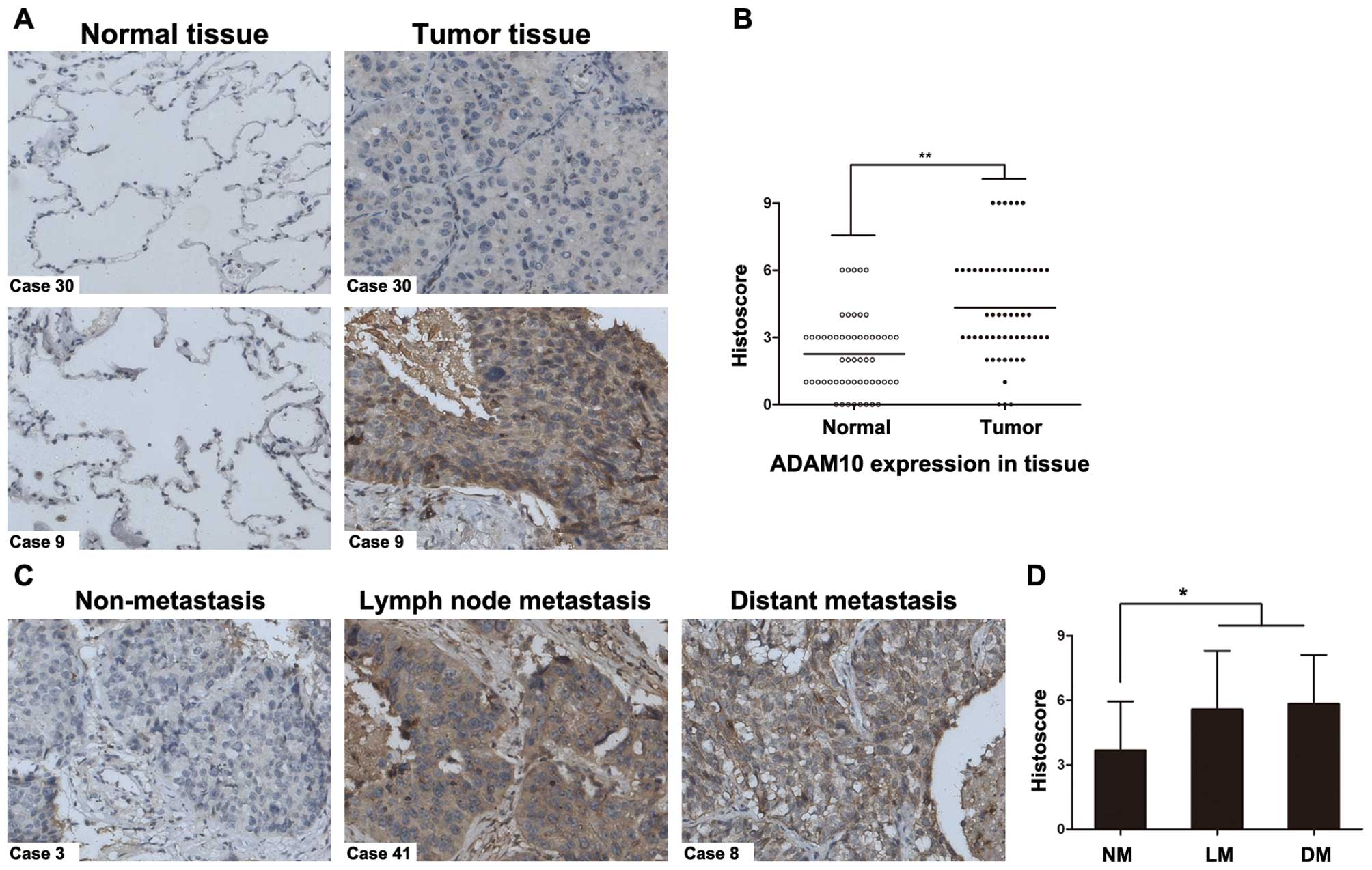 | Figure 1Expression of the ADAM10 protein in
NSCLC clinical specimens. (A) Immunohistochemical analysis of
ADAM10 in paired paraffin-embedded human NSCLC tissues and the
adjacent normal ones. ADAM10 staining in 2 representative cases,
the cells with brown staining were considered positively stained,
ADAM10 expression showed predominately membranous and cytoplasmic
localization. Normal tissues (left panels) showed negative or weak
staining, while tumor tissues showed moderate to strong ADAM10
staining (right panels). (B) Statistical analysis with histoscore
(area stained multiplied by intensity) of ADAM10 staining in
tissues from NSCLC patients (n=56). The ADAM10 expression levels
were significantly higher in tumor tissues compared with
non-affected normal ones. Original magnification, ×200.
**P<0.01. (C) Immunohistochemical staining of ADAM10
in non-metastatic and metastatic malignant tissues from human NSCLC
patients. Representative non-metastatic NSCLC tissues (left panel)
showing weak membrane and cytoplasm ADAM10 staining (histoscore,
3). Carcinomas with lymph node and distant metastasis (middle and
right) showing strong membrane and cytoplasm ADAM10 staining
(histoscore, 6 and 9, respectively); (D) Statistical analysis
revealed that the histoscores of ADAM10 were significantly elevated
in metastatic carcinomas (including lymph node and distant
metastasis, n=38) compared with non-metastatic tissues (n=18). NM,
non-metastasis; LM, lymph node metastasis; DM, distant metastasis.
Original magnification, ×200. *P<0.05. |
To determine the clinical significance of ADAM10
expression in NSCLC, the correlation of its expression pattern in
patient tumor samples with clinical features was analyzed. The
results are summarized in Table I.
Among patients with NSCLC, a high ADAM10 expression was not
associated with age, gender, tumor size and histological types.
However, the overexpression of ADAM10 showed a significant
correlation with poor differentiation, lymph node and distant
metastasis (P<0.05). These results further suggest that the
overexpression of ADAM10 correlates with the aggravated progression
of NSCLC, particularly with metastasis. This protein may be a
clinically significant player in NSCLC metastasis.
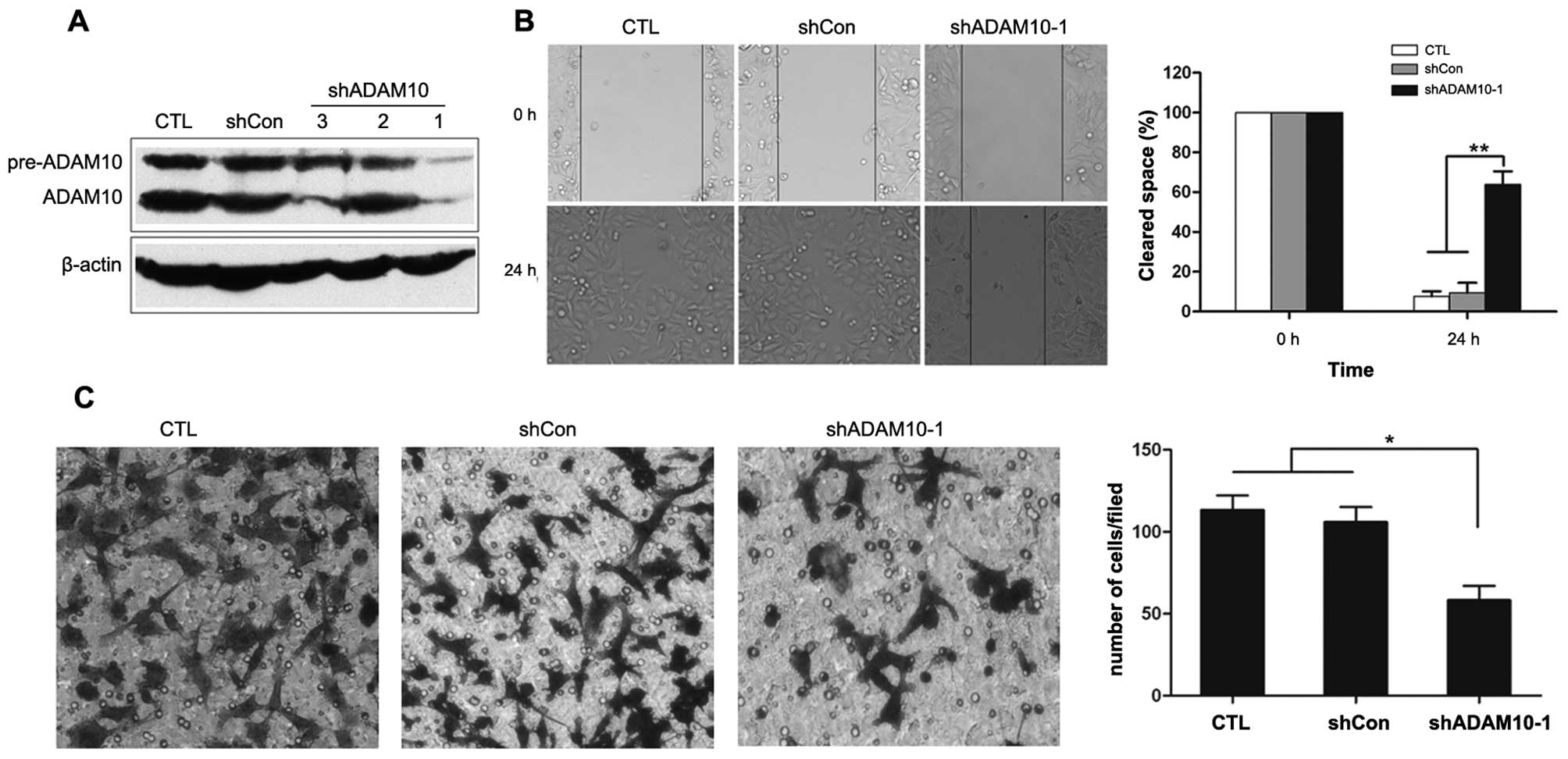
Knockdown of ADAM10 inhibits the
migration and invasion of A549 NSCLC cells
To directly examine whether ADAM10 is functionally
important for NSCLC metastasis, ADAM10 shRNA was used to silence
ADAM10 expression in A549 cells, a highly metastatic NSCLC cell
line with a high expression of ADAM10 (Fig. 2A). As shown in Fig. 2A, 3
A549-derived stable cell lines were selected that expressed
different shRNA, and only 1 shRNA construct targeting ADAM10
(shADAM10-1) effectively inhibited its expression at the protein
level, thus prompting us to select this A549 stable cell line for
further study.
The migration and invasion of tumor cells are
usually considered a prerequisite of tumor metastasis. To assess
whether the downregulation of ADAM10 in A549 cells reduces cell
migration, a wound healing migration assay was performed. After 24
h, a significant delay in the wound closure rate was observed with
the microscopic examination of shADAM10-1 cells compared to the
control cells (P<0.01; Fig.
2B).
To examine the influence of silencing ADAM10
expression on the inhibition of NSCLC cell invasion, standardized
Matrigel invasion assay was employed. As was expected, the invasion
number of shADAM10-1 cells was significantly less compared to the
control cells (P<0.05; Fig. 2C).
These results suggest that the repression of ADAM10 expression in
NSCLC cells inhibits cellular migration and invasion. As a
consequence, ADAM10 plays a pivotal role in NSCLC metastasis.
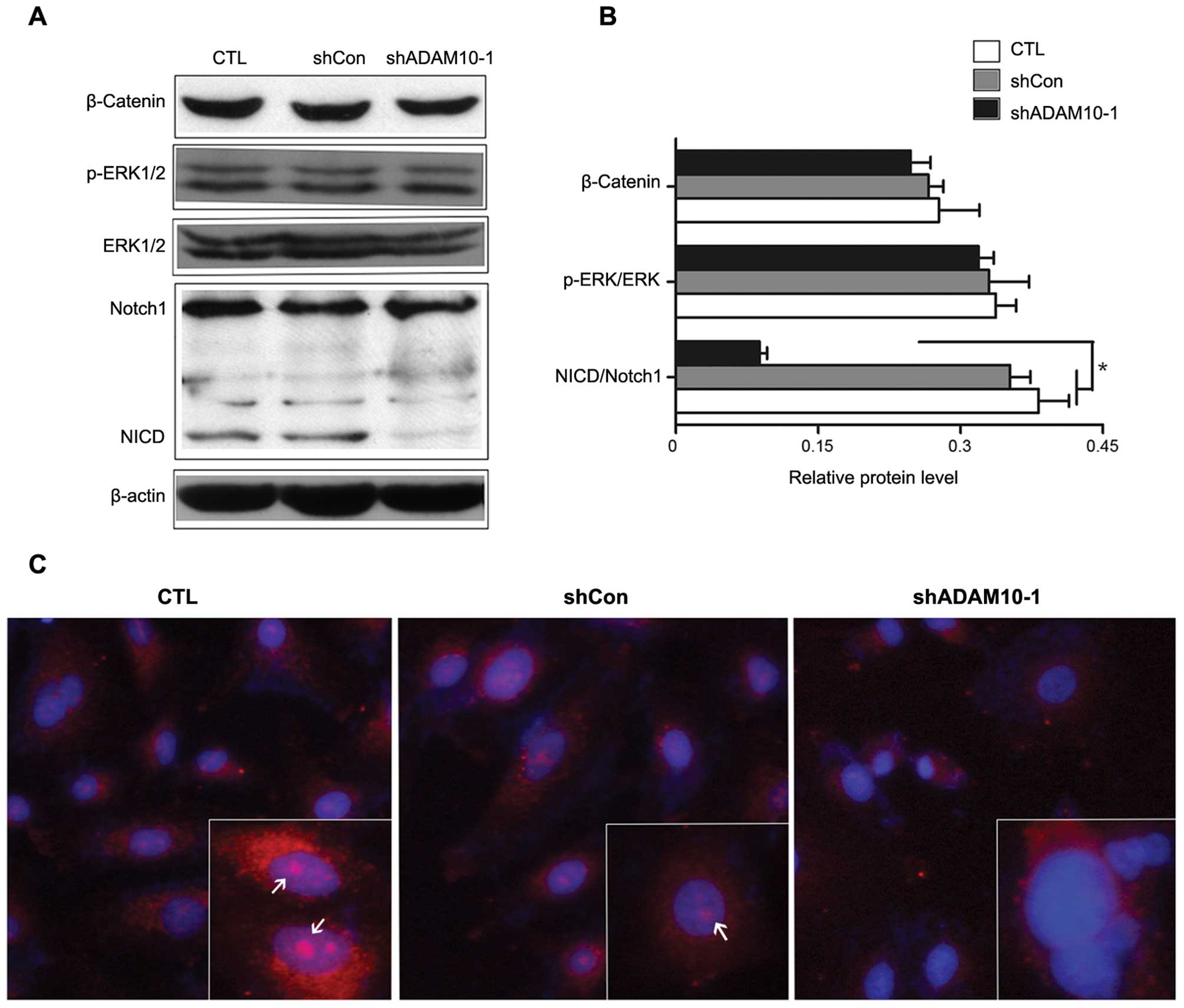
Silencing ADAM10 expression inhibits the
cleavage of the intracellular domain of Notch1 in A549 NSCLC
cells
To explain the above effect of ADAM10 on the
migration and invasion of NSCLC, we selectively analyzed several
signaling factors which are related to NSCLC metastasis. Previous
studies have shown that the ERBB/ERK, E-cadherin/β-catenin and
Notch1 signaling pathways play key roles in NSCLC formation and
metastasis progression. Of note, the proteolytic activated function
of ADAM10 has been implicated in these pathways (10). In line with this, the ADAM10
regulation of ERK1/2 and β-catenin expression was first
investigated by western blot analysis. As a result, the stable
silencing of ADAM10 failed to suppress β-catenin expression and
ERK1/2 phosphorylated activation in A549 cells (Fig. 3A). Thus, additional consideration
was given to the possible role of ADAM10 in Notch1 signaling due to
its proteolytic function contributing to the cleavage of Notch1 to
release the Notch1 intracellular domain (NICD), the activated form
of Notch1 that translocates to the nucleus to modulate the
downstream gene expression to facilitate cancer progression
(26). Of note, when the knockdown
of ADAM10 occurred in A549 cells, the expression levels of NICD
significantly decreased compared with the control cells (Fig. 3A and B). Consistently, the cellular
immunofluorescence assay indicated the similar effect. As shown in
Fig. 3C, the fluorescence intensity
of NICD located in the nucleus was weakened in the shADAM10-1
cells. These results suggest that silencing ADAM10 expression in
NSCLC cells inhibits the expression of NICD, the activated form of
Notch1, and this effect may further inhibit downstream gene (such
as Hey1) transcription (data not shown).
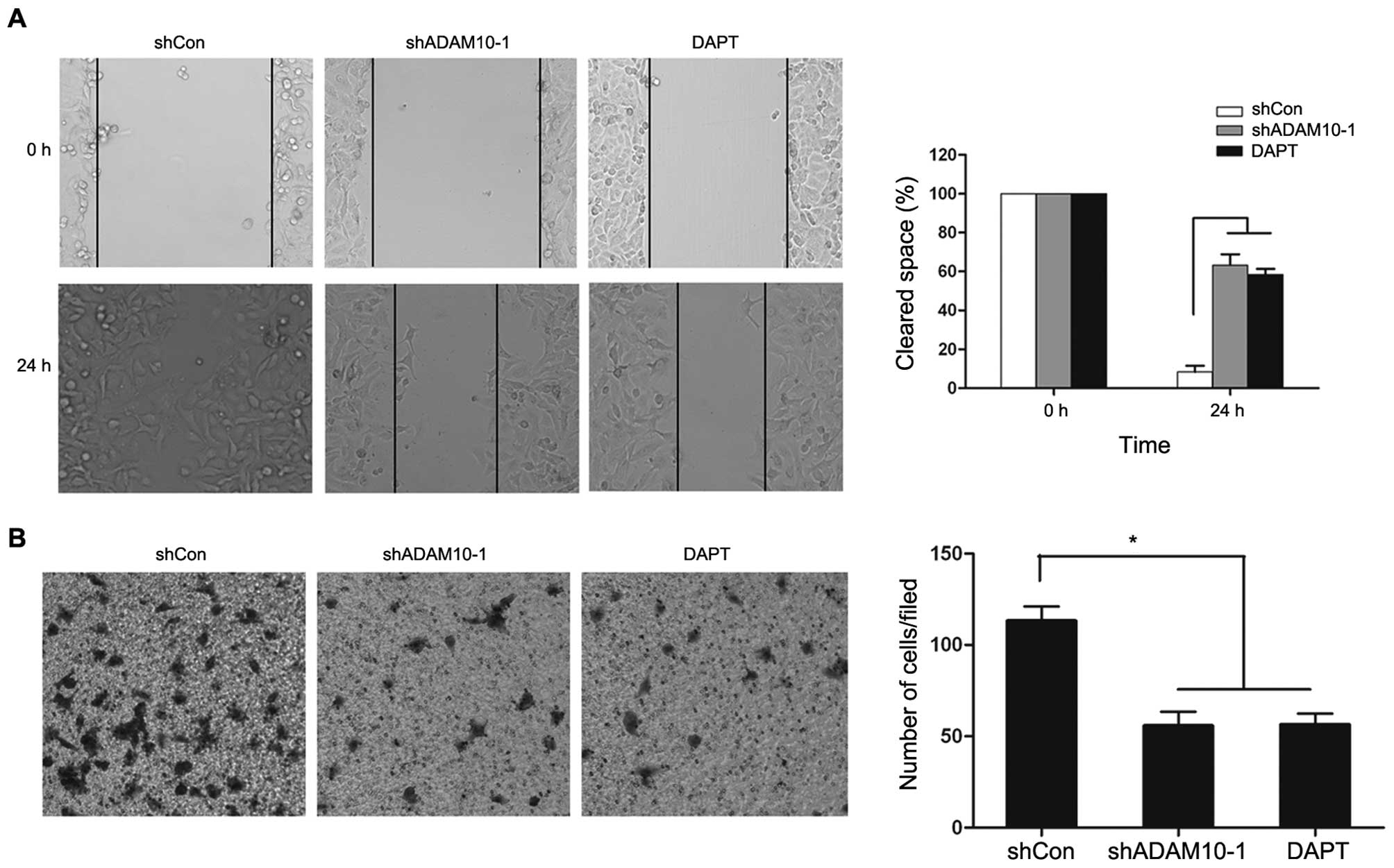
Notch1 signaling inhibitor suppresses the
migration and invasion of A549 NSCLC cells
The above results suggest that the knockdown of
ADAM10 suppresses metastasis through the inhibition of Notch1
activation in NSCLC cells. We then wished to examine whether the
inhibition of Notch signaling can reduce NSCLC cell migration and
invasion. To determine this, we examined the inhibition of Notch
signaling with DAPT, a γ-secretase inhibitor (GSI) in A549 cells.
Compared to the control cells, wound healing migration and Matrigel
invasion assay revealed that the repression of Notch signaling by
DAPT significantly decreased the migration and invasion rate of
shADAM10-1 cells (Fig. 4). However,
we did not observe any difference between the 2 groups treated with
DAPT or shADAM10-1, respectively (Fig.
4). These results suggest that either ADAM10 siRNA or GSI
inhibit the Notch1 signaling pathway, and further suppress the
migration and invasion of NSCLC cells.
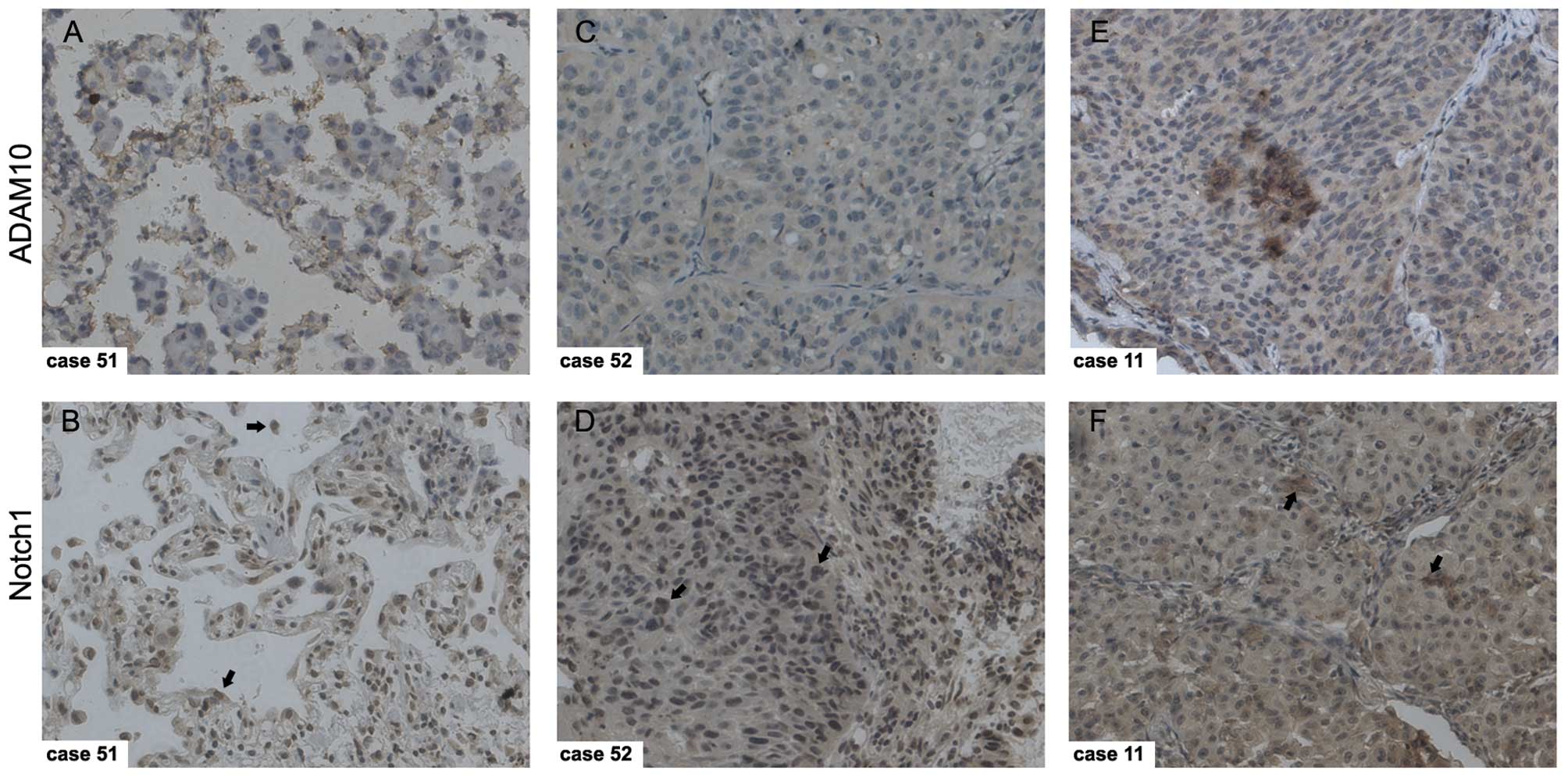
Significantly positive correlation
between ADAM10 and Notch1 expression in NSCLC
The above results indicated that the knockdown of
ADAM10 inactivated the Notch1 signaling pathway via inhibition of
NICD release from Notch1 in NSCLC cells, and then further
suppressed downstream gene expression. This prompted us to
investigate whether ADAM10 and Notch1 are co-expressed in NSCLC
tissues. To determine this, the co-expression of ADAM10 and Notch1
was also detected with IHC in NSCLC samples. The strong
immunostaining of ADAM10 correspondingly displayed a high
expression of Notch1 in the same NSCLC sample, which was
overexpressed in both the cytoplasm and nucleus (Fig. 5). It was concluded that ADAM10
expression showed a highly significant positive correlation with
Notch1 in the 56 cancer samples (Table
II). Taken together, these results suggest that ADAM10 is an
endogenous Notch1 activator in NSCLC.
 | Table IICorrelation between ADAM10 and Notch1
expression in tumor tissues from 56 patients with NSCLC. |
Table II
Correlation between ADAM10 and Notch1
expression in tumor tissues from 56 patients with NSCLC.
| | ADAM10 | |
|---|
| |
| |
|---|
| n | Low histoscore
(0–4) | High histoscore
(6–9) | Spearman’s
test |
|---|
| Total | 56 | 25 | 31 |
P<0.001
r=0.547 |
| Notch1 |
| Low histoscore
(0–4) | 20 | 14 | 6 | |
| High histoscore
(6–9) | 36 | 11 | 25 | |
Discussion
The protease, ADAM10, has been shown to be
overexpressed in various malignancies and to influence the
progression and metastasis of cancer cells. Gavert et al
reported that the expression of ADAM10 was detected at the invasive
front of human colorectal tumor tissues (27). Recently, ADAM10 was detected to be
overexpressed in pancreatic cancer and to promote the invasiveness
and migration of cancer cells via the inhibition of the cleavage of
CXCL16 (9). In adenoid cystic
carcinoma, blocking the expression of ADAM10 with ADAM10-specific
siRNA inhibited cancer cell proliferation and metastasis (28). For NSCLC, the active form of ADAM10
protein was higher in tumor tissues compared with the corresponding
normal tissues from 14 patients. It has been hypothesized that
ADAM10 plays a role in angiogenesis and/or metastasis in lung
cancer (14). However, reports
relating to this hypothesis are very limited. Therefore, in this
study, we aimed to investigate the impact of ADAM10 expression on
the invasive and metastatic potential of NSCLC cells. We
characterized the protein expression of ADAM10 in NSCLC tissues
from 56 patients. Immunohistochemical analysis indicated that
ADAM10 was overexpressed in malignant tissues compared to matched
normal tissues from NSCLC patients. Furthermore, ADAM10 expression
was markedly increased in metastatic lymph nodes/or distant
metastatic tissues compared with corresponding primary tumors.
Based on the results from clinical correlation analysis and
previous reports, it is reasonable to speculate that ADAM10 plays a
role in promoting the migration and invasion of NSCLC cells. In
this context, we investigated the effects of silencing ADAM10 on
in vitro cell migration and invasion. Our results indicated
that the knockdown of ADAM10 expression by specific shRNA
significantly inhibited the migration and invasion of A549 cells,
which have an abnormal Notch expression, and showed very strong
ADAM10 protein expression. The data confirm the importance of
ADAM10 expression in the process of NSCLC metastasis. The above
results support the hypothesis proposed by Zou et al
(14).
It is imperative to determine the mechanism by which
the knockdown of ADAM10 expression represses the migration and
invasion of NSCLC cells. Multiple mechanisms proposed have been
associated with the shedding activity of ADAM10 and that it may
promote NSCLC progression via the cleavage of its substrates, such
as the Notch1 receptor, ERBB2 and E-cadherin to activate the
different signaling pathways. We analyzed the protein expression of
ERK1/2 and p-ERK in both ADAM10 knockdown and mock-transfected A549
cells and no significant change was observed. The results are
consistent with those from a previous report indicating that
INCB3619, the inhibitor of both ADAM10 and 17, induces apoptosis in
A549 cells and has antitumor activity in a A549 xenograft model;
however, ADAM10 did not play a key role in affecting AKT and ERK
activity (14). ADAM17 plays more
important roles in activating ErbB signaling. A similar result was
obtained for the detection of β-catenin protein expression in both
ADAM10 knockdown and mock-transfected A549 cells. Although we could
not rule out the function of β-catenin in NSCLC cell metastasis, we
focused on the Notch1 signaling pathway which is one of the most
common signaling pathways contributing to lung cancer (24,25).
The biological role of Notch signaling in cancer was
not recognized until 1991 when Notch was suspected to be casually
related to the development of T-cell acute lymphocytic leukemia
(29). To date, the aberrantly
activated Notch1 signaling pathway, an important member of the
Notch receptor family, has been observed to mediate tumor
metastasis. It has been reported that the downregulation of Notch1
and Jagged1 suppresses the invasion of cervical carcinoma and
choriocarcinoma cells (30). Nam
et al reported that the γ-secretase DAPT and RNA
interference-mediated knockdown of Notch1 inhibited the migration
and invasion of the MDA-MB-435 breast cancer cell line (31). The downregulation of Notch1
inhibited invasion by the inactivation of NF-κB, VEGF and MMP-9 in
pancreatic cancer cells (32). It
was also hypothesized that δ-tocotrienol inhibited cell migration
and invasion via inactivation of the Notch1 signaling pathway in
NSCLC cells (22). Of note, the
increased expression of Notch1 protein was examined in patient
tissues and NSCLC stem cells which suggests a role of the Notch1
signaling pathway in NSCLC progression (25,33).
ADAM10 and ADAM17 was identified in previous studies
as the major sheddase of the Notch receptor (34,35).
Specifically, it was shown that ADAM10 was absolutely required for
Notch1 signaling induced by ligands, while signaling independent of
ligands required ADAM17 (36). In
A549 cells, there was a very strong Notch ligand Jagged1 protein
expression (14). A previous report
showed that the Notch ligand Jagged2 promoted lung adenocarcinoma
metastasis, but the corresponding Notch receptor remains unknown
(19). Based on the above reports,
we speculated that ADAM10 may be involved in the cleavage of the
Notch1 receptor in NSCLC. First, we detected Notch1 and ADAM10
protein co-expression in both tumor and matched normal tissues from
NSCLC patients with IHC and the results showed that there was a
significantly positive correlation between Notch1 and ADAM10
protein expression. Moreover, the nuclear expression of Notch1 was
also observed in several tumor tissues. Second, the effects of
silencing ADAM10 on the shedding of Notch1 were also analyzed in
vitro. Of note, we found that the knockdown of ADAM10
significantly inhibited the cleavage of NICD which is the activated
form of Notch1 in NSCLC cells, using both western blot analysis and
immunofluorescence assay. This effect decreased the amount of NICD
in the nucleus, which regulates the downstream gene expression. Our
results suggest that silencing ADAM10 expression represses the
migration and invasion of NSCLC cells by disrupting Notch1
activation. Our findings support a manifest paradigm that the
Notch1 signaling pathway activated by ADAM10 shedding plays an
important role in the metastasis of NSCLC.
However, the detailed mechanism by which Notch1
signaling influences cancer cell migration and invasion remains
unknown. Recently, Xie et al demonstrated that the
activation of Notch1 enhanced epithelial mesenchymal transition
(EMT) in gefitinib-acquired resistant lung cancer cells (37). Their results indicated that NICD
promoted the EMT phenotype by inhibiting the expression of
E-cadherin in gefitinib-resistant PC9/AB2 lung cancer cells. Their
results suggested that the activation of the Notch1 signaling
pathway is critical in gefitinib-acquired resistance and the EMT
phenotype in lung adenocarcinoma cells. Their results support our
conclusion that ADAM10 promotes migration and invasion via the
activation of the Notch1 signaling pathway and may facilitate EMT.
The A549 NSCLC cell line is a type of gefitinib resistance cancer
cell showing very low E-cadherin expression. Thus, the inhibition
of Notch1 may be a novel strategy for the reversal of the EMT
phenotype, thereby potentially increasing the therapeutic drug
sensitivity of lung cancer cells. McGowan et al recently
reported that Notch1 inhibition reduced the formation of brain
metastasis from breast cancer via reducing the proportion of the
cancer stem cell (CSC) surface markers, CD44hi/CD24lo (38). CSCs are thought to be responsible
for the failure of the current chemotherapy of lung cancer
(15). There has been a growing
body of evidence underscoring the importance of Notch signaling
which may impact the CSC phenotype and tumorigenicity (39,40).
For example, in ALDH+ lung cancer cells (lung CSCs), the
elevated expression of Notch1, Notch2 and Notch3 was also observed
and the decreased protein expression of the Notch signaling
downstream genes, such as Hes1, Hey1 and Hey2 was also detected
while treatment with GSIs (33).
Further studies are required to determine the underlined mechanism
of the inhibition of NSCLC cell metastasis via the inactivation of
the Notch1 signaling pathway. In conclusion, our results suggest
that the knockdown of ADAM10 by using RNAi technology is not only
an experimental technology, but may also serve as a therapeutic
tool for the treatment of NSCLC metastasis by targeting CSCs.
Patients with NSCLC have a poor prognosis due to
metastasis and drug resistance. EMT plays a role in EGFR-TKI
acquired resistant lung adenocarcinoma and cancer metastasis. Notch
signaling is emerging as a potentially invaluable molecular pathway
to affect NSCLC stem cells and acting as a target for novel NSCLC
treatment options (33). Based on
previous reports and our results, the Notch1 signaling pathway may
be inhibited using different strategies. GSIs, working as a
double-edged sword, are anticancer agents being tested in clinical
trials. Since GSIs may cause abnormalities in the gastrointestinal
tract, thymus and spleen in rodents (41), their toxicity to normal tissues has
attracted increasing attention. Several studies have highlighted
the potential of targeting ADAMs as a new approach for anticancer
therapy. In fact, ADAM inhibitors are also in preclinical
development and ADAM inhibitors seem to have less toxicity than
GSIs. Standard chemotherapy in combination with ADAM10 or Notch1
targeting may eliminate both bulk tumor cells and CSCs, and
therefore be considered a curative therapy.
In conclusion, we demonstrate that ADAM10 may serve
as a potential target for the therapeutic intervention of NSCLC
metastasis. This molecule, combined with the Notch1 protein, was
significantly co-overexpressed in most NSCLC tissues we examined.
Importantly, we provide preclinical evidence for the specific
silencing of ADAM10 expression with ADAM10 shRNA as an therapeutic
strategy against NSCLC metastasis by targeting Notch1 signaling.
Additional extensive research must be implemented on this topic in
order to further intensify the understanding of the correlation
between ADAM10 and NSCLC cancer progression.
Acknowledgements
This study was supported by the Doctor Fund Project
of the Chinese Ministry of Education (No. 20090142110014) and the
Doctor Innovation Fund of the Huazhong University of Science and
Technology.
References
|
1
|
Wu X, Piper-Hunter MG, Crawford M, Nuovo
GJ, Marsh CB, Otterson GA and Nana-Sinkam SP: MicroRNAs in the
pathogenesis of lung cancer. J Thorac Oncol. 4:1028–1034. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: recent
advances and future directions. Oncologist. 15:5–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curran WJ Jr: Treatment of locally
advanced non-small cell lung cancer: what we have and have not
learned over the past decade. Semin Oncol. 32:S2–S5. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar
|
|
5
|
Duffy MJ, McKiernan E, O’Donovan N and
McGowan PM: Role of ADAMs in cancer formation and progression. Clin
Cancer Res. 15:1140–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saftig P and Reissb K: The ‘A Disintegrin
and metalloproteases’ ADAM10 and ADAM17: novel drug targets with
therapeutic potential? Eur J Cell Biol. 90:527–535. 2011.
|
|
7
|
Shintani Y, Higashiyama S, Ohta M, et al:
Overexpression of ADAM9 in non-small cell lung cancer. Cancer Res.
64:4190–4196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai WC, Hsu PW, Lai TC, et al:
MicroRNA-122, a tumor suppressor microRNA that regulates
intrahepatic metastasis of hepatocellular carcinoma. Hepatology.
49:1571–1582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaida MM, Haag N, Günther F, Tschaharganeh
DF, Schirmacher P, Friess H, Giese NA, Schmidt J and Wente MN:
Expression of A disintegrin and metalloprotease 10 in pancreatic
carcinoma. Int J Mol Med. 26:281–288. 2010.PubMed/NCBI
|
|
10
|
Crawford HC, Dempsey PJ, Brown G, Adam L
and Moss ML: ADAM10 as a therapeutic target for cancer and
inflammation. Curr Pharm Des. 15:2288–2299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko SY, Lin SC, Wong YK, Liu CJ, Chang KW
and Liu TY: Increase of disintergin metalloprotease 10 (ADAM10)
expression in oral squamous cell carcinoma. Cancer Lett. 245:33–43.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fogel M, Gutwein P, Mechtersheimer S, et
al: L1 expression as a predictor of progression and survival in
patients with uterine and ovarian carcinomas. Lancet. 362:869–875.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SB, Schramme A, Doberstein K, et al:
ADAM10 is upregulated in melanoma metastasis compared with primary
melanoma. J Invest Dermatol. 130:763–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou BB, Peyton M, He B, et al: Targeting
ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in
non-small cell lung cancer. Cancer Cell. 10:39–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galluzzo P and Bochetta M: Notch signaling
in lung cancer. Expert Rev Anticancer Ther. 11:533–540. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
López-Malpartida AV, Ludeña MD, Varela G
and García Pichel J: Differential ErbB receptor expression and
intracellular signaling activity in lung adenocarcinomas and
squamous cell carcinomas. Lung Cancer. 65:25–33. 2009.PubMed/NCBI
|
|
17
|
Pelosi G, Scarpa A, Puppa G, et al:
Alteration of the E-cadherin/β-catenin cell adhesion system is
common in pulmonary neuroendocrine tumors and is an independent
predictor of lymph node metastasis in atypical carcinoids. Cancer.
103:1154–1164. 2005.
|
|
18
|
Lai EC: Notch signaling: control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Ahn YH, Gibbons DL, et al: The
Notch ligand Jagged2 promotes lung adenocarcinoma metastasis
through a miR-200-dependent pathway in mice. J Clin Invest.
121:1373–1385. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: γ-secretase inhibitor prevents
Notch3 activation and reduces proliferation in human lung cancers.
Cancer Res. 67:8051–8057. 2007.
|
|
21
|
Chen Y, Li D, Liu H, et al: Notch-1
signaling facilitates survivin expression in human non-small cell
lung cancer cells. Cancer Biol Ther. 11:14–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with Notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Westhoff B, Colaluca IN, D’Ario G, et al:
Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci
USA. 106:22293–22298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donnem T, Andersen S, Al-Shibli K, Al-Saad
S, Busund LT and Bremnes RM: Prognostic impact of Notch ligands and
receptors in nonsmall cell lung cancer. Cancer. 116:5676–5685.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartmann D, de Strooper B, Serneels L, et
al: The disintegrin/metalloprotease ADAM 10 is essential for Notch
signalling but not for α-secretase activity in fibroblasts. Hum Mol
Genet. 11:2615–2624. 2002.PubMed/NCBI
|
|
26
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gavert N, Conacci-Sorrell M, Gast D,
Schneider A, Altevogt P, Brabletz T and Ben-Ze’ev A: L1, a novel
target of β-catenin signaling, transforms cells and is expressed at
the invasive front of colon cancers. J Cell Biol. 168:633–642.
2005.
|
|
28
|
Xu Q, Liu X, Chen W and Zhang Z:
Inhibiting adenoid cystic carcinoma cells growth and metastasis by
blocking the expression of ADAM 10 using RNA interference. J Transl
Med. 8:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grabher C, von Boehmer H and Look AT:
Notch 1 activation in the molecular pathogenesis of T-cell acute
lymphoblastic leukaemia. Nat Rev Cancer. 6:347–359. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pang RT, Leung CO, Ye TM, et al:
MicroRNA-34a suppresses invasion through downregulation of Notch1
and Jagged1 in cervical carcinoma and choriocarcinoma cells.
Carcinogenesis. 31:1037–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nam DH, Jeon HM, Kim S, et al: Activation
of Notch signaling in a xenograft model of brain metastasis. Clin
Cancer Res. 14:4059–4066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Banerjee S, Li Y, et al:
Down-regulation of Notch-1 inhibits invasion by inactivation of
nuclear factor-κB, vascular endothelial growth factor, and matrix
metalloproteinase-9 in pancreatic cancer. Cancer Res. 66:2778–2784.
2006.
|
|
33
|
Sullivan JP, Spinola M, Dodge M, et al:
Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Tetering G, van Diest P, Verlaan I,
van der Wall E, Kopan R and Vooijs M: Metalloprotease ADAM10 is
required for Notch1 site 2 cleavage. J Biol Chem. 284:31018–31027.
2009.PubMed/NCBI
|
|
35
|
Brou C, Logeat F, Gupta N, et al: A novel
proteolytic cleavage involved in Notch signaling: the role of the
disintegrin-metalloprotease TACE. Mol Cell. 5:207–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bozkulak EC and Weinmaster G: Selective
use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol
Cell Biol. 29:5679–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie M, Zhang L, He CS, et al: Activation
of Notch-1 enhances epithelial-mesenchymal transition in
gefitinib-acquired resistant lung cancer cells. J Cell Biochem.
113:1501–1513. 2012.PubMed/NCBI
|
|
38
|
McGowan PM, Simedrea C, Ribot EJ, et al:
Notch1 inhibition alters the CD44hi/CD24lo population and reduces
the formation of brain metastases from breast cancer. Mol Cancer
Res. 9:834–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bolós V, Blanco M, Medina V, Aparicio G,
Díaz-Prado S and Grande E: Notch signaling in cancer stem cells.
Clin Transl Oncol. 11:11–19. 2009.
|
|
40
|
Pannuti A, Foreman K, Rizzo P, Osipo C,
Golde T, Osborne B and Miele L: Targeting Notch to target cancer
stem cells. Clin Cancer Res. 16:3141–3152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barten DM, Meredith JE Jr, Zaczek R,
Houston JG and Albright CF: Gamma-secretase inhibitors for
Alzheimer’s disease: balancing efficacy and toxicity. Drugs R D.
7:87–97. 2006.
|