Introduction
Metastatic renal cell carcinoma (RCC) is associated
with a poor prognosis, with a median survival of 8 months (1). At the time RCC are identified, in most
cases the tumors have already progressed and are generally
resistant to chemotherapy and radiotherapy. Although some patients
respond to immunotherapy, many cases are resistant to interleukin-2
or interferon-α (IFN-α) therapy and the overall response rate is
less than 20% (2). The factors
leading to this lack of response remain under investigation.
Therefore, it is necessary to find more effective, universal
treatments for this disease. Molecular targeting of abnormal signal
transduction pathways is promising for the treatment of many
diseases. Many studies have suggested that chemokine played a
significant role, not only in immune and inflammatory responses,
but also in malignant cell growth and progression (3,4). The
CXCR4 chemokine receptor belongs to the group of seven
transmembrane G protein-coupled receptors (GPCRs) which are
ubiquitously expressed in various normal cells and tissues,
including neurons, lymphatic tissues, microglia and hematopoietic
cells (3,5). CXCR4 and its ligand, stromal
cell-derived factor-1 (SDF-1), currently termed CXCL12, are
believed to play significant roles in tumorigenesis and metastasis
(6). Based on these studies,
downregulation of CXCR4 may provide a therapeutic strategy for
inhibiting tumors metastasis and for enhancing the survival of
patients with RCC. AMD3100 is a specific CXCR4 antagonist that is
used in the clinic to improve the success of stem cell
transplantation in cancer patients (7). However, drug resistance may limit the
use of AMD3100 in clinical applications (8). In another clinical study, two patients
treated with 40 and 160 mg/kg/h AMD3100 had unexpected premature
ventricular contractions, which resulted in the discontinuation of
the drug (9). Surprisingly, AMD3100
reduced growth of lymphomas in vivo when administered three
times weekly, but enhanced tumor growth after continuous drug
infusions (6,10). However, recently the advent of
RNAi-directed ‘knock-down’ has sparked a revolution in somatic cell
genetics, allowing for inexpensive and rapid analysis of gene
function in mammals, and might be exploited for gene therapy in the
future (11).
Our previous experiment has confirmed that the RCC
A-498 cell line, which expresses high level of CXCR4, was
associated with increased invasiveness (12). In the present study, we employed
RNAi to inhibit CXCR4 expression in A-498 cells and investigated
the effect of CXCR4 suppression on the proliferation and apoptosis
of those cells in vitro.
Materials and methods
Cell line and culture
A-498 cells (ATCC, Manassas, VA, USA), a human renal
cell carcinoma cell line, were grown at 37°C in a humidified
atmosphere of 95% air and 5% CO2 in ATCC-formulated
Eagle’s minimum essential medium (EMEM, cat. no. 30-2003, ATCC)
supplemented with 10% fetal bovine serum (Hyclone Corp., USA).
Construction of the CXCR4 specific short
hairpin RNA (shRNA) expression vector and stable transfection
Double chains of oligonucleotide with complementary
sequences that can code short hairpin RNA (shRNA) were obtained
from GenePharma Co., Ltd. (Shanghai, China). The siRNA recognized
nucleotides 525–543 of CXCR4 mRNA (GAAGCAT GACGGACAAGTA) of the
transcript according to the NCBI database (GeneID7852). CXCR4-shDNA
oligos are listed below: 5′-CACCGAAGCATGACGGACAAGTATTCAAGA
GATACTTGTCCGTCATGCTTCTTTTTTTG-3′ (sense) and
5′-GATCCAAAAAAGAAGCATGACGGACAAGTATCTC TTGAATACTTGTCCGTCATGCTTC-3′
(antisense). The target sequence of the negative control group
named shRNA-control was 5′-GTTCTCCGAACGTGTCACGT-3′ (sense) and
5′-ACGTGACACGTTCGGAGAAT-3′ (antisense), which has no homology with
that of human or mice. The hairpin loop region was annealed with
its complementary strand and was cloned into the pGPU6/GFP/Neo
plasmid vector (GenePharma Co., Ltd.) carrying a green fluorescent
protein (GFP) reporter gene and genes for ampicillin and neomycin
resistance. The shRNA expression vectors were transfected into
A-498 cells using the Lipofectamine 2000 transfection reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. G418 was added into the culture medium (500 mg/ml,
Gibco Bio-Cult) after 48 h. Stable G418-resistant clones were
obtained after 4 weeks. The expanded cells were then used for
subsequent studies.
RNA isolation and reverse transcription
polymerase chain reaction (PCR)
Total RNA was isolated from cells by using TRIzol
Reagent (Invitrogen). cDNA synthesis was performed using reverse
transcription reagents (Promega, Madison, WI, USA). Real-time PCR
was performed using SYBR-Green I Mix (ABI, Foster City, CA, USA)
and an ABI Prism 7700 Sequence Detection system (ABI) according to
the manufacturer’s instructions. The primer sequences for the genes
and expected product sizes were as follows: 5′-GGAAAAGAGG
GGAGGAGAG-3′ (sense) and 5′-CACTTCCAATTCAG CAAGCA-3′ (antisense)
for CXCR4, 5′-ACCACCATGAGA AGGCTGG-3′ (sense) and
5′-CTCAGTGTAGCCCAGGA TGC-3′ (antisense) for GAPDH.
Western blotting
Stable transfected A-498 cells were lysed with a
denaturing SDS-PAGE sample buffer using standard methods, and the
resulting lysates were cleared by centrifugation. Proteins were
transferred onto a nitrocellulose membrane, blocked with
Tris-buffered saline plus 0.1% Tween-20 (TTBS) containing 5%
non-fat milk for 2 h. The membrane was incubated overnight with the
rabbit anti-human primary polyclonal antibody against CXCR4 (Abcam,
USA) at 1:1000 dilution at 4°C, then washed and incubated with the
HRP-conjugated goat anti-rabbit IgG (Pierce, Rockford, IL, USA) at
1:2000 for 1 h at 37.0°C. Actin was probed using a rabbit
monoclonal antibody against human protein and an anti-rabbit IgG as
secondary antibody (Santa Cruz Biotechnology). Finally, the bands
were visualized by chemiluminescence using a chemiluminescence kit
(Invitrogen) and the specific bands were recorded on X-ray film.
BandScan 5.0 software was used for gray scale scan to evaluate the
relative value of protein expression.
Cell proliferation assay
Cells in the log-growth phase were harvested,
suspended at a density of ~1×104 cells/well. After 24 h
of culture, 20 μl MTT (5 mg/ml, Sigma) was added to each
well. The plates were then incubated at 37°C for 4 h. Then culture
medium was removed and 150 μl of DMSO (Sigma) was added and
thoroughly mixed for 10 min. Absorbance of each well was measured
at a wavelength of 490 nm and the numbers of surviving cells were
calculated.
Invasion and migration assay
The invasion assay was performed using an 8
μm pore size transwell chamber in 24-well plates (Corning
Costar, Cambridge, MA, USA). A-498 cells (1.0×105) in
500 μl of serum-free MEM medium were loaded into the top
chamber with fetal bovine serum placed in the bottom chamber as a
chemoattractant. After further incubation at 37°C for 10 h, the
cells on the top of the filters were removed with cotton swabs. The
cells on the lower surface of the filters were fixed in 4%
paraformaldehyde and stained with 0.1% crystal violet. The crystal
violet was removed and the cells were washed three times with PBS,
and then the remaining crystal violet that had stained the migrated
cells was eluted with one wash with 33% acetic acid. The OD540 nm
of the eluted crystal violet was determined as a measure of
migrated cells. The cell migration assay was performed in a similar
mode, except that cells were seeded into the uncoated filter and
incubated for 24 h.
Analysis of apoptosis by flow
cytometry
Apoptosis was determined with an Annexin
V-FITC/propidium iodide Apoptosis Detection kit (Bipec Biopharma
Corp., Cambridge, MA) according to the manufacturer’s protocol. The
cells were washed with PBS and subsequently incubated for 5 min at
room temperature in the dark in 500 μl of 1X binding buffer
containing 5 μl of Annexin V-FITC and 10 μl of
propidium iodide. Afterwards, apoptosis was analyzed by flow
cytometry (FCM).
Statistical analysis
All measurements were carried out with the same
instrument under the same experimental condition. Data are
expressed as means ± standard deviation (SD). All data were
analyzed statistically by one-way analysis of variance (ANOVA) and
P<0.05 was considered significant.
Results
Assessment of the effect of CXCR4 siRNA
in A-498 cells
After the plasmid vector with green fluorescent
protein (GFP) was transfected into the cells using Lipofectamine
2000 transfection reagent, it produced a green fluorescent wave.
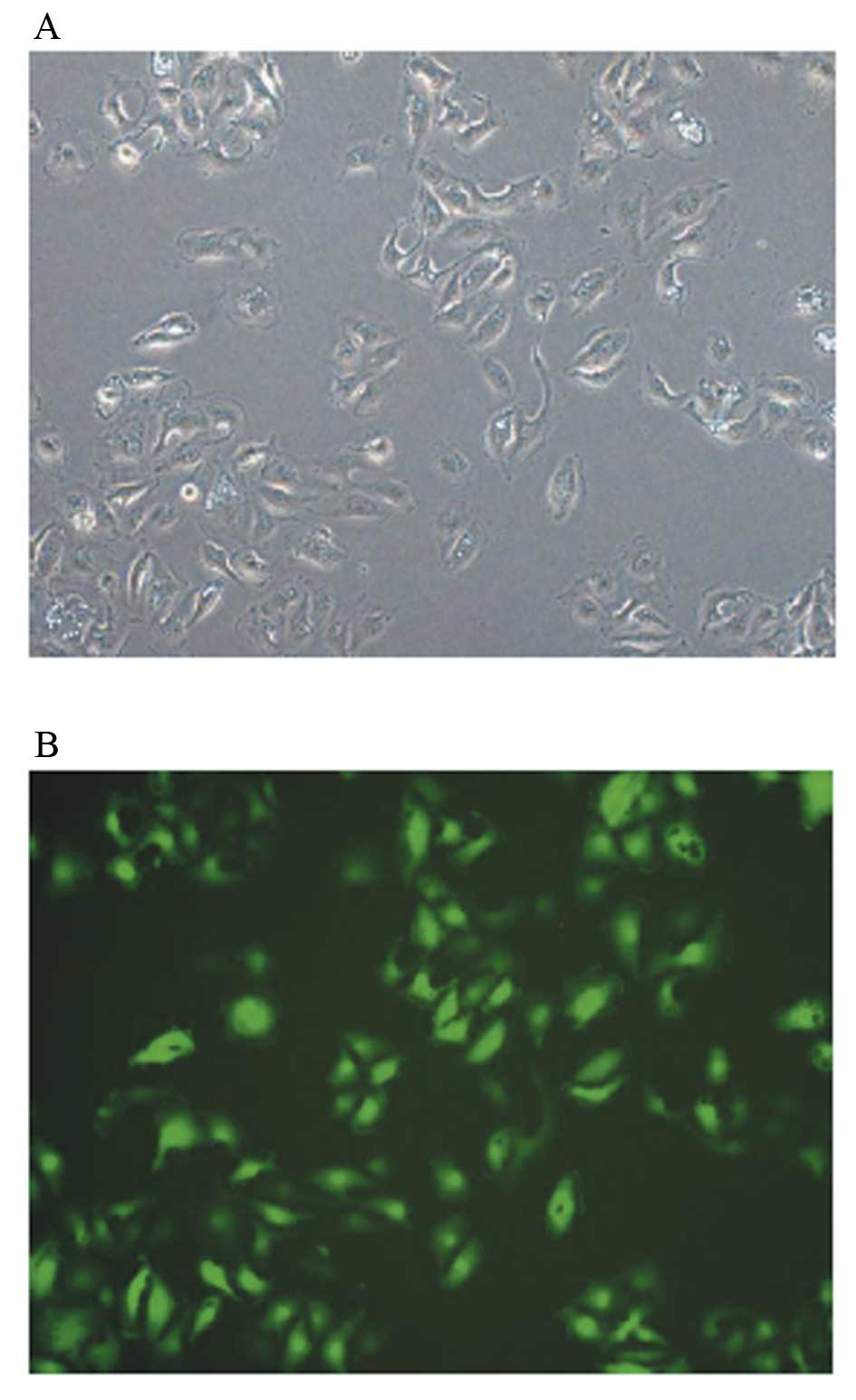
Microscopic analysis revealed that CXCR4 RNAi induced morphological
changes in A-498 cells characterized by irregular morphology, less
dense structure and were smaller in volume compared with
untransfected cells (Fig. 1A). The
fluorescent microphoto indicated that transfection was successful
(Fig. 1B).
RNAi inhibits CXCR4 mRNA expression in
A-498 cells
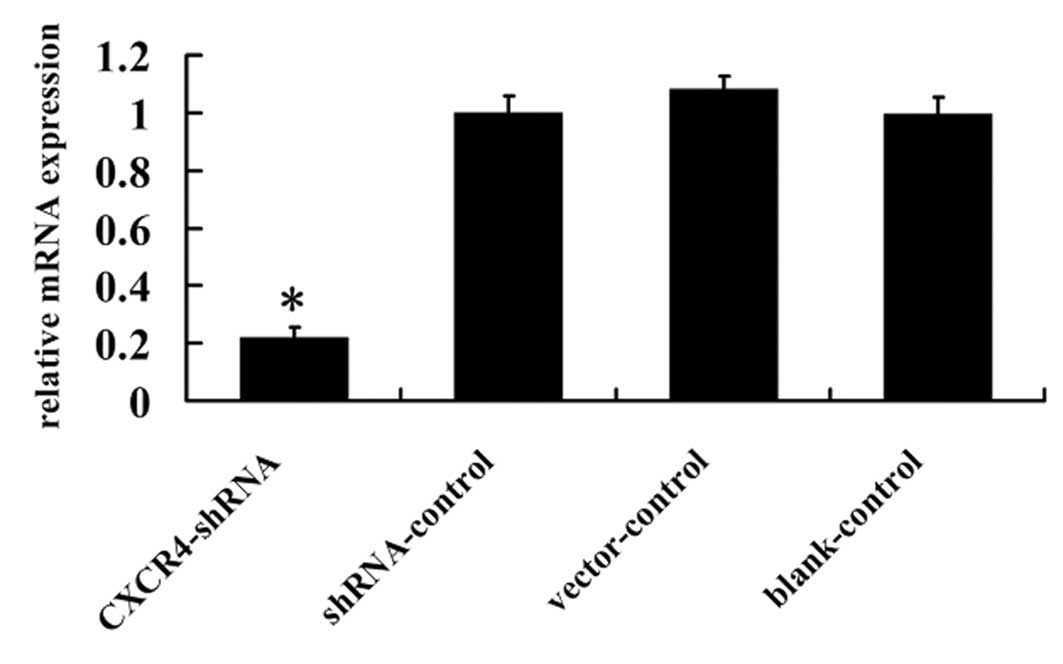
To study the silencing effect of CXCR4 shRNA, CXCR4
expression was evaluated by RT-PCR. As shown in Fig. 2, CXCR4 mRNA expression in
CXCR4-shRNA transfected A-498 cells was reduced 78.9%, compared to
shRNA-control, vector-control and blank-control cells (P<0.01),
indicating that the corresponding mRNA sequence for CXCR4 RNAi was
specific to the intended target.
RNAi inhibits CXCR4 protein expression in
A-498 cells
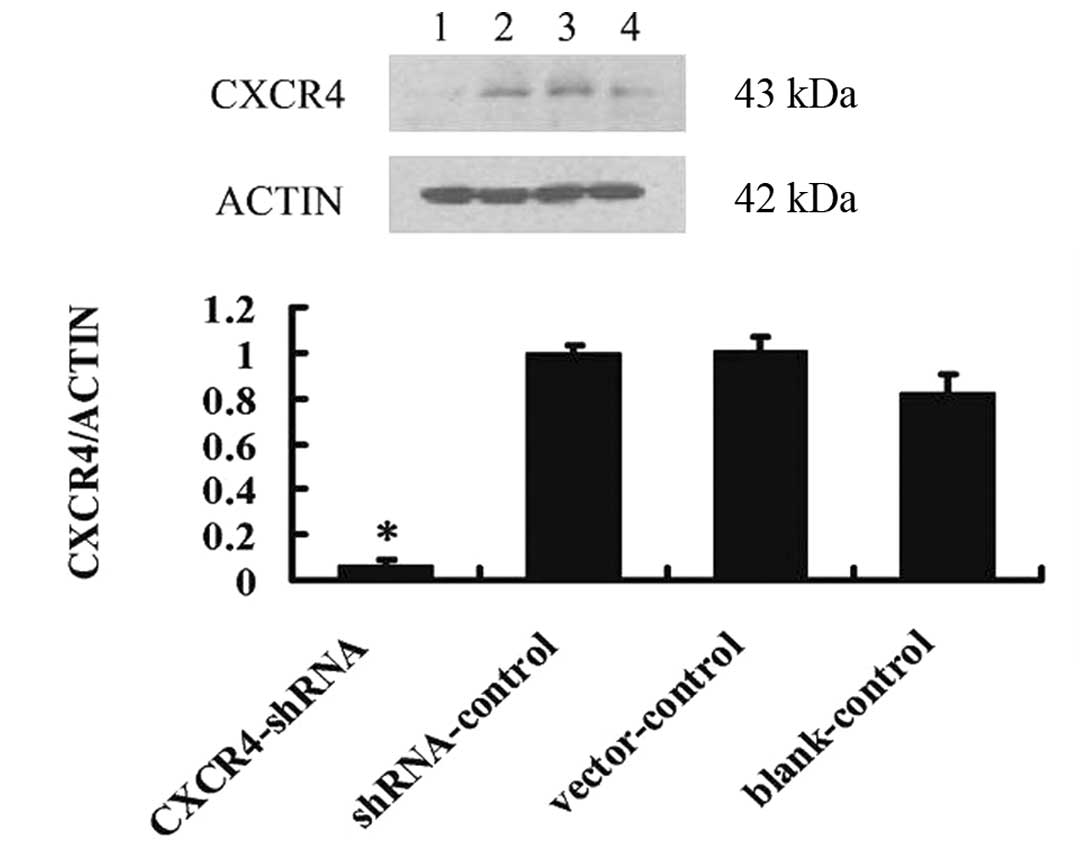
CXCR4-shRNA resulted in a significant decrease in
CXCR4 protein levels (11.67±3.09% normalized to β-actin) (Fig. 3) compared with shRNA-control cells
(55.50±3.72% normalized to β-actin), vector-control (53.44±5.65%
normalized to β-actin) and blank-control cells (50.63±8.17%
normalized to β-actin) (P<0.01), suggesting that CXCR4-shRNA
strongly inhibits CXCR4 expression.
CXCR4 shRNA inhibits A-498 cell
proliferation
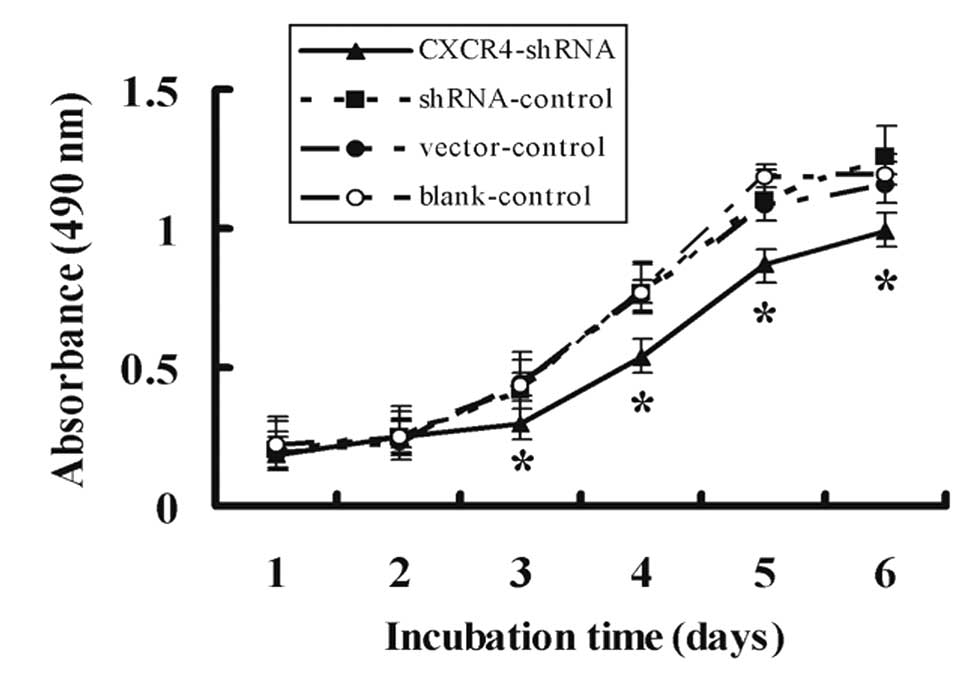
The growth rate of cells after transfection was
examined using MTT assay for 6 days. As shown in Fig. 4, CXCR4-shRNA reduced the growth of
A-498 cells significantly as compared to shRNA, vector, and
blank-controls (P<0.01). Cell proliferation was inhibited
notably in a time-dependent manner for CXCR4-shRNA cells and the
highest inhibitory rate was 31.33±1.78% on Day 3. There was no
difference in inhibition among shRNA, empty vector, or
blank-controls (P>0.05).
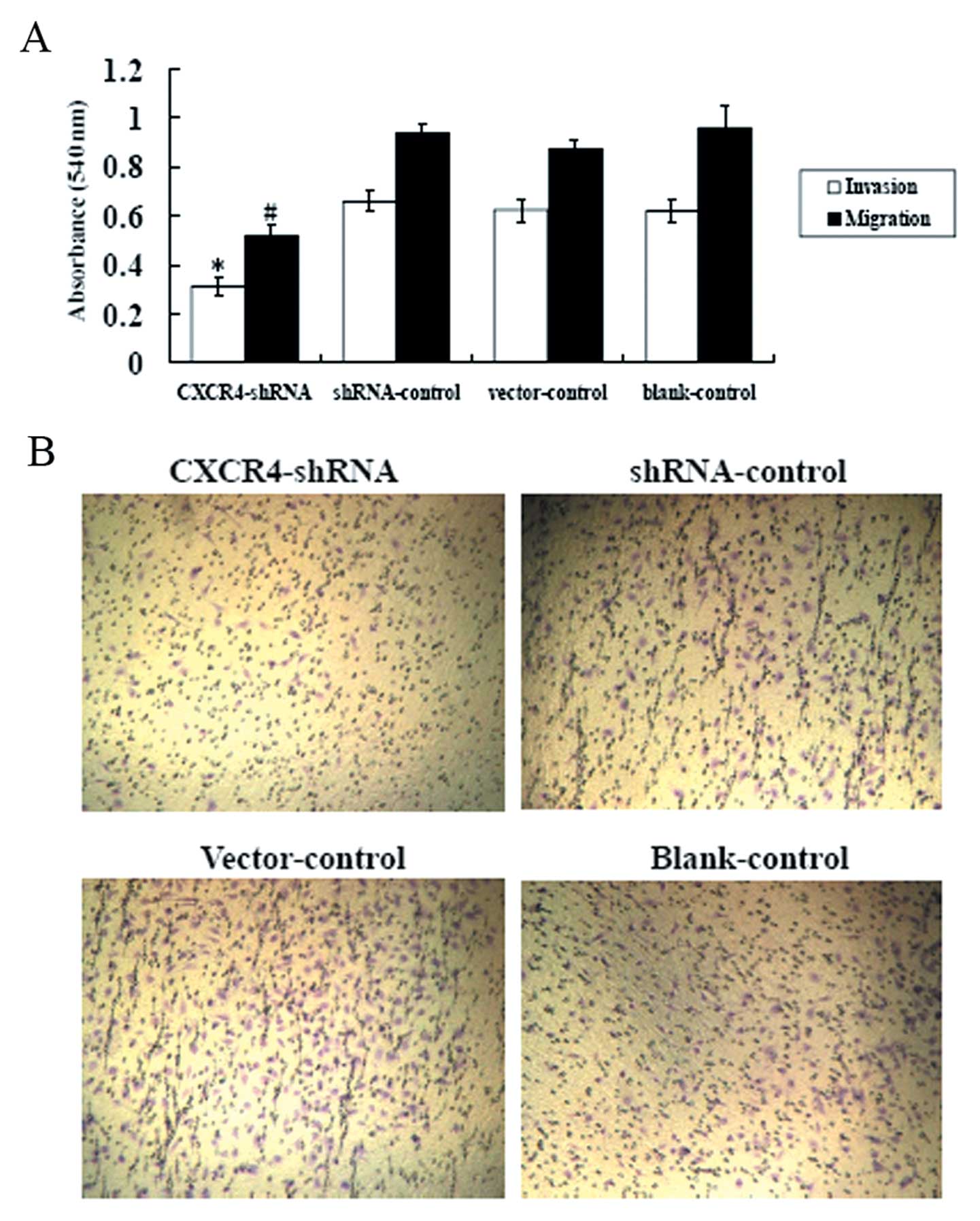
CXCR4 shRNA inhibits A-498 cell invasion
and migration
We evaluated whether suppression of CXCR4 altered
the motility of A-498 cells across transwell polycarbonate
membranes. As shown in Fig. 5A,
compared with shRNA, vector, and blank controls, the cell
invasiveness and migration of CXCR4-shRNA cells were reduced by
51.28±1.64% and 43.73±2.75%, respectively (P<0.01). In contrast,
there were no differences observed between shRNA, vector, and blank
controls (P>0.05). Fewer CXCR4-shRNA-transfected cells than
shRNA, vector, and blank control-tansfected cells were observed
when the polycarbonate filters were stained with crystal violet
(Fig. 5B). Our data suggested that
silencing by CXCR4-shRNA can inhibit the invasive potential of
A-498 cells.
Effect of CXCR4 shRNA on cell
apoptosis
The cell apoptosis assay was measured by FCM.
Apoptosis rate was 32.76±0.56% in CXCR4 shRNA cells, 11.72±0.70% in
shRNA-control cells, 10.99±0.50% in vector-control cells, and
12.83±0.90% in blank-control cells. As shown in Table I, compared with control or untreated
cells, more necrotic cells were detected in the cells treated with
plasmid-mediated CXCR4 shRNA (P<0.01).
 | Table IEffect of CXCR4 gene depletion on
A-498 cell apoptosis. |
Table I
Effect of CXCR4 gene depletion on
A-498 cell apoptosis.
| Apoptosis rate
(%) |
|---|
|
|
|---|
| Groups | Early phase | Late phase | Total |
|---|
| Negative-control | 3.65±0.21 | 8.06±0.48 | 11.72±0.47 |
| Vector-control | 3.58±0.16 | 7.41±0.34 | 10.99±0.38 |
| Blank-control | 4.53±0.32 | 8.28±0.58 | 12.83±0.69 |
| CXCR4 shRNA | 17.74±0.31a | 15.01±0.26a | 32.76±0.38a |
Discussion
Chemokines are 8 to 10-kDa cytokines that are
classified into four groups (CXC, CC, C and CX3C) based on the
position of the first two cysteines (13). CXCR4, a GPCR, is constitutively
expressed in a wide range of normal tissues and its expression is
enhanced in many solid tumors (14). Quantitative analysis of CXCR4 gene
expression has been proposed as a prognostic marker and a predictor
of potential metastasis in colorectal cancer (15), breast cancer (16), gastric cancer (17), acute myelogenous leukemia (18), osteosarcoma (19) and prostate cancer (20). Zagzag et al found that CXCR4
mRNA is upregulated in kidney cancer samples compared to adjacent
normal tissue indicating a closely relation between CXCR4 and tumor
cell dissemination and invasion (21). In addition, it was found that strong
CXCR4 expression in renal cell carcinoma is associated with
advanced T status (22). However,
few reports have investigated the effects of siRNA directed
inhibition of CXCR4 in RCC. Accordingly, to gain more insight into
the effect of CXCR4 in A-498 cells, we selectively knocked down
CXCR4 expression using RNAi and observed the subsequent effects in
A-498 cells.
In this study, we successfully constructed a plasmid
expression vector that contained a CXCR4 short hairpin RNA
expression cassette. Our study showed that in stable transfected
A-498 cells, the shRNA was introduced into the cytoplasm, forming
an RNA-induced silencing complex with other nucleus enzymes. We
found that CXCR4 mRNA and protein production were downregulated. In
contrast, CXCR4 expression was unchanged in the control groups,
which confirmed that high specific gene suppression by RNAi was
achieved.
We previously reported that overexpression of CXCR4
in A-498 cells is associated with increased Matrigel matrix
invasion (12). In this study we
observed that RNAi-mediated silencing of CXCR4 decreased cell
proliferation by MTT and Matrigel invasion assays. Our results
suggest that CXCR4 is a positive regulator in the growth of A-498
cells and thus supports A-498 cell proliferation. Previous studies
have found that CXCR4 nuclear localization may be responsible for
these metastatic changes (12,23).
The nuclear localization of CXCR4 is comparable to the mechanism
exhibited by the epidermal growth factor receptor (EGFR), another
membrane receptor, which translocates to the nucleus after binding
to epidermal growth factor, and increases the promoter region of
the cyclin D1 gene transcription (24). Cyclin D1 is a regulatory kinase
critical for progression through the G1 to S phase transition of
the cell cycle and is involved in cancer cell growth (25), and thus nuclear translocation of
EGFR leads to increased cell proliferation.
Increased resistance to apoptosis is a common
characteristic of cancer cells, and alterations in the apoptotic
pathway contribute to tumorigenesis and chemotherapy resistance in
carcinoma cells. In this study, we also observed that A-498 RCC
cells treated with CXCR4 shRNA had an increased apoptotic rate.
Previous studies indicated that suppression of CXCR4 reduces the
induction of apoptosis in other types of cancer cells such as
neuroectodermal tumors (26),
antagonists medulloblastoma (27),
and breast cancer cell lines in vitro (28). Furthermore, another report showed
that crosstalk between CXCR4 and integrin signaling increased
adhesion of small cell lung cancer (SCLC) cells to stromal cells,
which protected these cells from chemotherapy-induced apoptosis
conferring chemoresistance (29).
CXCR4 blockade reduced adhesion and survival signals from the tumor
microenvironment to SCLC cells and increased sensitivity to
chemotherapy induced apoptosis (30). As an anti-apoptotic factor, Bcl-2
protects cells from apoptosis by regulating mitochondria membrane
potential and preventing mitochondrial cytochrome c release
(31). Carmen et al reported
that Bcl-2 activity decreases after addition of CXCR4 antagonists,
leading to A-498 cell death through a mitochondrial apoptotic
pathway (32). After binding to
CXCR4, SDF-1 causes mobilization of calcium, decrease of cyclic AMP
within the cells, and activates multiple signal transduction
pathways such as PI3K/Akt/eNOS pathway, which can enhance cell
proliferation, migration, survival, and angiogenesis signals by
inducing eNOS activity (33). It
has also been reported that a number of Akt downstream target
molecules such as nitric oxide (34), and Bcl-2 family members including
Bcl-xL, Bcl-w, Bad and Bax (35)
are involved in regulating tumor cell apoptosis (36). In summary, these findings provide
evidence that downregulation of CXCR4 expression has an apoptotic
effect in A-498 cells and this mechanism could lead to an
anti-tumor effect.
In conclusion, our present study shows that
silencing of CXCR4 using RNA interference could effectively inhibit
proliferation, invasion and metastasis in A-498 RCC cells.
Traditionally, the standard treatment for patients with RCC is
surgical resection in the case of localized disease (37). However, for metastatic RCC, gene
therapy based on RNA interference-mediated silencing of CXCR4 might
be a promising and innovative anticancer therapy. In addition, more
studies are required to assess the pharmacokinetics and improve the
tissue specific targeting of certain genes with siRNA.
Acknowledgements
This study was supported by the Science and
Technology key project of basic research of Shanghai, China (no.
10JC1417800).
References
|
1
|
Godley P and Kim SW: Renal cell carcinoma.
Curr Opin Oncol. 14:280–285. 2002. View Article : Google Scholar
|
|
2
|
Fishman M and Seigne J: Immunotherapy of
metastatic renal cell cancer. Cancer Control. 9:293–304.
2002.PubMed/NCBI
|
|
3
|
Vicari AP and Caux C: Chemokines in
cancer. Cytokine Growth Factor Rev. 13:143–154. 2002. View Article : Google Scholar
|
|
4
|
Navarini-Meury AA and Conrad CC: Melanoma
and innate immunity-active inflammation or just erroneous
attraction? Melanoma as the source of leukocyte-attracting
chemokines. Semin Cancer Biol. 19:84–91. 2009.
|
|
5
|
Skommer J, Wlodkowic D and Pelkonen J:
CXCR4 expression during tumour cell death. Leuk Res. 31:1155–1156.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Broxmeyer HE, Orschell CM and Clapp DW:
Rapid mobilization of murine and human hematopoietic stem and
progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med.
201:1293–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Labrosse B, Brelot A and Heveker N:
Determinants for sensitivity of human immunodeficiency virus
coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 72:6381–6388.
1998.PubMed/NCBI
|
|
9
|
Hendrix CW, Collier AC, Lederman MM, et
al: Safety, pharmacokinetics, and antiviral activity of AMD3100, a
selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir
Immune Defic Syndr. 37:1253–1262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paul S, Mancuso P, Rabascio C, et al: In
Vitro and Preclinical Activity of the Novel AMD3100 CXCR4
Antagonist in Lymphoma Models. In: American Society of Hematology
Annual Meeting; 2002; Philadelphia, PA, USA. Blood. 2002
|
|
11
|
Duxbury MS and Whang EE: RNA interference:
a practical approach. J Surg Res. 117:339–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Wang Z, Yang B, et al: CXCR4
nuclear localization follows binding of its ligand SDF-1 and occurs
in metastatic but not primary renal cell carcinoma. Oncol Rep.
22:1333–1339. 2009.PubMed/NCBI
|
|
13
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim J, Takeuchi H, Lam ST, et al:
Chemokine receptor CXCR4 expression in colorectal cancer patients
increases the risk for recurrence and for poor survival. J Clin
Oncol. 23:2744–2753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hiller DJ, Li BD and Chu QD: CXCR4 as a
predictive marker for locally advanced breast cancer
post-neoadjuvant therapy. J Surg Res. 166:14–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie L, Wei J, Qian X, et al: CXCR4, a
potential predictive marker for docetaxel sensitivity in gastric
cancer. Anticancer Res. 30:2209–2216. 2010.PubMed/NCBI
|
|
18
|
Spoo AC, Lubbert M and Wierda WG: CXCR4 is
a prognostic marker in acute myelogenous leukemia. Blood.
109:786–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laverdiere C, Hoang BH and Yang R:
Messenger RNA expression levels of CXCR4 correlate with metastatic
behavior and outcome in patients with osteosarcoma. Clin Cancer
Res. 11:2561–2567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun YX, Wang J, Shelburne CE, et al:
Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers
(PCa) in vivo. J Cell Biochem. 89:462–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zagzag D, Krishnamachary B, Yee H, et al:
Stromal cell-derived factor-1alpha and CXCR4 expression in
hemangioblastoma and clear cell-renal cell carcinoma: von
Hippel-Lindau loss-of-function induces expression of a ligand and
its receptor. Cancer Res. 65:6178–6188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas CW, Claudine G, Stefan B, et al:
Strong expression of chemokine receptor CXCR4 by renal cell
carcinoma correlates with advanced disease. J Oncol. 2008:1–6.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang LH, Liu Q, Xu B, et al:
Identification of nuclear localization sequence of CXCR4 in renal
cell carcinoma by constructing expression plasmids of different
deletants. Plasmid. 63:68–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin SY, Makino K, Xia W, et al: Nuclear
localization of EGF receptor and its potential new role as a
transcription factor. Nat Cell Biol. 3:802–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan MZ, Shimizu S, Patel JP, et al:
Regulation of neuronal P53 activity by CXCR 4. Mol Cell Neurosci.
30:58–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerlach LO, Skerlj RT, Bridger GJ, et al:
Molecular interactions of cyclam and bicyclam non-peptide
antagonists with the CXCR4 chemokine receptor. J Biol Chem.
276:14153–14160. 2001.PubMed/NCBI
|
|
28
|
Dewan MZ, Ahmed S, Iwasaki Y, et al:
Stromal cell-derived factor-1 and CXCR4 receptor interaction in
tumor growth and metastasis of breast cancer. Biomed Pharmacother.
60:273–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burger M, Glodek A, Hartmann T, et al:
Functional expression of CXCR4 (CD184) on small-cell lung cancer
cells mediates migration, integrin activation, and adhesion to
stromal cells. Oncogene. 22:8093–8101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hartmann TN, Burger JA, Glodek A, et al:
CXCR4 chemokine receptor and integrin signaling co-operate in
mediating adhesion and chemoresistance in small cell lung cancer
(SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chao DT and Korsmeyer SJ: Bcl-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
32
|
Carmen HL, Jaris V, Laura Hi, et al:
CXCL12/CXCR4 signaling promotes human thymic dendritic cell
survival regulating the Bcl-2/Bax ratio. Immunol Lett. 120:72–78.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng H, Dai T, Zhou B, et al:
SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis
under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis.
201:36–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao F, Gao E, Yue TL, et al: Nitric oxide
mediates the antiapoptotic effect of insulin in myocardial
ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial
nitric oxide synthase phosphorylation. Circulation. 105:1497–1502.
2002. View Article : Google Scholar
|
|
35
|
Yin G, Li LY, Qu M, Luo HB, Wang JZ and
Zhou XW: Upregulation of AKT attenuates amyloid-β-induced cell
apoptosis. J Alzheimers Dis. 25:337–345. 2011.
|
|
36
|
New DC, Wu K, Kwok AW, et al: G
protein-coupled receptor-induced Akt activity in cellular
proliferation and apoptosis. FEBS J. 274:6025–6036. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ljungberg B, Cowan NC, Hanbury DC, et al:
EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol.
58:401–402. 2010. View Article : Google Scholar : PubMed/NCBI
|