Introduction
Cancer Statistics 2010 indicated that breast cancer
incidence is the highest one in female cancer (counts for 23%) and
the main cause of cancer death for women (counts for 14%) (1). Large number of epidemiological studies
(2) have shown that, breast cancer
incidence or mortality was significantly negatively correlated with
sun exposure, suggested that lacking of sunlight causes the
reduction of vitamin D synthesis which in turns closely related
with the disease; additionally, there is evidence that (3) high levels of plasma vitamin D can
reduce the risk of breast cancer. Vitamin D is a multifunctional
hormone, besides its indispensable role in calcium and phosphorus
metabolism, recent research have found that it can inhibit a
variety of tumor cells. New research shows that vitamin D has
anti-inflammatory and anticancer mechanisms (4).
The role of inflammatory microenvironment in tumor
development has been paid increased attention. The interation of
the amounts of inflammatory cytokines, growth factors and the
oncogene activation engage in fast induction of cyclooxygenase-2
(COX-2) expression during carcinogenesis, COX-2 affects tumor
progression by participating in the malignant proliferation,
invasion and metastasis. COX-2 is overexpressed in a variety of
malignancies, including breast cancer, which is estrogen-dependent.
It has been reported that (5) once
COX-2/PGE2 pathway was activated, it can elevate the activity of
estrogen synthesis enzyme, thus in turn increase the level of
estrogen in breast cancer, correspondingly promoting breast cancer
development. In 2010, a study (6)
was reported on the adjustment of cytochrome P4501B1 (CYP1B1)
expression by PGE2 in breast cancer cells. CYP1B1 belongs to the
cytochrome P450 (CYP) super-gene family and is the only member of
CYP1B subtribe, its main function is to catalyze hydroxylation of
estrogen, generating the genotoxic carcinogens. These results
indicated that, there exists an important relationship between
COX-2/PGE2 pathway and the carcinogenic effect of
estrogen-metabolizing enzymes (CYP1B1). The anti-inflammatory and
anticancer effect of vitamin D is relevant to its negative
regulation of CYP1B1 expression followed by interference with the
COX-2/PGE2 pathway.
In this study, human breast cancer tissues from
different patients and estrogen receptor α (ERα)-positive breast
cancer cell line MCF-7 were used. First we investigated the protein
expression of COX-2, p-ERα and CYP1B1 in human breast cancer tissue
samples for analyzing the relevance between each of them; besides,
1,25-dihydroxyvitamin D3
[1,25(OH)2D3] which is the active metabolite
of vitamin D, was used to treat MCF-7 cells. After treatment, the
expression of COX-2, PGE2, p-ERK, p-ERα and CYP1B1 were detected,
cell proliferation and cell cycle transformation were observed. We
also disscuss the mechanism of 1,25(OH)2D3
affecting CYP1B1 through COX-2/PGE2 pathway during human breast
cancer tumorigenesis.
Materials and methods
Chemicals and reagents
1α,25-dihydroxyvitamin D3
[1,25(OH)2D3] and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma- Aldrich, Inc. (St. Louis, MO, USA).
Phenol red-free Dulbecco’s modified Eagle’s medium (DMEM) was
purchased from SAFC Biosciences, Inc. (Lenexa, KS, USA). Newborn
calf serum (NBCS) was obtained from Life Technologies, Inc.; rabbit
anti-human CYP1B1, phospho-ERα(ser118) and ERK polyclonal
antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA). Rabbit anti-human COX-2, phospho-ERK1/2 (Thr202/Tyr204)
and β-actin polyclonal antibodies; goat anti-rabbit IgG/HRP, goat
anti-rabbit IgG/FITC and human PGE2 ELISA kit were from Bioss, Inc.
(Beijing, China). Enhanced chemiluminescence (ECL) system and goat
anti-rabbit IgG/Cy3 were from Beyotime, Inc. Polyvinylidene
difluoride (PVDF) membrane from Millipore, Inc. PCR oligonucleotide
primers were synthesized by Sangon Biotech Co. (Shanghai, China).
PrimeScript RT reagent kit was from Takara, Inc. (Dalian,
China).
Tissue samples
Formalin-fixed, paraffin-embedded sections from 42
surgically removed breast tumors were analyzed, after local ethics
committee approval. All samples were obtained from patients that
had not undergone any chemotherapy or radiotherapy before surgical
resection. All patients were diagnosed and treated in the First
Affiliated Hospital of Chongqing Medical University during the
period from 2010 to 2011. All patients with breast carcinoma (35
invasive ductal carcinomas, 3 mucinous adenocarcinomas, 3
intraductal carcinomas, 1 malignant myoepithelioma and 1 atypical
medullary carcinoma) were woman. The mean patient age was 52 years
(range, 36–75). All specimens obtained at surgery or outside
histology slides were reviewed by a senior pathologist.
Immunostaining for COX-2,
phospho-ERα(ser118) and CYP1B1 protein expression
The streptavidin-biotin peroxidase complex method
was used for immunohistochemistry. Briefly, tissue sections (4-μm
thick) were dewaxed and antigen retrieval was performed in citrate
buffer using a microwave. Sections were incubated for 10 min with a
3% hydrogen peroxide solution to quench endogenous peroxidase
activity and then were incubated overnight at 4°C with specific
primary antibodies and developed using 3,3-diaminobenzidine for 3
min. The anti-COX-2, anti-phospho-ERα(ser118) and anti-CYP1B1
antibodies were used at 1:100 dilution.
Evaluation of immunohistochemical
staining
Evaluation of the COX-2 and CYP1B1 expression was
considered as positive when the results of immunohistochemistry in
the tumor cells demonstrated an unequivocal granular staining of
the cytoplasm. Only nuclear staining was considered as positive for
p-ERα(ser118) expression. Each marker immunostain was recorded
according to stain intensity (intensity score) and percentage of
cancerous cells that stained positively (quantity score). Intensity
score was evaluated as negative (0), weak (1), moderate (2) or strong (3), and quantity score was categorized as
follows: 0, negative; 1, <10%; 2, 11–50%; 3, 51–75%; and 4,
>75%. By multiplying both components, a score (0–12) was
obtained. Marker was considered to be positive if it had a score of
≥3 and as negative if it had a score of <3.
Removal of sex hormones by
Charcoal/Dextran-treated newborn calf serum (7)
Charcoal was washed twice with cold sterile water
immediately before use. A 5% charcoal suspension in 0.5% dextran
T40 of the same volume as serum was centrifuged at 1000 × g for 10
min. Supernatants were aspirated, and the serum aliquot was mixed
with the charcoal pellets. This charcoal-serum mixture was
maintained in suspension by mixing 4 times/min at 56°C for 30 min.
This suspension was centrifuged at 1000 × g for 20 min. This
procedure was repeated twice, and the supernatant was filtered
through a 0.22 μm cellulose acetate-filter. The charcoal
dextran-treated NBCS (CDNBCS) was stored at −20°C until needed.
Cell culture and treatment
Human breast cancer cell line MCF-7 was obtained
from the Department of Pathophysiology, Chongqing Medical
University. MCF-7 was grown in phenol red-free medium (DMEM),
supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and
10% CDNBCS at 37°C in an atmosphere containing 5% CO2.
Stock solutions of 1,25(OH)2D3 was prepared
in ethanol and added directly to the culture medium for incubation.
Control cells were treated only with ethanol. The final ethanol
concentration was always <0.5%.
Cell proliferation assay
The cells were made into suspension and added to
sterilized 96-well plates at a density of 2×104/ml.
There groups were set up on this study: the blank control group
containing only 200 μl DMEM medium, the negative control group
consisting of only 200 μl cell suspension and the
1,25(OH)2D3 treatment group. In the treatment
group, 1,25(OH)2D3 solution of the same
volume but various concentration was added into wells separately so
that the final concentration of 1,25(OH)2D3
ranged between 1–100 nmol/l. The cells were incubated for 24, 48
and 72 h, respectively. MTT (5 mg/ml) was added to each aspirated
well. Cells were incubated for a further 4 h at 37°C. After 4 h,
media were aspirated. Then cells were added with 150 μl DMSO and
incubated for a further 10 min at 37°C with gentle shaking. Cells
viability was asessed by absorbance of the end of the experimental
time. The resulting optical density at 570 nm was measured on
computer-controlled microplate analyzer. The experiment was
repeated for 3 times, and average of 3 results was taken as a final
value.
Cell cycle analysis
Cells were collected, washed, suspended in cold PBS,
fixed in 75% ethanol at −20°C overnight, washed and resuspended in
PBS with RNAase (20 mg/l). Cellular DNA was stained with PI and
cell samples were analyzed on Becton-Dickinson Flow Cytometer (BD
Biosciences, USA).
Reverse transcription-polymerase chain
reaction analysis
Total RNA was extracted from MCF-7 cells using
TRIzol (Takara). The first-strand cDNA was synthesized from 1 μg of
total RNA using a Prime Script kit (Takara). COX-2 gene expression
was quantified by semi-quantitative reverse transcriptase
polymerase chain reaction (RT-PCR). β-actin was uesd as an
endogenous control. RT-PCRs were performed with primers listed in
Table I. The PCR conditions were:
94°C for 3 min, followed by 35 cycles of 95°C for 50 sec, 58°C for
50 sec and 72°C for 50 sec. Five microliters of the PCR product was
separated by electrophoresis in 2% agarose gel and visualized by
ethidium bromide staining. Gene expression analysis was performed
with the Quantity One Software (Bio-Rad, Hercules, CA, USA).
 | Table ISequences of the primer pairs for
COX-2 and human housekeeping genes used for RT-PCR. |
Table I
Sequences of the primer pairs for
COX-2 and human housekeeping genes used for RT-PCR.
| Target name | Accession no.
(Ref-seq) | Primer
sequence |
|---|
| COX-2 | NM-000963.2 | Forward:
5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′
Reverse: 5′-AGATCATCTCTGCCTGAGTATCTT-3′ |
| β-actin | NM-001101.3 | Forward:
5′-CTGGGACGACATGGAGAAAA-3′
Reverse: 5′-AAGGAAGGCTGGAAGAGTGC-3′ |
Measurement of PGE2 by enzyme-linked
immunosorbent assay
MCF-7 cells were seeded in 6-well tissue culture
plates (5×105 cells/well). After 24 h the growth media
were replaced with phenol red-free DMEM medium containing vehicle
or 100 nmol/l 1,25(OH)2D3. After 72 h, PGE 2
concentrations were measured in the conditioned media using an
ELISA kit.
Western blot analysis
Cells were treated with either vehicle or 100 nmol/l
1,25(OH)2D3 for 72 h. Then they were
harvested, and whole-cell extracts were used. Cell lysates were
resolved by 8% SDS-polyacrylamide gel electrophoresis followed by
electroblotting onto a PVDF membrane. The membrane was probed with
the appropriate primary antibody followed by incubation with
secondary antibody. The immunoreactive bands were visualized using
an ECL kit, according to the manufacturer’s instructions. Proteins
expression analysis was performed with the Quantity One Software
(Bio-Rad).
Immunofluorescence analysis
MCF-7 Cells, grown on coverslips and incubated with
vehicle or 100 nmol/l 1,25(OH)2D3 for 72 h,
were fixed with 4% paraformaldehyde in PBS for 30 min and
permeabilized with 0.3% Triton X-100 for additional 10 min at 25°C.
Cells were then incubated overnight at 4°C with the following
primary antibodies: anti-COX-2 (1:20 in PBS),
anti-phospho-ERα(ser118) (1:20 in PBS) and anti-CYP1B1 (1:20 in
PBS). The primary antibodies were visualized, after appropriate
washing with PBS, using goat anti-rabbit IgG-FITC (1:50 in PBS) or
goat anti-rabbit IgG-Cy3 (1:50 in PBS) for 30 min at 37°C. Nuclei
were stained with DAPI. Coverslips were finally mounted with
antifade medium for observation using a fluorescence microscope.
Fluorescent density parameter was performed using software
Image-Pro Plus 6. 0 (Media Cybernetics, Inc., MD, USA).
Statistical analysis
Results are expressed as means ± SD. Statistical
correlation between COX-2 expression, p-ERα(ser118) expression and
CYP1B1 expression in cancer tissues were calculated using the
χ2 test. All statistical analyses were performed by SPSS
13.0 using the independent sample t-test for comparing the two
sample groups. For all tests, P<0.05 was considered
statistically significant.
Results
Association of COX-2 staining with
p-ERα(ser118) and CYP1B1 staining in breast cancer tissues
In breast cancer tissues, 78.6% of the tumors were
positive for COX-2 expression, 66.7% of the tumors were postive for
p-ERα(ser118) expression and 73.8% of the tumors were postive for
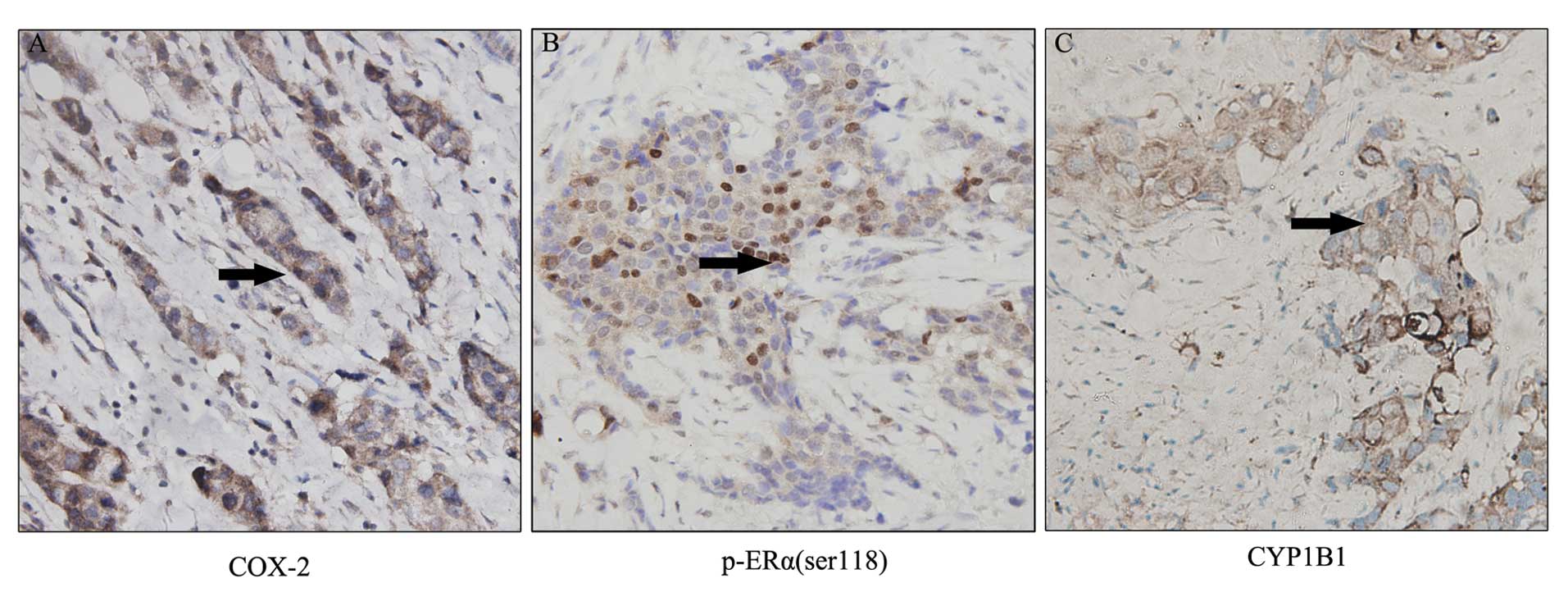
CYP1B1 expression. As demonstrated in Fig. 1, abundance of COX-2 and CYP1B1 were
perdominantly observed in cell cytoplasm of breast cancer tissues,
whereas p-ERα(ser118) displayed nuclear staining. Thus three groups
were formed, COX-2 protein expression correlated significantly with
p-ERα(ser118) (P<0.001) and CYP1B1 (P<0.01), the expression
of p-ERα(ser118) correlated to CYP1B1 (P<0.05) (Table II).
 | Table IICorrelation between COX-2,
p-ERα(ser118) and CYP1B1 protein expression in breast cancer
tissues. |
Table II
Correlation between COX-2,
p-ERα(ser118) and CYP1B1 protein expression in breast cancer
tissues.
| p-ERα(ser118) | CYP1B1 |
|---|
|
|
|
|---|
| +
n (%) | −
n (%) | r | P-value | +
n (%) | −
n (%) | r | P-value |
|---|
| COX-2 |
| + | 27 (82) | 6 (18) | 0.615 | 0.000 | 28 (85) | 5 (15) | 0.481 | 0.001 |
| − | 1 (11) | 8 (89) | | | 3 (33) | 6 (67) | | |
| p-ERα(ser118) |
| + | | | | | 24 (86) | 4 (14) | 0.383 | 0.012 |
| − | | | | | 7 (50) | 7 (50) | | |
Inhibitive effect of
1,25(OH)2D3 on the growth of MCF-7 cells
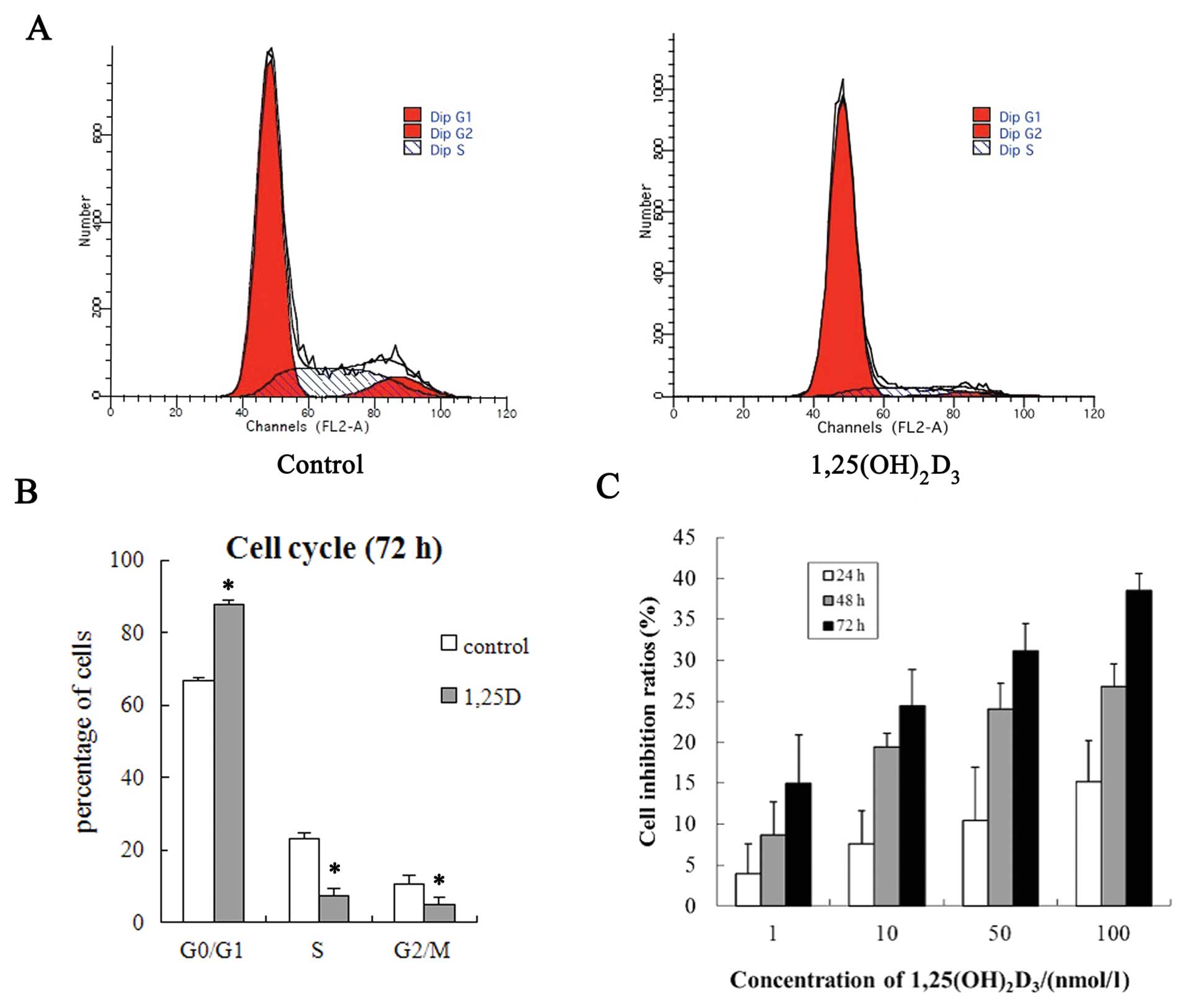
The results of the MTT assay showed that
1,25(OH)2D3 significantly inhibited the
proliferation of MCF-7 cells, and the inhibitive effect was time-
and dose-dependent (Fig. 2C). The
average growth inhibition ratios were 14.9, 24.4, 31.1 and 38.5%
after the cells were treated with 1,25(OH)2D3
of 1, 10, 50 and 100 nmol/l for 72 h. The ratios increased with the
increase of 1,25(OH)2D3 concentration.
1,25(OH)2D3 induces
MCF-7 cells arrest in the G0/G1 phase
MCF-7 cells were treated for 72 h with 100 nmol/l
1,25(OH)2D3, the percentage of G0/G1 phase
cells increased from 66.65±0.77 to 87.64±1.23, (P<0.001), but S
phase decreased from 22.94±1.79 to 7.49±1.98 (P<0.01), whereas
G2/M phase decreased from 10.40±2.57 to 4.87±2.11 (P<0.05).
These results suggest that 1,25(OH)2D3
blocked the cells at G0/G1 phase (Fig.
2A and B).
Suppression of the COX-2/PGE2 pathway by
1,25(OH)2D3 in MCF-7 cells
1,25(OH)2D3 regulation of the
expression of COX-2, the key enzymes involved in PGE2 metabolism.
After 72 h, 100 nmol/l 1,25(OH)2D3
significantly decreased total COX-2 mRNA levels (Fig. 3A) and COX-2 protein levels (Fig. 3B) in MCF-7 cells. As the result of
1,25(OH)2D3 reduced COX-2 protein levels,
1,25(OH)2D3 significantly decreased PGE2
levels secreted into the medium from MCF-7 cells (Fig. 3C).
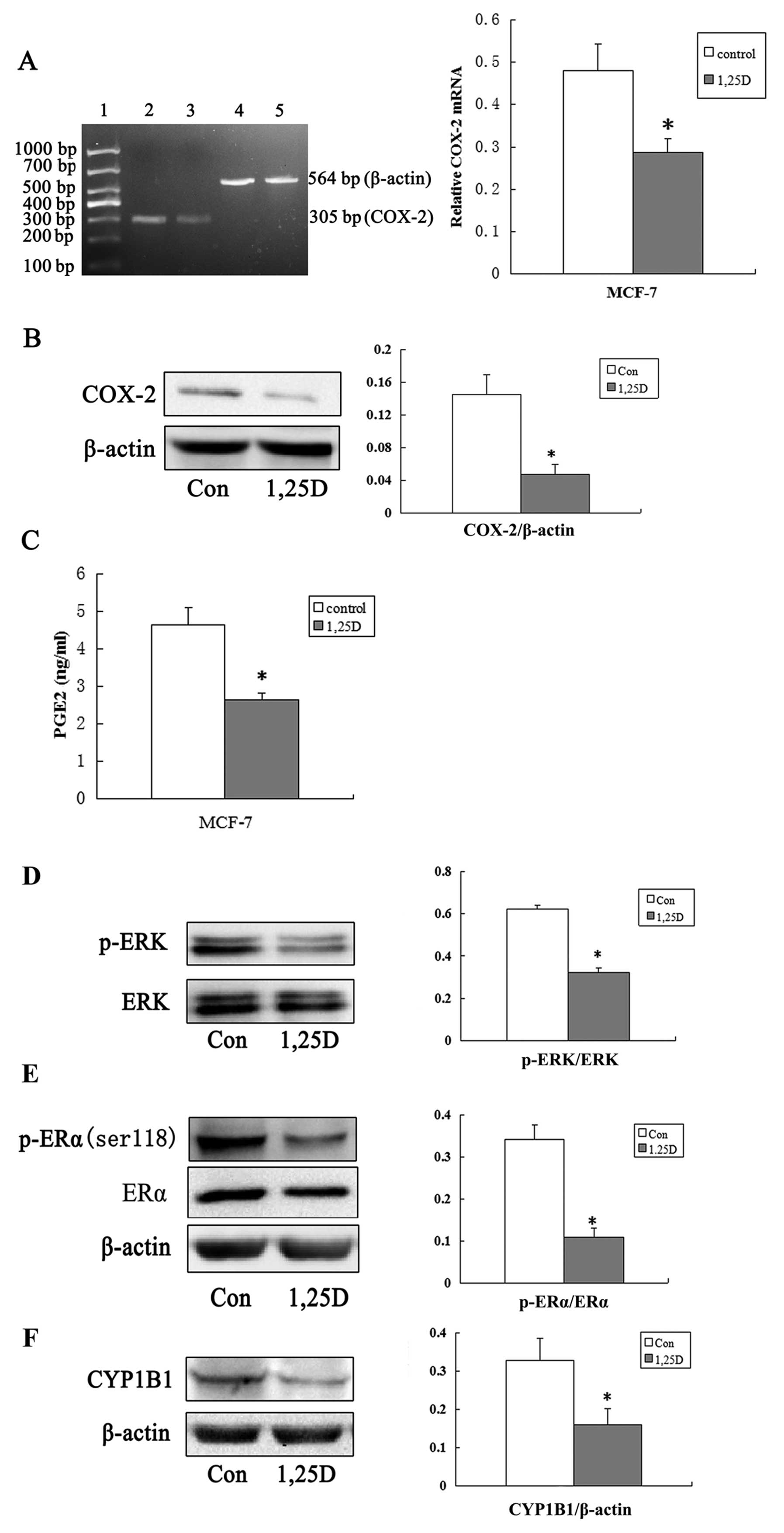 | Figure 3Interference with the COX-2/PGE2
pathway molecules and downregulation of CYP1B1 by
1,25(OH)2D3. (A) The
1,25(OH)2D3 decreases COX-2 mRNA levels in
MCF-7 cells. MCF-7 cells were treated with
1,25(OH)2D3 as described in Fig. 2A and COX-2 mRNA levels were
determined. (*P<0.05). Expression levels of COX-2 in
control (lane 2) and 1,25(OH)2D3 group (lane
3) were measured using RT-PCR. β-actin levels were used as internal
positive controls (lanes 4 and 5). Lane 1 is DNA marker ladder. (B)
1,25(OH)2D3 decreases COX-2 protein levels.
Western blot analysis showing COX-2 expression in MCF-7 cells
treated with 100 nmol/l 1,25(OH)2D3 (1,25D)
or vehicle (control) for 72 h. β-actin was used to normalise
relative expressions among groups. The y-axis represents the
relative protein expression level (ratio of protein/β-actin)
(*P<0.05). (C) 1,25(OH)2D3
decreases PGE2 levels in MCF7 cells. MCF-7 cells were treated with
1,25(OH)2D3 as described in Fig. 2A. PGE2 levels in medium were
measured (*P<0.05). (D–F) Effect of
1,25(OH)2D3 on the levels of phosphorylated
ERK, phosphorylated p-ERα and CYP1B1 proteins in MCF-7 cells. Cells
were treated with 100 nmol/l 1,25(OH)2D3 for
72 h. Cells were lysed and immunoblotted. The blot was probed with
β-actin antibody for normalization and with (D)anti-ERK and
anti-phospho-ERK antibodies, (E) anti-phospho-ERα-ser118 antibody
and (F) anti-CYP1B1 antibody. Each blot representative of three
independent experiments. *P<0.05 significant
difference between control and 1,25(OH)2D3
group. |
1,25(OH)2D3 reduces
CYP1B1 expression through decreased PGE2-induced ERK and ERα
activation
Based on the previous demonstration, PGE2-induced
CYP1B1 expression is mediated by PGE2-induced phosphorylation of
the ERα at serine residues 118 via the activation of ERK signaling
pathway (6). MCF-7 cells were
treated with 100 nmol/l 1,25(OH)2D3 for 72 h
and 1,25(OH)2D3 decreased CYP1B1 protein
levels (Fig. 3F). To evaluate the
upstream signal for CYP1B1 expression after
1,25(OH)2D3 treatment, we examined the
activation of ERK kinases and phosphorylated ERα(ser118) in MCF-7
cells. Western blot assay showed that as
1,25(OH)2D3-reduced PGE2 levels,
phosphorylated ERK (Fig. 3D) and
phosphorylated ERα(ser118) (Fig.
3E) decreased. Similar results were observed through
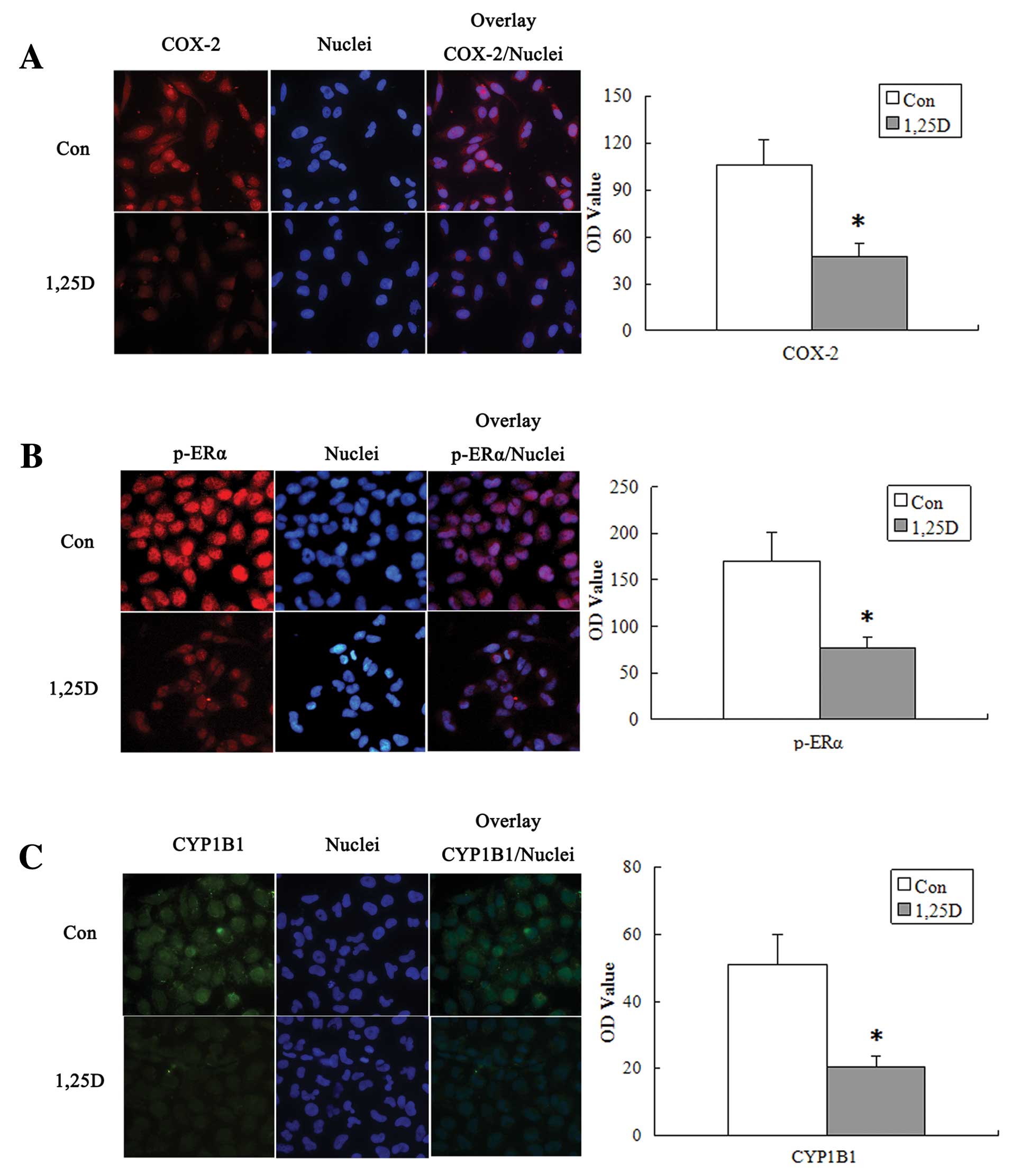
immunofluorescence (Fig. 4).
Discussion
Inflammation is one of the hallmarks of a tumor
(8). COX-2 is a key initiation
factor in inflammatory response, stimulated to expression by
inflammatory cytokines, growth factors, oncogenes and other
stimuli, and plays an pivotal role in inflammation response and
tumor development. Elevated COX-2 expression in breast cancer is
associated with higher histological grade, much greater
angiogenesis, increased invasion and metastasis, and decreased
overall survival time (9). This
phenomenon is mainly considered to be mediated via PGE2, the
predominant product of COX-2. High expression of COX-2/PGE2 in
breast cancer has been shown to correlate with the expression of
enzymes such as aromatase and 17β-hydroxysteroid dehydrogenase
(17β-HSD), the estrogen synthetases in human breast cancer
(10), consequently resulted in
high levels of estrogen in breast cancer and improved the risk of
this disease. Recently, Han et al (6) found that in the estrogen receptor
(ER)-positive breast cancer cells, estrogen α receptor (ERα) was
phosphorylated by PGE2 in mutiple loci through activated multiple
signaling pathways, and then phosphorylated ERα (p-ERα) combined
with the estrogen receptor response element (ERE) on CYP1B1
promoter for transcriptional activation the expression of CYP1B1.
In our study, serine residue 118, a vital phosphorylated sites on
ERα, was used as a target for detection of p-ERα. Both MCF-7 cell
line and tumor samples were analyzed. We found that all of COX-2,
p-ERα and CYP1B1 were expressed in vivo and in vitro.
There were significant positive correlation between any two of them
in human breast cancer tissues, displaying that the mechanism of
COX-2/PGE2 pathway regulate CYP1B1 expression may exist in
vivo. We verified this mechanism in breast cancer cell line
MCF-7. COX-2/PGE2 pathway that regulate estrogen-metabolizing
enzyme CYP1B1 expression level influences the carcinogenic effects
of estrogen, possibly playing a considerable role in breast cancer
tumorigenesis.
Vitamin D is a steroid hormone mediating biological
effect via its active metabolite form of 1,25-dihydroxyvitamin
D3 [1,25(OH)2D3] in the body.
1,25(OH)2D3 is the active metabolite form of
vitamin D. 1,25(OH)2D3 has anti-inflammatory
and anticancer effect, which is closely related to its inhibition
function in COX-2/PGE2 pathway. Krishnan et al have reported
that in human prostate cancer cells (11) and breast cancer cells (12), 1,25(OH)2D3
downregulated COX-2 both in transcription and translation levels,
accompanied with the decline of PGE2 levels, followed by
inactivation of downstream signaling cascade reaction by PGE2
(13). Consistent with the previous
report, our experiments demonstrated that
1,25(OH)2D3 downregulated the COX-2 both in
gene and protein levels, therewith the decline of PGE2 levels in
MCF-7 cell line. Further more, MTT assay confirmed that, within the
1–100 nmol/l range, 1,25(OH)2D3 inhibited
MCF-7 cell proliferation in an time-and dose-dependent manner,
while arrested the cell cycle in G0/G1 phase.
PGE2 is an inflammatory factor secreted in autocrine
or paracrin manner, contributing to stimulation of cancer cell
growth through a number of distinct ways. It stimulates cell
proliferation through G protein-coupled pathway; activating the
nuclear peroxisome proliferator-activated receptor (PPAR);
activating several proliferation stimulation signaling pathways
such as RAS/MAPK and PI3K/AKT (14). Among those pathways, ERK activation
pathway in MAPK family is the most representative and
indispensable. We found that in MCF-7 cells, when treated with
1,25(OH)2D3, there was a significant
reduction on PGE2 level as well as phosphorylated ERK, indicating
that 1,25(OH)2D3 downregulated the activity
of MAPK pathway. ERα is a transcription factor, which facilitates
its function by phosphorylating the serine residues in the
N-terminal region. The phosphorylation can be either
estrogen-dependent or estrogen-independent. In the absence of
estrogen, three serine residues in the N-terminal region of ERα can
be phosphorylated: 118, by ERK (15); 167, by AKT (16); and 305, by PKA (17). To further investigate whether
1,25(OH)2D3 downregulates the phosphorylation
of ERα at Ser118 [p-ERα(ser118)] via ERK pathway, MCF-7 cell line
was selected for its high expression of ERα; estrogen-free serum
and phenol red-free medium was chosen to exclude the estrogen and
estrogen-like activity of phenol on the phosphorylation effect of
ERα. Results show that, 1,25(OH)2D3 inhibited
the expression of PGE2 and its downstream ERK pathway, then reduced
the protein level of p-ERα(ser118). Thus,
1,25(OH)2D3 interfered with COX-2/PGE2
pathway reduced ERK activity, and then downregulated the
transcription factor activity of ERα, leading to the negative
adjustment of CYP1B1 expression.
CYP1B1, a member of the cytochrome P450 enzyme
family, an extrahepatic enzyme in tumor initiation, promotion and
drug resistance. It catalyzes the hydroxylation of estradiol in the
C4 site, then the metabolites 4-hydroxy-estradiol (4-OH-E2) is
futher oxidized into estradiol-3,4-quinone (E2–3,4-Q). E2–3,4-Q can
reacted with the purine on DNA forming apurinic adducts, finally
leading to DNA mutations (18). The
oxygen free radicals, which were generated from the process of
4-OH-E2 oxidated into estradiol-3,4-quinone, could cause DNA
damage. CYP1B1 itself can activate other carcinogens and metabolize
a number of anticancer drugs, which lead to the development of drug
resistance in tumors (19). CYP1B1
gene expression is mainly through activation of the phosphorylation
of ERα (20). As CYP1B1 is
important in tumor initiation and progression, and highly expressed
on numerous tumors, thus, it is a good target for cancer
prevention, diagnosis and treatment. Our research suggested that,
in estrogen receptor-positive breast cancer cells, COX-2/PGE2
pathway may play a vital role on the control of CYP1B1 expression.
1,25(OH)2D3 interferes with the COX-2/PGE2
pathway, inhibites the activity levels of the phosphorylation of
ERK and ERα, then downregulates the expression of CYP1B1,
consequently inhibiting the proliferation of breast cancer cells.
1,25(OH)2D3 restrained the expression of
CYP1B1, which is significant for its anti-breast cancer
effects.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Bertone-Johnson ER: Vitamin D and breast
cancer. Ann Epidemiol. 19:462–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bertone-Johnson ER, Chen WY, Holick MF, et
al: Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk
of breast cancer. Cancer Epidemiol Biomarkers Prev. 14:1991–1997.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krishnan AV, Swami S and Feldman D:
Vitamin D and breast cancer: inhibition of estrogen synthesis and
signaling. J Steroid Biochem Mol Biol. 121:343–348. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Subbaramaiah K, Hudis C, Chang SH, Hla T
and Dannenberg AJ: EP2 and EP4 receptors regulate aromatase
expression in human adipocytes and breast cancer cells. Evidence of
a BRCA1 and p300 exchange. J Biol Chem. 283:3433–3444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han EH, Kim HG, Hwang YP, Song GY and
Jeong HG: Prostaglandin E2 induces CYP1B1 expression via
ligand-independent activation of the ERα pathway in human breast
cancer cells. Toxicol Sci. 114:204–216. 2010.PubMed/NCBI
|
|
7
|
Han D, Denison MS, Tachibana H and Yamada
K: Effects of estrogenic compounds on immunoglobulin production by
mouse splenocytes. Biol Pharm Bull. 25:1263–1267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ristimaki A, Sivula A, Lundin J, et al:
Prognostic significance of elevated cyclooxygenase-2 expression in
breast cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
10
|
Gunnarsson C, Jansson A, Holmlund B, et
al: Expression of COX-2 and steroid converting enzymes in breast
cancer. Oncol Rep. 16:219–224. 2006.PubMed/NCBI
|
|
11
|
Krishnan AV, Shinghal R, Raghavachari N,
Brooks JD, Peehl DM and Feldman D: Analysis of vitamin D-regulated
gene expression in LNCaP human prostate cancer cells using cDNA
microarrays. Prostate. 59:243–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krishnan AV, Swami S, Peng L, Wang J,
Moreno J and Feldman D: Tissue-selective regulation of aromatase
expression by calcitriol: implications for breast cancer therapy.
Endocrinology. 151:32–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moreno J, Krishnan AV, Swami S, Nonn L,
Peehl DM and Feldman D: Regulation of prostaglandin metabolism by
calcitriol attenuates growth stimulation in prostate cancer cells.
Cancer Res. 65:7917–7925. 2005.PubMed/NCBI
|
|
14
|
Greenhough A, Smartt HJ, Moore AE, et al:
The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and
adaptation to the tumour microenvironment. Carcinogenesis.
30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kushner PJ, Agard DA, Greene GL, et al:
Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol.
74:311–317. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng W, Webb P, Nguyen P, et al:
Potentiation of estrogen receptor activation function 1 (AF-1) by
Src/JNK through a serine 118-independent pathway. Mol Endocrinol.
15:32–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen D, Pace PE, Coombes RC and Ali S:
Phosphorylation of human estrogen receptor α by protein kinase A
regulates dimerization. Mol Cell Biol. 19:1002–1015. 1999.
|
|
18
|
Cavalieri E, Chakravarti D, Guttenplan J,
et al: Catechol estrogen quinones as initiators of breast and other
human cancers: implications for biomarkers of susceptibility and
cancer prevention. Biochim Biophys Acta. 1766:63–78.
2006.PubMed/NCBI
|
|
19
|
Bruno RD and Njar VC: Targeting cytochrome
P450 enzymes: a new approach in anti-cancer drug development.
Bioorg Med Chem. 15:5047–5060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuchiya Y, Nakajima M, Kyo S, Kanaya T,
Inoue M and Yokoi T: Human CYP1B1 is regulated by estradiol via
estrogen receptor. Cancer Res. 64:3119–3125. 2004. View Article : Google Scholar : PubMed/NCBI
|