Introduction
Osteosarcoma is the most prevalent primary bone
malignancy, and incidence peaks during the adolescent growth spurt
(1). Numerous studies have sought
to improve our understanding of osteosarcoma, and epidemiological
studies have reported significant findings, such as the effects of
puberty, disorders of bone growth and remodeling, and genetic
predisposition (2–4). However, as with several types of
cancer, the etiology of osteosarcoma remains unclear. Although
neoadjuvant chemotherapy and limb salvage surgery greatly improve
long-term survival and quality of life, recurrence and metastasis
remain major challenges for clinicians.
Spindle assembly checkpoint provides a surveillance
mechanism responsible for the fidelity of mitotic chromosome
segregation (5,6); it inhibits the onset of premature
anaphase until all chromosomes are properly attached to the mitotic
spindle apparatus and located at the metaphase plate, and defects
in this process contribute to chromosomal instability and
aneuploidy (5,7,8).
Mad2 is a key component in the spindle assembly
checkpoint (9). Our previous study
demonstrated that Mad2 was overexpressed in human osteosarcoma
tissue, and that increased expression of Mad2 was associated with
early metastasis and poor survival (10). The findings of several reports are
in accordance with our studies in many different malignant tumors
(8,11–13).
The exact relationship between Mad2 expression and clinical outcome
remains unclear.
In this study, we found that Mad2 overexpression
conferred osteosarcoma cells with an enhanced ability to cause
early dyscrasia, promote pulmonary metastasis and decrease
survival. The underlying mechanisms involve an increased
invasiveness and enhanced cancer stem cell-like properties.
Materials and methods
Chemicals and reagents
The following antibodies for immunoblot and
immunohistochemistry analysis were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA): Nanog, POU5F1, Sox2,
ABCG2 and β-actin. We obtained primary antibody of Mad2, CXCR4,
MMP-1 from Abcam (Cambridge, MA, USA), and the horseradish
peroxidase-labeled goat anti-rabbit IgG (H+L) were obtained from
Cell Signaling Technology (Danvers, MA, USA). Anti-Stro-1 and CD117
primary antibodies used for FACScan analysis of cell surface
expression were supplied by Becton-Dickinson Pharmingen (San Diego,
CA, USA). B-27 serum-free supplement (50X) was obtained from Gibco
(Grand Island, NY, USA). Recombinant EGF and bFGF were purchased
from PeproTech (Rocky Hill, NJ, USA).
Cell culture
The human osteosarcoma cell line MNNG/HOS was
purchased from the Shanghai Institute for Biological Sciences of
the Chinese Academy of Sciences. All the cells were cultured in
RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10%
(vol/vol) fetal bovine serum (FBS) (Invitrogen) and 1% (vol/vol)
penicillin-streptomycin (Invitrogen). Cells were propagated in a
humidified environment at 37°C with 5% CO2 and 100%
humidity. Primary osteosarcoma cells were isolated from the
xenotransplanted mice at the end of the observation point. In
brief, tumor tissues were cut into small pieces and digested with
0.25% trypsin and 0.1% type II collagenase for 30 min and 2 h,
respectively. The released cells were cultured in RPMI-1640 medium
supplemented with 10% FBS and antibiotics. Cell viability was
determined using trypan blue stain.
Adenoviral vector construction and
infection
The AdV-Mad2-GFP was constructed by Shanghai
GeneChem Co., Ltd. Titers of viral particles were determined
according to Darling et al (14). For all experiments, cells were
infected for 72 h at 37°C in 5% CO2 using a multiplicity
of infection (MOI) of 10 before subsequent detection. AdV-GFP
infection was set as vehicle control, and a pseudo-infection acted
as blank control.
Sarcosphere formation assay
The sphere formation assay followed procedures
previously described (15). Fresh
aliquots of EGF and bFGF were added every other day. After
culturing for 14 days, colonies containing >50 cells were
regarded as sarcosphere and the spheres were processed to form the
next generation of spheres every 14 days for three more times.
Proliferation and drug toxicity
assay
For proliferation detection, cells were seeded at
1×104 cells/well in 96-well plates for 2, 4, 6 and 8
days and the cells were then harvested and counted. For drug
resistance assay, cells were cultured (5×104 per well)
in 96-well plates for 1 day, treated with increasing concentrations
of doxorubicin and methotrexate for 24 h and then sent for MTT
assay. MTT assay was performed according to the manufacturer’s
recommendations (Roche Diagnostics GmbH, Mannheim, Germany).
Optical intensities were read on a multi-well scanning
spectrophotometer at OD492 (Molecular Devices, Sunnyvale, CA, USA).
All the experiments were repeated three times.
Transwell assay
Cell invasion assay was performed in dual-chambered
invasion plates (BD Biosciences, San Jose, CA, USA) as previously
described. The polyethylene terephthalate membranes had an 8-μm
pore size and were coated with a layer of reconstituted
extracellular matrix Matrigel. Assayed cells were placed in the
upper chamber (1×105 cells/well) in serum-free RPMI. The
lower chambers were filled with RPMI medium with 10% FBS. At
termination of the assay (24 h), the inserts were removed and the
inner side was wiped with cotton swabs. The filters were stained
with Harris’s hematoxylin solution (Sigma-Aldrich, Inc., St. Louis,
MO, USA) and were peeled off after washing and mounted on the
slides. The migrated cells were counted under a light
microscope.
Animals and orthotopic transplantation
assay
To determine tumorigenicity and to establish
orthotopic osteosarcoma animal models, 24 male BALB/C nude mice
~4–6 weeks old were purchased from and maintained at the Wuhan
University Center for Animal Experiment. The care and use of
animals were in accordance with the recommendations and guidelines
of the National Institutes of Health and were reviewed and approved
by the Institutional Animal Care and Use Committee (IACUC)
(approval number: 2011006). The mice were randomly divided into
AdV-Mad2-GFP and AdV-GFP groups according to their injected cells,
with 12 mice in each group. For orthotopic injections, cells
(either transfected with AdV-Mad2-GFP or AdV-GFP) in log-phase
growth were harvested, washed and resuspended with
phosphate-buffered saline (PBS). BALB/C nude mice were anesthetized
and cells (5×105 cells in 0.1 ml PBS) were then injected
into both distal femoral bone marrow cavities of each mouse. Mice
were monitored daily until they reached the humane endpoint
criteria. The criteria were defined as two months after injection
or hunched abnormal posture for >48 h. Once the criteria were
met, the mice were euthanized, and a section of the xenografted
osteosarcoma tissue was obtained for primary cell culture; a second
section was sent for pathological examination, and a third was sent
for total-RNA and protein isolation for further molecular
biological manipulation.
Western blots
For immunoblot analysis, cells were first infected
with either empty AdV-GFP adenovirus or AdV-Mad2-GFP as described
above; a pseudo-transfection group was set as blank control.
Protein was extracted from subconfluent cultures using lysis buffer
containing 1 mM PMSF (Sigma-Aldrich, Inc.) and quantified using the
BCA methods. Aliquots of 40 μg protein from each sample were then
resolved using SDS-PAGE gels and subsequently transferred to PVDF
membranes. Membranes were blocked in 5% milk solution, incubated
with primary antibody at 4°C, overnight. They were then washed and
incubated with horseradish peroxidase-conjugated secondary
antibody. The immunoreactivity was detected by chemiluminescence.
Statistical analyses of western blotting data were performed on the
densitometric values obtained with NIH Image 1.61 software.
Immunohistochemistry
All tissues were subjected to routine pathological
examination; in brief, the specimens were fixed and embedded.
Serial sections (5 μm) were cut and mounted on silane-coated glass
slides. The sections were deparaffinized and rehydrated, then
antigen retrieval was performed by boiling the slides in 10 mM
citrate buffer (pH 6.0) in a microwave oven for 10 min. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 30
min. The slides were incubated in a humid chamber with respective
primary antibodies, at 4°C, overnight and were then incubated for
45 min at 37°C with horseradish peroxidase-conjugated secondary
antibody. The slides were then developed using a DAB color kit
(ProteinTech Group), counterstained with hematoxylin, and
dehydrated. Primary antibodies were substituted with PBS as
negative controls.
FACScan analysis of Stro-1 and CD117
expression
For analysis of cell surface markers, subconfluent
cultures were infected as described above for 72 h. The cells were
harvested with fresh 0.25% trypsin solution (Sigma-Aldrich, Inc.)
and then resuspended in PBS/0.5% normal rabbit serum
(Sigma-Aldrich, Inc.), and blocked on ice for 15 min. Cells were
subsequently labeled with anti-Sto-1 and/or anti-CD117 for 60 min
and maintained on ice until analysis. The expression was assessed
by flow cytometry using a Becton-Dickinson FACSort (San Jose, CA,
USA), and data were analyzed using WinMDI software (Scripps
Research Institute, La Jolla, CA, USA).
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analysis was performed by analysis of variance or Student’s t-test
using the SPSS13.0 statistical program with significance at
P<0.05. Survival was calculated by the Kaplan-Meier method, and
the Log-rank test was performed with significance at P<0.05.
Results
Mad2 upregulation promotes early
dyscrasia and decreases survival
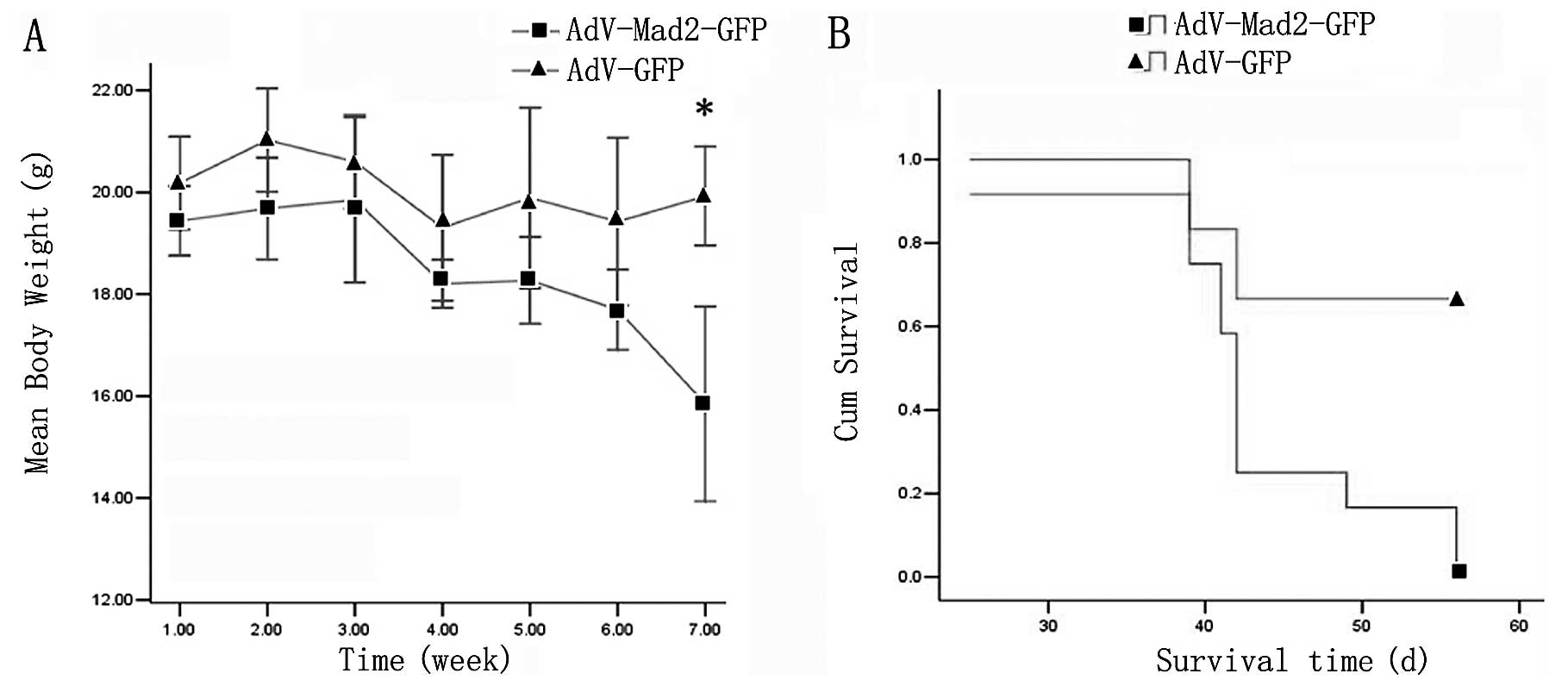
We found that the AdV-Mad2-GFP group exhibited early
dyscrasia. The average body weight was 15.6±1.08 g for the
AdV-Mad2-GFP xenografted group, while it was ~20±2.52 g for the
control group. We found that the AdV-Mad2-GFP xenografted group
also exhibited early death; most of the mice from the AdV-Mad2-GFP
xenograft group died at approximately the sixth week
post-injection, whereas the mock group exhibited a higher survival
rate at the terminal observation point (Fig. 1A and B).
Transient Mad2 overexpression is
associated with increased invasiveness and enhanced pulmonary
metastasis
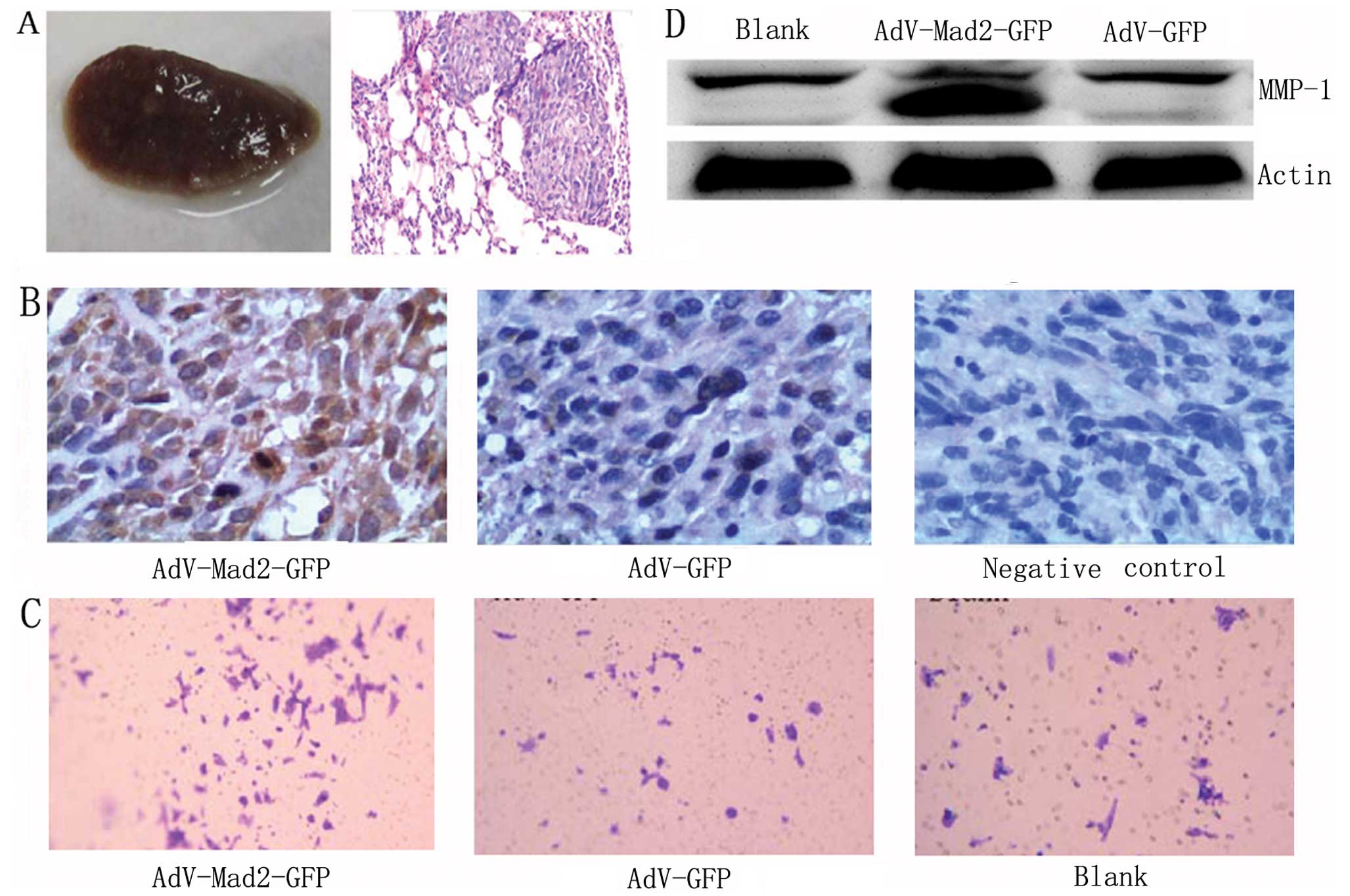
We observed an enhanced ability toward pulmonary
metastasis in the AdV-Mad2-GFP xenograft group compared with the
mock transfection group (Fig. 2A).
In the mouse model, the CXCR4 expression was also upregulated in
the primary tumor tissues from the AdV-Mad2-GFP xenograft group
compared with the mock group (Fig.
2B). We further investigated whether Mad2 overexpression
promoted invasiveness and found that AdV-Mad2-GFP cells exhibited
an increased ability to invade following transient infection using
the in vitro transwell assay (Fig. 2C). Moreover, we found that MMP-1 was
markedly upregulated in the AdV-Mad2-GFP group following transient
infection (Fig. 2D).
Mad2 upregulation promotes stem
cell-related properties
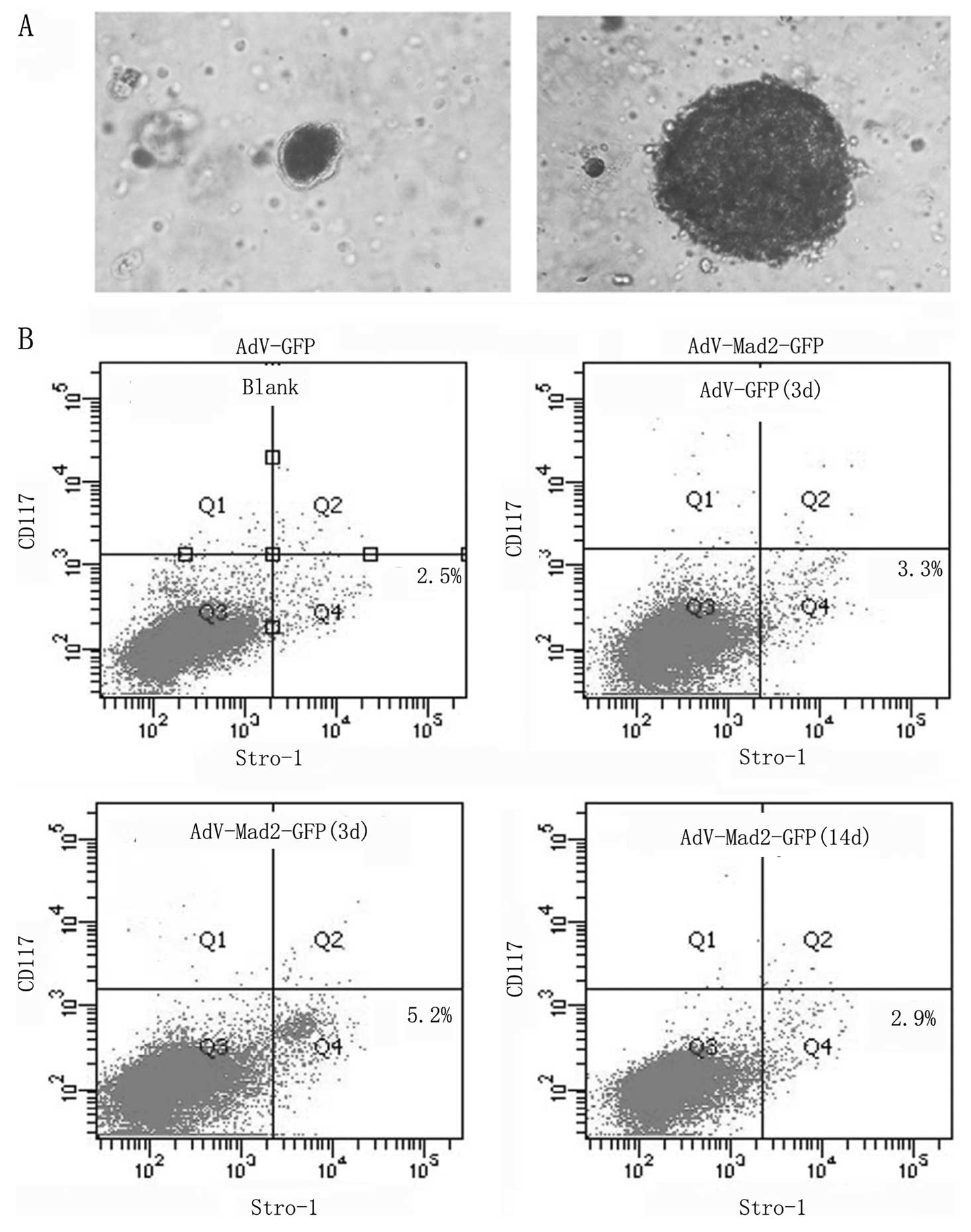
After 14 days of culture in sphere-specific
conditions, the AdV-Mad2-GFP group exhibited an enhanced ability to
form spheres compared with the other two groups (Fig. 3A). The spherical cells were
collected and subjected to the culture conditions above for three
more generations, which confirmed the ability of these cells to
self-renew. Stro-1 and CD117 were successfully used to identify
osteosarcoma stem cells. Flow cytometry revealed that the
AdV-Mad2-GFP group demonstrated an increased number of Stro-1
positive cells, whereas no effect was observed regarding CD117. The
Stro-1-positive cells diminished 14 days after infection (Fig. 3B). In the present study, we did not
find any significant change in the expression of stem cell-related
genes among different infection groups using both qRT-PCR and
western blot assay (data not shown).
Mad2 upregulation has no effect on
osteosarcoma proliferation and drug resistance
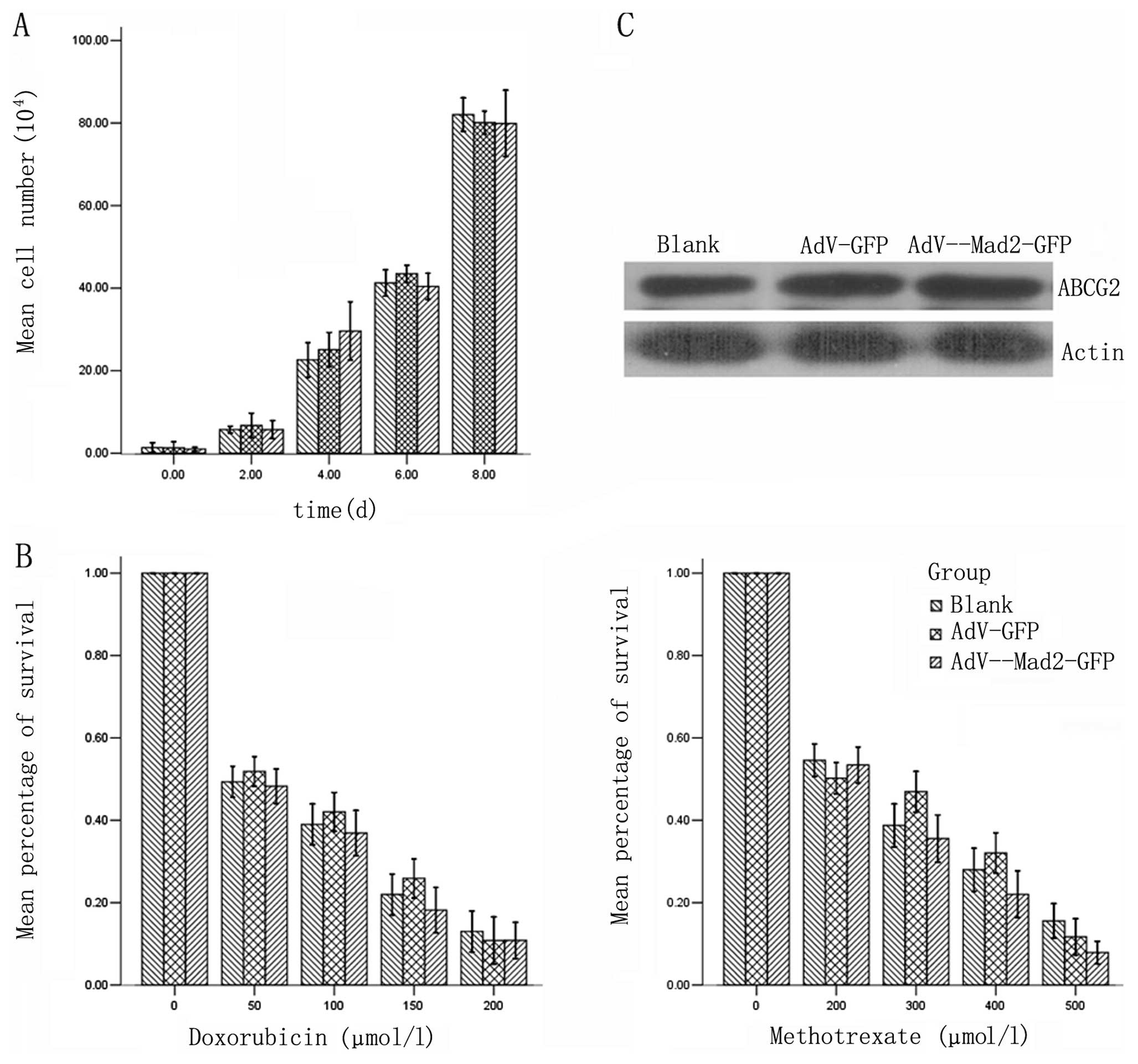
We examined the proliferation potential among the
different experimental groups, and found no significant differences
(Fig. 4A). The similarity among the
volumes and weights of the primary tumors in the different
xenograft groups also confirmed our findings (Table I). We further investigated the
drug-resistant properties and we did not find any significant
differences following transient infection. The expression of ABCG2
also showed no significant differences (Fig. 4B and C).
 | Table IVolume and weight of primary tumors in
xenotransplantation models. |
Table I
Volume and weight of primary tumors in
xenotransplantation models.
| Volume
(cm3) | Weight (g) |
|---|
|
|
|
|---|
| Group | Mean ± SD | P-value | Mean ± SD | P-value |
|---|
| AdV-GFP | 1.792±0.273 | 0.082 | 2.628±0.450 | 0.076 |
| AdV-Mad2-GFP | 1.656±0.262 | | 2.391±0.456 | |
Discussion
In order to upregulate Mad2 expression, we first
examined the basal expression levels of Mad2 in osteosarcoma cell
lines, and found that HOS/MNNG exhibited relatively low Mad2
expression and was tumorigenic, thus, it was suitable for our study
(data not shown). We transfected the osteosarcoma cell line
HOS/MNNG with AdV-Mad2-GFP and AdV-GFP, respectively. We used
different concentrations of virus for infection, and found that
transfection can be achieved with both low toxicity and high
efficiency when using 10 MOI. The transfection efficacy was
determined by fluorescence microscopy and flow cytometry three days
post-infection, and the Mad2 expression was clearly elevated in the
AdV-Mad2-GFP group compared with the other two groups (data not
shown).
Mad2 is aberrantly expressed in many malignant types
of cancer, such as gastric, colon, and lung cancer (12,13,16).
Our previous study demonstrated that Mad2 is overexpressed in human
osteosarcoma and that increased expression of Mad2 correlated with
a poorer clinical outcome. The transgenic animal model established
by Sotillo et al was not tissue specific, therefore, they
did not report any tumor primarily derived from bone (8). We utilized an orthotopic
xenotransplanted mouse osteosarcoma model to detect whether Mad2
overexpression promotes osteosarcoma carcinogenesis. The mice were
divided into two groups according to the different cells
transplanted. In this study, we also found that Mad2 upregulation
promoted early dyscrasia, enhanced pulmonary metastasis and
decreased survival using a xenograft mouse model.
We found that Mad2 overexpression caused immediately
enhanced invasive abilities, which we observed by the transwell
invasion assay and MMP-1 expression levels following transient
infection. We also found that, in the xenotransplantation model,
CXCR4 was elevated in the tissue of the AdV-Mad2-GFP xenograft
group compared with the control group. These results strongly
support that Mad2 overexpression is involved in regulating invasion
and metastasis.
Sotillo et al found that transgenic mice
exhibiting Mad2 overexpression are more susceptible to tumor
formation, accompanied by increased chromosome instability.
Moreover, they reported that continued overexpression of Mad2 is
not required for tumor maintenance, and overexpression of Mad2 in
mice does not affect regression of Kras-driven lung tumors when
Kras is inhibited; however, tumors that experienced transient Mad2
overexpression recurred at markedly elevated rates (8,17). The
above studies indicated that Mad2 is unlikely to act as an
oncogene, thus, we further investigated the cancer stem cell
hypothesis. Cancer stem cells possess the ability to self-renew,
which is manifested by the serial formation of spheres in
anchorage-independent serum free conditions. In our study, this
property was promoted in the AdV-Mad2-GFP infection group.
Cancer stem cells share many cell surface markers
with normal stem cells. Since Stro-1 is a traditional marker used
to identify mesenchymal stem cells, it may be able to discriminate
osteosarcoma stem cells from non-stem like cancer cells. Adhikari
et al successfully isolated osteosarcoma stem cells that
were Stro-1 and CD117 double positive (18). In our study, we found an increased
percentage of Stro-1-positive cells in the AdV-Mad2-GFP infected
group; however, we did not observe any significant changes in CD117
expression, for a reason that remains to be elucidated. We further
detected the expression of many stem cell-related genes including
Nanog, POU5F1 and Sox2; however, we did not find any significant
changes (data not shown). We hypothesized that this is due to the
relatively smaller portion of cancer stem cells, and western blot
analysis is not an ideal technology under these conditions. Cancer
stem cells possess the ability to differentiate. In the present
study, we detected the population of Stro-1-positive cells 14 days
after infection, and the percentage decreased to a level similar to
the control group; since the adenovirus was not likely to recombine
into the host genome, the effect gradually diminished, suggesting
that Stro-1 positive cells again differentiated into
Stro-1-negative cells when Mad2 decreased.
The role of Mad2 under normal conditions is to
arrest mitosis in metaphase when abnormal chromosome aggregation
occurs. We investigated whether Mad2 overexpression inhibits the
cell cycle and therefore affects the potential for proliferation,
and we found that there was no significant difference in the
proliferation curve among the different groups. The in vivo
transplantation study demonstrated that primary tumor volume and/or
weight exhibited no difference between the two groups; this finding
also supports the possibility that Mad2 expression does not impact
proliferation.
To investigate the drug-resistant properties
further, cells were exposed to methotrexate and doxorubicin, two
widely-used chemotherapy agents for osteosarcoma treatment. We
found no significant difference after transient infection using MTT
assay. ABCG2 is closely related to drug resistance, however, there
was no evident difference between the different transfection
groups.
Carcinogens not only induce gene mutations but also
cause aneuploid lesions. Furthermore, normal cells exposed to
chemical carcinogens can potentially become aneuploid (19,20),
and may become cancerous following the introduction of certain
chromosomes (21,22). However, only a fraction of these
cells give rise to cancer, thus, it is possible that chromosomal
derangements are important initial stages of tumor development, and
may be involved in the advancement of cancer. The origin of cancer
stem cells remains under investigation, and many researchers have
demonstrated that the transformation of tissue by stem cells leads
to cancer stem cell generation. In our study, we hypothesized that
chromosome instability is a bridge between Mad2 overexpression and
osteosarcoma stem cells, and this is in accordance with previous
reports that indicated that genomic instability is another
potential mechanism resulting in cancer stem cells (23).
In conclusion, our findings demonstrated that Mad2
overexpression exhibited a strong tendency toward metastasis and
poor survival in a transplantation model. In the in vitro
assay, we found that Mad2 upregulation closely correlated with
invasiveness and stem cell-like properties, however, further
investigation into the precise mechanism is warranted.
Acknowledgements
This study was supported in part by Grants from the
Hubei Natural Science Foundation of China (302131702), and the
Fundamental Research Funds for the Central Universities (no.
201130202020002).
Abbreviations:
|
Mad2
|
mitotic arrest defective protein 2
|
|
PI
|
propidium iodide
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savage SA and Mirabello L: Using
epidemiology and genomics to understand osteosarcoma etiology.
Sarcoma. 2011:5481512011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottaviani G and Jaffe N: The etiology of
osteosarcoma. Cancer Treat Res. 152:15–32. 2009. View Article : Google Scholar
|
|
4
|
Pritchard DJ, Finkel MP and Reilly CA Jr:
The etiology of osteosarcoma. A review of current considerations.
Clin Orthop Relat Res. 14–22. 1975. View Article : Google Scholar
|
|
5
|
Dobles M and Sorger PK: Mitotic
checkpoints, genetic instability, and cancer. Cold Spring Harb Symp
Quant Biol. 65:361–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silva P, Barbosa J, Nascimento AV, Faria
J, Reis R and Bousbaa H: Monitoring the fidelity of mitotic
chromosome segregation by the spindle assembly checkpoint. Cell
Prolif. 44:391–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weaver BA and Cleveland DW: Decoding the
links between mitosis, cancer, and chemotherapy: The mitotic
checkpoint, adaptation, and cell death. Cancer Cell. 8:7–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sotillo R, Hernando E, Diaz-Rodriguez E,
et al: Mad2 overexpression promotes aneuploidy and tumorigenesis in
mice. Cancer Cell. 11:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eytan E, Braunstein I, Ganoth D, et al:
Two different mitotic checkpoint inhibitors of the
anaphase-promoting complex/cyclosome antagonize the action of the
activator Cdc20. Proc Natl Acad Sci USA. 105:9181–9185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu L, Guo WC, Zhao SH, Tang J and Chen JL:
Mitotic arrest defective protein 2 expression abnormality and its
clinicopathologic significance in human osteosarcoma. APMIS.
118:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hisaoka M, Matsuyama A and Hashimoto H:
Aberrant MAD2 expression in soft-tissue sarcoma. Pathol Int.
58:329–333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka K, Nishioka J, Kato K, et al:
Mitotic checkpoint protein hsMAD2 as a marker predicting liver
metastasis of human gastric cancers. Jpn J Cancer Res. 92:952–958.
2001. View Article : Google Scholar
|
|
13
|
Wang L, Yin F, Du Y, et al: MAD2 as a key
component of mitotic checkpoint: A probable prognostic factor for
gastric cancer. Am J Clin Pathol. 131:793–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darling AJ, Boose JA and Spaltro J: Virus
assay methods: accuracy and validation. Biologicals. 26:105–110.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato T, Daigo Y, Aragaki M, et al:
Overexpression of MAD2 predicts clinical outcome in primary lung
cancer patients. Lung Cancer. 74:124–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sotillo R, Schvartzman JM, Socci ND and
Benezra R: Mad2-induced chromosome instability leads to lung tumour
relapse after oncogene withdrawal. Nature. 464:436–440. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adhikari AS, Agarwal N, Wood BM, et al:
CD117 and Stro-1 identify osteosarcoma tumor-initiating cells
associated with metastasis and drug resistance. Cancer Res.
70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duesberg P: Does aneuploidy or mutation
start cancer? Science. 307:412005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duesberg P, Fabarius A and Hehlmann R:
Aneuploidy, the primary cause of the multilateral genomic
instability of neoplastic and preneoplastic cells. IUBMB Life.
56:65–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bachoo RM, Maher EA, Ligon KL, et al:
Epidermal growth factor receptor and Ink4a/Arf: convergent
mechanisms governing terminal differentiation and transformation
along the neural stem cell to astrocyte axis. Cancer Cell.
1:269–277. 2002. View Article : Google Scholar
|
|
22
|
Ilyas M, Straub J, Tomlinson IP and Bodmer
WF: Genetic pathways in colorectal and other cancers. Eur J Cancer.
35:335–351. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang Y, Zhong Z, Huang Y, et al:
Stem-like cancer cells are inducible by increasing genomic
instability in cancer cells. J Biol Chem. 285:4931–4940. 2010.
View Article : Google Scholar : PubMed/NCBI
|