Introduction
Pancreatic cancer is known for its high mortality
rate. It is one of the most common causes of cancer mortality in
developed countries (1). Due to the
lack of effective-specific diagnosis methods, 26% of patients are
in the middle-late stage when being diagnosed and only <10% of
patients has a surgery opportunity, and even if the patients have
the operation, they still need a series of comprehensive therapies,
including chemotherapy (2). In the
past decades, gemcitabine was prefered as the first-line drug for
pancreatic cancer chemotherapy, but the benefits were very limited,
so that new chemotherapy regimens for pancreatic cancer were
explored and tested. But new chemotherapy results did not achieve
considerable achievement and still could not replace gemcitabine as
the gold standard for clinical treatment (3). Thus, it is extremely urgent to look
for agents that have low toxicity and high-efficency.
Emodin, l,3,8-trihydroxyl-6-methyl anthraquinone, is
a monomer of Chinese Herb separated from Rhubarb Genera and
Polygonum and Rhamnus and Folium Sennae. A
number of studies show that emodin has a good effect on treating
prostate (4), colorectal (5) and pancreatic cancer (6). In this study, we found that the Panc-1
cells’ MMP declined and apoptosis rate increased dose-dependently
with emodin treatment and the cell proliferation of each group was
inhibited in a dose-and time-dependent manner. The feeding stuff
curve did not decline significantly in the group of the mice
treated with emodin at the dose of 40 mg/kg and the apoptosis rate
of the tumor cells was higher than the lower-dose groups. These
results demonstrated that emodin could induce Panc-1 cells
apoptosis via declining the MMP and a moderate dose of emodin
improved the living state of the model mice.
Materials and methods
Chemicals and reagents
Emodin and MTT were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Emodin was dissolved in dimethyl sulfoxide
(DMSO). The final concentration of DMSO used in vitro was
<0.1% and that in vivo <1%. Dulbecco’s modified
Eagle’s medium (DMEM), fetal bovine serum (FBS),
penicillin-streptomycin, trypsin-EDTA were obtained from Gibco-BRL
(Invitrogen, Grand Island, NY, USA).
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1) kit was purchased from Beyotime Biotechnology
(Haimen, China). Annexin V-FITC cell apoptosis detection kit was
purchased from Nanjing KeyGen Biotechology Co., Ltd., (Nanjing,
China). Terminal deoxynucleotidyl transferase-mediated deoxyuridine
triphosphate nick-end labeling (TUNEL) kit was purchased from Roche
Co. (Mannheim, Germany).
Cell line and cell culture
Human pancreatic cancer cell line Panc-1 was
purchased from the American Type Culture Collection and cultured in
DMEM with 10% FBS, 100 units/ml penicillin, and 100 μg/ml
streptomycin at 37°C under a humidified 5% CO2
atmosphere. Cells were passaged at 80–90% confluence.
JC-1 analysis
Panc-1 cells were placed in 6-well plates and
cultured overnight. Then, Panc-1 cells were cultured in the medium
with 0, 10, 20, 40 or 80 μmol/l emodin for 24 h. After that,
Panc-1 cells were stained with 5 mg/ml JC-1 for 20 min at 37°C in
the dark. Then MMP depletion was observed under a fluorescence
microscope.
FCM analysis
Panc-1 cells (4×105) were seeded to each
6-well plate. When cells adhered to the culture flask wall, they
were treated with 0, 10, 20, 40 or 80 μmol/l emodin for 24 h
before collected for trypsin digestion. The cell apoptosis were
then detected according to the instructions of Annexin V-FITC cell
apoptosis detection kit and assessed by FCM (Becton-Dickinson, San
Jose, CA, USA).
MTT analysis
Panc-1 cells were cultured in the medium with
various concentrations of emodin (0, 10, 20, 40 or 80
μmol/l) for different time (12, 24, 48 or 72 h) and then the
viability of Panc-1 cells was determined by MTT. Briefly, cells
were plated at a density of 5×103 cells/well in 96-well
microtiter plates. After treatment, 20 μl of MTT solution [5
mg/ml in phosphate-buffered saline (PBS)] was added to each well
and the plates were incubated. The supernatant was aspirated and
the MTT formazan was dissolved in 150 μl of DMSO. The plates
were mixed for 10 min on a gyratory shaker and absorbance was
measured with an ELISA reader (Bio-Tek ELx800, Winooski, VT, USA)
at a wavelength of 490 nm. Experiment was repeated thrice.
Inhibition rate (%) = [1 - (dosing absorbance - blank
absorbance)/(control absorbance - blank absorbance)] × 100%.
Experimental animals
Male nude mice [4–5 weeks old, BALB/c (nu/nu)),
weight 17–18 g) were purchased from Shanghai Cancer Institute for
Tumor Implantation and maintained in a specific pathogen free (SPF)
environment in the Animal Experiment Center of the Wenzhou Medical
College. All animal studies were approved by the Animal Research
and Ethics Committee of the Wenzhou Medical College.
Model establishment and experiment
scheme
Suspensions consisting of Panc-1 cells in serum-free
medium, with >90% viability, were used for model establishment.
Mice were anesthetized with 2% pentobarbital sodium solution and a
small left abdominal flank incision was made. Panc-1 cells
(5×106) in 100 μl serum-free medium were injected
into the subcapsular region of the pancreas using a 27-gauge
needle. The abdominal wound was closed in one layer.
After three weeks, the model mice were divided into
five groups randomly to receive different treatment: control group
(N, physiological saline), E10 group (emodin, 10 mg/kg),
E20 group (emodin, 20 mg/kg), E40 group
(emodin, 40 mg/kg), E80 group (emodin, 80 mg/kg). Each
mouse was treated 5 times by intraperitoneal injection of emodin
every 3 days. The feeding stuff intake was recorded before every
treatment. One week after the last treatment, the body weight was
measured and the mice were euthanized with 2% sodium pentobarbital,
followed by measuring the largest diameter of tumors. Finally,
implanted tumors were formalin-fixed and paraffin-embedded for
subsequent TUNEL assay.
TUNEL analysis
We assessed the degree of tumor apoptosis with the
TUNEL assay after the nude mice were sacrificed. TUNEL staining of
paraffin-embedded tumor sections was done with the TUNEL kit
according to the instructions. Laser scanning confocal microscope
(Olympus BX51, Japan) under 400-fold observation camera was used,
with excitation wavelength 488 nm and emission wavelength 568 nm.
We observed 10 field visions of the strongest fluorescence on each
slice.
Statistical analysis
SPSS 17.0 was used for statistical analysis. Data
are presented as the means ± SD. Differences among groups of cells
or mice were analyzed by one-way ANOVA followed by unpaired
Student’s t-test. P<0.05 was considered statistically
significant.
Results
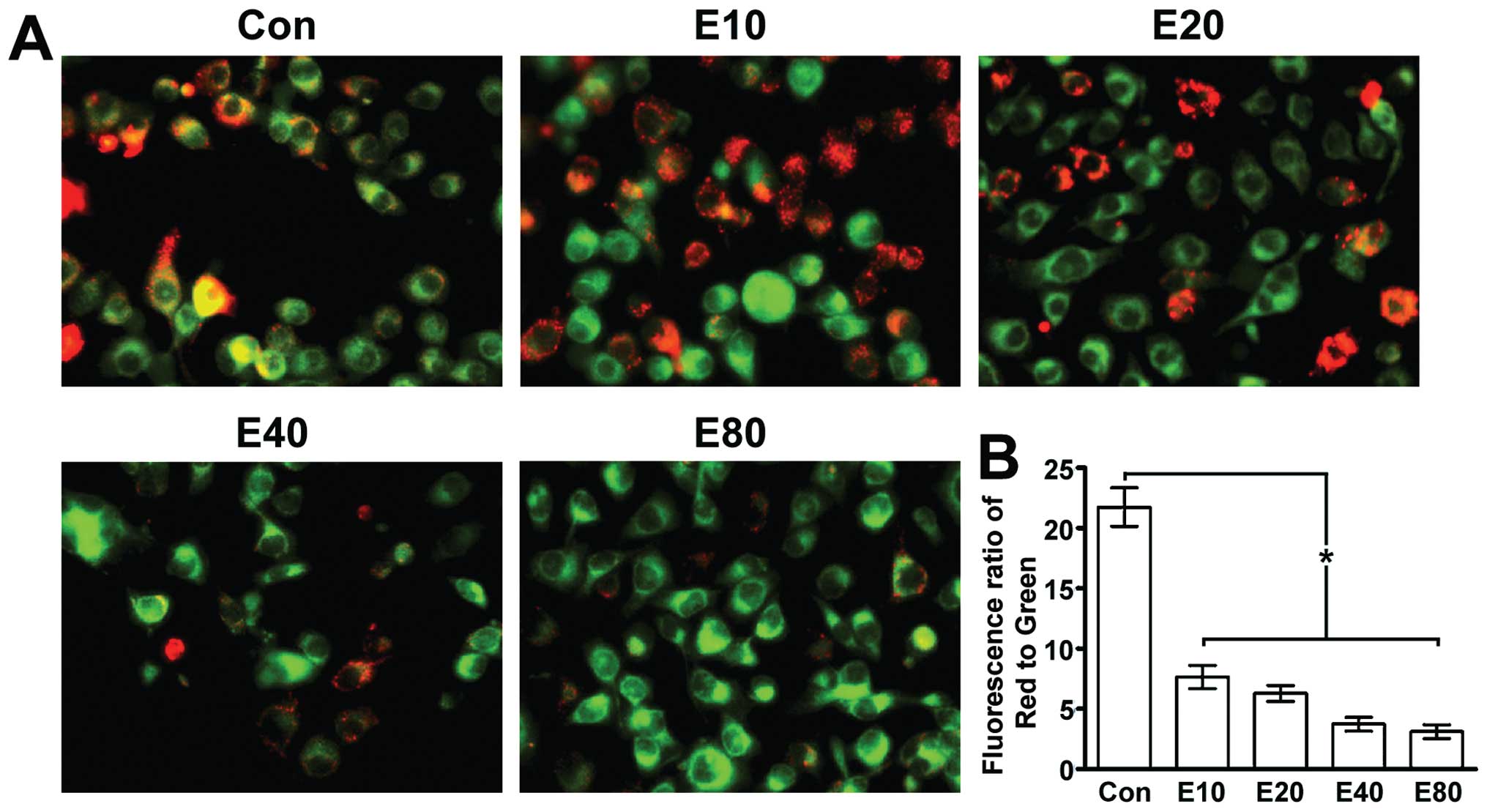
Emodin induces MMP decline in human
pancreatic cancer cell line Panc-1
JC-1 fluorescent dyes can gather in the matrix of
mitochondria and produce red fluorescence. If the MMP is reduced,
JC-1 can not gather the matrix so that JC-1 exists in the matrix as
a monomer, producing green fluorescence. JC-1 fluorescent color
changed from red to green along with the increasing concentration
of emodin, suggesting that MMP declined along with the increasing
concentration of emodin (Fig.
1).
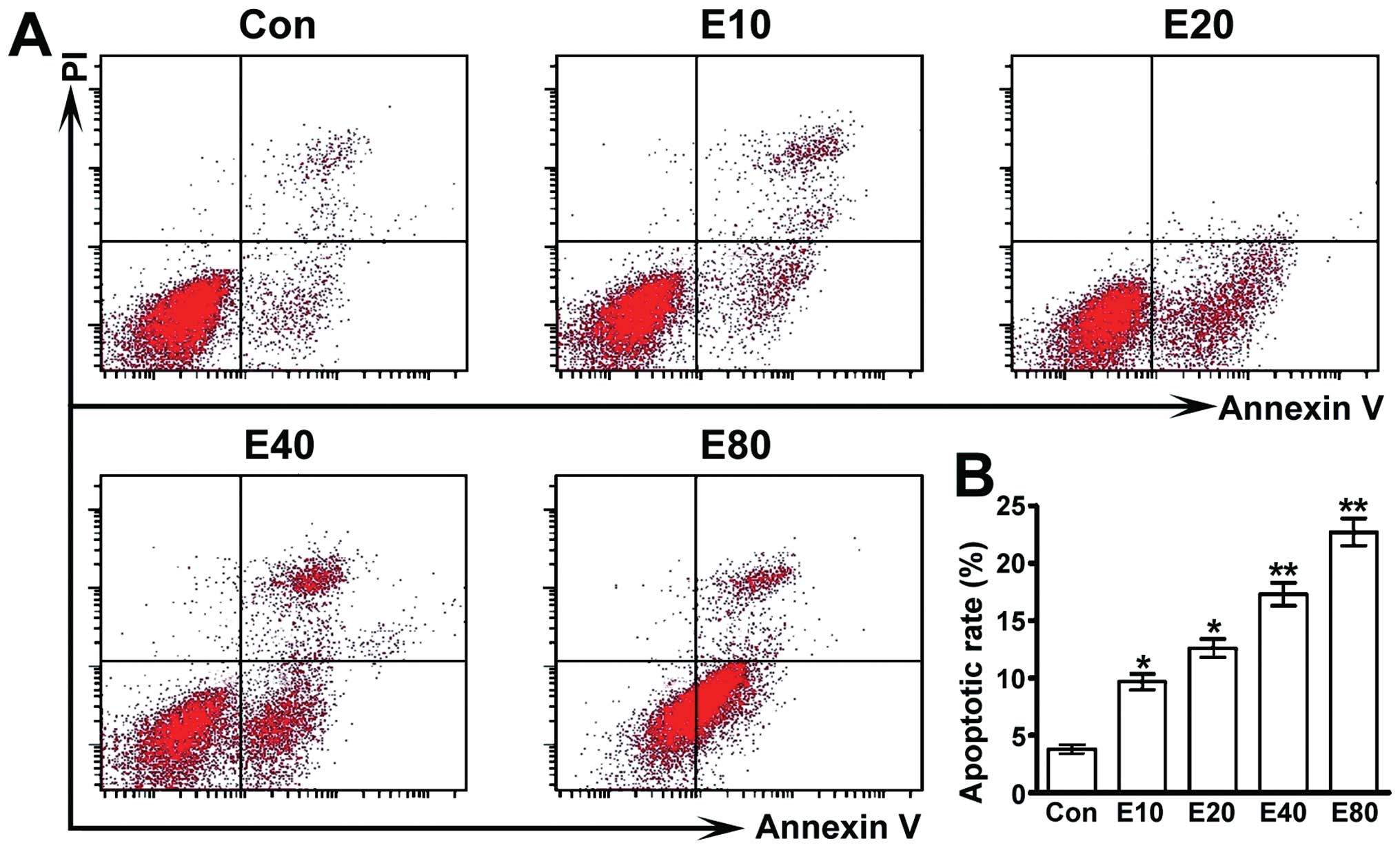
Emodin induces apoptosis of human
pancreatic cancer Panc-1 cells
We employed Annexin V-FITC cell apoptosis detection
kit to detect cell apoptosis. Our study showed that cell apoptosis
rate of each group was upregulated dose-dependently by emodin
(Fig. 2).
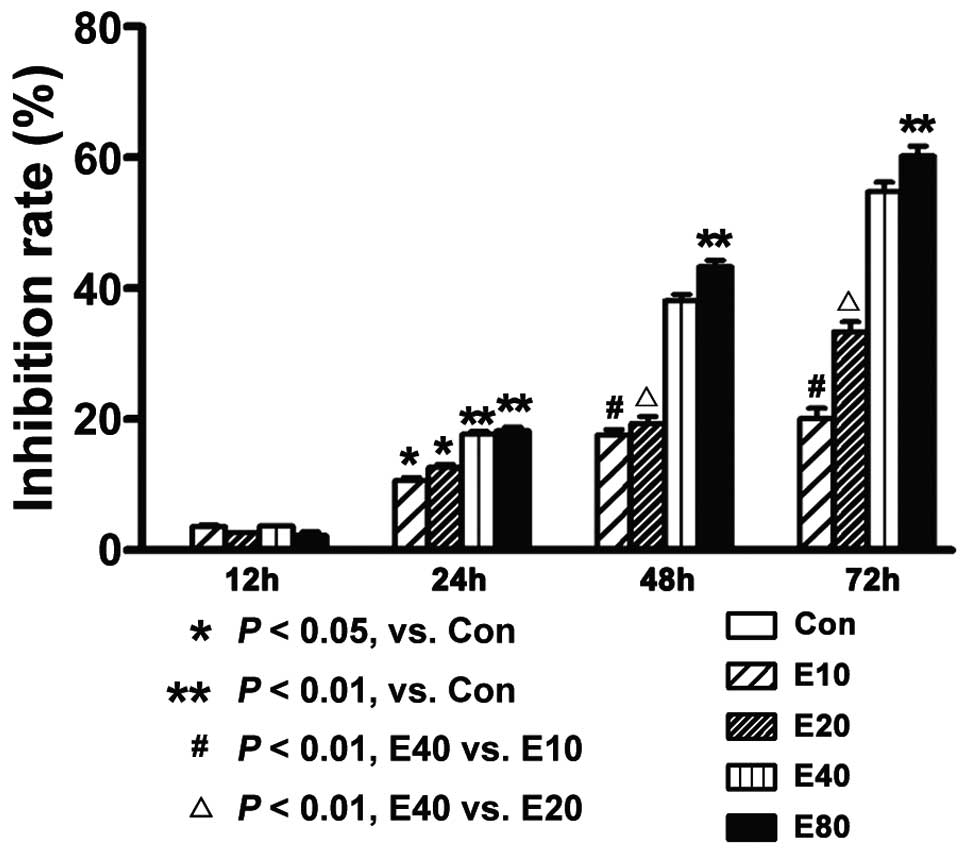
Emodin inhibits the proliferation of
human pancreatic cancer cell Panc-1
The cell proliferation-inhibition rate was detected
by MTT assay. We found that cell proliferation was inhibited dose-
and time-dependently. With treatment of 40 μmol/l emodin,
the inhibition rate of each time point (12, 24, 48 or 72 h) is
3.66±0.99%, 17.67±0.49%, 38.13±0.11% and 54.73±0.83%, respectively
(Fig. 3).
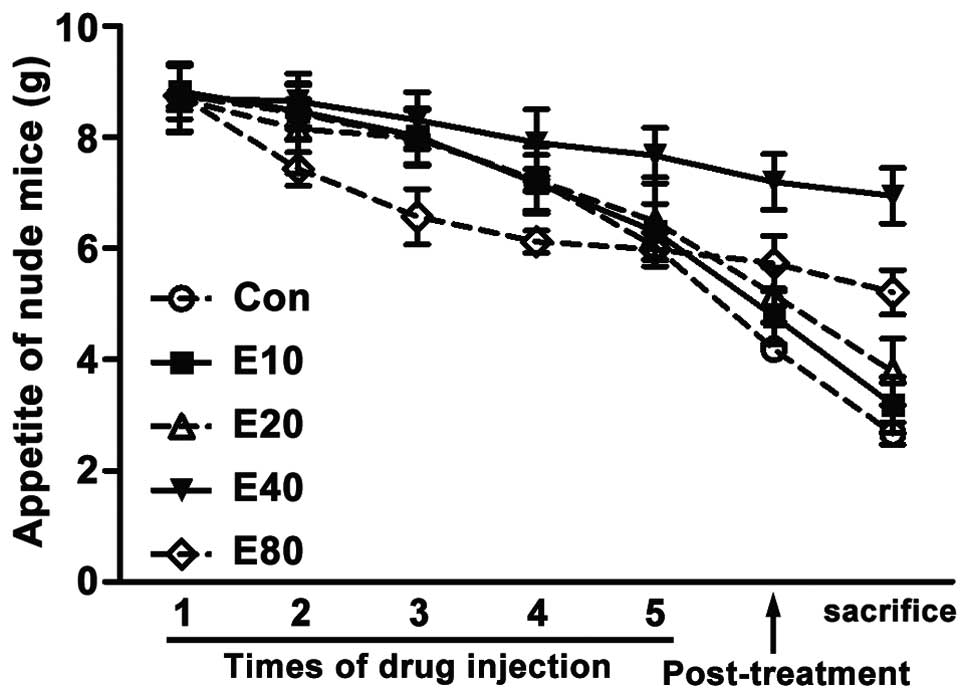
Emodin increases the amount of feed of
model mice
We recorded the feeding stuff eaten by each mouse
each day. Data showed that at the begining of experiment, feeding
stuff intake of each group was equal. As treatment progressing,
feeding stuff intake of all groups decreased day by day. But the
feeding stuff intake of E40 group declined less than the
other groups. In the initial stage, feeding stuff intake of
E80 group dramatically declined, but it changed gently
in the last stage (Fig. 4).
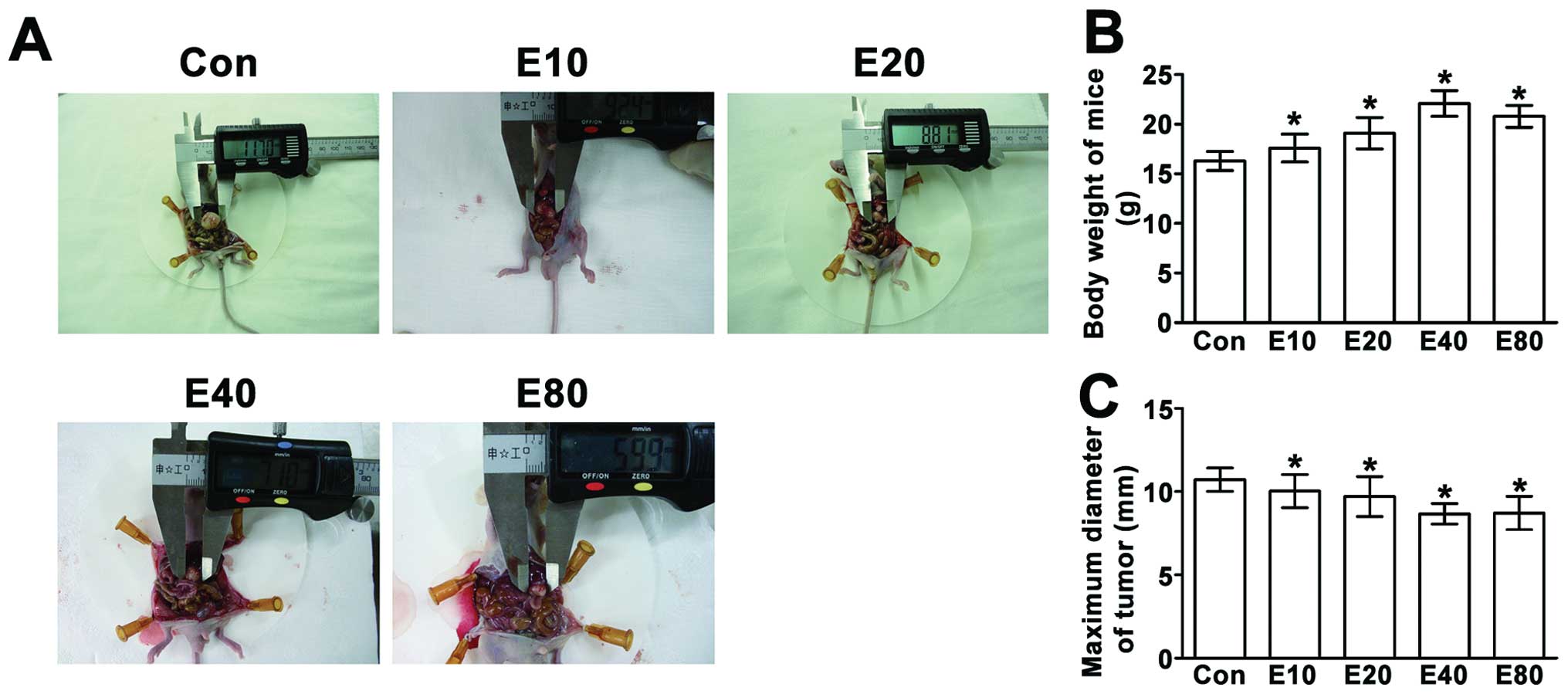
Emodin inhibits the growth of tumor and
increased the body weight of model mice
Compared to E40 and E80
groups, body weight in other groups was obviously reduced. The
tumor diameter of E80 group was smaller than
E40 group, but the body weight is smaller than
E40 group (Fig. 5).
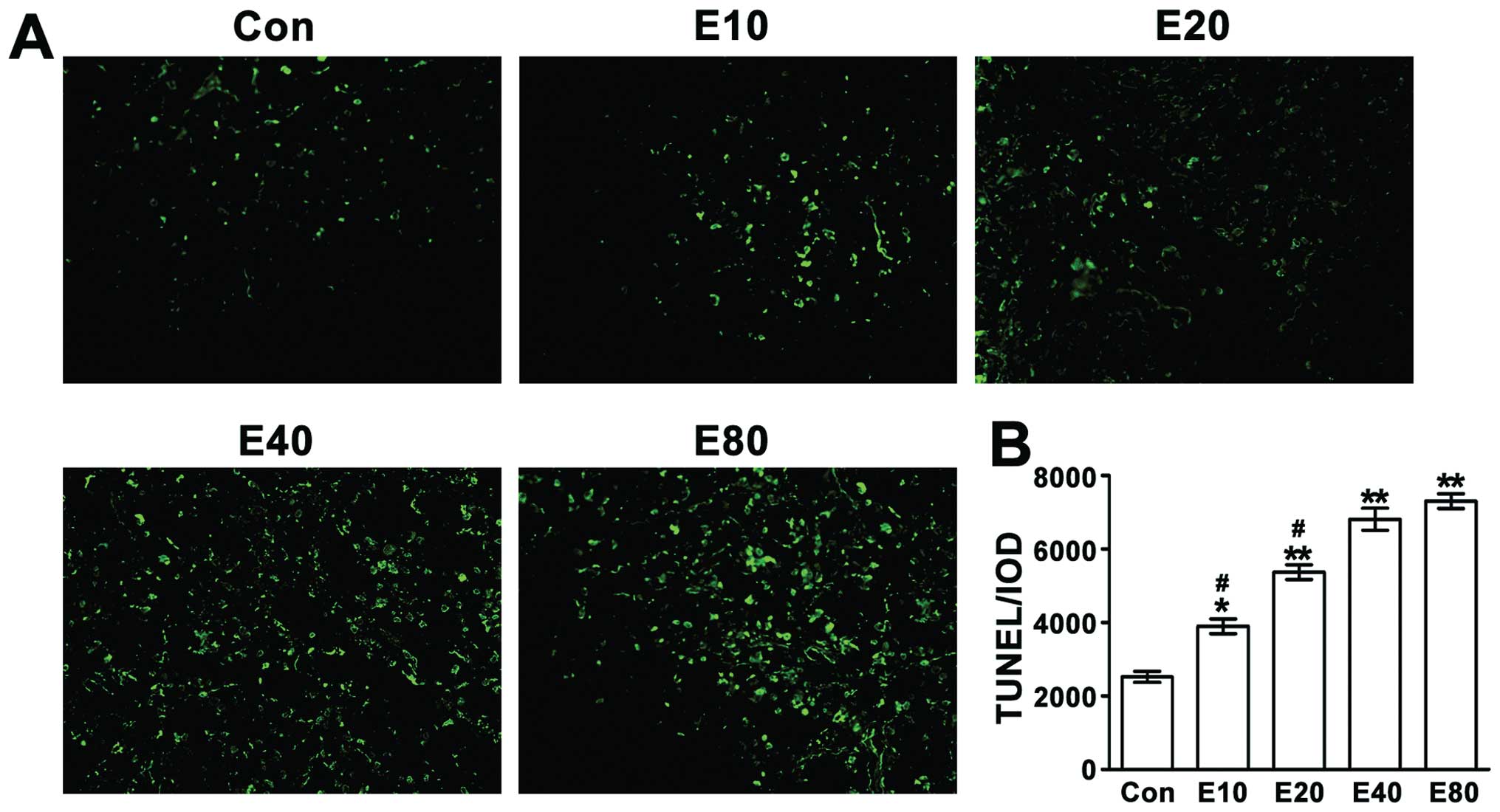
Emodin induces Panc-1 cell apoptosis in
mouse tumors
Apoptosis of the tumor cells was detected by TUNEL
assay. TUNEL-positive cells were observed with laser scanning
confocal microscope (magnification, ×400) (Fig. 6). With the increasing dose of
emodin, the cell apoptosis increased. Apoptosis of tumor cells in
E40 group and E80 group was significantly
increased compared to the control group (P<0.01).
Discussion
Traditional Chinese medicine (TCM) used for tretment
of cancer still have several controversies, unclear treatment
mechanism and lack of theoretical basis of combination therapy
(7,8). Up to now, only few kinds of TCM are
accredited and applied in international society. Even so, a survey
report shows that approximately 40% of American cancer patients
take TCM (9).
In recent years, more and more Chinese medicine
monomers are extracted. These drugs are of high purity, with single
chemical properties, such as emodin. Emodin is a kind of kinase
inhibitor II (10), which could
combine with DNA and prevent the proliferation and differentiation
of tumor cells (11). Previous
studies suggest that emodin may downregulate the nuclear factor-κB
(NF-κB) that could improve the proliferation, inhibit apoptosis,
enhance cancer cell drug-resistance (12) and decrease the Bax/Bcl-2 ratio
(13).
Mitochondria is not only the cells’ energy factory,
but also a ‘suicide weapon’ store because multiple apoptosis
pathways stay there. Changing the tumor cells’ metabolism of
mitochondria or stimulating the MMP decline can significantly
improve the efficiency of the cancer treatment (14). Mitochondria disorders are proved to
be an important part of cell apoptosis, leading to many
physiological changes (15). For
example, the MMP changes early when the cell is interfered with by
the apoptosis factor. The inner mitochondrial membrane
permeabilization causes disruption of MMP (16). In recent years, many experiments
suggest that keeping the MMP steady could prevent cells from
tending to apoptosis (17) and once
the MMP dissipates, the cell apoptosis will be irreversible
(18). Keeping MMP normal is
necessary for mitochondrial function. The decline of MMP is
associated with the mitochondrial membrane permeability (MPT)
changing, and MPT results from the opening of a mitochondrial
permeability transition pore, also known as the MPT pore or MPTP (a
protein pore that is formed in the inner membrane of the
mitochondria). MPTP allows water and solutes to come into the
matrix, causing the expansion of the mitochondria which results in
the rupture of outer mitochondrial membrane (MOM) so that the
apoptosis factors such as cyt c, Smac/DIABLO, AIF, release to
cytoplasm, finally leading to cell apoptosis (19,20).
Our study showed that even a low concentration of emodin led to MMP
decline. As the drug concentration increased, the MMP declined,
with the cell apoptosis rate and cell proliferation inhibition rate
rising. Our results suggest that emodin could induce MMP decline in
Panc-1 cells, thereby leading to cell apoptosis and cell
proliferation inhibition.
Gemcitabine was used to treat advanced pancreatic
cancer as a standard drug. Most patients develop resistance to
gemcitabine (21), which has strong
toxic side effect and is very expensive. The success of this
treatment is poor and overall survival has not improved for decades
(22). In recent years, much
attention was paid to Chinese traditional medicine monomers (CTMM).
CTMM promotes apoptosis, inhibits proliferation, reverses
drug-resistance in chemotherapy and improves the effect of
chemotherapy on cancer therapy (23). Emodin is one of these drugs which
has been shown to play a therapeutic role in the gastrointestinal
tract, and genitourinary cancer (5,24–26).
Our study showed that emodin suppressed tumor growth and induced
tissue cell apoptosis. Emodin at the dose of 80 mg/kg still did not
show strong drug toxicity as no unceasing decline of appetite of
mice in this group was seen. At the end the body weight was more
than control, E10 and E20 groups
demonstrating that emodin may inhibit tumor growth and the mice can
tolerate the drug toxicity.
In conclusion, our study suggests that emodin has a
good effect on declining the MMP of Panc-1 cells, promoting
apoptosis and inhibiting cell proliferation. This drug improved the
life quality of the mice with implanted tumors.
Acknowledgments
We are grateful for the funding support from the
Administration of Traditional Chinese Medicine of Zhengjing
Province, China (grant no. 2011ZZ010); the Zhejiang Provincial
Science Fund for Distinguished Young Scholars (grant no.
LR12H280001) and the National Natural Science Foundation of China
(grant no. 81173606). We thank the entire staff of the Animal
Experimental Center in Wenzhou Medical College and the scientific
research platform of the Second Afiliated Hospital of Wenzhou
Medical College for helpful assistance.
References
|
1
|
Maisonneuve P and Lowenfels AB:
Epidemiology of pancreatic cancer: an update. Dig Dis. 28:645–656.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Triano LR, Chang BW and Saif MW: New
developments in the treatment of locally advanced pancreatic
cancer. In: Highlights from the 45th ASCO annual meeting; Orlando,
FL JOP. 10. pp. 366–372. 2009, PubMed/NCBI
|
|
3
|
Conroy T, Desseigne F, Ychou M, et al:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu CX, Zhang XQ, Kang LD, et al: Emodin
induces apoptosis in human prostate cancer cell LNCaP. Asian J
Androl. 10:625–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Damodharan U, Ganesan R and Radhakrishnan
UC: Expression of MMP2 and MMP9 (gelatinases A and B) in human
colon cancer cells. Appl Biochem Biotechnol. 165:1245–1252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo HC, Bu HQ, Luo J, et al: Emodin
potentiates the antitumor effects of gemcitabine in PANC-1
pancreatic cancer xenograft model in vivo via inhibition of
inhibitors of apoptosis. Int J Oncol. 40:1849–1857. 2012.PubMed/NCBI
|
|
7
|
Baumann S, Fas SC, Giaisi M, et al:
Wogonin preferentially kills malignant lymphocytes and suppresses
T-cell tumor growth by inducing PLCgamma1- and
Ca2+-dependent apoptosis. Blood. 111:2354–2363. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Zhang B, Jiang D, Wei Y and Zhang N:
Herb network construction and co-module analysis for uncovering the
combination rule of traditional Chinese herbal formulae. BMC
Bioinformatics. 11:S62010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh B, Hu G, Kao S, Gebski V, Walls R,
Truong L, Beale P and Clarke S: The safety and tolerability of
Chinese herbal medicine in cancer patients receiving chemotherapy:
pilot study. WebmedCentral Chinese Medicine. 2:WMC0016712011.
|
|
10
|
Yim H, Lee YH, Lee CH and Lee SK: Emodin,
an anthraquinone derivative isolated from the rhizomes of Rheum
palmatum, selectively inhibits the activity of casein kinase II as
a competitive inhibitor. Planta Med. 65:9–13. 1999. View Article : Google Scholar
|
|
11
|
Wang L, Lin L and Ye B: Electrochemical
studies of the interaction of the anticancer herbal drug emodin
with DNA. J Pharm Biomed Anal. 42:625–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu A, Chen H, Tong H, et al: Emodin
potentiates the antitumor effects of gemcitabine in pancreatic
cancer cells via inhibition of nuclear factor-kappaB. Mol Med Rep.
4:221–227. 2011.PubMed/NCBI
|
|
13
|
Chen H, Wei W, Guo Y, et al: Enhanced
effect of gemcitabine by emodin against pancreatic cancer in
vivo via cytochrome C-regulated apoptosis. Oncol Rep.
25:1253–1261. 2011.PubMed/NCBI
|
|
14
|
Fulda S, Galluzzi L and Kroemer G:
Targeting mitochondria for cancer therapy. Nat Rev Drug Discov.
9:447–464. 2010. View
Article : Google Scholar
|
|
15
|
Leber B, Lin J and Andrews DW: Still
embedded together binding to membranes regulates Bcl-2 protein
interactions. Oncogene. 29:5221–5230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang CL, Ma YG, Xue YX, Liu YY, Xie H and
Qiu GR: Curcumin induces small cell lung cancer NCI-H446 cell
apoptosis via the reactive oxygen species-mediated mitochondrial
pathway and not the cell death receptor pathway. DNA Cell Biol.
31:139–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis: an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian X, Luo Y, Liu YP, et al:
Downregulation of Bcl-2 and survivin expression and release of
Smac/DIABLO involved in bufalin-induced HL-60 cell apoptosis.
Zhonghua Xue Ye Xue Za Zhi. 27:21–24. 2006.(In Chinese).
|
|
19
|
Halestrap AP: Calcium, mitochondria and
reperfusion injury: a pore way to die. Biochem Soc Trans.
34:232–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lopez M, Welsh K, Yuan H, et al:
Antagonists of IAP-family anti-apoptotic proteins BTI. Probe
Reports from the NIH Molecular Libraries Program. 2010
|
|
21
|
Kim MP and Gallick GE: Gemcitabine
resistance in pancreatic cancer: picking the key players. Clin
Cancer Res. 14:1284–1285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long J, Zhang Y, Yu X, et al: Overcoming
drug resistance in pancreatic cancer. Expert Opin Ther Targets.
15:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan W, Lu J, Huang M, et al: Anti-cancer
natural products isolated from Chinese medicinal herbs. Chin Med.
6:272011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei WT, Chen H, Ni ZL, et al: Antitumor
and apoptosis-promoting properties of emodin, an anthraquinone
derivative from Rheum officinale Baill, against pancreatic cancer
in mice via inhibition of Akt activation. Int J Oncol.
39:1381–1390. 2011.PubMed/NCBI
|
|
25
|
Wang QJ, Cai XB, Liu MH, Hu H, Tan XJ and
Jing XB: Apoptosis induced by emodin is associated with alterations
of intracellular acidification and reactive oxygen species in
EC-109 cells. Biochem Cell Biol. 88:767–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Sun Y, Li X, et al: Emodin
potentiates the anticancer effect of cisplatin on gallbladder
cancer cells through the generation of reactive oxygen species and
the inhibition of survivin expression. Oncol Rep. 26:1143–1148.
2011.PubMed/NCBI
|