Introduction
Retinoblastoma is the most common intraocular
malignancy in children, with an incidence rate of 11.8 per million
children aged 0–4 years in the United States (1). Histopathologically, retinoblastoma
tumor shows perivascular sleeves consisting of viable neoplastic
cells and necrotic tissue peripherally (2), which shows a close association of
tumor cells with blood vessels. Marback et al suggested
relatively high vascular area of the tumor could be a poor
prognostic factor of retinoblastoma (3). Moreover, intraocular extension to the
choroid and the optic nerve, a well-known poor prognostic factor of
retinoblastoma, was found to be associated with extensive tumor
necrosis, which was accompanied by thrombosis of the central
retinal vessels (4,5). The majority of cancer patients are in
the hyper-coagulated status and activation of coagulation might be
involved in tumor progression (6).
Therefore, therapeutic agents modulating tumor angiogenesis and
thrombosis at the same time could be a good treatment modality for
retinoblastoma.
Tissue factor (TF), a 47-kDa membrane-bound
glycoprotein, is a well-known cellular activator of the coagulation
cascade (7), which primarily binds
to factor VII (FVII) and forms the TF:FVIIa complex (8). TF itself also can simulate
angiogenesis-independent FVIIa, and the TF:FVIIa complex is
involved in signaling pathway through cell-bound protease-activated
receptors (PARs), inducing proangiogenic and immune modulating
cytokines, chemokines and growth factors (7,9). In
addition, TF is a well-known tumor pro-coagulant, and is also
considered to be associated with tumor angiogenesis (10,11).
Therefore, tumor angiogenesis could be effectively inhibited by
blockade of the tissue factor pathway.
Tissue factor pathway inhibitor (TFPI), which is
mainly synthesized in the vascular endothelium and present mainly
in the endothelium and plasma, consists of Kunitz-type domains,
where the first domain binds to FVIIa, the second to activated
factor X (FXa), and inactivates the TF:FVIIa complex and FXa
(12). Therefore, the dual
inhibitory effect of TFPI on the TF:FVIIa complex and FXa could be
a promising candidate modulating thrombosis and tumor angiogenesis
at the same time.
We previously reported that TF is expressed in tumor
cells of retinoblastoma cells, whose expression is closely
associated with proliferation of tumor cells in retinoblastoma
(13). However, it remains to be
elucidated whether TF is involved in tumor angiogenesis of
retinoblastoma.
Herein, we demonstrated for the first time that TF
regulates tumor angiogenesis of retinoblastoma. We found that TF is
expressed by endothelial cells of retinoblastoma, whose expression
is upregulated with the proliferation of endothelial cells. In
addition, blockade of the TF pathway by TFPI effectively inhibits
FGF-2-induced proliferation of endothelial cells. Moreover,
FGF-2-induced angiogenic processes of migration and tumor formation
of endothelial cells are suppressed by TFPI, which would be
mediated by blockade of the extracellular signal-regulated kinase
(ERK) pathway.
Materials and methods
Orthotopic transplantation mouse model of
retinoblastoma
BALB/c female nude mice were purchased from Oriental
(Korea). Care, use and treatment of all animals in this study were
in agreement with the ARVO statement for the Use of Animals in
Ophthalmic and Vision Research. As in our previous report (14), cultivated SNOUT-Rb1 cells
(1×106 cells) were harvested, suspended in cold
phosphate-buffered saline and injected into the intravitreal cavity
of BALB/c nude mice. Tumor development was observed by indirect
ophthalmoscopic examination twice a week for 4 weeks. Four weeks
after inoculation, the mice were sacrificed and enucleated.
Immunofluorescence staining
The enucleated eyes were formalin-fixed,
paraffin-embedded and then sectioned (4 μm). The slides were
de-paraffinized and incubated with proteinase K at 37°C. After
blocking endogenous peroxidase activity with hydrogen peroxide and
non-specific binding with blocking kit (Zymed Laboratories Inc.,
South San Francisco, CA, USA), slides were incubated overnight with
anti-TF (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-CD31 (1:200; BD Biosciences, San Jose, CA, USA) or anti-Ki67
(BD Biosciences) at 4°C. Alexa Fluor 594 donkey IgG (1:500;
Molecular Probes, Eugene, OR, USA), Alexa Fluor 594 donkey IgG
(1:200; Molecular Probes), Alexa Fluor 546 donkey IgG (1:100;
Molecular Probes) were used as secondary antibodies. The nuclei
were stained with 4′,6-diamidino-2-phenylindole (DAPI;
Sigma-Aldrich Co., St. Louis, MO, USA). The slides were mounted
Faramount Aqueous mounting medium (Dako, Glostrup, Denmark) and
observed under a fluorescence microscope (BX50, Olympus, Tokyo,
Japan).
Immunohistochemistry
Slides were prepared as for immunofluorescence
staining and incubated with anti-TF (1:200; Santa Cruz
Biotechnology) at 4°C for 12 h. Then, a biotinylated goat antibody
(Dako) was used for the avidin/biotin complex (Vectastain kit;
Vector Laboratories, Burlingame, CA, USA) and the
3-amino-9-ethyl-carbazole chromogen (AEC, Dako). The slides were
mounted Faramount Aqueous mounting medium (Dako) and observed under
a light microscope (Carl Zeiss, Chester, VA, USA). Primary antibody
was omitted for negative control.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from Lonza (Basel, Switzerland) and cultured on 0.15%
gelatin-PBS coated plates in M199 medium (Gibco-BRL, Rockville, MD,
USA) supplemented with 20% fetal bovine serum (Gibco-BRL), 3 ng/ml
fibroblast growth factor-2 (FGF-2; Millipore, Bedford, MA, USA), 10
U/ml heparin (Sigma-Aldrich) and 1% antibiotic-antimycotic solution
(Invitrogen, Carlsbad, CA, USA) at 37°C in a moist atmosphere of
95% air and 5% CO2. The medium was changed every third
day. Cultured cells were observed daily under a phase-contrast
microscope (Carl Zeiss). HUVECs used in this study were taken from
passage 4 to 8. When required, FGF-2 (10 ng/ml; Sigma-Aldrich) or
TFPI (0.1–1 nM; American Diagnostica GmbH, Pfungstadt, Deutschland)
treatment was carried out.
Western blot analysis
Cells were harvested, washed with ice-cold phosphate
buffer solution, and lysed with buffer containing 50 mM of Tris-HCl
(pH 7.4), 150 mM of NaCl, 1% Nonidet P40, 2 mM of sodium
orthovanadate and a protease inhibitor cocktail (Roche). An equal
amount (15 μg) of the samples was separated on sodium dodecyl
sulfate-polyacrylamide gel and then transferred onto nitrocellulose
filters (Bio-Rad Laboratories, Hercules, CA, USA). The membranes
were immunoblotted with primary antibodies against TF (1:1000;
Santa Cruz Biotechnology), ERK 1/2 (1:1000; Cell Signaling
Technology, Beverly, MA, USA) or phospho-ERK 1/2 (1:1000; Cell
Signaling Technology). To ensure the equal loading of protein in
each lane, the blots were stripped and re-probed with an antibody
against β-actin.
Cell proliferation assay
Cell proliferation was evaluated with the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay modified from our previous description (15). HUVECs (5×103 cells) were
seeded onto 96-well plates and cultured overnight. The cells were
treated with FGF-2 (10 ng/ml; Sigma-Aldrich) under various
concentrations of TFPI (0.1–1 nM; American Diagnostica GmbH) for 24
h. Following incubation, the medium was carefully removed from the
plate, and dimethyl sulfoxide was added to solubilize formazan
produced from MTT by the viable cells. Absorbance was measured at
540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA,
USA).
Wound migration assay
The migration of endothelial cells was evaluated
with wound migration assay modified from our previous study
(16). HUVECs (1×106
cells) were seeded and cultured onto gelatin-coated 60-mm culture
dishes at 90% confluence and monolayers of cells were wounded with
a micropipette tip. After rinsing with serum-free medium, the
wounded monolayers were incubated with treatment of 0.5 or 1.0 nM
TFPI (American Diagnostica GmbH) and 10 ng/ml FGF-2 (Sigma-Aldrich)
for 12 h. Migration was measured by counting the number of cells
that moved across the reference line under a light microscope (Carl
Zeiss).
Tube formation assay
The tube formation of endothelial cells was assayed
as previously described (17).
HUVECs (1×105 cells) were inoculated on the surface of
the Matrigel with treatment of 0.5 or 1.0 nM TFPI (American
Diagnostica GmbH) and with 10 ng/ml FGF-2 (Sigma-Aldrich) for 12 h.
Tube formation was observed under a light microscope (Carl Zeiss)
and photographed at a ×400 magnification. Tube formation was
quantified by counting the number of connected cells divided by the
total number of cells in randomly selected fields at a ×400
magnification.
Statistical analysis
Statistical differences between groups were
evaluated with the Mann-Whitney U test. Data were recorded as the
mean ± SD. P-values of ≤0.05 were considered to indicate
statistically significant differences.
Results
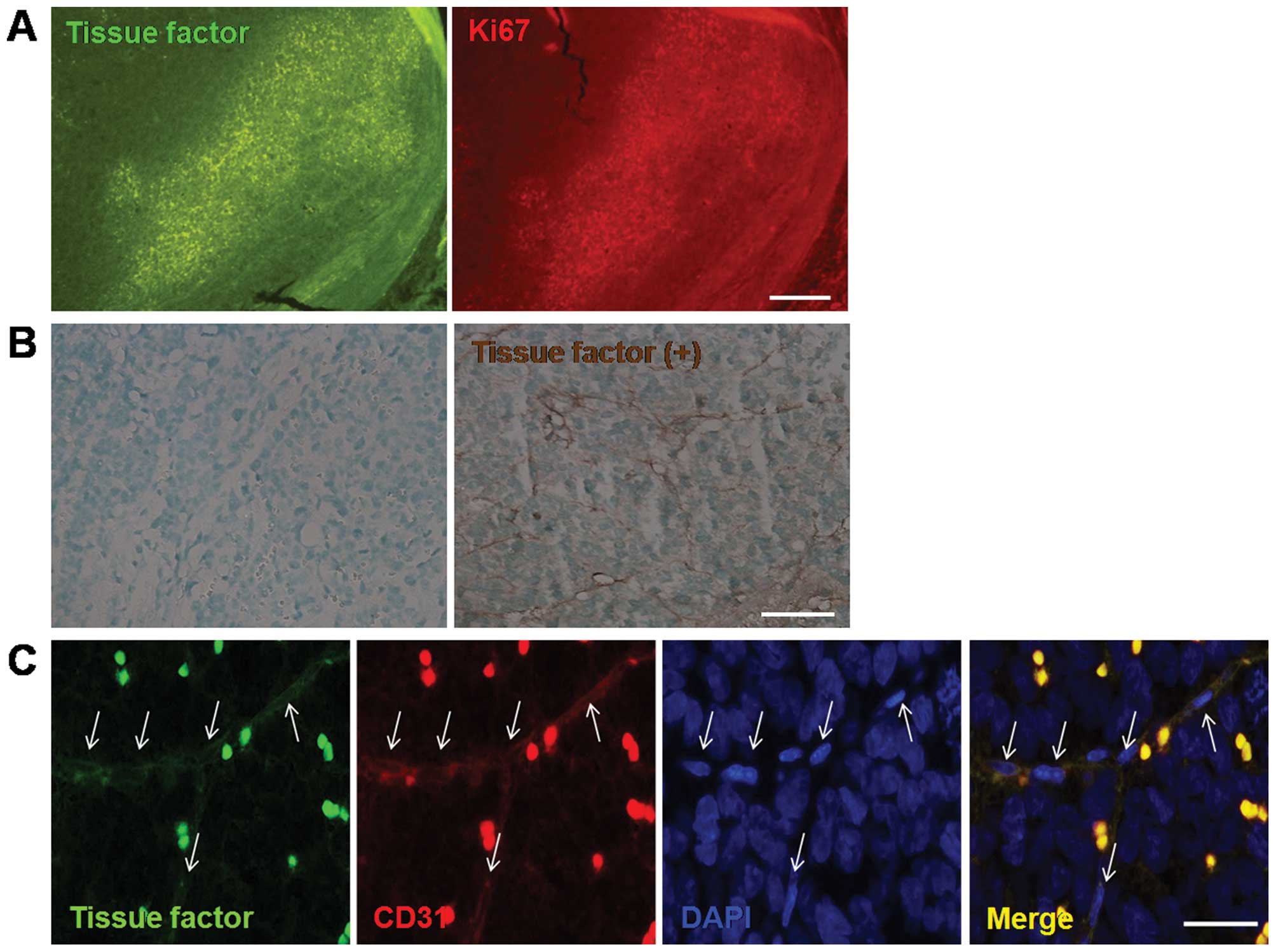
TF is expressed on tumor vessels of the
orthotopic transplantation model of retinoblastoma
Four weeks after the inoculation of retinoblastoma
cells, the vitreous cavity was almost completely occupied by the
tumor. Based on our recent report that TF immunopositive cells are
mainly detected in the proliferative area of retinoblastoma
(13), immunoreactivity for TF in
retinoblastoma was analyzed with Ki67, a proliferation marker in
the orthotopic transplantation mouse model of retinoblastoma. As
shown in Fig. 1A, TF in
retinoblastoma was prominently expressed in the area of highly
proliferative activity. In addition, TF expression was confirmed in
retinoblastoma tissue through immunohistochemistry, markedly where
we found that TF is expressed on the tumor vessels as well as
around tumor cells of the mouse model of retinoblastoma (Fig. 1B).
Therefore, given that proliferative tumor cells of
high metabolic activity in retinoblastoma are supplied by abundant
tumor angiogenesis (2,3), we investigated whether TF is expressed
on tumor vessels of retinoblastoma. As expected, there was strong
immunoreactivity of TF on tumor vessels of retinoblastoma, which
was furthermore co-localized with CD31, as an endothelial cell
maker (Fig. 1C). Thus, TF
expression on tumor vessels of retinoblastoma suggests a
correlation of TF with tumor angiogenesis of retinoblastoma.
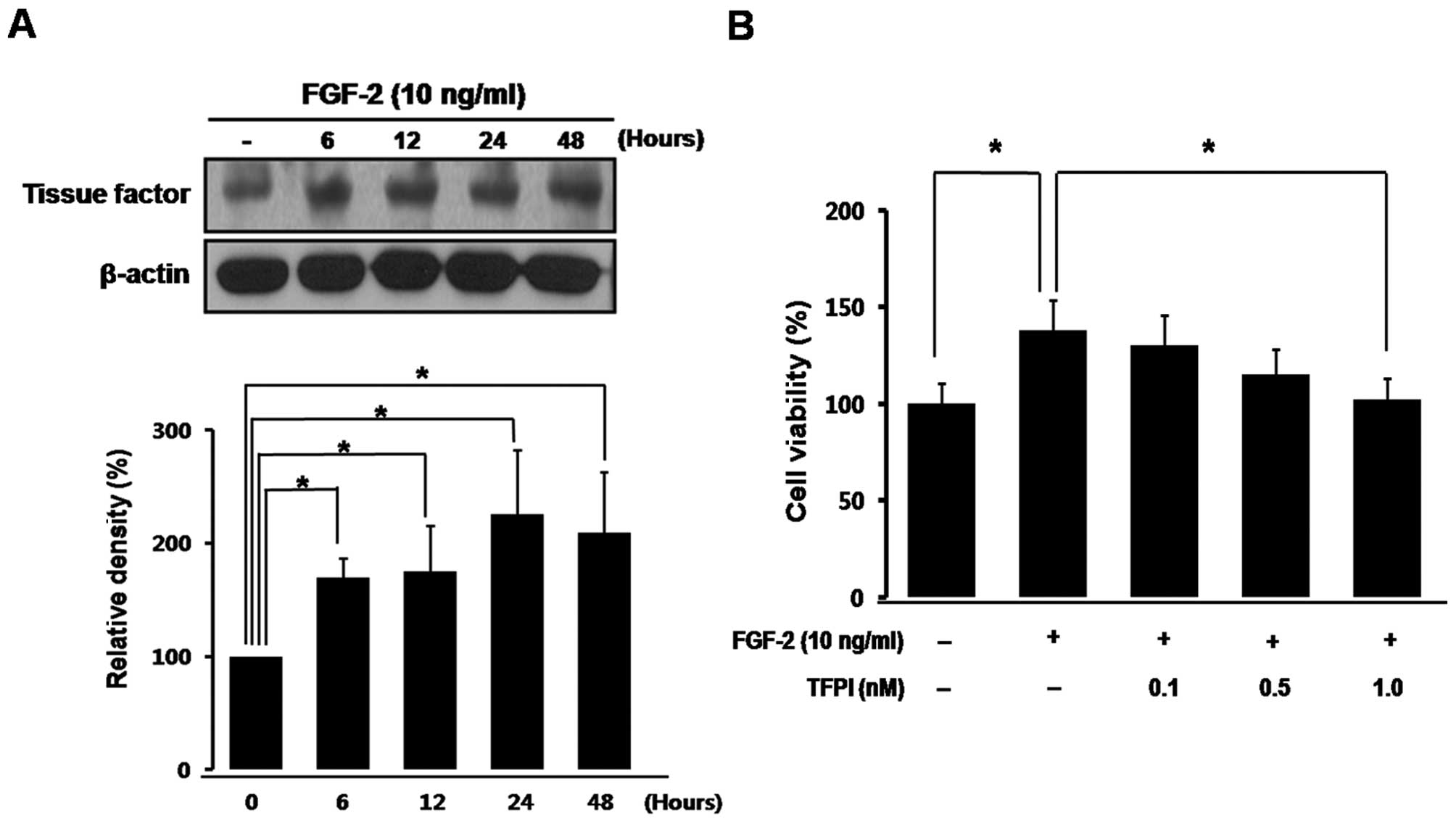
TF regulates proliferation of vascular
endothelial cells
To examine whether TF is related to tumor
angiogenesis of retinoblastoma, we determined temporal expression
of TF in the proliferation of vascular endothelial cells. As
demonstrated in Fig. 2A, TF
expression in HUVECs progressively and significantly increased with
proliferation induced by FGF-2, a well-known mitogen of
retinoblastoma tumor (*P<0.05) (18).
Next, we evaluated whether proliferation of vascular
endothelial cells could be directly inhibited by blockade of the TF
pathway. As shown in Fig. 2B,
proliferation of HUVECs was significantly increased by FGF-2
treatment (*P<0.05), which was prevented by
co-treatment with TFPI in a dose-dependent manner
(*P<0.05).
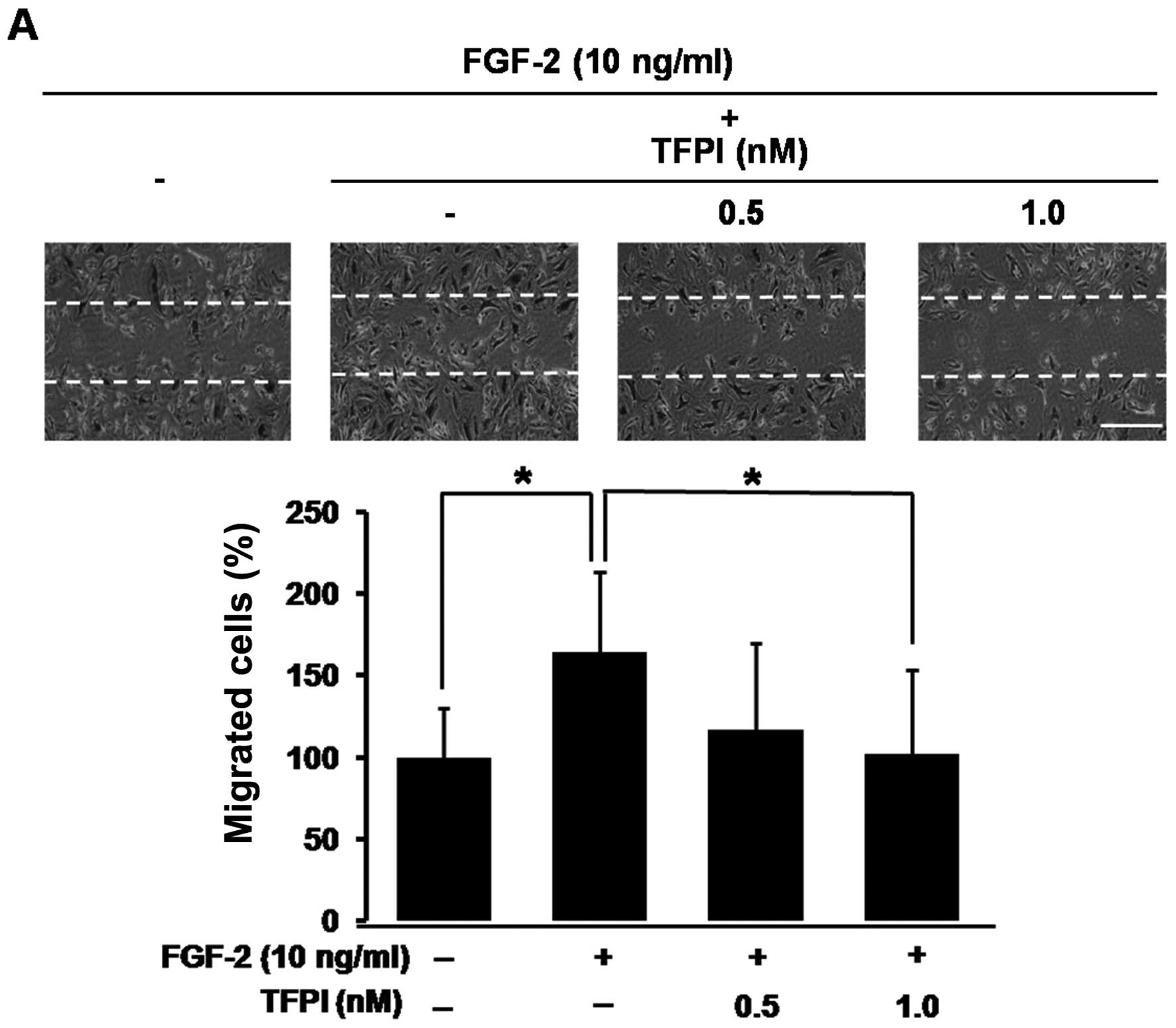
TF regulates angiogenic processes of
migration and tube formation of vascular endothelial cells
Based on our data that proliferation of vascular
endothelial cells is regulated by the TF pathway, we investigated
whether the TF pathway is involved in the regulation of angiogenic
processes of migration and tube formation of vascular endothelial
cells. The migration of HUVECs increased 1.7-fold with FGF-2
treatment (*P<0.05), whereas the migratory activity
was completely inhibited by co-treatment with TFPI
(*P<0.05, Fig. 3A).
Moreover, FGF-2 induced extensive formation of capillary-like
networks, which was 1.6-fold compared to control
(*P<0.05), was nearly abolished by co-treatment with
TFPI (*P<0.05, Fig.
3B). These results suggest that in addition to FGF-2 mediated
proliferation of vascular endothelial cells, FGF-2 mediated
angiogenic processes of migration and tube formation could be
regulated by the TF pathway.
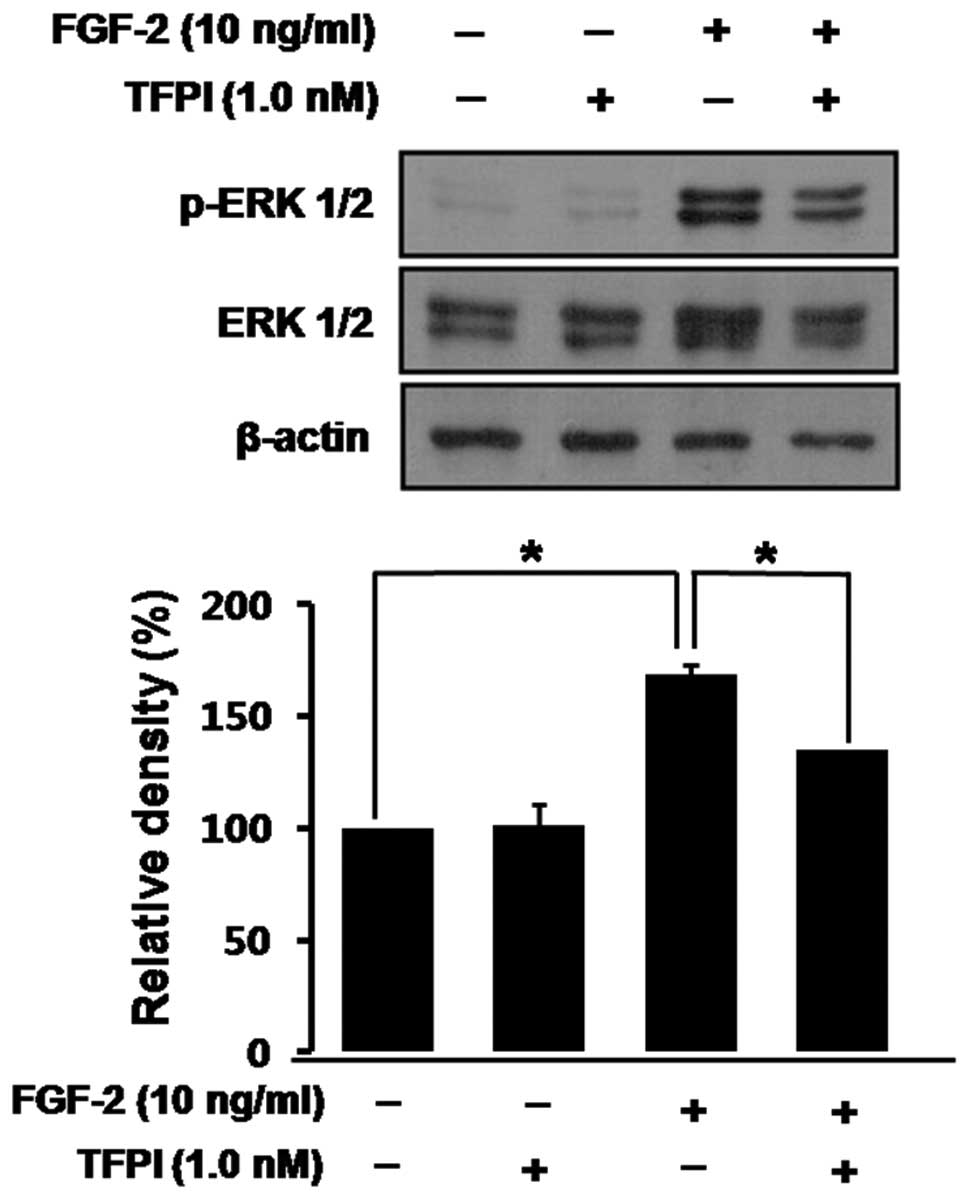
TF regulates FGF-2-induced angiogenic
processes through ERK activation
Given that the sustained activation of the ERK
pathway is required for FGF-2-induced angiogenesis (19), we confirmed whether TF could
regulate FGF-2-induced angiogenic processes via ERK activation.
ERK-1/2 phosphorylation was significantly increased with FGF-2
treatment in HUVECs (*P<0.05), which was completely
blocked by co-treatment with TFPI (*P<0.05, Fig. 4). Therefore, it was clearly
demonstrated that blockade of the TF pathway could effectively
inhibit tumor angiogenesis mediated by FGF-2 of retinoblastoma.
Discussion
Although TF has been reported to be expressed in
several malignant tumors such as colon cancer, breast cancer,
leukemia and small cell lung cancer (20,21),
the role of TF in retinoblastoma had yet to be elucidated. Our
findings show that TF is expressed in retinoblastoma cells, and
could be involved in the proliferation of tumor cells (13). In addition to tumor cells, it was
also indicated that TF expression in tumor could be related to
tumor angiogenesis (9–11). Herein, we demonstrated for the first
time that TF regulates tumor angiogenesis of retinoblastoma.
Growth factors in tumor are known to be involved in
growth, progression and drug resistance (22). Among variable grow factors, FGF-2
was reported to be produced in retinoblastoma cells to contribute
to tumor progression as a major underlying mitogen of
retinoblastoma (18,22). FGF-2 was found in a vascular pattern
of a transgenic mouse model of retinoblastoma, which suggests that
FGF-2 in the tumor microenvironment of retinoblastoma plays a
direct role in supporting tumor angiogenesis (23). Moreover, FGF-2 expression in the
transgenic mouse model of retinoblastoma is prominently localized
to the tumor vasculature (23).
First, we discovered that TF is selectively expressed in the
proliferative area of retinoblastoma including the tumor vessels as
well as tumor cells. Then we showed that in addition to
FGF-2-induced proliferation of vascular endothelial cells,
FGF-2-induced angiogenic processes of migration and tube formation
of vascular endothelial cells were directly regulated by the TF
pathway, which would be mediated by the ERK pathway.
Although it is not clear how TF is involved in tumor
angiogenesis, many supporting results has been reported. For
example, increased expression of TF in pancreatic cancer was
associated with VEGF expression and increased microvessel density
(9), whereas Low-TF mice showed
reduced tumor blood vessel size in B16F1 melanoma (24). In addition, TF is believed to play a
role in angiogenesis indirectly by clotting-dependent mechanisms or
by modulating angiogenic properties of tumor cells, or directly by
clotting-independent mechanisms (25). In particular, specific inhibitors to
the TF:FVIIa complex impaired angiogenesis, in contrast to the FXa
inhibitor that could not (26).
Thus, a direct mechanism through TF:VIIa complex mediated signaling
may play a crucial role in angiogenesis, which suggest that the
inhibitory effects of TFPI on angiogenic processes are probably
mediated by inhibiting TF:VIIa-mediated signaling, not by FXa.
Based on the fact that TFPI could inhibit FGF-2-stimulated
angiogenic processes of vascular endothelial cells (27,28),
we demonstrated that blockade of the TF pathway by TFPI could
inhibit FGF-2-induced angiogenic processes of migration and tube
formation as well as proliferation of vascular endothelial cells
via the ERK pathway. The TF:VIIa complex leads to activation of the
G-protein coupled receptor called protease-activated receptor 2
(PAR2) (29), whose activation
could activate several mitogen-activated protein kinase pathways
(25). Therefore, the
anti-angiogenic activity of TFPI would be mediated by
TF:VIIa-PAR2-MAPK signaling.
In addition to our previous report that TF
expression is increased in proliferating tumor cells of
retinoblastoma, which could be inhibited by blockade of the TF
pathway, TFPI (13), in our current
study we demonstrated that TF is also expressed on tumor vessels of
retinoblastoma, which could be involved in the angiogenic processes
of tumor angiogenesis in retinoblastoma. Given that, TF is
expressed in tumor vessels of retinoblastoma as well as in tumor
cells, which is directly involved both in the proliferation of
tumor cells and in tumor angiogenesis. In conclusion, our results
suggest that blockade of the TF pathway by TFPI could effectively
inhibit tumor growth by suppressing tumor cell proliferation and
tumor angiogenesis at the same time. Therefore, TFPI could be
considered to be applied to retinoblastoma as an effective
therapeutic targeting tumor cell proliferation and tumor
angiogenesis.
Acknowledgements
We thank Mr. Myoung Seok Jeong for his technical
assistance. This study was supported by the Global Core Research
Center (GCRC) grant from NRF/MEST, Republic of Korea
(2012-0001187), the Bio-Signal Analysis Technology Innovation
Program of MEST/NRF, Republic of Korea (2012-0006058), and the
Mid-Career Researcher Program of MEST/NRF, Republic of Korea
(2012-0004931).
References
|
1
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009.
|
|
2
|
Burnier MN, McLean IW, Zimmerman LE and
Rosenberg SH: Retinoblastoma. The relationship of proliferating
cells to blood vessels. Invest Ophthalmol Vis Sci. 31:2037–2040.
1990.PubMed/NCBI
|
|
3
|
Marback EF, Arias VE, Paranhos A Jr,
Soares FA, Murphree AL and Erwenne CM: Tumour angiogenesis as a
prognostic factor for disease dissemination in retinoblastoma. Br J
Ophthalmol. 87:1224–1228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubin CM, Robison LL, Cameron JD, et al:
Intraocular retinoblastoma group V: an analysis of prognostic
factors. J Clin Oncol. 3:680–685. 1985.PubMed/NCBI
|
|
5
|
Chong EM, Coffee RE, Chintagumpala M,
Hurwitz RL, Hurwitz MY and Chevez-Barrios P: Extensively necrotic
retinoblastoma is associated with high-risk prognostic factors.
Arch Pathol Lab Med. 130:1669–1672. 2006.PubMed/NCBI
|
|
6
|
Falanga A: Thrombophilia in cancer. Semin
Thromb Hemost. 31:104–110. 2005. View Article : Google Scholar
|
|
7
|
Schaffner F and Ruf W: Tissue factor and
PAR2 signaling in the tumor microenvironment. Arterioscler Thromb
Vasc Biol. 29:1999–2004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van den Berg YW, Osanto S, Reitsma PH and
Versteeg HH: The relationship between tissue factor and cancer
progression: insights from bench and bedside. Blood. 119:924–932.
2012.PubMed/NCBI
|
|
9
|
Khorana AA, Ahrendt SA, Ryan CK, et al:
Tissue factor expression, angiogenesis, and thrombosis in
pancreatic cancer. Clin Cancer Res. 13:2870–2875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Pedrera C, Barbarroja N, Dorado G,
Siendones E and Velasco F: Tissue factor as an effector of
angiogenesis and tumor progression in hematological malignancies.
Leukemia. 20:1331–1340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Contrino J, Hair G, Kreutzer DL and
Rickles FR: In situ detection of tissue factor in vascular
endothelial cells: correlation with the malignant phenotype of
human breast disease. Nat Med. 2:209–215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lwaleed BA and Bass PS: Tissue factor
pathway inhibitor: structure, biology and involvement in disease. J
Pathol. 208:327–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee BJ, Kim JH, Woo SH, Kim DH and Yu YS:
Tissue factor is involved in retinoblastoma cell proliferation via
both the Akt and extracellular signal-regulated kinase pathways.
Oncol Rep. 26:665–670. 2011.PubMed/NCBI
|
|
14
|
Kim JH, Kim JH, Yu YS, Kim DH, Kim CJ and
Kim KW: Establishment and characterization of a novel,
spontaneously immortalized retinoblastoma cell line with adherent
growth. Int J Oncol. 31:585–592. 2007.PubMed/NCBI
|
|
15
|
Jun HO, Kim Y, Kwon YW, et al: Wondonin, a
novel compound, inhibits hypoxia-induced angiogenesis through
hypoxia-inducible factor 1 alpha. FEBS Lett. 581:4977–4982. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lim Y, Jo DH, Kim JH, et al: Human
apolipoprotein(a) kringle V inhibits ischemia-induced retinal
neovascularization via suppression of fibronectin-mediated
angiogenesis. Diabetes. 61:1599–1608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min JK, Cho YL, Choi JH, et al: Receptor
activator of nuclear factor (NF)-kappaB ligand (RANKL) increases
vascular permeability: impaired permeability and angiogenesis in
eNOS-deficient mice. Blood. 109:1495–1502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schweigerer L, Neufeld G and Gospodarowicz
D: Basic fibroblast growth factor is present in cultured human
retinoblastoma cells. Invest Ophthalmol Vis Sci. 28:1838–1843.
1987.PubMed/NCBI
|
|
19
|
Eliceiri BP, Klemke R, Strömblad S and
Cheresh DA: Integrin alphavbeta3 requirement for sustained
mitogen-activated protein kinase activity during angiogenesis. J
Cell Biol. 140:1255–1263. 1998. View Article : Google Scholar
|
|
20
|
Callander NS, Varki N and Rao LV:
Immunohistochemical identification of tissue factor in solid
tumors. Cancer. 70:1194–1201. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito T, Koyama T, Nagata K, Kamiyama R
and Hirosawa S: Anticoagulant effects of retinoic acids on leukemia
cells. Blood. 87:657–665. 1996.PubMed/NCBI
|
|
22
|
Zunyan D, Ying H and Sadee W: Growth
factor signaling and resistance to cancer chemotherapy. Curr Topic
Med Chem. 4:1345–1354. 2004. View Article : Google Scholar
|
|
23
|
Cebulla CM, Jockovich ME, Piña Y, et al:
Basic fibroblast growth factor impact on retinoblastoma progression
and survival. Invest Ophthalmol Vis Sci. 49:5215–5221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, May L, Milsom C, et al: Contribution
of host-derived tissue factor to tumor neovascularization.
Arterioscler Thromb Vasc Biol. 28:1975–1981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bluff JE, Brown NJ, Reed MW and Staton CA:
Tissue factor, angiogenesis and tumour progression. Breast Cancer
Res. 10:2042008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hembrough TA, Swartz GM, Papathanassiu A,
et al: Tissue factor/factor VIIa inhibitors block angiogenesis and
tumor growth through a nonhemostatic mechanism. Cancer Res.
63:2997–3000. 2003.PubMed/NCBI
|
|
27
|
Hembrough TA, Ruiz JF, Papathanassiu AE,
Green SJ and Strickland DK: Tissue factor pathway inhibitor
inhibits endothelial cell proliferation via association with the
very low density lipoprotein receptor. J Biol Chem.
276:12241–12248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Provencal M, Michaud M, Beaulieu E, et al:
Tissue factor pathway inhibitor (TFPI) interferes with endothelial
cell migration by inhibition of both the Erk pathway and focal
adhesion proteins. Thromb Haemost. 99:576–585. 2008.PubMed/NCBI
|
|
29
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|