Introduction
Osteosarcoma is the most common primary malignant
bone tumor; it occurs during adolescence and is ranked eighth in
general incidence among childhood cancers. The overall 5-year
survival rate for osteosarcoma ranges from 50 to 80% (1–5).
Despite adequate surgical removal and chemotherapy at an early
stage of osteosarcoma, some patients experience distant metastasis
to the lung, bone or other organs; therefore, prevention of the
invasion and metastasis of osteosarcoma is crucial in improving the
prognosis of patients. To combat the aggressive potential of
osteosarcoma, various factors associated with invasion and
metastasis have been identified. Among them, autocrine motility
factor (AMF) plays an important role in metastasis. AMF is secreted
by tumor cells and stimulates the potential of proliferation
(6), migration (7,8),
angiogenesis (9,10) and resistance to apoptosis (11,12).
In previous studies, molecular cloning and sequencing identified
phosphoglucose isomerase (PGI) as AMF (13). PGI is an essential cytosolic enzyme
of sugar metabolism and plays a key role in both glycolysis and the
gluconeogenesis pathway, catalyzing the interconversion of glucose
6-phosphate and fructose 6-phosphate in both normal and tumor cells
(14). PGI is secreted
extracellularly from various tumors, not from normal cells, and
behaves as AMF (15–17). Elevated serum AMF levels in
malignant tumors, including the gastrointestinal tract (18), colorectum (19), breast (20), and lung cancer (21), are associated with cancer
progression and metastasis. In addition, recent studies indicated
that upregulated AMF expression is involved in the metastasis of
osteosarcoma (22). Moreover,
silencing AMF causes mesenchymal-to-epithelial transition and
completely prevents pulmonary metastasis of osteosarcoma (23). Thus, suppressing AMF appears to be
an appropriate treatment to control tumor invasion and metastasis;
however, inhibition of AMF expression throughout the body may have
a risk. AMF works as PGI, which is a critical molecule for glucose
metabolism in all kinds of cells and, therefore, suppressing AMF in
the whole body may inhibit a patient’s ability to metabolize
glucose. This is one of the reasons why clinical trials to suppress
AMF have not been performed.
Hyperthermia is an effective local adjuvant therapy
for carcinomas and sarcomas. A randomized phase III trial showed
that regional hyperthermia combined with neo-adjuvant chemotherapy
for soft tissue sarcomas had better local progression-free survival
than chemotherapy alone (24).
Several reports have suggested that hyperthermia for osteosarcoma
achieved an effective response, including the induction of
apoptosis (25) and inhibition of
tumor proliferation (26,27) and DNA synthesis (27)in vitro. Regional hyperthermia
using an alternating magnetic field reduced the pulmonary
metastasis of osteosarcoma in an in vivo study (28). In the present study, we examined the
involvement of AMF and heat shock genes including heat shock
protein (HSP) and tumor cell motility in osteosarcoma cells under
normal and hyperthermic conditions.
Materials and methods
Antibodies and reagents
Anti-AMF/PGI mouse monoclonal antibody was purchased
from ProMab Biotechnologies Inc. (Richmond, CA, USA) and
anti-β-actin mouse monoclonal antibody was purchased from
Sigma-Aldrich Inc. (St. Louis, MO, USA). 17-AAG, a heat shock
protein (HSP)90 inhibitor, KNK437, an HSP70/72/105 inhibitor, and
KRIBB-III, an HSP27 inhibitor were purchased from Selleck Chemicals
Inc. (Houston, TX, USA), Merck Inc. (Darmstadt, Germany) and
Sigma-Aldrich Inc., respectively. The horseradish peroxidase
(HRP)-conjugated goat anti-mouse antibody was purchased from Zymed
Inc. (South San Francisco, CA, USA). The enzyme-linked
immunosorbent assay kit for human glucose 6 phosphate isomerase was
purchased from Uscn Life Science Inc. (Wuhan, China).
Cell culture
The human osteosarcoma cell line HuO9 was kindly
provided by Dr T. Hotta (Niigata University, Niigata, Japan) and
grown in RPMI-1640 supplemented with 10% heat-inactivated fetal
bovine serum (FBS). The cells were maintained at 37°C in a
humidified atmosphere of 5% CO2 and 95% air.
Treatment with hyperthermia and HSP
inhibitors
Culture with hyperthermia was carried out at 41°C
for 24 h in a 5% CO2 incubator. Prior to hyperthermia
exposure, cells were washed with phosphate-buffered saline (PBS),
and fresh medium was added. The concentrations of HSP inhibitors
were less than the cytotoxic level shown in previous reports, with
10 nM for 17-AAG (29) and
KRIBB-III (30) and 10 μM for
KNK437 (31).
DNA microarray analysis
HuO9 cells were separated into two conditions, 41
and 37°C. The isolated total-RNA of the cells in each condition was
used for synthesis of cDNA, which was labeled with biotin and
hybridized with the GeneChip Array, Human Genome U133 Plus 2.0
Array (Affymetrix Inc., Santa Clara, CA, USA). The array was
scanned with a GeneChip 3000 scanner. The signal intensities from
hybridized cDNA were quantified. The final processed data were
obtained by the global normalization method using GCOS
software.
RT-PCR analysis
Total-RNA was isolated from hyperthermia-treated
HuO9 cells with or without HSP inhibitors for 24 h using Isogen
(Wako Pure Chemical Industries, Osaka, Japan). The cDNA was
generated using a SuperScript III First-strand Synthesis SuperMix
(Invitrogen Inc., Carlsbad, CA, USA) as recommended in the
manufacturer’s protocol. The products of reverse transcription
reactions were used for PCR. β-actin was used as an internal
control. The number of amplification cycles for PGI/AMF, β-actin
genes, was 25, respectively, which was selected to allow linear
amplification of the cDNA under study. The primer sequences and
their respective PCR fragment lengths were: PGI/AMF,
5′-AATGCAGAGACGGCGAAGAAG-3′ (forward) and
5′-ACGAGAAGAGAAAGGGGAGTC-3′ (reverse) (1066 bp); β-actin,
5′-TGACGCGGTCACCCACACTGTGCCCAT-3′ (forward) and
5′-CTAGAAGCATTTGCGGTGGGAGGG-3′ (reverse) (610 bp). PCR products
were electrophoresed on 1% agarose gels, stained with ethidium
bromide and photographed.
Sampling intracellular AMF from cell
cultures
HuO9 cells cultured on 10-cm dishes were treated by
hyperthermia with or without HSP inhibitors for 24 h and then
transferred to 37°C for 24 h in a 5% CO2 incubator.
Intracellular proteins were collected by scraping and lysed in
radioimmune precipitation assay buffer (20 mM Tris-HCl, pH 7.4, 150
mM NaCl, 10 mM EDTA, 1% of NP-40, Triton X-100, sodium
deoxycholate) containing 1 mM phenylmethylsulfonyl fluoride. After
cell lysates were centrifuged, the supernatants were subjected to
SDS-PAGE to investigate the expression of intracellular AMF/PGI and
β-actin. The protein concentration of each sample was determined
using Bio-Rad protein assay reagent (Bio-Rad Laboratories Inc.,
Hercules, CA, USA).
Western blot analysis
All protein samples were separated on 10% SDS-PAGE
gels and transferred to a polyvinylidene difluoride membrane
(Millipore Inc., Billerica, MA, USA). Western blotting was carried
out by the SNAP-id protein detection system (Millipore Inc.)
according to the manufacturer’s instructions. The membrane was
blocked with Bløk, a noise-cancelling reagent (Millipore Inc.), for
30 sec at room temperature. The blocked membrane was incubated with
diluted primary antibodies (AMF/PGI 1:1,000, β-actin 1:1,000) for
10 min. Following extensive washing, anti-mouse HRP-conjugated
secondary antibody (1:1,000) was added and incubated for 10 min.
Proteins were visualized using a chemiluminescence (ECL)
system.
ELISA
To examine the concentration of secreted AMF, an
enzyme-linked immunosorbent assay kit for human glucose 6 phosphate
isomerase (Uscn Life Science Inc.) was used according to the
manufacturer’s instructions. The secreted protein was isolated from
RPMI with 10% FBS. HuO9 cells were expanded on 10-cm dishes as a
confluent monolayer. After the cells had been exposed to
hyperthermia with or without HSP inhibitors for 24 h, the medium
was replaced with 5 ml RPMI with 10% FBS. Supernatants were
collected for ELISA after the cells had been incubated at 37°C for
24 h.
Wound healing assay
Horizontal motility was measured by the
wound-healing assay. The surface of the cultured HuO9 cell
monolayer in each prepared well was wounded by a pipette tip and
the medium was replaced. After 24 and 48 h of incubation, the
wounded area was photographed and the wound area filled was
calculated using the formula: % wound area filled = (average wound
width before incubation - average wound width after incubation /
average wound width before incubation) × 100.
Phagokinetic track assay
Random cell motility was measured by the
phagokinetic track assay as previously described. Uniform carpets
of gold particles were prepared on coverslips coated with 1.0%
bovine serum albumin (BSA) by fixing with 100% ethanol and warm air
drying. The treated coverslips were then embedded with colloidal
gold particles and placed in 35-mm tissue culture dishes. Then,
3,000 cells in suspension culture were added to the plates. After
24 h, the phagokinetic tracks were visualized using dark field
illumination with a Nikon inverted microscope. The area cleared of
gold particles by ≥30 cells was measured using NIH Image J.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Wound healing and
phagokinetic track motility assays were analyzed using analysis of
variance (ANOVA) and the significance of individual differences was
evaluated using the Tukey HSD if ANOVA was significant.
Results
Upregulated heat shock proteins in
osteosarcoma cells under hyperthermia
HSP has several subtypes, HSP110/105, HSP90,
HSP72/70, HSP60, HSP40, HSP27, HSP22, and HSP10. We confirmed the
molecular responses of HuO9 cells under hyperthermia using a
microarray. More than 2-fold upregulated genes among HSPs compared
to the control were HSP105, HSP70, HSP27 and HSP22 (Table I). Since there are no reports
showing that HSP22 is involved in cell migration, cancer
metastasis, invasion, or proliferation, KNK437, an HSP70/72/105
inhibitor, and KRIBB-III, an HSP27 inhibitor, were selected in this
study. 17AAG, an HSP90 inhibitor, was also selected as this subtype
has been well examined as a molecular target drug for cancer
therapy (32,33), although the increase of HSP90 gene
expression was slight (1.82-fold).
 | Table IHSP genes >2-fold upregulated by
hyperthermia compared with the control sample. |
Table I
HSP genes >2-fold upregulated by
hyperthermia compared with the control sample.
| Gene name | Gene symbol | GenBank accession
no. | Fold change |
|---|
| Heat shock
105-kDa/110kDa protein 1 | HSPH1 | NM_006644 | 2.77 |
| Heat shock 70-kDa
protein 1A | HSPA1A | NM_005345 | 4.45 |
| Heat shock 70-kDa
protein 1B | HSPA1B | NM_005346 | 4.4 |
| Heat shock 70-kDa
protein 6 (HSP70B′) | HSPA6 | NM_002155 | 6.67 |
| Heat shock 27-kDa
protein 1 | HSPB1 | NM_001540 | 2.84 |
| Heat shock 22-kDa
protein 8 | HSPB8 | AF133207 | 2.41 |
Hyperthermia reduces mRNA level and
secretion of AMF
To analyze the effect of hyperthermia on AMF
expression, the HuO9 osteosarcoma cell line was exposed to
hyperthermia at 41°C and to normal conditions at 37°C for 24 h. The
level of mRNA, intracellular protein and secreted protein was
determined by RT-PCR, western blotting and ELISA, respectively. The
HuO9 osteosarcoma cell line showed a decrease in the mRNA level of
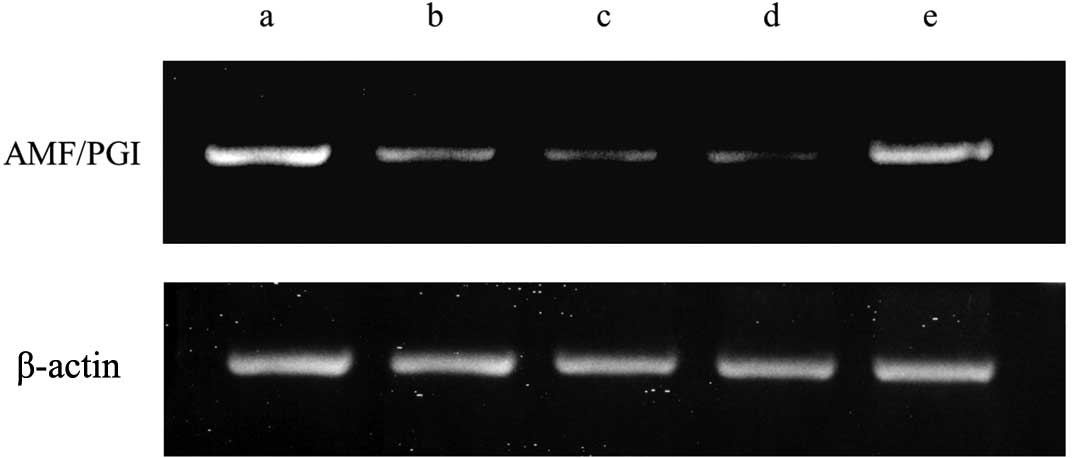
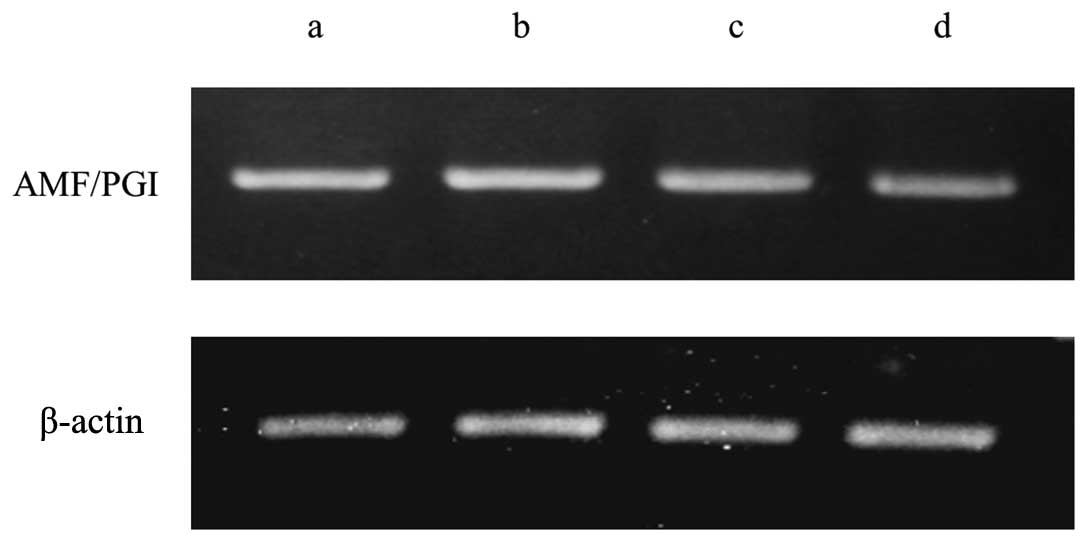
AMF under hyperthermic conditions (Fig.
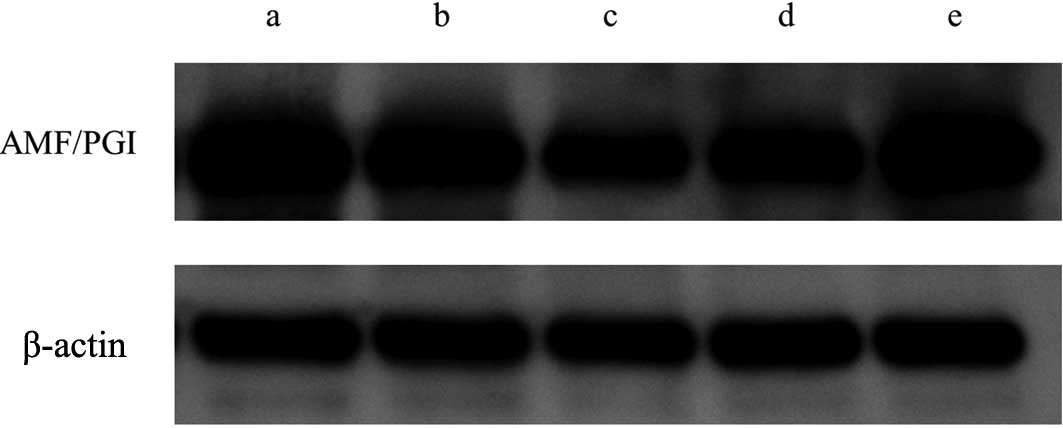
1). Western blotting revealed slight downregulation of
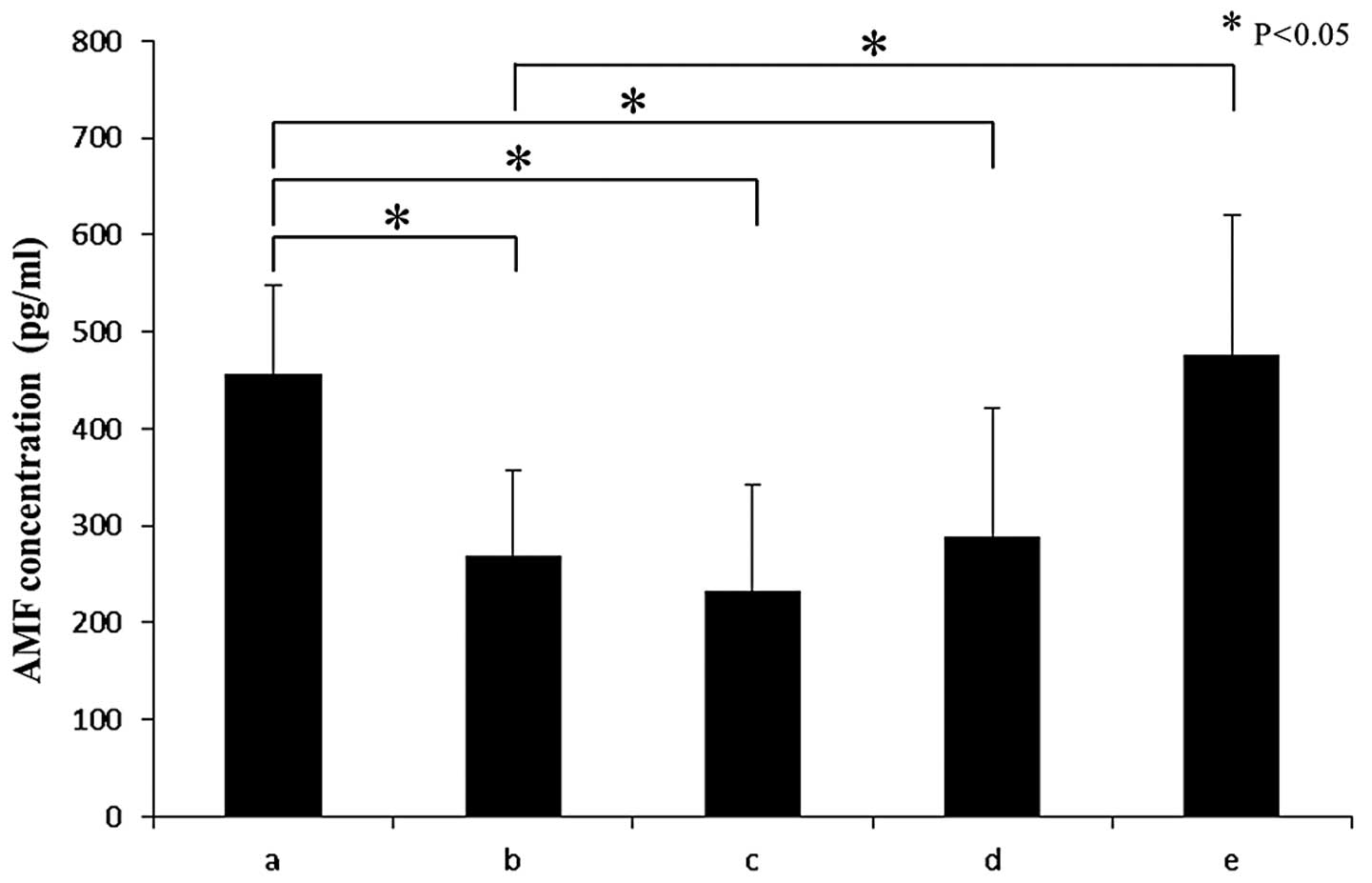
intracellular AMF compared with the control sample (Fig. 2). Secreted AMF measured with ELISA
was significantly downregulated by hyperthermia compared with the
control [hyperthermia; mean 269.0±87.4 (SD) vs control; mean
456.7±90.5 p=0.047] (Fig. 3).
Previous reports showed that the amount of secreted AMF was mainly
dependent on the mRNA level, not on the intracellular protein level
(15–17); therefore, we concluded that
hyperthermia reduced the expression of mRNA of AMF and subsequently
suppressed the secretion of AMF.
Hyperthermia reduces the motility of
osteosarcoma
The motility of osteosarcoma cells was significantly
decreased under hyperthermia compared to the control sample at a
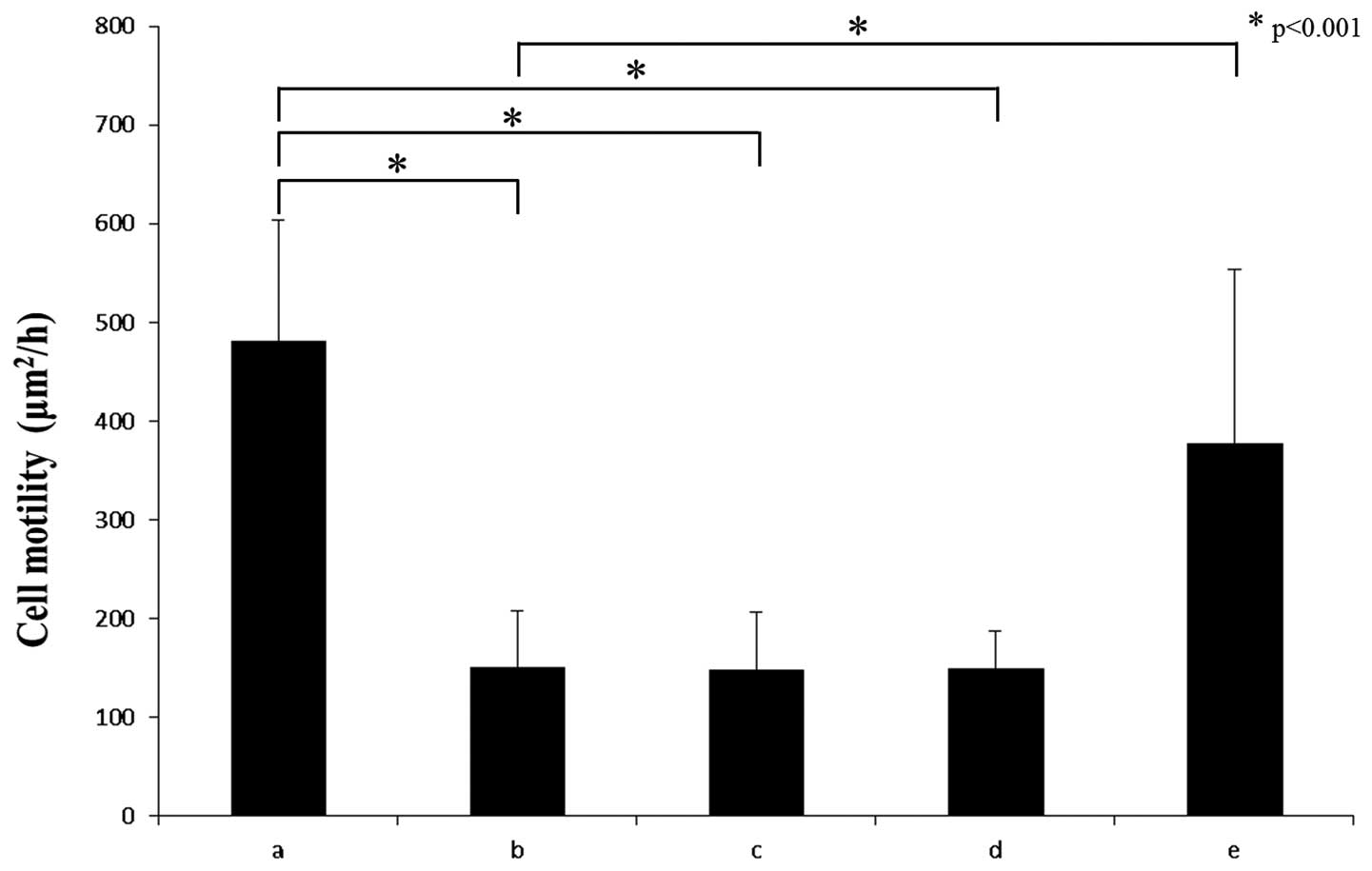
normal temperature. The phagokinetic track assay showed that random
motility of HuO9 cells was significantly suppressed by hyperthermia
(p<0.001) (Fig. 4). Furthermore,
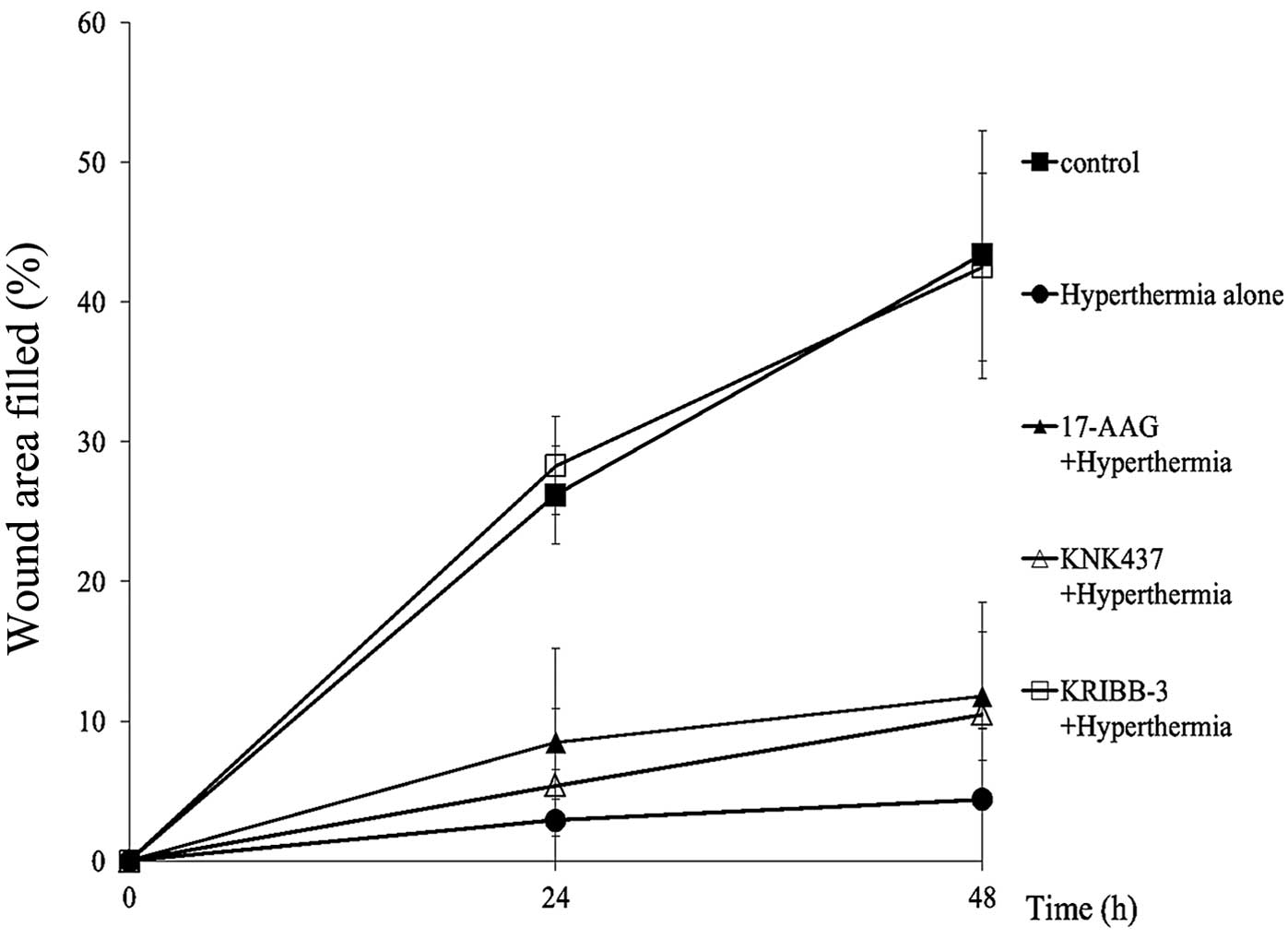
the wound-healing assay revealed the significant suppression of
horizontal motility under hyperthermia. The difference was apparent
at 24 h (hyperthermia; mean 2.9±3.6 vs control; mean 26.2±3.5
p=0.004) and at 48 h (hyperthermia; mean 4.4±5.1 vs control; mean
43.4±8.9 p=0.001) after hyperthermia (Fig. 5).
Effect of HSP inhibitors on AMF
expression and motility under hyperthermic and normal
conditions
To explore which HSP pathways play an important role
in the regulation of AMF under hyperthermia, we treated HuO9 cells
with various HSP inhibitors during hyperthermic exposure. The AMF
mRNA level downregulated by hyperthermia was recovered to almost
the same level at 37°C by the addition of an HSP27 inhibitor,
KRIBB-III (Fig. 1). Western
blotting and ELISA also showed the recovery of intracellular and
secreted AMF by the addition of KRIBB-III under hyperthermic
conditions, as expected (p=0.024) (Figs. 2 and 3). These results suggest that the
downregulation of AMF expression under hyperthermia is associated
with HSP27. As shown in Figs. 4 and
5, the recovery of cell motility
was observed under hyperthermic conditions with KRIBB-III by the
phagokinetic track (p<0.001) and wound-healing assay,
respectively. Meanwhile, an HSP90 inhibitor, 17-AAG, and an
HSP70/72/105 inhibitor, KNK437, had no statistically significant
effect on AMF expression and motility compared with hyperthermia
alone. No HSP inhibitors affected AMF expression under normal
conditions (Fig. 6).
Discussion
In the present study, we discovered that
hyperthermia reduced the mRNA level of AMF, secreted AMF and the
motility of osteosarcoma cells. The difference in the amount of
secreted AMF between the hyperthermia-treated sample and control
was approximately 180 pg/ml, which appeared sufficient to suppress
cell motility since a previous study indicated that addition of AMF
100 pg/ml enhanced motility by 1.5-fold (7,13). A
few reports have described the mechanisms of how hyperthermia
affects tumor cell migration, although there have been many reports
on thermal therapy preventing tumor cell proliferation. Sato et
al reported that hyperthermia suppressed the invasion of
fibrosarcoma by inhibiting the production of membrane type-1 matrix
metalloproteinase (MMP) and proMMP-2 activity (34). In the present study, we found that a
new mechanism was involved in the suppression of osteosarcoma cell
motility under hyperthermia via AMF downregulation.
To date, no clinically available agents inhibiting
AMF have been reported, although some articles have reported that
silencing AMF by RNA interference (35,36) or
hammerhead ribozyme (23) could
inhibit metastasis and the invasion of malignant tumors. Since AMF
works as PGI, which plays an important role in glucose metabolism
not only in tumors but also in normal cells (15,37,38),
complete block of AMF/PGI throughout the body may be harmful or
even lethal for normal cells, compelling us to identify procedures
that regulate AMF expression locally. Hyperthermia is well known as
a clinically available modality for cancer therapy. Regional
hyperthermia combined with neo-adjuvant chemotherapy for
soft-tissue sarcomas showed better local progression-free survival
than chemotherapy alone in a randomized study (24). Hyperthermic isolated limb perfusion
with pre-operative chemotherapy for osteosarcoma patients achieved
a good response compared to chemotherapy without hyperthermia
(39). We expect that regional
hyperthermia of osteosarcomas can control AMF secretion from tumor
cells without affecting the glucose metabolism throughout the body
and prevent tumor invasion and metastasis.
Little is known about the regulatory mechanism of
AMF expression as promoter region analysis of AMF remains
insufficient, except for one report showing that a minisatellite in
intron 9 of human PGI genes stimulated transcription from PGI
promoter (40). Hypoxia induces AMF
expression via HIF-1α (41);
however, there have been no reports on the proteins or conditions
that downregulate AMF expression. In our study, reduced AMF
expression by hyperthermia was recovered by the addition of the
HSP27 inhibitor, which indicates that HSP27 inhibits AMF expression
and tumor cell motility under hyperthermia.
HSPs are induced not only by heat shock but also by
other pathological conditions and work as molecular chaperones in
maintaining cellular homeostasis and contributing to cell survival
(42). Mammalian HSPs have been
classified into six groups and HSP27 belongs to small HSPs. Largely
oligomerized HSP27 works as a chaperone preventing aggregation
while a small oligomer of this molecule stabilizes actin filaments
(43). In immunohistochemical
studies, overexpression of HSP27 was associated with poor prognosis
(44) and distant metastasis
(45) in osteosarcoma patients and
was found at a higher rate in high-grade than low-grade
osteosarcomas (45). HSP27
expression was associated with favorable prognosis only in
malignant fibrous histiocytoma among many types of cancer (46). Shin et al reported that the
HSP27 inhibitor inhibited cancer proliferation and migration by
blocking HSP27 phosphorylation under normal conditions (30). There have been several reports on
the use of the HSP27 inhibitor under normal conditions, but there
are no reports on its use under hyperthermia. We speculated that
the discrepancy in the effects of HSP27 on tumor cell migration
between our results and previous reports is likely due to a
difference in HSP27 function between normal and hyperthermic
conditions. Hyperthermia treatment presents a theoretical dilemma;
heat shock stress induces various heat shock proteins, the
expression of which is related to poor prognosis and the inhibitors
of which are clinically used for cancer therapy (43), although thermal treatments have
achieved favorable treatment results. Our hypothesis that HSP27
functions depend on temperature (normal or hyperthermia) may
resolve the dilemma of hyperthermia treatments. In addition,
hyperthermia is expected to be more effective for HSP27-expressing
osteosarcoma as HSP27 has a propensity to suppress tumor migration
under hyperthermic conditions.
In conclusion, hyperthermia suppressed the
expression of AMF and the motility of the HuO9 osteosarcoma cell
line via HSP27. Our results suggest that hyperthermia is effective
in preventing the invasion and metastasis of osteosarcoma by
reducing AMF, and HSP27 regulates AMF expression under
hyperthermia. Therefore, hyperthermia may be a clinical therapeutic
modality for osteosarcoma in, for example, adjuvant and palliative
therapies.
Abbreviations:
|
AMF
|
autocrine motility factor
|
|
PGI
|
phosphoglucose isomerase
|
|
HSP
|
heat shock protein
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G, et
al: Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: an analysis of 1,702 patients treated on
neoadjuvant cooperative osteosarcoma study group protocols. J Clin
Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
2
|
Whelan JS, Jinks RC, McTiernan A, et al:
Survival from high-grade localised extremity osteosarcoma: combined
results and prognostic factors from three European Osteosarcoma
Intergroup randomised controlled trials. Ann Oncol. Oct
19–2011.(Epub ahead of print). View Article : Google Scholar
|
|
3
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006.PubMed/NCBI
|
|
4
|
Ferrari S, Smeland S, Mercuri M, et al:
Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose
methotrexate, cisplatin, and doxorubicin for patients with
localized osteosarcoma of the extremity: a joint study by the
Italian and Scandinavian Sarcoma Groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar
|
|
5
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsutsumi S, Yanagawa T, Shimura T, et al:
Regulation of cell proliferation by autocrine motility
factor/phosphoglucose isomerase signaling. J Biol Chem.
278:32165–32172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silletti S, Watanabe H, Hogan V, Nabi IR
and Raz A: Purification of B16-F1 melanoma autocrine motility
factor and its receptor. Cancer Res. 51:3507–3511. 1991.PubMed/NCBI
|
|
8
|
Watanabe H, Kanbe K and Chigira M:
Differential purification of autocrine motility factor derived from
a murine protein-free fibrosarcoma. Clin Exp Metastasis.
12:155–163. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Funasaka T, Haga A, Raz A and Nagase H:
Tumor autocrine motility factor is an angiogenic factor that
stimulates endothelial cell motility. Biochem Biophys Res Commun.
284:1116–1125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Funasaka T, Haga A, Raz A and Nagase H:
Autocrine motility factor secreted by tumor cells upregulates
vascular endothelial growth factor receptor (Flt-1) expression in
endothelial cells. Int J Cancer. 101:217–223. 2002. View Article : Google Scholar
|
|
11
|
Tsutsumi S, Hogan V, Nabi IR and Raz A:
Overexpression of the autocrine motility factor/phosphoglucose
isomerase induces transformation and survival of NIH-3T3
fibroblasts. Cancer Res. 63:242–249. 2003.PubMed/NCBI
|
|
12
|
Haga A, Funasaka T, Niinaka Y, Raz A and
Nagase H: Autocrine motility factor signaling induces tumor
apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome
expression. Int J Cancer. 107:707–714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe H, Takehana K, Date M, Shinozaki
T and Raz A: Tumor cell autocrine motility factor is the
neuroleukin/phosphohexose isomerase polypeptide. Cancer Res.
56:2960–2963. 1996.PubMed/NCBI
|
|
14
|
Faik P, Walker JI, Redmill AA and Morgan
MJ: Mouse glucose-6-phosphate isomerase and neuroleukin have
identical 3′ sequences. Nature. 332:455–457. 1988.
|
|
15
|
Niinaka Y, Paku S, Haga A, Watanabe H and
Raz A: Expression and secretion of neuroleukin/phosphohexose
isomerase/maturation factor as autocrine motility factor by tumor
cells. Cancer Res. 58:2667–2674. 1998.PubMed/NCBI
|
|
16
|
Yanagawa T, Watanabe H, Takeuchi T,
Fujimoto S, Kurihara H and Takagishi K: Overexpression of autocrine
motility factor in metastatic tumor cells: possible association
with augmented expression of KIF3A and GDI-beta. Lab Invest.
84:513–522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagawa T, Funasaka T, Tsutsumi S, Raz T,
Tanaka N and Raz A: Differential regulation of phosphoglucose
isomerase/autocrine motility factor activities by protein kinase
CK2 phosphorylation. J Biol Chem. 280:10419–10426. 2005. View Article : Google Scholar
|
|
18
|
Baumann M, Kappl A, Lang T, Brand K,
Siegfried W and Paterok E: The diagnostic validity of the serum
tumor marker phosphohexose isomerase (PHI) in patients with
gastrointestinal, kidney, and breast cancer. Cancer Invest.
8:351–356. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filella X, Molina R, Jo J, Mas E and
Ballesta AM: Serum phosphohexose isomerase activities in patients
with colorectal cancer. Tumour Biol. 12:360–367. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bodansky O: Serum phosphohexose isomerase
in cancer. II. As an index of tumor growth in metastatic carcinoma
of the breast. Cancer. 7:1200–1226. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel PS, Raval GN, Rawal RM, et al:
Comparison between serum levels of carcinoembryonic antigen, sialic
acid and phosphohexose isomerase in lung cancer. Neoplasma.
42:271–274. 1995.PubMed/NCBI
|
|
22
|
Dobashi Y, Watanabe H, Matsubara M, et al:
Autocrine motility factor/glucose-6-phosphate isomerase is a
possible predictor of metastasis in bone and soft tissue tumours. J
Pathol. 208:44–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niinaka Y, Harada K, Fujimuro M, et al:
Silencing of autocrine motility factor induces
mesenchymal-to-epithelial transition and suppression of
osteosarcoma pulmonary metastasis. Cancer Res. 70:9483–9493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Issels RD, Lindner LH, Verweij J, et al:
Neo-adjuvant chemotherapy alone or with regional hyperthermia for
localised high-risk soft-tissue sarcoma: a randomised phase 3
multicentre study. Lancet Oncol. 11:561–570. 2010. View Article : Google Scholar
|
|
25
|
Alcaide M, Ramírez-Santillán C, Feito MJ,
et al: In vitro evaluation of glass-glass ceramic
thermoseed-induced hyperthermia on human osteosarcoma cell line. J
Biomed Mater Res A. 100:64–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trieb K, Blahovec H and Kubista B: Effects
of hyperthermia on heat shock protein expression, alkaline
phosphatase activity and proliferation in human osteosarcoma cells.
Cell Biochem Funct. 25:669–672. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shui C and Scutt A: Mild heat shock
induces proliferation, alkaline phosphatase activity, and
mineralization in human bone marrow stromal cells and Mg-63 cells
in vitro. J Bone Miner Res. 16:731–741. 2001. View Article : Google Scholar
|
|
28
|
Shido Y, Nishida Y, Suzuki Y, Kobayashi T
and Ishiguro N: Targeted hyperthermia using magnetite cationic
liposomes and an alternating magnetic field in a mouse osteosarcoma
model. J Bone Joint Surg Br. 92:580–585. 2010. View Article : Google Scholar
|
|
29
|
Bagatell R, Beliakoff J, David CL, Marron
MT and Whitesell L: Hsp90 inhibitors deplete key anti-apoptotic
proteins in pediatric solid tumor cells and demonstrate synergistic
anticancer activity with cisplatin. Int J Cancer. 113:179–188.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin KD, Lee MY, Shin DS, et al: Blocking
tumor cell migration and invasion with biphenyl isoxazole
derivative KRIBB3, a synthetic molecule that inhibits Hsp27
phosphorylation. J Biol Chem. 280:41439–41448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yokota S, Kitahara M and Nagata K:
Benzylidene lactam compound, KNK437, a novel inhibitor of
acquisition of thermotolerance and heat shock protein induction in
human colon carcinoma cells. Cancer Res. 60:2942–2948.
2000.PubMed/NCBI
|
|
32
|
Bagatell R, Gore L, Egorin MJ, et al:
Phase I pharmacokinetic and pharmacodynamic study of
17-N-allylamino-17-demethoxygeldanamycin in pediatric patients with
recurrent or refractory solid tumors: a pediatric oncology
experimental therapeutics investigators consortium study. Clin
Cancer Res. 13:1783–1788. 2007. View Article : Google Scholar
|
|
33
|
Koga F, Kihara K and Neckers L: Inhibition
of cancer invasion and metastasis by targeting the molecular
chaperone heat-shock protein 90. Anticancer Res. 29:797–807.
2009.PubMed/NCBI
|
|
34
|
Sato T, Sawaji Y, Matsui N, et al: Heat
shock suppresses membrane type 1-matrix metalloproteinase
production and progelatinase A activation in human fibrosarcoma
HT-1080 cells and thereby inhibits cellular invasion. Biochem
Biophys Res Commun. 265:189–193. 1999. View Article : Google Scholar
|
|
35
|
Funasaka T, Hu H, Yanagawa T, Hogan V and
Raz A: Down-regulation of phosphoglucose isomerase/autocrine
motility factor results in mesenchymal-to-epithelial transition of
human lung fibrosarcoma cells. Cancer Res. 67:4236–4243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Funasaka T, Hogan V and Raz A:
Phosphoglucose isomerase/autocrine motility factor mediates
epithelial and mesenchymal phenotype conversions in breast cancer.
Cancer Res. 69:5349–5356. 2009. View Article : Google Scholar
|
|
37
|
Kugler W, Breme K, Laspe P, et al:
Molecular basis of neurological dysfunction coupled with haemolytic
anaemia in human glucose-6-phosphate isomerase (GPI) deficiency.
Hum Genet. 103:450–454. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yanagawa T, Funasaka T, Tsutsumi S, Hu H,
Watanabe H and Raz A: Regulation of phosphoglucose
isomerase/autocrine motility factor activities by the
poly(ADP-ribose) polymerase family-14. Cancer Res. 67:8682–8689.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakano H, Tateishi A, Miki H, et al:
Hyperthermic isolated regional perfusion for the treatment of
osteosarcoma in the lower extremity. Am J Surg. 178:27–32. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Williams RR, Hassan-Walker AF, Lavender
FL, Morgan M, Faik P and Ragoussis J: The minisatellite of the
GPI/AMF/NLK/MF gene: interspecies conservation and transcriptional
activity. Gene. 269:81–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Funasaka T, Yanagawa T, Hogan V and Raz A:
Regulation of phosphoglucose isomerase/autocrine motility factor
expression by hypoxia. FASEB J. 19:1422–1430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khalil AA, Kabapy NF, Deraz SF and Smith
C: Heat shock proteins in oncology: diagnostic biomarkers or
therapeutic targets? Biochim Biophys Acta. 1816:89–104.
2011.PubMed/NCBI
|
|
43
|
Jego G, Hazoumé A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
Nov 13–2010.(Epub ahead of print).
|
|
44
|
Uozaki H, Horiuchi H, Ishida T, Iijima T,
Imamura T and Machinami R: Overexpression of resistance-related
proteins (metallothioneins, glutathione-S-transferase pi, heat
shock protein 27, and lung resistance-related protein) in
osteosarcoma. Relationship with poor prognosis. Cancer.
79:2336–2344. 1997. View Article : Google Scholar
|
|
45
|
Moon A, Bacchini P, Bertoni F, et al:
Expression of heat shock proteins in osteosarcomas. Pathology.
42:421–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Têtu B, Lacasse B, Bouchard HL, Lagacé R,
Huot J and Landry J: Prognostic influence of HSP-27 expression in
malignant fibrous histiocytoma: a clinicopathological and
immunohistochemical study. Cancer Res. 52:2325–2328.
1992.PubMed/NCBI
|