Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer worldwide and the third most common cause of
cancer-related mortality, resulting in approximately 500,000 deaths
per annum. Most HCC cases occur in either Eastern Asia
(particularly in China) or sub-Saharan Africa (1). Currently, the prognosis for HCC is
poor, as no effective therapy has yet been developed (2). Therefore, developing effective
therapeutic agents to treat HCC are of paramount importance.
Casticin is one of the main components of the fruits
of Vitex rotundifolia L. Casticin has been widely used in
Chinese traditional medicine as an anti-inflammatory drug for
thousands of years (3). In recent
years, increasing experimental evidence has been provided that
casticin exhibits anti-carcinogenic activity in breast (4), cervical (5–7), lung
(8), colon cancer (9) and HCC (10). It has been proposed that cell cycle
arrest and apoptosis induced by casticin are the possible
mechanisms for its anticancer effects. However, the precise
underlying mechanisms have not been fully elucidated.
Forkhead box class O (FOXO) subfamily of forkhead
transcription factors include FOXO1a/FKHR, FOXO3a/FKHRL1 and
FOXO4/AFX (11,12). FOXO3a possesses a large number of
functions, including cellular proliferation, transformation,
differentiation and longevity. Recent studies suggest that the
phosphorylation of FOXO3a at threonine-32 plays an important role
in deciding the function of FOXO3a. The phosphorylation results in
impairment of DNA binding ability and results in an increased
binding affinity for the 14-3-3 protein (13). Newly formed 14-3-3-FOXO complexes
are then exported from the nucleus (14), thereby inhibiting FOXO-dependent
transcription. Dephosphorylation of active FOXO3a induces cell
cycle arrest and apoptosis (15).
It has been reported that FOXO3a may be a potentially important
prognostic factor in HCC (16). Fei
et al(17) demonstrated that
arsenic trioxide induced the growth arrest of HCC cells involving
FOXO3a expression and phosphorylation. Forkhead box M1 (FOXM1)
belongs to the forkhead box transcription factor family and is a
downstream target of FOXO3a (18).
FoxM1 is a proliferation-associated transcription factor that is
frequently upregulated in different types of cancers including HCC
(19). Wang et al(20) demonstrated that FOXM1 was a novel
target of a natural agent in pancreatic cancer. However, the
intracellular mechanisms by which casticin inhibits growth in HCC
cells through regulation of the FOXO3a/FOXM1 pathway have never
been investigated.
In the present study, we demonstrated that casticin
induced FOXO3a dephosphorylation and FOXM1 inactivation, leading to
growth inhibition and cell cycle arrest in HCC cells. These results
suggest that forkhead transcription factor FOXM1 is a downstream
cellular target and a potential novel marker for casticin action
and that casticin activates FOXO3a to suppress FOXM1 expression in
HCC cells.
Materials and methods
Chemicals
Casticin was purchased from Chengdu Biopurify
Phytochemicals Ltd. (Chengdu, China). It has a molecular weight of
374.3 ku, appears as yellow crystals and has a purity of 98.0%.
Casticin was prepared in dimethyl sulfoxide (DMSO) as a 10 mmol/l
stock solution and diluted in medium to the indicated concentration
before use. The following items were purchased from Hunan
Clonetimes Biotech Co., Ltd. (Changsha, China): RPMI-1640 medium
(Invitrogen Life Technologies, Carlsbad, CA, USA), fetal bovine
serum (Invitrogen Life Technologies), propidium iodide (PI;
Sigma-Aldrich, St. Louis, MO, USA), antibodies against FOXO3a,
phospho-FOXO3a-Thr32 (Millipore), FoxM1, cyclin dependent kinase
(CDK1), cyclin B, p27KIP1, cdc25B and β-actin (Santa
Cruz Biotechnology, Inc.). Lipofectamine 2000 was purchased from
Invitrogen Life Technologies. Protease inhibitor cocktail and all
other chemicals were obtained from Sigma.
Cells and cell culture
Hep G2 (p53 wild-type) and PLC/PRF/5 (p53 mutant)
cells were obtained from American Type Culture Collection
(Rockville, MD, USA). They were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 μg/ml streptomycin (Invitrogen Life Technologies) in an
incubator containing 50 ml/l CO2 at 37˚C.
Clonogenic assay
Cells were plated in 24-well plates at a density of
300 cells/well for 24 h, prior to the addition of various
concentrations of casticin (2.5, 5.0 and 10.0 μmol/l). After 24 h
of treatment, the drug-containing medium was removed and replaced
with complete growth medium. Medium was changed every three days
for 10 days until visible colonies formed. Colonies were
simultaneously fixed and stained with Wright-Giemsa solution in
methanol and manually counted. Individually stained colonies in
each well were counted. The colony formation fraction was
calculated as follows: Colony number/(number of cells seeded ×
plating efficiency), where plating efficiency was equivalent to the
colony number divided by the number of cells seeded in the
drug-free medium.
Cell cycle analysis
Cell cycle analysis was performed using PI staining
as described previously (21).
Briefly, cells were washed in PBS and fixed in 90% ethanol. Fixed
cells were then washed twice in PBS and stained in 50 μM PI
containing 5 μg/ml DNase-free RNase for 1 h. They were analyzed by
flow cytometry (FCM) using a FACScan (Coulter Epics XL-MSL; Beckman
Coulter, Fullerton, CA, USA) and winMDI software.
RNA interference
A control non-specific small interfering RNA (siRNA)
(UUCUCCGAACGUGUCACGUdTdT) was purchased from Qiagen. FOXO3a siRNA
(ACUCCGGGUC CAGCUCCAC) was synthesized by Shanghai GenePharma Co.
(Shanghai, China). Transfection of siRNA was carried out with
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
procedure recommended by the manufacturer. Twenty-four hours after
transfection, the cells were treated with DMSO (control) or
casticin at the indicated concentrations for 24 h. The cells were
then collected and processed for western blotting and clonogenic
assay.
Western blot analysis
Desired cells (1×106) were seeded in
100-mm culture dishes, allowed to attach by overnight incubation
and treated with DMSO (control) or 2.5, 5.0 and 10.0 μmol/l
casticin for specified time periods. The cell lysates were prepared
as described by us previously (22). The cell lysates were cleared by
centrifugation at 14,000 rpm for 30 min. The lysate proteins were
resolved by 10 or 12.5% SDS-PAGE and were transferred onto a
polyvinylidene fluoride membrane. The membrane was incubated with
Tris-buffered saline containing 0.05% Tween-20 and 5% (w/v) non-fat
dry milk. The membrane was then treated with the desired primary
antibody for 1 h at room temperature or overnight at 4˚C. Following
treatment with the appropriate secondary antibody, the
immunoreactive bands were visualized using the enhanced
chemiluminescence method. The blots were stripped and re-probed
with anti-actin antibody to normalize for differences in protein
loading. Change in the level of desired protein was determined by
densitometric scanning of the immunoreactive band and was corrected
for β-actin loading control. Immunoblotting for each protein was
performed at least twice using independently prepared lysates to
ensure reproducibility of the results.
Statistical analysis
The database was set up with the SPSS 15.0 software
package (SPSS Inc., Chicago, IL, USA) to be analyzed. Data are
represented as means ± SD. The means of multiple groups were
compared with one-way ANOVA, after the equal assessment of
variance. The comparisons among the means were performed using the
LSD method. Statistical comparison was also performed with the
two-tailed t-test when appropriate. A P<0.05 was considered to
indicate a statistically significant result.
Results
Casticin induces growth inhibition and
cell cycle arrest in HCC cells
Since the previous study demonstrated that casticin
inhibited the viability of HCC cells using an MTT assay (10), we first examined the effect of
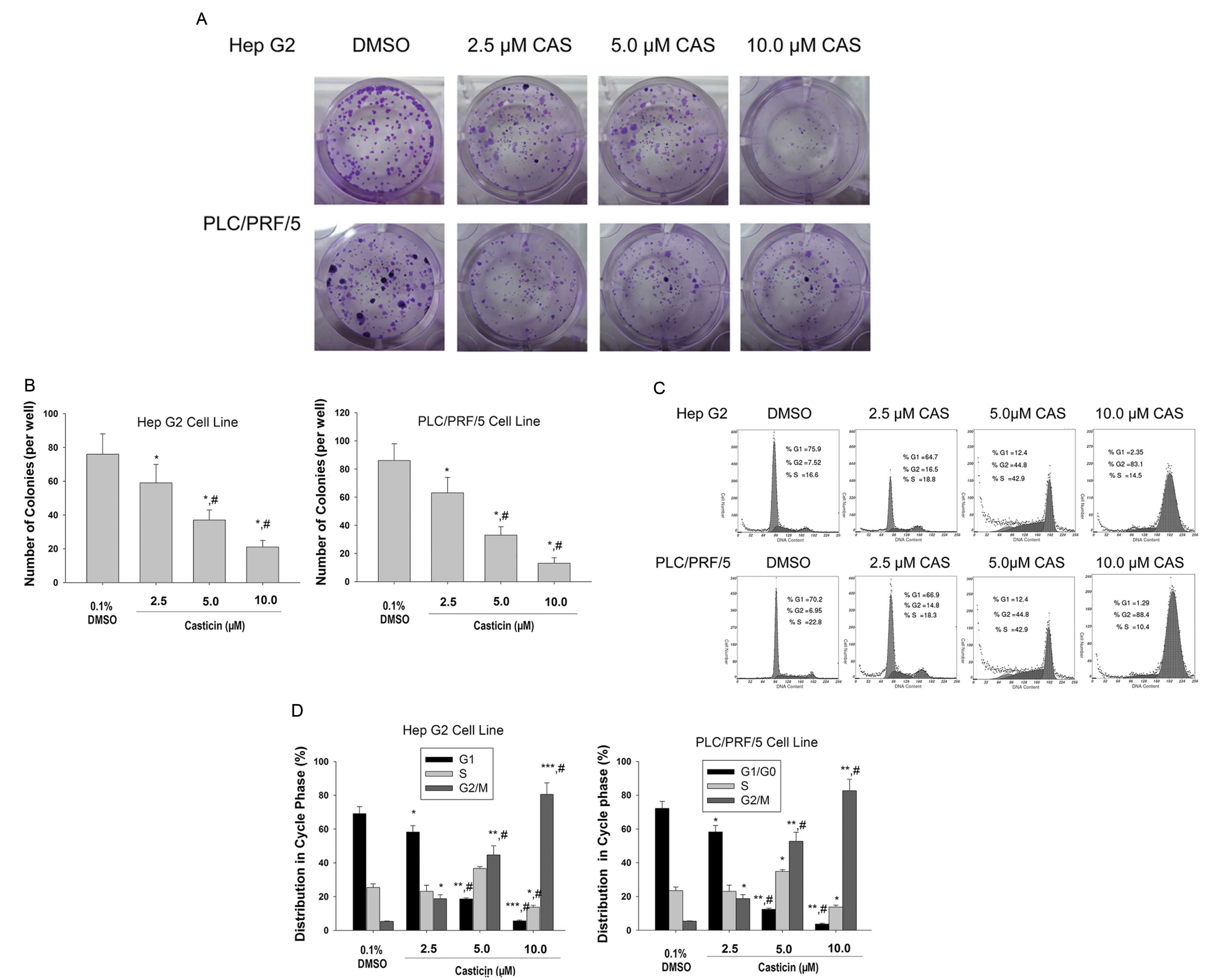
casticin on the cell growth by a clonogenic assay. Fig. 1A and B shows that casticin treatment
resulted in a significant inhibition of colony formation of Hep G2
and PLC/PRF/5 cells when compared to the control.
We next sought to evaluate the effects of casticin
treatment on the phase distribution of the cell cycle using FCM
after PI staining. As shown in Fig. 1C
and D, casticin treatment caused a significant accumulation of
cells in the G2/M phase and a marked decrease of cells in the G1/G0
phase when compared to control cells. These results revealed that
casticin induced the growth inhibition and cell cycle arrest in the
G2/M phase in HCC cells.
Casticin downregulates the expression of
FOXM1 in HCC cells
It has been previously reported that the loss of
FOXM1 expression induces the growth inhibition and cell cycle
arrest in HCC cells (23). We
investigated whether casticin regulates FOXM1 expression during
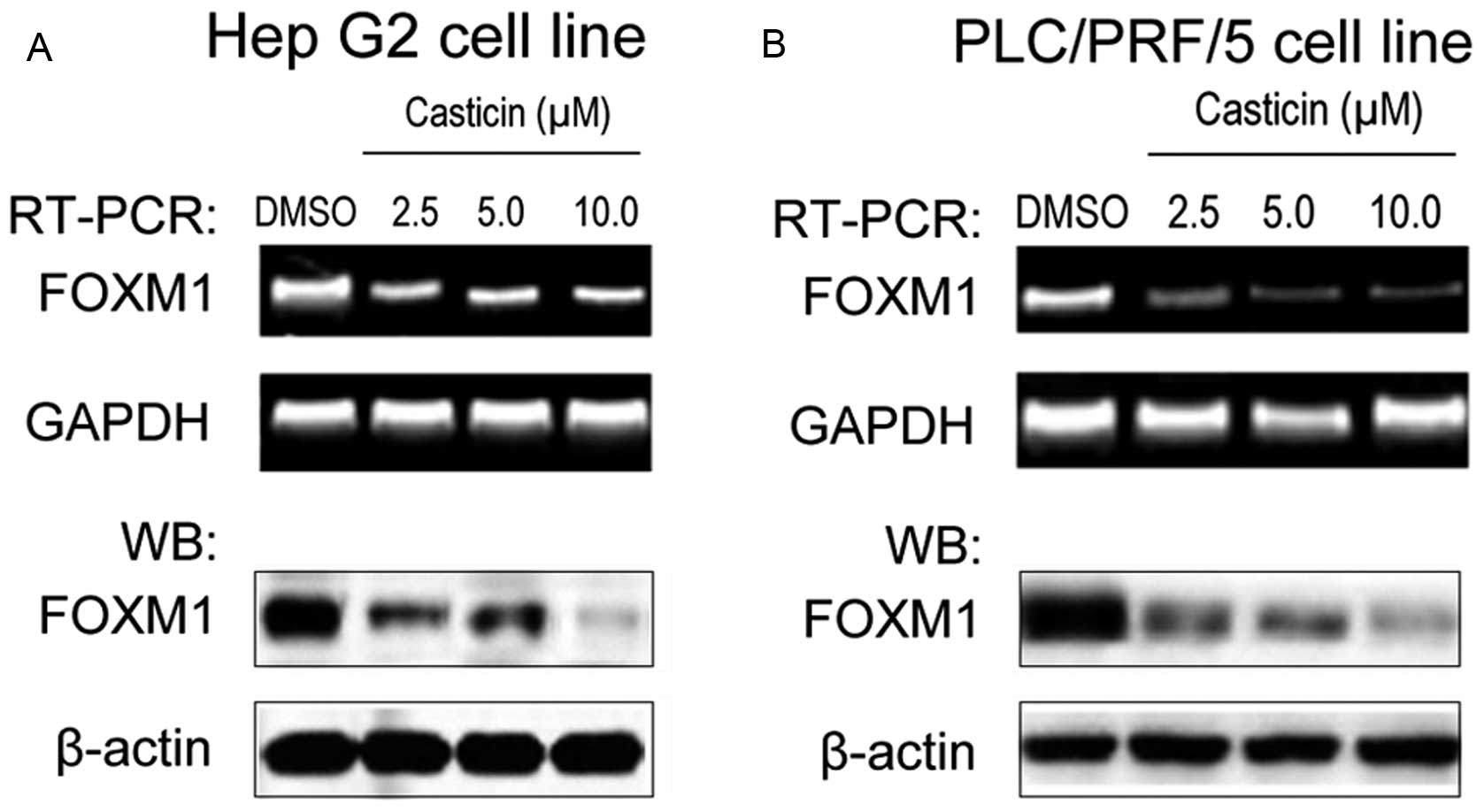
casticin-induced growth inhibition in HCC cells. The results
revealed that FOXM1 was overexpressed in the Hep G2 (Fig. 2A) and PLC/PRF/5 cell lines (Fig. 2B). Exposure of cells to 2.5, 5.0,
10.0 μmol/l casticin for 24 h significantly reduced the expression
of FoxM1 at the mRNA and protein levels (Fig. 2).
Casticin modulates the expression of
downstream targets of FOXM1 in HCC cells
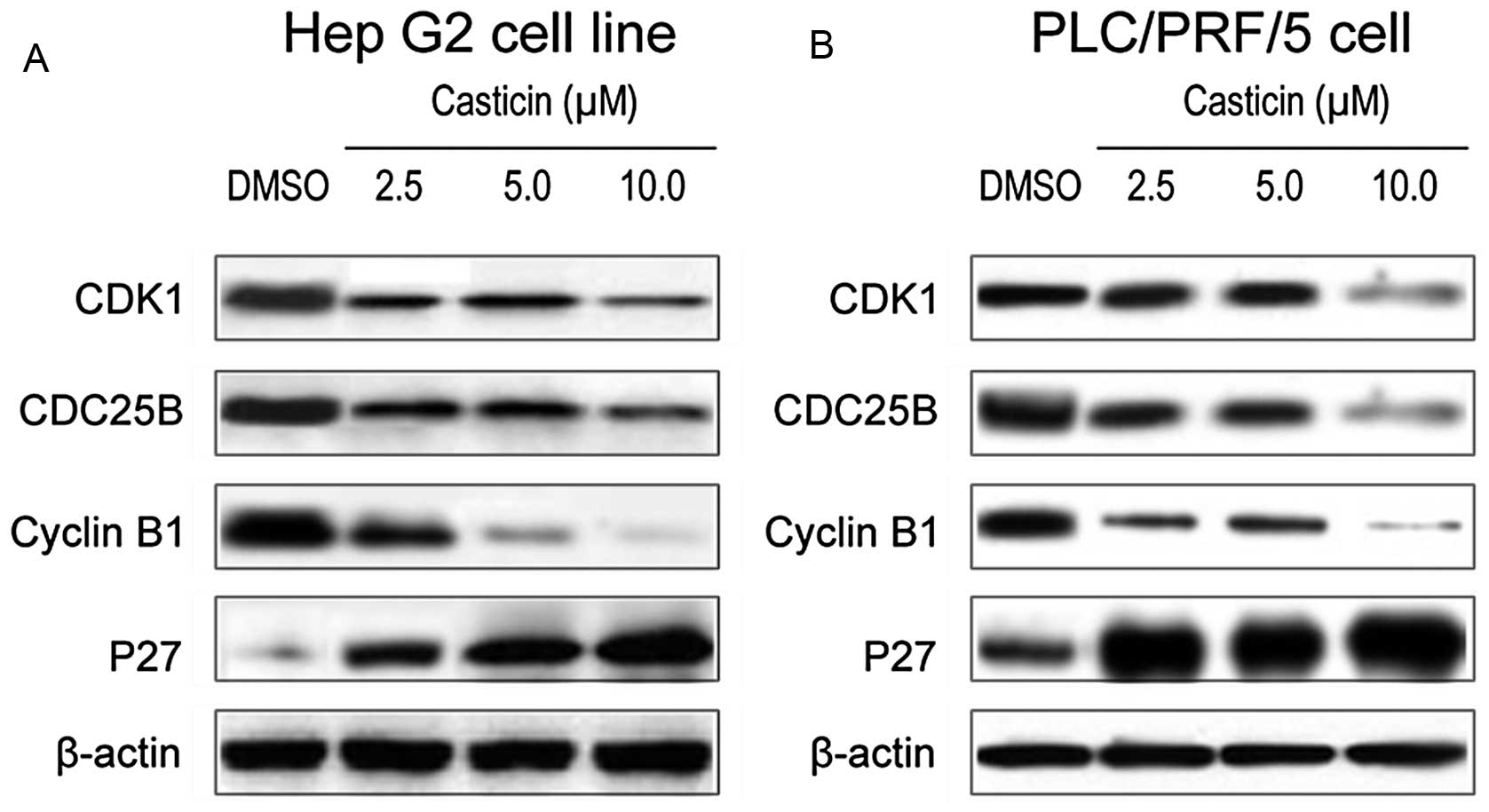
To further confirm the effect of casticin on FOXM1
functional regulation, we assessed the expression of downstream
target genes of FOXM1 in HCC cells after casticin treatment. It is
well known that FOXM1 has several downstream target genes, such as
CDK1, CDC25B, cyclin B1 and p27KIP1, for the regulation
of growth and cell cycle arrest in cells. Western blot analysis
results showed that casticin inhibited the expression of CDK1,
CDC25B, cyclin B1 and increased p27KIP1 in Hep G2
(Fig. 3A) and PLC/PRF/5 (Fig. 3B) cells at the protein level. These
results provide molecular evidence suggesting that the
casticin-induced growth inhibition and cell cycle arrest in HCC
cells may be mediated via inactivation of the FoxM1 function.
Casticin decreases the phosphorylation
level of FOXO3a protein in HCC cells
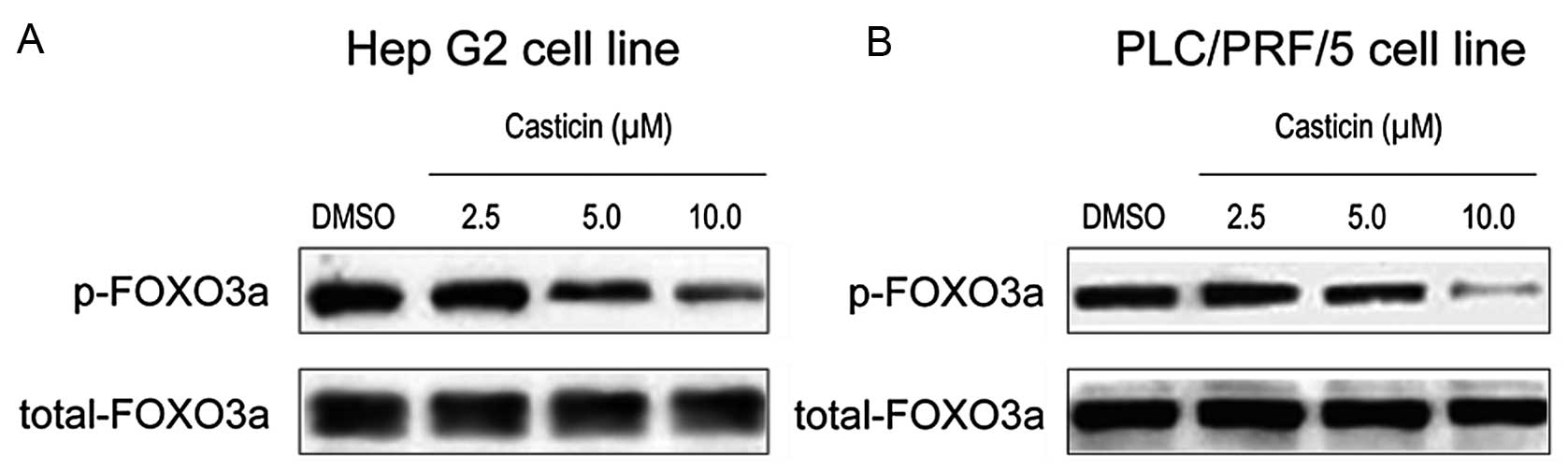
Since FOXO3a is the upstream regulator of the FOXM1
transcription factor (24), we
sought to examine the expression of phosphorylated FOXO3a protein
in order to explain the mechanism for the effect of casticin on
FOXM1 inhibition. Western blotting showed that treatment with
casticin led to a decrease in the phosphorylation level of FOXO3a
and a corresponding reduction in the expression of FOXM1 in HCC
cells (Fig. 4A and B). These
results indicate that the inhibition of FOXM1 expression by
casticin may be associated with the inhibition of FOXO3a
phosphorylation.
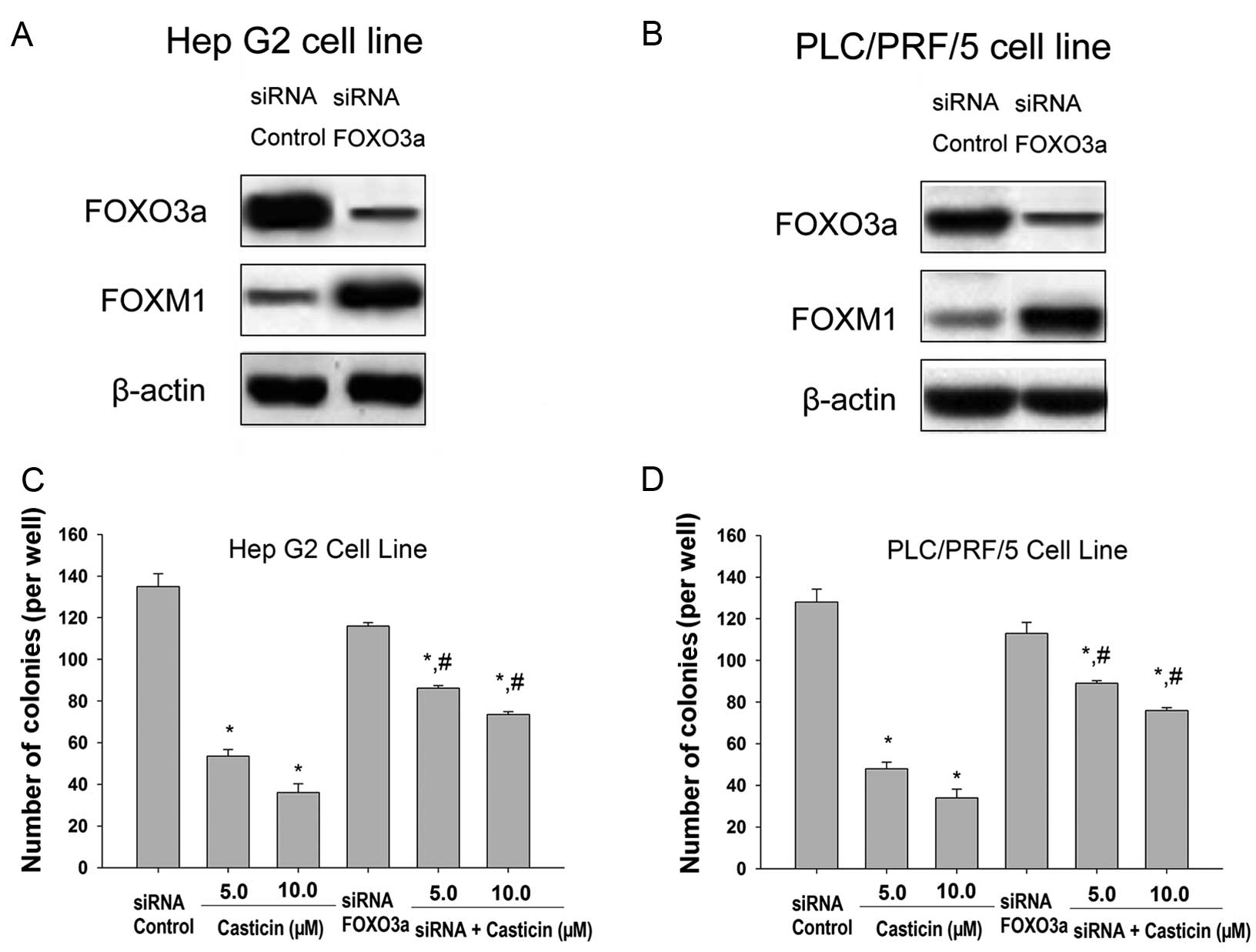
Silencing of the FOXO3a gene attenuates
casticin-mediated growth inhibition in HCC cells
In order to confirm the relevance of the FOXO3a
factor in the cellular growth inhibition response to casticin, we
decided to perform gene silencing experiments. To this end, cells
were generated in which FOXO3a protein expression was abrogated
using siRNA technology (Fig. 5A and
B). A clear increase in the expression level of FOXM1 in the
FOXO3a siRNA-transfected cells was noted (Fig. 5A and B). In addition, we found that
the knockdown of FOXO3a significantly attenuated casticin-induced
inhibition of growth of HCC cells (Fig.
5C and D). These findings indicate that casticin induces HCC
cell growth inhibition by repressing FOXM1 through inducing FOXO3a
activity.
Discussion
Abnormal cell proliferation is an important
characteristic of malignant tumors including HCC. This suggests
that cell cycle arrest could be an effective method in the
treatment of malignant tumors. We previously showed that casticin
inhibits the viability of HCC cells (10). In this study, to evaluate the effect
of casticin on HCC cells, we investigated cell growth inhibition,
cell cycle arrest and the molecular mechanisms following treatment
of human HCC cells with casticin in vitro. We detected HCC
cell growth by the clonogenic assay. We further demonstrated that
casticin significantly inhibited the colony formation in Hep G2 and
PLC/PRF/5 cell lines. In addition, cell cycle analysis showed that
the percentage of G2/M phase cells was significantly elevated in
casticin-treated cells compared with DMSO-treated cells, indicating
a G2/M phase cell cycle arrest. These findings are in line with
those of other reports concerning casticin treatment in other types
of cancer cells such as cervical cancer cells (5–7). For
this reason, casticin-induced cancer cell growth inhibition was
hypothesized to be mediated through an induction of cell cycle
arrest in the G2/M phase in all cancers studied.
FOXM1, one member of the forkhead box transcription
factor family, is a critical regulator of cell cycle progression
and functions in cell proliferation, organogenesis, aging and
carcinogenesis (25). In
vitro loss of FOXM1 is associated with cell cycle arrest and
leads to defective mitotic spindle integrity. Furthermore, in
vivo, loss of FOXM1 has been reported to be embryonic lethal
due to a failure to enter mitosis (26–28).
Recent studies have shown that FOXM1 is overexpressed in several
types of cancer, such as lung cancer, glioblastomas and gastric
cancer. It is reported to have an important role in the development
and progression of these cancers (29,30).
FOXM1 expression is tightly associated with proliferation and is
extinguished in differentiated or resting cells that have exited
the cell cycle. In normal tissues, the expression of FOXM1 is
restricted to embryonic tissues and a few adult endocrine glands
(31). FOXM1 is strongly expressed
in mouse fetal liver but not in the adult one. However, FoxM1 is
highly expressed in HCC and HCC cell lines.
The above findings indicate that FOXM1 is a
potential specific target for HCC therapy. Notably, a recent study
showed that lower expression of FOXM1 in HCC was associated with
prolonged disease-free survival after curative liver resection and
validated FOXM1 as a prognostic marker for HCC (32). In the present study, we investigated
the role and regulation of FOXM1 in response to casticin treatment
in HCC cells. We found that casticin suppressed the expression of
the transcription factor FOXM1 in Hep G2 and PLC/PRF/5 cells. The
suppression of FOXM1 by casticin was also associated with the
downregulation of FOXM1 activity as revealed by the concomitant
decrease in expression of the FOXM1 downstream targets,
cyclin-dependent kinase (CDK1), cyclin B1, CDC25B and an increase
in p27KIP1. These results suggest that FOXM1 affects the
cell cycle of HCC cells by regulating the expression levels of
CDK1, cyclins (cyclin B1) and CDKI (p27). In addition to the above
mechanisms, a recent study suggested that cellular senescence
caused by FOXM1 depletion may be involved in the inhibition of cell
survival (28).
FOXO3a is a member of the forkhead box class O
(FOXO) transcription factor family and an important regulator of
FOXM1 activity and function (33).
This study aimed to elucidate the involvement of FOXO3a during
casticin-induced growth inhibition and cell cycle arrest in HCC
cells. In this study, we showed that casticin inhibited FOXO3a
phosphorylation in a concentration-dependent manner. Importantly,
the silencing of the FOXO3a gene by siRNA transfection clearly
attenuated the induction of cell growth and FOXM1 expression
inhibition by casticin. These results showed that activation of
FOXO3a contributed to HCC growth inhibition by casticin through
downregulation of FOXM1 expression and inactivation of FOXM1
function. Emerging evidence has been provided that FOXO3a
activation induces cell cycle arrest resulting in tumor suppression
(34,35). Agents that activate FOXO3a may be
novel therapeutic agents that can inhibit and prevent tumor growth
and development in various cancer types. Moreover, activation of
FOXO3a could enhance the effects of a series of chemotherapeutic
drugs such as cisplatin and paclitaxel in various types of cancer
(36,37). However, whether casticin enhances
the sensitivity of cancer cells to chemotherapeutic drugs needs
further investigation.
In conclusion, these results showed that
downregulation of the expression levels of phosphorylated FOXO3a
and FOXM1 in HCC cells by casticin decreased the colony formation
ability and induced G2/M phase cell cycle arrest. Furthermore, a
decrease in the FOXM1 expression level resulted in downregulation
of CDK1, CDC25B and cyclins B1 along with upregulation of p27. The
depletion of FOXO3a also reduced the effects of casticin. Our study
provides clearly evidence that the FOXO3a/FOXM1 signaling pathway
may serve as a new target for the natural flavonoid casticin in HCC
therapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation (81172375) and the Municipal Bureau of
Science and Technology of Changsha, Hunan, China (K1104060-31).
References
|
1
|
Purushotham AD, Lewison G and Sullivan R:
The state of research and development in global cancer surgery. Ann
Surg. 255:427–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao LQ, Chen XL, Wang Q, et al:
Upregulation of PTEN involved in rosiglitazone-induced apoptosis in
human hepatocellular carcinoma cells. Acta Pharmacol Sin.
28:879–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng X, Fang Z, Wu Y and Zhang H: Chemical
constituents of the fruits of Vitex trifolia L. Zhongguo
Zhong Yao Za Zhi. 21:167–168. 1911996.(In Chinese).
|
|
4
|
Haidara K, Zamir L, Shi QW and Batist G:
The flavonoid casticin has multiple mechanisms of tumor
cytotoxicity action. Cancer Lett. 242:180–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wojcinski S, Farrokh A, Hille U, et al:
The Automated Breast Volume Scanner (ABVS): initial experiences in
lesion detection compared with conventional handheld B-mode
ultrasound: a pilot study of 50 cases. Int J Womens Health.
3:337–346. 2011. View Article : Google Scholar
|
|
6
|
Zeng F, Tian L, Liu F, Cao J, Quan M and
Sheng X: Induction of apoptosis by casticin in cervical cancer
cells: reactive oxygen species-dependent sustained activation of
Jun N-terminal kinase. Acta Biochim Biophys Sin (Shanghai).
44:442–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Csupor-Loffler B, Hajdu Z, Zupko I, et al:
Antiproliferative effect of flavonoids and sesquiterpenoids from
Achillea millefolium s. l on cultured human tumour cell
lines. Phytother Res. 23:672–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayakawa J, Sato-Nishimori F, Moriyasu M
and Matsukawa Y: G2-M arrest and antimitotic activity mediated by
casticin, a flavonoid isolated from Viticis Fructus (Vitex
rotundifolia Linne fil). Cancer Lett. 208:59–64. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: a potential natural
anti-cancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Yang Y, Tian L, Sheng XF, Liu F
and Cao JG: Casticin-induced apoptosis involves death receptor 5
upregulation in hepatocellular carcinoma cells. World J
Gastroenterol. 17:4298–4307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galili N, Davis RJ, Fredericks WJ, et al:
Fusion of a fork head domain gene to PAX3 in the solid tumour
alveolar rhabdomyosarcoma. Nat Genet. 5:230–235. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson MJ, Viars CS, Czekay S, Cavenee
WK and Arden KC: Cloning and characterization of three human
forkhead genes that comprise an FKHR-like gene subfamily. Genomics.
47:187–199. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo S, Rena G, Cichy S, He X, Cohen P and
Unterman T: Phosphorylation of serine 256 by protein kinase B
disrupts transactivation by FKHR and mediates effects of insulin on
insulin-like growth factor-binding protein-1 promoter activity
through a conserved insulin response sequence. J Biol Chem.
274:17184–17192. 1999. View Article : Google Scholar
|
|
14
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura N, Ramaswamy S, Vazquez F,
Signoretti S, Loda M and Sellers WR: Forkhead transcription factors
are critical effectors of cell death and cell cycle arrest
downstream of PTEN. Mol Cell Biol. 20:8969–8982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu M, Ma J, Xue W, et al: The expression
and prognosis of FOXO3a and Skp2 in human hepatocellular carcinoma.
Pathol Oncol Res. 15:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fei M, Lu M, Wang Y, et al: Arsenic
trioxide-induced growth arrest of human hepatocellular carcinoma
cells involving FOXO3a expression and localization. Med Oncol.
26:178–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park TJ, Kim JY, Oh SP, et al: TIS21
negatively regulates hepatocarcinogenesis by disruption of cyclin
B1-Forkhead box M1 regulation loop. Hepatology. 47:1533–1543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: a novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao XC, Tian L, Cao JG and Liu F:
Induction of apoptosis by 5,7-dihydroxy-8-nitrochrysin in breast
cancer cells: the role of reactive oxygen species and Akt. Int J
Oncol. 37:1345–1352. 2010.PubMed/NCBI
|
|
22
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu QF, Liu C, Tai MH, et al: Knockdown of
FoxM1 by siRNA interference decreases cell proliferation, induces
cell cycle arrest and inhibits cell invasion in MHCC-97H cells in
vitro. Acta Pharmacol Sin. 31:361–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shankar S, Chen Q and Srivastava RK:
Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to
enhance antiangiogenic effects of EGCG through activation of FOXO
transcription factor. J Mol Signal. 3:72008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laoukili J, Stahl M and Medema RH: FoxM1:
at the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
26
|
Krupczak-Hollis K, Wang X, Kalinichenko
VV, et al: The mouse Forkhead Box m1 transcription factor is
essential for hepatoblast mitosis and development of intrahepatic
bile ducts and vessels during liver morphogenesis. Dev Biol.
276:74–88. 2004. View Article : Google Scholar
|
|
27
|
Wonsey DR and Follettie MT: Loss of the
forkhead transcription factor FoxM1 causes centrosome amplification
and mitotic catastrophe. Cancer Res. 65:5181–5189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laoukili J, Kooistra MR, Bras A, et al:
FoxM1 is required for execution of the mitotic programme and
chromosome stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng J, Wang L, Li Q, et al: FoxM1 is
up-regulated in gastric cancer and its inhibition leads to cellular
senescence, partially dependent on p27 kip1. J Pathol. 218:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu M, Dai B, Kang SH, et al: FoxM1B is
overexpressed in human glioblastomas and critically regulates the
tumorigenicity of glioma cells. Cancer Res. 66:3593–3602. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korver W, Roose J and Clevers H: The
winged-helix transcription factor Trident is expressed in cycling
cells. Nucleic Acids Res. 25:1715–1719. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Calvisi DF, Pinna F, Ladu S, et al:
Forkhead box M1B is a determinant of rat susceptibility to
hepatocarcinogenesis and sustains ERK activity in human HCC. Gut.
58:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yilmaz OH and Morrison SJ: The PI-3kinase
pathway in hematopoietic stem cells and leukemia-initiating cells:
a mechanistic difference between normal and cancer stem cells.
Blood Cells Mol Dis. 41:73–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han CY, Cho KB, Choi HS, Han HK and Kang
KW: Role of FoxO1 activation in MDR1 expression in
adriamycin-resistant breast cancer cells. Carcinogenesis.
29:1837–1844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernandez de Mattos S, Villalonga P,
Clardy J and Lam EW: FOXO3a mediates the cytotoxic effects of
cisplatin in colon cancer cells. Mol Cancer Ther. 7:3237–3246.
2008.PubMed/NCBI
|
|
37
|
Sunters A, Fernandez de Mattos S, Stahl M,
et al: FoxO3a transcriptional regulation of Bim controls apoptosis
in paclitaxel-treated breast cancer cell lines. J Biol Chem.
278:49795–49805. 2003. View Article : Google Scholar : PubMed/NCBI
|