Introduction
Lung cancer is the leading cause of cancer-related
morbidity and mortality, resulting in more than 1.1 million deaths
worldwide (1,2). Approximately 75–80% of lung cancer
cases are non-small cell lung cancer (NSCLC), and 65% of these
patients have advanced-stage disease at diagnosis (3). Despite the aggressive approaches
achieved in the therapy of lung cancer in the past decades, the
prognosis of NSCLC remains poor, with 5-year survival rates of
5–14%, even when patients are treated with surgery, radiotherapy
and/or chemotherapy (4–6). Therefore, efforts to develop new and
less toxic therapeutic approaches for the treatment of lung cancer
are ongoing.
Measles virus, one member of the Paramyxoviridae
family, is a negative strand RNA virus. The typical cytopathic
effect of Measles virus is the formation of multinuclear cell
aggregates (syncytia), which result from the fusion of infected
cells. The wild-type virus enters cells exclusively via the SLAM
receptor, one of the surface hemagglutinin (H) glycoproteins
predominantly found on activated B and T lymphocytes (9–11). In
contrast, the attenuated measles vaccine virus Edmonston strain
preferentially infects cells with the CD46 receptor (12,13),
which is frequently overexpressed in tumor cells (14). In addition, measles vaccine strains
are attenuated and have an excellent safety record with several
hundred millions of vaccine doses having been safely administered
in over 40 years of use. These preferences enable the attenuated
measles vaccine virus to be tumor-selective and result in minimal
cytopathic effects on normal tissue.
Previous studies have shown that live attenuated
measles virus Edmonston B strain has potent and specific oncolytic
activity against a variety of human tumor types, including
lymphoma, multiple myeloma, epithelial ovarian cancer and glioma
(15–20). Thus, the present study was designed
to evaluate the therapeutic effects of live attenuated measles
vaccine virus Hu-191 strain (MV) for the treatment of lung
carcinoma. This study demonstrated that the oncolytic therapy
exhibits powerful antitumor effects against murine lung carcinoma
and provides potential implications for the treatment of human lung
cancer.
Materials and methods
Cell lines
LLC, A549 and HEK 293T cells were purchased from the
American Type Culture Collection (Rockville, MD, USA). They were
maintained in monolayer cultures in DMEM, supplemented with 10%
heat-inactivated fetal bovine serum (FBS), at 37°C in a humidified
atmosphere containing 5% CO2. Live attenuated measles
vaccine virus Hu-191 strain was obtained from Chengdu Company of
Biological Products.
MTT colorimetric assay
Survival of cells after treatment was quantified
using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT; Sigma, St. Louis, MO, USA) colorimetric assay (21). Briefly, LLC and A549 cells were
seeded in 96-well plates (1×104/ml). They were treated
with MV or PBS. Each well was supplemented with 20 μl of 5 mg/ml
MTT in complete media and incubated at 37°C for 4 h. The medium and
MTT solution were then removed, and 150 μl of dimethyl sulfoxide
(DMSO) was added to each well. 293T cells were also treated as a
non-transformed cell control. Absorbance was read at 490 nm using a
microplate reader. Data were the average of 6 wells, and the
experiment was repeated 3 times with similar results. Medium
only-treated cells served as the indicator of 100% cell
viability.
Assessment of apoptosis in vitro
Cell apoptosis was analyzed by DNA ladder and flow
cytometry (Annexin V-FITC Apoptosis Detection kit; Sigma). The
pattern of DNA cleavage was analyzed by agarose gel electrophoresis
as described (22). Briefly, cells
(1×106) were lysed with 0.1 ml lysis buffer containing
50 mM Tris-HCl (pH 8.0), 1% Nonidet P-40 and 20 mM EDTA, followed
by the addition of RNase A (Sigma) at a final concentration of 10
μg/μl and incubated at 37°C for 1 h. Cells were then treated with
2.5 μg/μl proteinase K overnight at 37°C. Samples (10 μl) in each
lane were subjected to electrophoresis on 1.5% agarose at 50 V for
3 h. DNA was stained with ethidium bromide.
Cells were collected and resuspended in 1X binding
buffer at a concentration of 1×106 cells/ml. Annexin
V-FITC conjugate (5 μl) and 10 μl of propidium iodide solution
(22–24) were added to each cell suspension.
After 10 min of incubation at room temperature, cells were analyzed
by flow cytometry. Cells undergoing early apoptosis were stained
with the Annexin V-FITC conjugate alone. Live cells showed no
staining by either the propidium iodide solution or Annexin V-FITC
conjugate. Necrotic cells were stained by both the propidium iodide
solution and Annexin V-FITC conjugate.
Murine tumor model and treatment
Lewis lung carcinoma (LLC) cells (1×106
to 1×107 cells) were inoculated subcutaneously in the
right flanks of C57BL/6 mice 6–8 weeks of age. To explore the
therapeutic efficacy of MV, we treated the mice on day 15 after the
implantation of tumor cells, when the size of tumors reached ~30
mm3. The mice were randomly divided into 4 groups (5
male and 5 female mice/group). Approximately 1×106,
1×105, 1×104 CCID50/ml of MV or
PBS were injected into the tumor every other day for 5 times. Tumor
volume was determined by the following formula: Tumor volume
(mm3) = π/6 × length (mm) × width (mm) × width (mm). All
of the studies involving mice were performed in accordance with
institutional guidelines concerning animal use and care. On day 4
after the completion of treatment as described above, the mice were
sacrificed by cervical dislocation. Mouse tissues of interest were
excised and fixed in 10% neutral buffered formalin solution or
frozen at 80°C (25).
Histological analysis
For the observations of potential side effects in
the treated mice, the tissues from each group were embedded in
paraffin. Sections (3–5 μm) were stained with hematoxylin and eosin
(H&E) (26). Immunostaining for
MV was performed as previously described (27). Briefly, paraffin-embedded tissue
sections from the treated mice were incubated with a polyclonal
rabbit antibody against MV at a 1:300 dilution at 4°C overnight.
Following washes, the secondary antibody, biotinylated goat
anti-rabbit antibody at a 1:100 dilution (Dako, Carpinteria, CA),
was added. Sections were then stained with streptavidin biotin
reagents (Dako LSAB kit; peroxidase). Immunofluorescence staining
was used to determine the infiltration of cytotoxic T lymphocytes
(CTLs) in the tumor tissue. The frozen sections were blocked (10%
FBS, 3% BSA) for 30 min before staining with the anti-CD4 antibody
(ab51312; Abcam, USA) and anti-CD8 antibody (ab22378; Abcam).
Fluorescence was visualized, and images were captured with
fluorescence microscopy.
Quantitative assessment of apoptosis
Tumor species were prepared as described above. The
presence of apoptotic cells within the tumor sections was
determined using the In Situ Cell Death Detection kit (Fluorescein;
Roche), following the manufacturer’s instructions. It is based on
the enzymatic addition of digoxigenin nucleotide to the nicked DNA
by terminal deoxynucleotidyl transferase (28). In the tissue sections, four
equal-sized fields were randomly chosen and analyzed. As a
proliferation marker, Ki67 is required for maintaining cell
proliferation. Immunohistochemical analysis with the anti-Ki67
antibody (ab16667; Abcam) was used to measure proliferation in the
tumor tissue.
Evaluation of potential adverse
effects
To evaluate the potential side effects or toxicity
on mice during the treatment, gross measures such as weight loss,
ruffling of fur, lifespan, behavior and feeding were investigated.
Tissues from the heart, liver, spleen, lung, kidney and brain were
fixed in 10% neutral buffered formalin solution, embedded in
paraffin, and stained with H&E.
Data analysis and statistics
In order for the comparison of individual time
points, data were assayed by variance (ANOVA) and an unpaired
Student’s t-test (29). Survival
analysis was computed by the Kaplan-Meier method and compared by
the log-rank test (30). A P-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
In vitro induction of apoptosis of lung
cancer cell lines with MV treatment
To study the ability of MV to augment the induction
of apoptosis of cells, we treated the various cell lines with MV.
A549, LLC and 293T cells were seeded in cell culture flasks
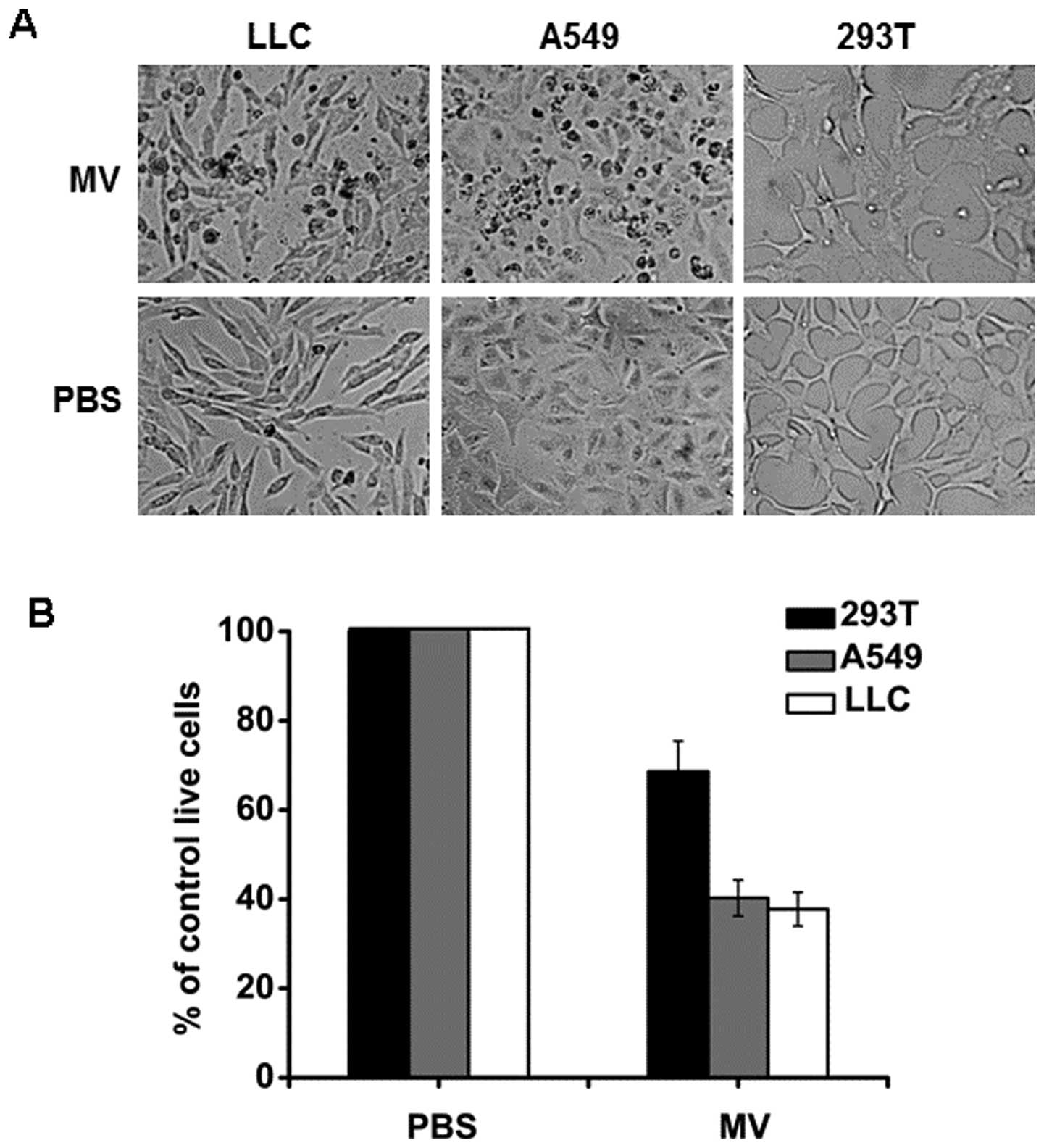
separately in appropriate culture. As shown in Fig. 1A, MV increased the cytopathic effect
on both the A549 and LLC cell lines compared with the control
group. Cell viability was also determined by MTT assay. As shown in
Fig. 1B, the treatment of MV
reduced the A549 and LLC cell viability by 60 and 62%,
respectively, whereas the treatment with MV reduced 293T cell
viability by 31%. These results indicated that the treatment of MV
resulted in additive cytotoxicity to A549 and LLC lung cancer
cells. However, the treatment showed less effect on 293T cell
viability when compared with the nontransformed cell control.
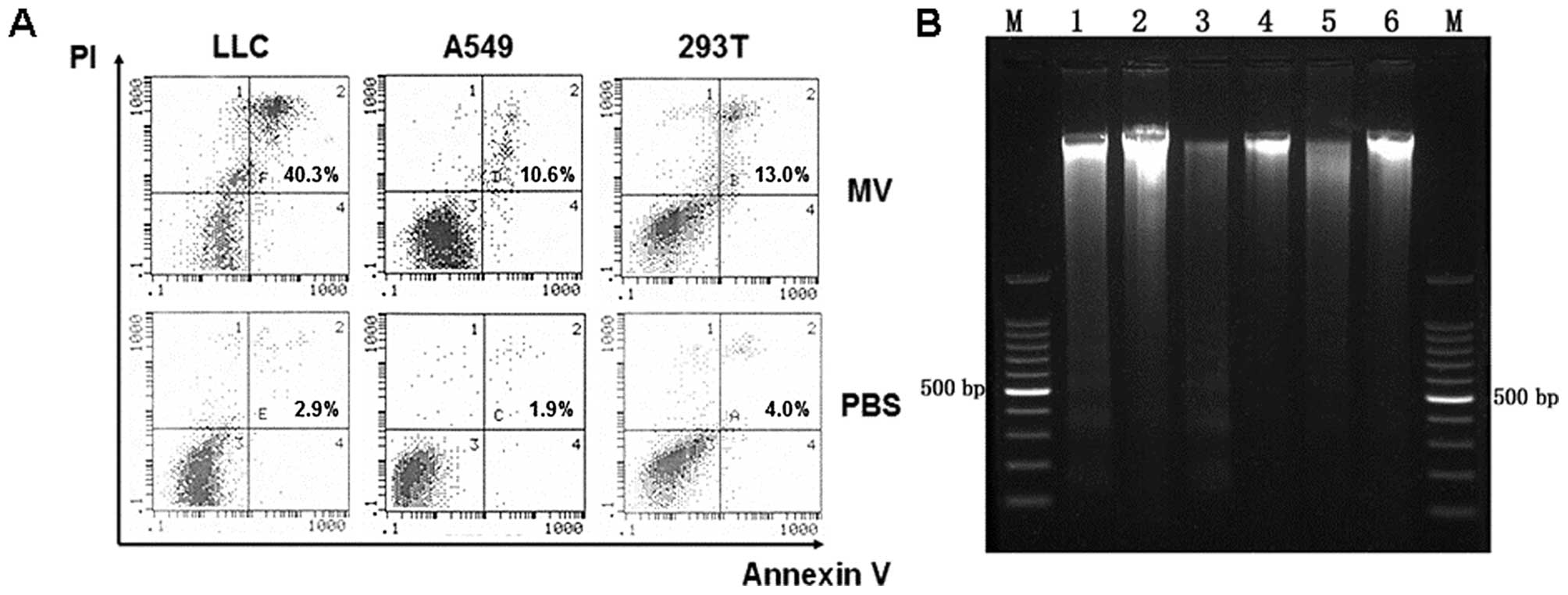
In the next set of experiments, we aimed to
determine increased cell apoptosis induced by MV. Flow cytometry
was used to estimate the number of apoptotic cells. As shown in
Fig. 2A, treatment with MV
increased the number of apoptotic cells compared with the control
groups. Furthermore, agarose gel electrophoresis of MV demonstrated
a ladder-like pattern of DNA fragments consisting of multiples of
~150–200 bp, consistent with internucleosomal DNA fragmentation
(Fig. 2B).
In vivo inhibition of growth of
established lung carcinoma in mice
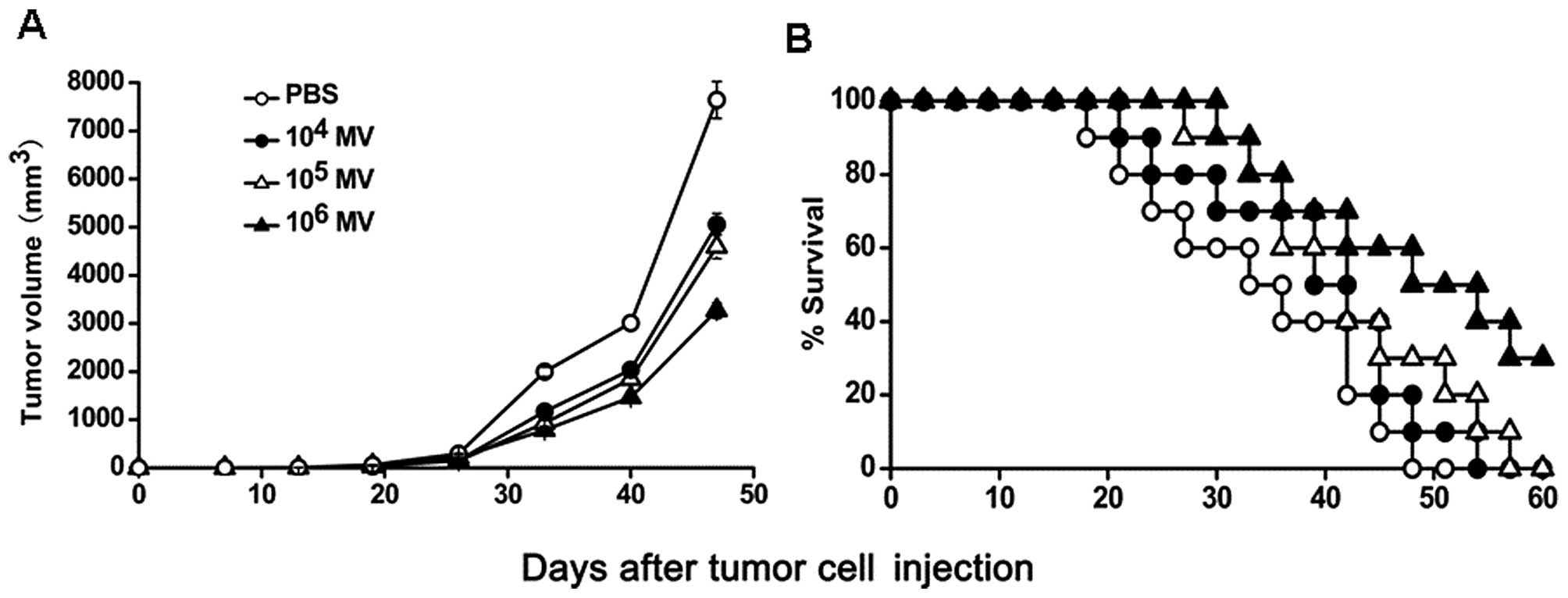
To determine the antitumor activity of MV in
vivo, immunocompetent C57BL/6 mice bearing LLC Lewis lung
carcinoma were treated with various amounts of MV or PBS. Our
findings showed that both 104 CCID50/ml MV
and 105 CCID50/ml MV resulted in effective
suppression of tumor growth. However, the treatment of
106 CCID50/ml MV had a superior antitumor
effect, resulting in >50% inhibition in tumor volume compared
with the PBS group. No significant difference in tumor volumes was
observed between the 104 CCID50/ml MV and
105 CCID50/ml MV groups (Fig. 3A). Moreover, a significant increase
in the survival rate was observed in the 106
CCID50/ml MV-treated mice (P<0.05, by log-rank test;
Fig. 3B). In addition,
106 CCID50/ml MV therapy effectively
prolonged the lifespan of the tumor-bearing animals.
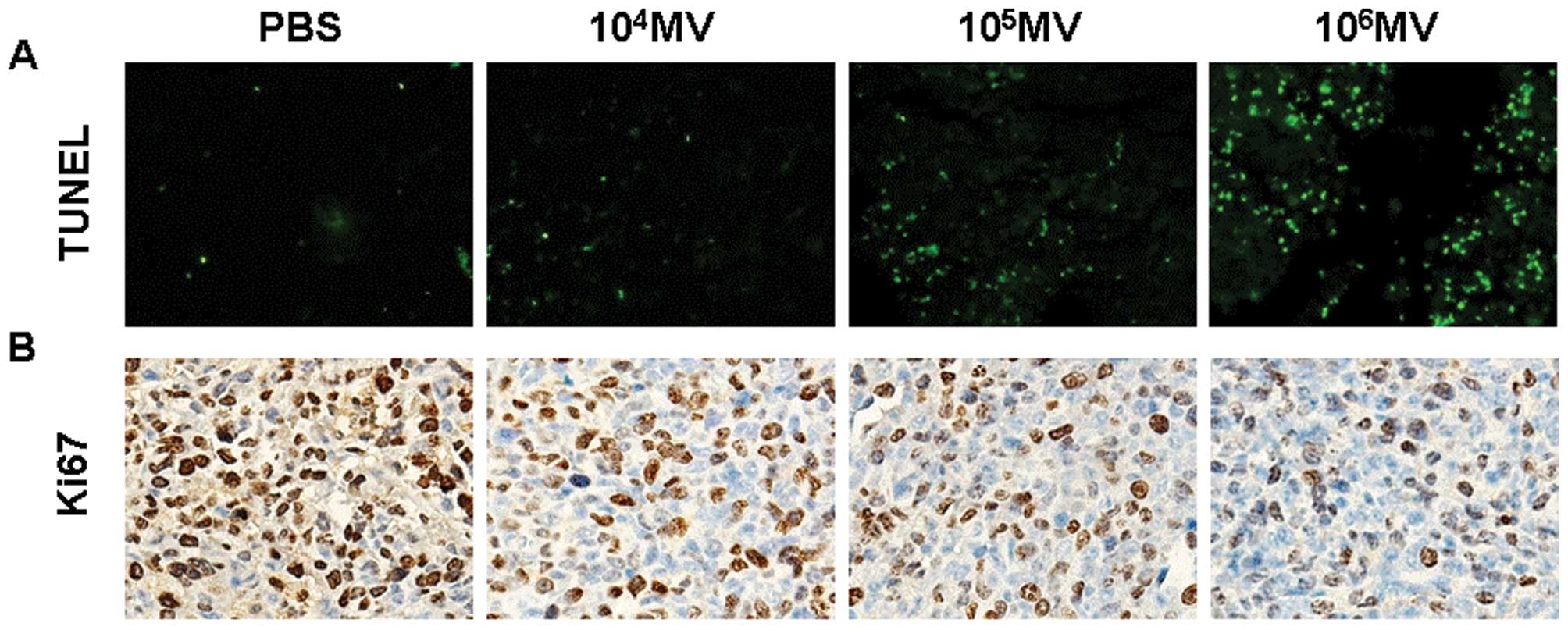
In vivo induction of apoptosis following
MV treatment
Having confirmed the antitumor activity in LLC Lewis
lung carcinoma models, we examined apoptosis-related molecular
markers in tumor sections. An apoptosis detection kit (TUNEL) was
used to detect early DNA fragmentation associated with apoptosis.
Apoptotic cells were noted within the tumors treated with
106 CCID50/ml MV, compared with the treatment
with 105 CCID50/ml MV or 104
CCID50/ml MV or PBS groups (Fig. 4A). We also tested the proliferation
index of tumor tissues. Immunohistochemical analysis with the
anti-Ki67 antibody, which is used to measure proliferation in tumor
tissues, showed less proliferative cells within the tumors treated
with 106 CCID50/ml MV, compared with the
other three groups (Fig. 4B).
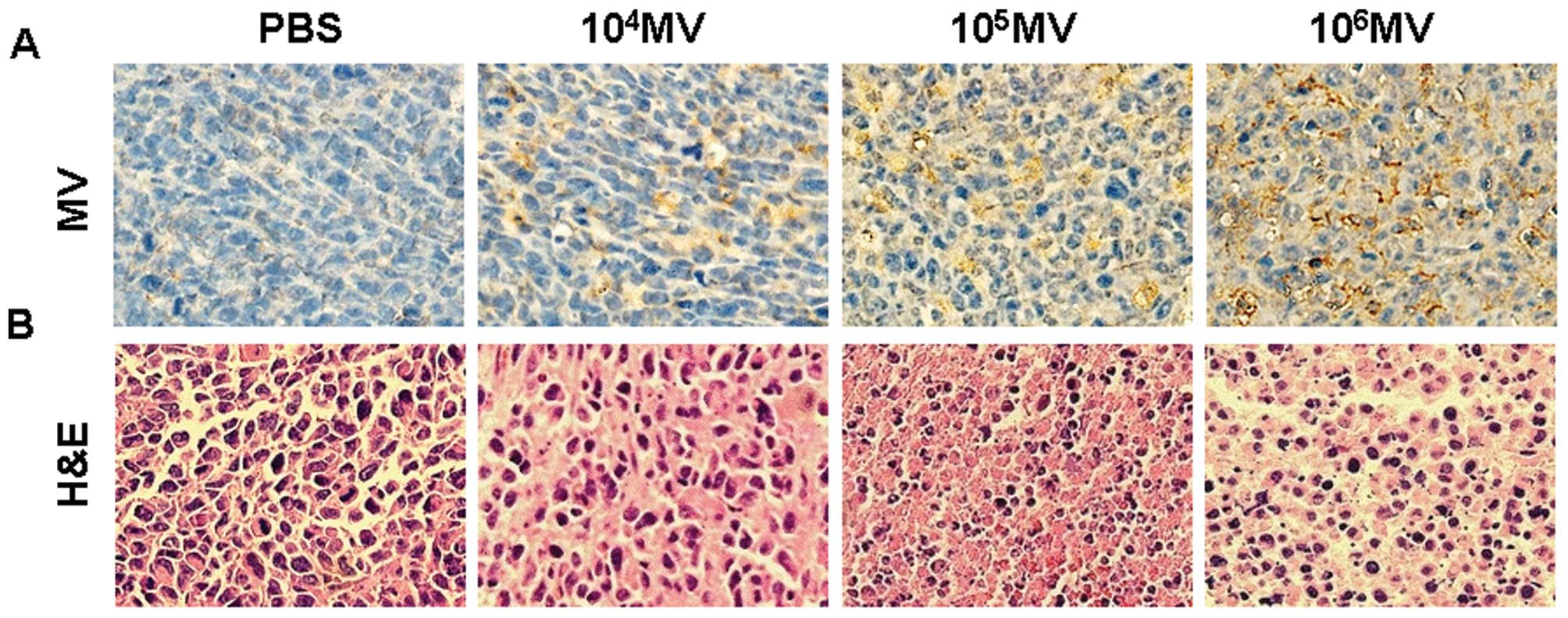
MV antigen-positive tumor cells were found within
the tumors treated with MV, which confirmed that MV was capable of
replicating in tumors. Virus antigen was detectable within either
apoptotic cells or the intact tumor cells (Fig. 5A).
Histological analysis of intratumoral
T-cell infiltration
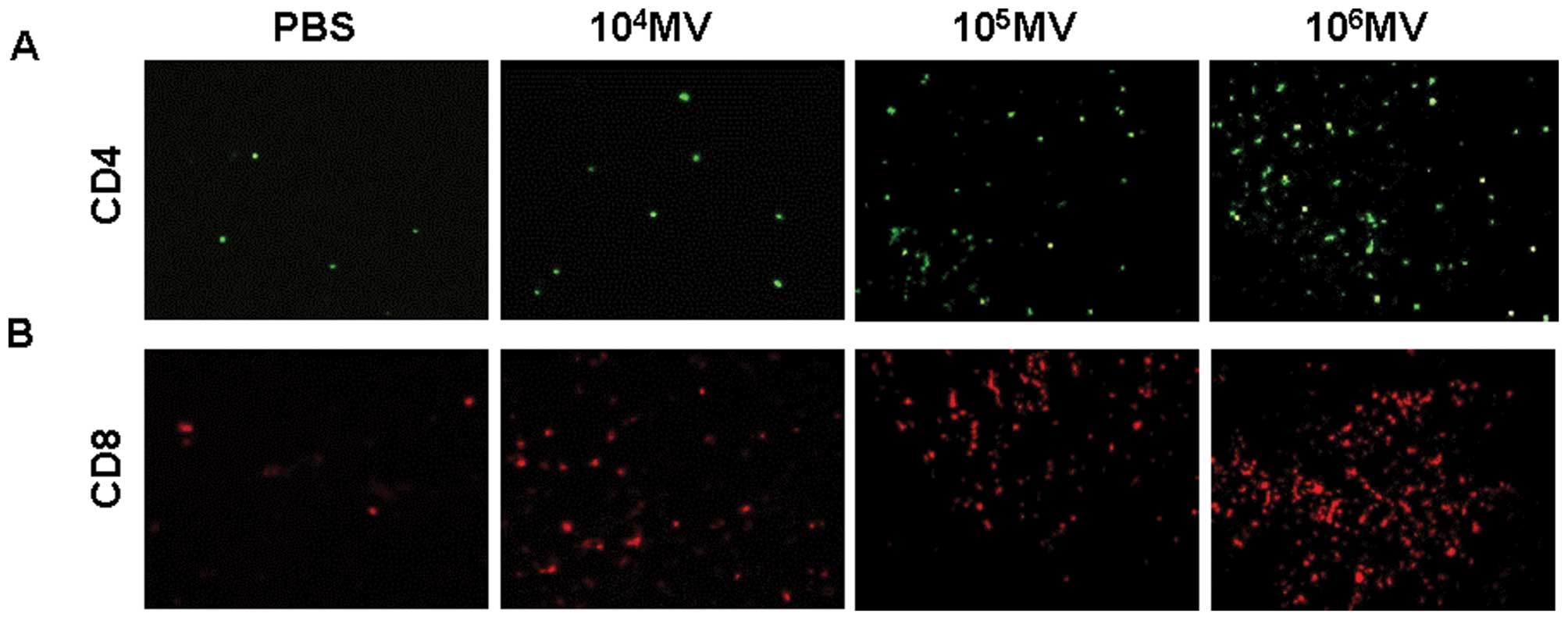
To determine whether the therapy improved the
infiltration of CD4 and CD8 lymphocytes into the tumors, anti-CD4
and anti-CD8 monoclonal antibodies were used in the
immunofluorescence staining. The results showed that 106
CCID50/ml MV or 105 CCID50/ml MV
significantly increased the infiltration of both CD4+
and CD8+ lymphocytes (Fig.
6).
Histological examination of tumor sections by
H&E staining showed that PBS-treated group tumor cells had
well-defined cell borders and hyperchromatic nuclei. The cytoplasm
of these cells was vesicular and eosinophilic, with evidence of
mitosis. In contrast, tumors from mice treated with MV showed
extensive necrosis, characterized by loss of nuclear staining,
increased cytoplasmic eosinophilia, and loss of cellular detail and
cell borders (Fig. 5B).
Observation of potential toxicity
To evaluate the possible adverse effects of the
treatments, the body weights of the mice were monitored every 7
days throughout the entire experiment and these values were
considered as a variable for the evaluation of systemic well-being
or cachexia. No significant differences in weights were found among
the four groups. No adverse consequences in other gross measures
such as ruffling of fur, behavior, feeding, or toxic death were
observed in the 106 CCID50/ml MV-treated
group. In the histopathological examination of the tissues, there
was also no significant differences. Therefore, we confirmed that
MV treatment is safe and effective.
Discussion
The beneficial effects of viral infections on cancer
patients have been known for decades. Numerous viruses, such as
adenovirus, vesicular stomatitis virus and measles virus, are
currently considered as potential cancer therapeutics (31,32).
Among them, measles virus was successfully used to treat multiple
tumors, including myeloma, ovarian cancer and glioma after either
intratumoral, intraperitoneal, or intravenous administration
(7,19,20).
Lung carcinoma is an aggressive tumor highly resistant to current
therapeutic approaches, such as chemotherapy or radiotherapy
(8). These studies suggest a
substantial antineoplastic potential for measles virus. The present
studies were designed to test the therapeutic efficacy of MV in
murine lung carcinoma.
The data in the present study showed that MV
oncolytic therapy not only specifically and significantly reduced
the growth of highly tumorigenic and poorly immunogenic LLC cells
but also effectively improved the survival of tumor-bearing
animals. Although the exact mechanism remains to be determined, the
antitumor efficacy of MV in vivo may in part result from the
increased induction of apoptosis following treatment. This
suggestion is supported by the present findings. In vitro
treatment with MV significantly reduced the viability (Fig. 1) and increased the apoptosis of
tumor cells compared with PBS treatment (Fig. 2). In addition, more apparent
apoptotic cells were found in the tumors treated with MV compared
with the PBS-treated group. The mechanism by which MV leads to
tumor cell apoptosis may be related to virus replication. This
feature of viral replication provides continuous amplification of
the input dose which continues until being stopped by the immune
response or a lack of susceptible cells.
Breaking of immune tolerance against self-tumor
antigen and induction of auto-immunity against tumors should be a
useful approach for the treatment of tumors. Previous studies have
shown that introduction of new antigens into tumor cells stimulates
immune responses against autologous malignant cells. In the present
study, intratumoral delivery of MV in tumor cells was intended to
modify the tumor cells and enhance the presentation ability of the
modified tumor cells to antigen-presenting cells (APCs) and then
cross-priming through APCs. Our findings found a significant
increasing infiltration. However, treatment with MV significantly
increased the infiltration of CD4+ and CD8+ T
lymphocytes in the tumor tissues of the MV-treated group coincident
with previous reports (Fig. 6).
The management of unresectable lung cancers remains
a major therapeutic challenge to medical oncologists. Our
observations may have potential implications for the treatment of
human lung cancer by MV, since MV is capable of repressing tumor
growth when administered.
Collectively, the present study showed that MV
treatment resulted in statistically significant reduction in tumor
growth, increased apoptosis of lung cancer cells in vitro
and in vivo, and increased infiltration of lymphocytes,
while significantly prolonging the survival of tumor-bearing
animals. In addition, there was no obvious undesired toxicity
following treatment. Our findings suggest that intratumoral
delivery of MV may be a promising strategy for the treatment of
lung cancer. Further studies involving this treatment strategy,
used alone or in combination with chemotherapy and biotherapy,
warrant consideration. Live attenuated measles vaccine may be used
as a novel type of anticancer drug.
References
|
1
|
Minna JD, Roth JA and Gazdar AF: Focus on
lung cancer. Cancer Cell. 1:49–52. 2002. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group. Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar
|
|
4
|
Khuri FR, Herbst RS and Fossella FV:
Emerging therapies in non-small-cell lung cancer. Ann Oncol.
12:739–744. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soria JC, Jang SJ, Khuri FR, Hassan K, Liu
D, Hong WK and Mao L: Overexpression of cyclin B1 in early-stage
non-small cell lung cancer and its clinical implication. Cancer
Res. 60:4000–4004. 2000.PubMed/NCBI
|
|
6
|
Vora SA, Daly BD, Blaszkowsky L, McGrath
JJ, Bankoff M, Supran S and Dipetrillo TA: High dose radiation
therapy and chemotherapy as induction treatment for stage III
nonsmall cell lung carcinoma. Cancer. 89:1946–1952. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng KW, Facteau S, Wegman T, O’Kane D and
Russell SJ: Non-invasive in vivo monitoring of trackable viruses
expressing soluble marker peptides. Nat Med. 8:527–531. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong H, Han B, Tourkova IL, Lokshin A,
Rosenbloom A, Shurin MR and Shurin GV: Low-dose paclitaxel prior to
intratumoral dendritic cell vaccine modulates intratumoral cytokine
network and lung cancer growth. Clin Cancer Res. 13:5455–5462.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erlenhoefer C, Wurzer WJ, Löffler S,
Schneider-Schaulies S, ter Meulen V and Schneider-Schaulies J:
CD150 (SLAM) is a receptor for measles virus but is not involved in
viral contact-mediated proliferation inhibition. J Virol.
75:4499–4505. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tatsuo H, Ono N, Tanaka K and Yanagi Y:
SLAM (CDw150) is a cellular receptor for measles virus. Nature.
406:893–897. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu EC, Iorio C, Sarangi F, Khine AA and
Richardson CD: CDw150 (SLAM) is a receptor for a lymphotropic
strain of measles virus and may account for the immunosuppressive
properties of this virus. Virology. 279:9–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dörig RE, Marcil A, Chopra A and
Richardson CD: The human CD46 molecule is a receptor for measles
virus (Edmonston strain). Cell. 75:295–305. 1993.PubMed/NCBI
|
|
13
|
Naniche D, Varior-Krishnan G, Cervoni F,
Wild TF, Rossi B, Rabourdin-Combe C and Gerlier D: Human membrane
cofactor protein (CD46) acts as a cellular receptor for measles
virus. J Virol. 67:6025–6032. 1993.PubMed/NCBI
|
|
14
|
Bjørge L, Hakulinen J, Wahlström T, Matre
R and Meri S: Complement-regulatory proteins in ovarian
malignancies. Int J Cancer. 70:14–25. 1997.
|
|
15
|
Grote D, Russell SJ, Cornu TI, Cattaneo R,
Vile R, Poland GA and Fielding AK: Live attenuated measles virus
induces regression of human lymphoma xenografts in immunodeficient
mice. Blood. 97:3746–3754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grote D, Cattaneo R and Fielding AK:
Neutrophils contribute to the measles virus-induced antitumor
effect: enhancement by granulocyte macrophage colony-stimulating
factor expression. Cancer Res. 63:6463–6468. 2003.
|
|
17
|
Peng KW, Ahmann GJ, Pham L, Greipp PR,
Cattaneo R and Russell SJ: Systemic therapy of myeloma xenografts
by an attenuated measles virus. Blood. 98:2002–2007. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dingli D, Peng KW, Harvey ME, Greipp PR,
O’Connor MK, Cattaneo R, Morris JC and Russell SJ: Image-guided
radiovirotherapy for multiple myeloma using a recombinant measles
virus expressing the thyroidal sodium iodide symporter. Blood.
103:1641–1646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phuong LK, Allen C, Peng KW, Giannini C,
Greiner S, TenEyck CJ, Mishra PK, Macura SI, Russell SJ and Galanis
EC: Use of a vaccine strain of measles virus genetically engineered
to produce carcinoembryonic antigen as a novel therapeutic agent
against glioblastoma multiforme. Cancer Res. 63:2462–2469.
2003.
|
|
20
|
Peng KW, TenEyck CJ, Galanis E, Kalli KR,
Hartmann LC and Russell SJ: Intraperitoneal therapy of ovarian
cancer using an engineered measles virus. Cancer Res. 62:4656–4662.
2002.PubMed/NCBI
|
|
21
|
Pumphrey CY, Theus AM, Li S, Parrish RS
and Sanderson RD: Neoglycans, carbodiimide-modified
glycosaminoglycans: a new class of anticancer agents that inhibit
cancer cell proliferation and induce apoptosis. Cancer Res.
62:3722–3728. 2002.PubMed/NCBI
|
|
22
|
Wei YQ, Zhao X, Kariya Y, Fukata H,
Teshigawara K and Uchida A: Induction of apoptosis by quercetin:
involvement of heat shock protein. Cancer Res. 54:4952–4957.
1994.PubMed/NCBI
|
|
23
|
Gorczyca W, Gong J, Ardelt B, Traganos F
and Darzynkiewicz Z: The cell cycle related differences in
susceptibility of HL-60 cells to apoptosis induced by various
antitumor agents. Cancer Res. 53:3186–3192. 1993.PubMed/NCBI
|
|
24
|
Barry MA, Reynolds JE and Eastman A:
Etoposide-induced apoptosis in human HL-60 cells is associated with
intracellular acidification. Cancer Res. 53:2349–2357.
1993.PubMed/NCBI
|
|
25
|
Sauter BV, Martinet O, Zhang WJ, Mandeli J
and Woo SL: Adenovirus-mediated gene transfer of endostatin in vivo
results in high level of transgene expression and inhibition of
tumor growth and metastases. Proc Natl Acad Sci USA. 97:4802–4807.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei YQ, Hang ZB and Liu KF: In situ
observation of inflammatory cell-tumor cell interaction in human
seminomas (germinomas): light, electron microscopic, and
immunohistochemical study. Hum Pathol. 23:421–428. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li G, Tian L, Hou JM, Ding ZY, He QM, Feng
P, Wen YJ, Xiao F, Yao B, Zhang R, Peng F, Jiang Y, Luo F, Zhao X,
Zhang L, Zhou Q and Wei YQ: Improved therapeutic effectiveness by
combining recombinant CXC chemokine ligand 10 with cisplatin in
solid tumors. Clin Cancer Res. 11:4217–4224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao F, Wei Y, Yang L, Zhao X, Tian L,
Ding Z, Yuan S, Lou Y, Liu F, Wen Y, Li J, Deng H, Kang B, Mao Y,
Lei S, He Q, Su J, Lu Y, Niu T, Hou J and Huang MJ: A gene therapy
for cancer based on the angiogenesis inhibitor, vasostatin. Gene
Ther. 9:1207–1213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peto R and Peto J: Asymptotically
efficient rank invariant test procedures. J R Stat Soc Ser A.
135:185–207. 1972. View
Article : Google Scholar
|
|
30
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Metastasis Rev. 23:367–387.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu TC and Kim D: Systemic efficacy with
oncolytic virus therapeutics: clinical proof-of-concept and future
directions. Cancer Res. 67:429–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parato KA, Senger D, Forsyth PA and Bell
JC: Recent progress in the battle between oncolytic viruses and
tumours. Nat Rev Cancer. 5:965–976. 2005. View Article : Google Scholar : PubMed/NCBI
|