Introduction
The cell membrane of mammalian cells comprises
glycolipids, glycoproteins and proteoglycans; these carbohydrate
structures undergo conformational changes during cellular
differentiation and transformation (1). In particular, protein glycosylation
accounts for the vast majority of post-translational processes that
affect protein folding, stability, solubility and function
(2). Glycosylation is catalyzed by
glycosyltransferases. Among them, fucosyltransferases transfer an
L-fucose sugar from a GDP-fucose donor substrate to an acceptor
substrate (3). The
fucosyltransferase gene family encodes enzymes that transfer fucose
from α(1,2), α(1,3/4) and α(1,6)
linkages to various glycans. FUT1 and FUT2 encode
α(1,2)-fucosyltransferases, which transfer a
terminal fucose residue from an α(1,2)-linkage to an existing galactose Type 1
or 2 precursor substance and form the H1 or H2 antigen as
precursors of soluble ABH antigens, respectively (4). FUT1 is ubiquitously expressed
in the human body and preferentially expressed in erythroid tissues
and vascular endothelial cells. FUT2 is mainly expressed in
the epithelial cells of the digestive and respiratory tracts
(4). FUT3-FUT7 and
FUT9 encode α(1,3)-fucosyltransferases, and their gene
products transfer a fucose residue from an α(1,3)-linkage to
galactose in H1 and H2 antigens to produce various Lewis antigens
(5,6).
Antigens of the ABH and Lewis histo-blood group
family can be found on the cell surface of various normal cells,
mainly epithelial cells. However, the expression of various
carbohydrate epitopes of this family is altered in carcinomas
(7). For example, Lewis Y antigen
(LeY), a Lewis antigen, is expressed in various cancer cells,
including breast, ovarian and colorectal cancer; its expression is
often associated with poor prognosis (8–12). In
addition, forced FUT1 and FUT2 expression in human
ovarian carcinoma-derived RMG-I cells increases activity of
α(1,2)-fucosyltransferase and LeY antigen and
promotes cell proliferation and resistance against anticancer
drugs, such as 5-FU and carboplatin (13,14).
The molecular mechanisms through which
overexpression of LeY antigen induces a malignant phenotype remain
to be elucidated. However, increased LeY expression induced by
FUT1 and FUT2 overexpression activates the epidermal
growth factor receptor (EGFR) signaling and induces increase in
mRNA expression and protein levels of human epidermal growth factor
receptor 2 (HER2), a member of EGFR family (15). Furthermore, an FUT1- and
FUT2-overexpressing cell line proliferates more aggressively
than the parent cell line (15),
suggesting that activation of EGFR and HER2 induces a malignant
phenotype in human cancer cells.
HER2 is a transmembrane glycoprotein that is
fucosylated by fucosyltransferase. It is involved in transmitting
signals that stimulate cell division (16). HER2-overexpression is caused by
amplification of the HER2 gene and it is observed in various
cancers (17,18), including breast and gastric cancer.
Clinically, it is a molecular target of trastuzumab, a monoclonal
antibody against HER2 (18,19). However, whether alteration of
glycosylation affects cell proliferation in HER2-overexpressing
cancer cells remains to be investigated. In this study, we examined
the effect of FUT1 suppression on the HER2 pathway and cell
proliferation.
Materials and methods
Cell lines and cell culture
In this study, we used four HER2-overexpressing
human cancer cell lines, NCI-N87 and MKN7 (derived from gastric
cancer) and SKBr3 and BT474 (derived from breast cancer), and two
cell lines, A431 and VMRC-LCD (derived from lung cancers), that do
not overexpress HER2. NCI-N87, SKBr3, BT474 and VMRC-LCD were
obtained from the American Type Culture Collection (Manassas, VA,
USA). MKN7 and A431 were provided by the Cell Resource Center for
Biomedical Research (Institute of Development, Aging and Cancer,
Tohoku University, Sendai, Japan). All cell lines were maintained
in RPMI-1640 medium (Sigma, St. Louis, MO, USA) supplemented with
10% heat-inactivated FBS (Gibco, Grand Island, NY, USA) and
incubated at 37°C in a 5% CO2 humidified atmosphere.
Preparation of siRNA and
transfection
The following three pairs of siRNA oligomers were
designed according to the sequence of human FUT1 (GenBank
accession number: NM_000148): FUT1-1 siRNA,
5′-AAAGGAUCUCUCAAGUC CGCGTT-3′ and 5′-CGCGGACUUGAGAGAUCCUUUTT-3′;
FUT1-2 siRNA, 5′-GCUACACCGUGGAAAGACUTT-3′ and
5′-AGUCUUUCCACGGUGUAGCTT-3′; FUT1-3 siRNA,
5′-UCGAUGUUUUCUUUACACCAC-3′ and 5′-GGUGUAA AGAAAACAUCGACA-3′.
FUT1-1 and FUT1-2 siRNAs were designed
based on a previous report (20)
and the resource of Open Biosystems (http://www.openbiosystems.com), respectively.
FUT1-3 siRNA was designed using a web-based online software
system (siDirect version 2.0, http://sidirect2.rnai.jp). These siRNAs were
chemically synthesized by Hokkaido System Science, Co., Ltd.
(Hokkaido, Japan).
Signal Silence EGF Receptor siRNA 1 and 2 (Santa
Cruz Biotechnology, Inc., CA, USA) were used as EGFR-siRNA1 and 2,
respectively. Negative control siRNA (Silencer Negative Control no.
1 siRNA) was obtained from Ambion, Inc. (Austin, TX, USA). Cells
were seeded in a 6- or 96-well plate. After 24 h, the cells were
transfected with siRNA (100 nM final concentration) using
Dharmafect 2 reagent (Dharmacon, Lafayette, CO, USA).
Measurement of mRNA expression by
real-time PCR
Real-time polymerase chain reaction (PCR) was used
to measure the mRNA expression of FUT1 in the cells. Cells
were plated at 1.5×104 in a 96-well plate and
transfected with siRNA (100 nM final concentration) as described
above. Twenty-four hours post-transfection, the total-RNA from the
cells was extracted using the RealTime ready cell lysis kit (Roche
Diagnostics GmbH, Mannheim, Germany) and reverse transcribed using
the Transcriptor First Strand cDNA synthesis kit (Roche Diagnostics
GmbH) with oligo(dt) primer. Real-time PCR was performed using
SsoFast™ EvaGreen Supermix (Bio-Rad, Richmond, CA, USA) and
gene-specific primers in a thermal cycler CFX96 real-time PCR
detection system (Bio-Rad). The primers used for amplification were
FUT1 F, 5′-AACGCCTCCTCTTCCTGTC-3′ and R, 5′-TGGGG
TAGACAGTCCAGGTG-3′; glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) F, 5′-GAAGGTGAAGGTCG GAGTC-3′ and R,
5′-GAAGATGGTGATGGGATTTC-3′ (GenBank accession no. NM_002046). The
designs of both primers have been previously described (21). Quantified data were normalized to
GAPDH. The PCR program included 45 cycles of 95°C for 1 sec and
60°C for 5 sec. After the PCR reaction was completed, a melting
curve analysis was performed. Each primer pair produced a single
and sharp peak, thereby indicating that the primers amplified only
one specific PCR product. No primer dimers were observed. All
samples were amplified in triplicate.
Western blotting
Cells were plated at 2.0×105 in a 6-well
plate and transfected with siRNA (100 nM final concentration) as
described above. After 72 h, the cells were washed with cold PBS
and harvested with lysis buffer [50 mM Tris-HCl (pH 8.0), 150 nM
NaCl, 5 nM EDTA, 1% NP-40, protease inhibitor cocktail (Roche
Diagnostics GmbH) and phosphatase inhibitor (Roche Diagnostics
GmbH)]. In the short-time EGF stimulation experiment, 72 h
post-transfection, the cells were starved in serum-free medium for
12 h and stimulated with EGF (10 ng/ml) for 10 min. The cells were
washed immediately and harvested as described above.
Cell lysates were separated by 12.5% SDS-PAGE and
blotted onto a PVDF membrane. The membranes were blocked with the
Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE, USA) and
then probed with polyclonal anti-HER2 antibody (Dako, Carpinteria,
CA, USA), monoclonal anti-phosphorylated HER2 antibody (Tyr1248;
Santa Cruz Biotechnology, Inc.), monoclonal anti-EGFR antibody
(Santa Cruz Biotechnology, Inc.), monoclonal anti-phosphorylated
EGFR antibody (Tyr1068), monoclonal anti-GAPDH antibody (Santa Cruz
Biotechnology, Inc.), anti-ERK1/2 antibody (Cell Signaling
Technology, Inc.), anti-phosphorylated ERK1/2 antibody
(Thr202/Tyr204; Cell Signaling Technology, Inc.) and anti-LeY
antibody (Abcam, Cambridge, UK), followed by incubation with a goat
anti-rabbit or a goat anti-mouse Alexa Fluor 680 IgG secondary
antibody (Invitrogen, Carlsbad, CA, USA). Protein bands were
detected and quantified using the Odyssey system (Li-Cor
Biosciences).
Cell proliferation assay
Cells were plated at 3.0×103 in a 96-well
plate and transfected with siRNA (100 nM final concentration) as
described above. At 0, 72 and 120 h the cells were harvested and
cell viability was determined using the Cell Counting kit-8 (Dojin
Laboratories, Kumamoto, Japan), which measures mitochondrial
succinate dehydrogenase activity. Briefly, 10 μl of
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
monosodium salt (WST-8) solution was added to each well. After a
2-h incubation at 37°C, the resulting water-soluble formazan dye
was assayed by a microplate autoreader SpectraMax (Molecular
Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm with a
reference of 630 nm. All experiments were performed in
triplicate.
Cell cycle analysis using
fluorescence-activated cell sorter (FACS)
Cells were plated at 2.0×105 in a 6-well
plate and transfected with siRNA (100 nM final concentration) as
described above. After a 72-h incubation, cells were treated with
trypsin and fixed with 70% ethanol in PBS overnight. The cells were
then washed once with PBS, incubated in the presence of RNase A
(0.25 mg/ml) for 30 min at 37°C, collected by centrifugation at 200
× g for 5 min and stained with propidium iodide (50 μl/ml). The
cells were filtered through a 50-μm pore size nylon mesh and
analyzed for cell cycle using a FACS system (Beckman Coulter,
Miami, FL, USA).
Statistical analysis
All experiments were performed independently and in
triplicate. Data are expressed as means ± standard error.
Statistical data were analyzed using Student’s t-test. Significance
was set at P<0.05.
Results
FUT1 knockdown by siRNA decreases LeY
antigen expression
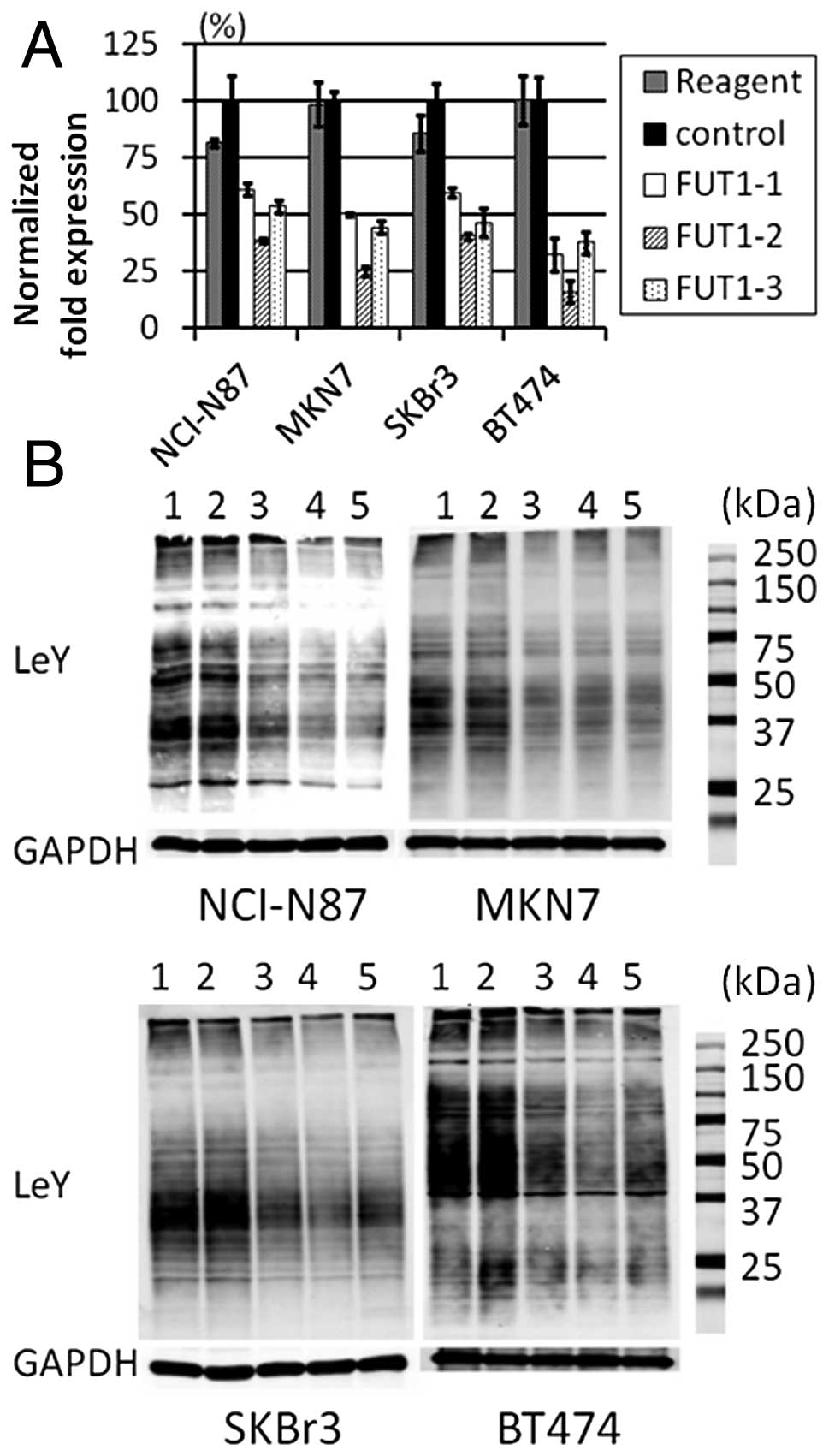
To detect the effects of FUT1 siRNAs, we
performed real-time PCR analysis (Fig.
1A) and western blotting of LeY antigen (Fig. 1B). FUT1 mRNA and LeY antigen
expression were reduced in all cells transfected with FUT1
siRNAs, but not in those transfected with control siRNA. Compared
with the cells transfected with control siRNA, the level of LeY
expression in the cells transfected with FUT1-1,
FUT1-2 or FUT1-3 siRNAs was FUT1-1 67.9,
FUT1-2 44.5 and FUT1-3 46.9% in NCI-N87 cells;
FUT1-1 50.5, FUT1-2 58.6 and FUT1-3 51.7% in
MKN7 cells; FUT1-1 49.2, FUT1-2 33.3 and
FUT1-3 62.3% in SKBr3 cells; and FUT1-1 54.1,
FUT1-2 41.1 and FUT1-3 58.8% in BT474 cells. These
results indicated that FUT1 siRNAs efficiently reduced the
levels of FUT1 mRNA and LeY antigen expression.
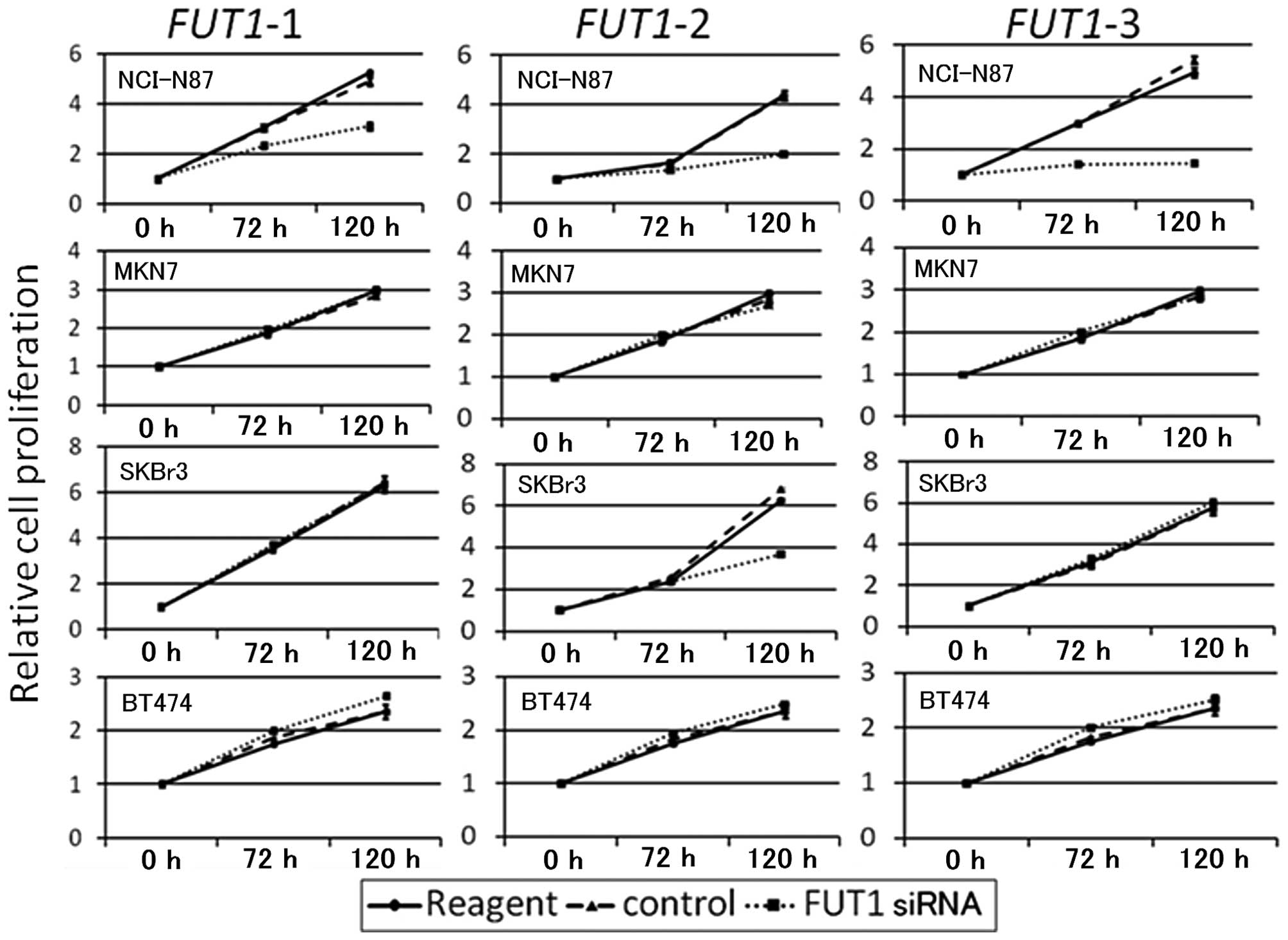
FUT1 knockdown inhibits cell
proliferation in NCI-N87 cells, but not in other cell lines
To examine the effect of siRNA-mediated FUT1
knockdown on cell growth, cell proliferation assays were performed
for the four HER2-overexpressing cell lines. Data are shown in
Fig. 2. FUT1 siRNAs
inhibited NCI-N87 cell proliferation 120 h post-transfection,
whereas they did not inhibit proliferation in MKN7 or BT474 cells.
Although FUT1-2 suppressed proliferation in SKBr3 cells,
FUT1-1 and FUT1-3 did not. Therefore this was
considered to be an off-target siRNA effect.
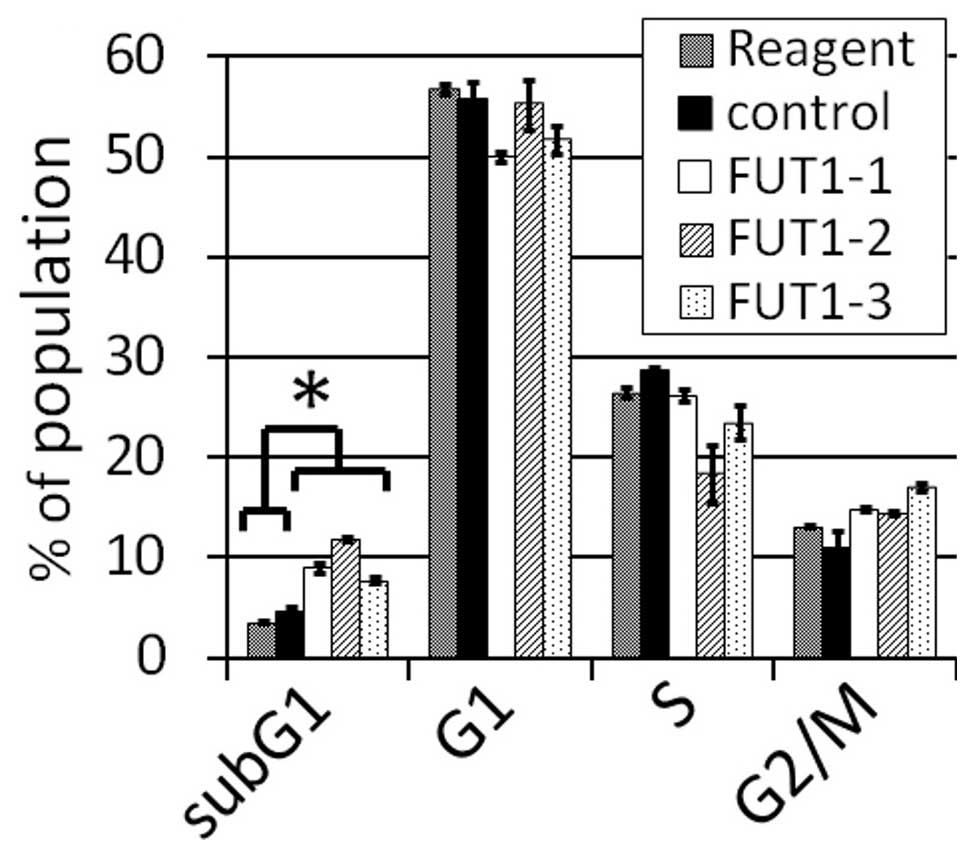
FUT1 knockdown leads to apoptosis in
NCI-N87 cells
To examine whether FUT1 knockdown changes the
proportion of cells in each cell cycle phase, FACS analysis was
performed for NCI-N87 cells (Fig.
3). All siRNAs significantly increased the subG1 fraction
(P<0.01). The G2/M fraction also increased, but not
significantly (P=0.09). These results indicate that the
downregulation of FUT1 mRNA and LeY antigen expression leads
to apoptosis in NCI-N87 cells.
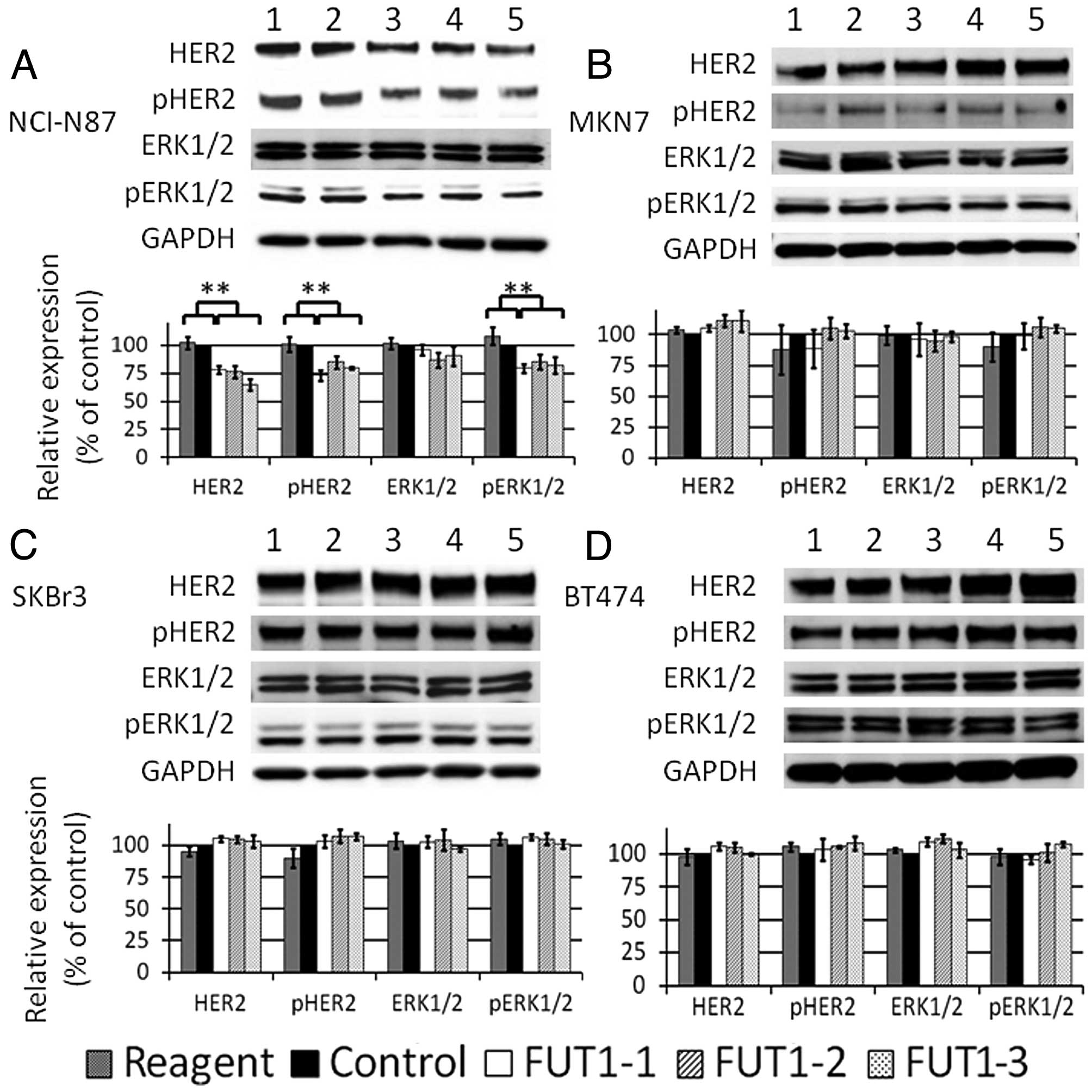
FUT1 knockdown downregulates the
expression of HER2 and the phosphorylated HER2 (pHER2) and
phosphorylated ERK1/2 (pERK) in NCI-N87 cells
To elucidate the mechanism of cell growth inhibition
induced by FUT1 knockdown, HER2, pHER2, ERK1/2 and pERK were
assessed by western blotting 72 h after transfection (we called it
‘normal cultural conditon’).
Representative western blotting data and bar charts
by triplicate experiments are shown in Fig. 4. FUT1 knockdown significantly
downregulated the total amount of HER2 and pHER2 in NCI-N87 cells
(Fig. 4A). The amount of pERK also
decreased, but the total amount of ERK remained unchanged. In
contrast to NCI-N87, no significant changes were observed in MKN7,
SKBr3 or BT474 cells (Fig.
4B-D).
FUT1 knockdown strongly downregulates
pHER2 and pERK following short-time EGF stimulation in NCI-N87
cells
To examine whether short-time EGF-stimulation alters
downregulation of HER2 and ERK1/2 by FUT1 knockdown, we
administered EGF for 10 min after starvation of the cells and
assessed the amount of HER2, pHER2, ERK1/2 and pERK. The amount of
pHER2 and pERK was markedly reduced in NCI-N87 cells (Fig. 5A). This reduction was more apparent
than that of normal culture condition (Fig. 4A). Alterations in HER2 and ERK1/2
levels were similar to those observed in normal culture condition.
In contrast, no significant changes were observed following EGF
stimulation in MKN7, SKBr3 or BT474 cells (Fig. 5B-D).
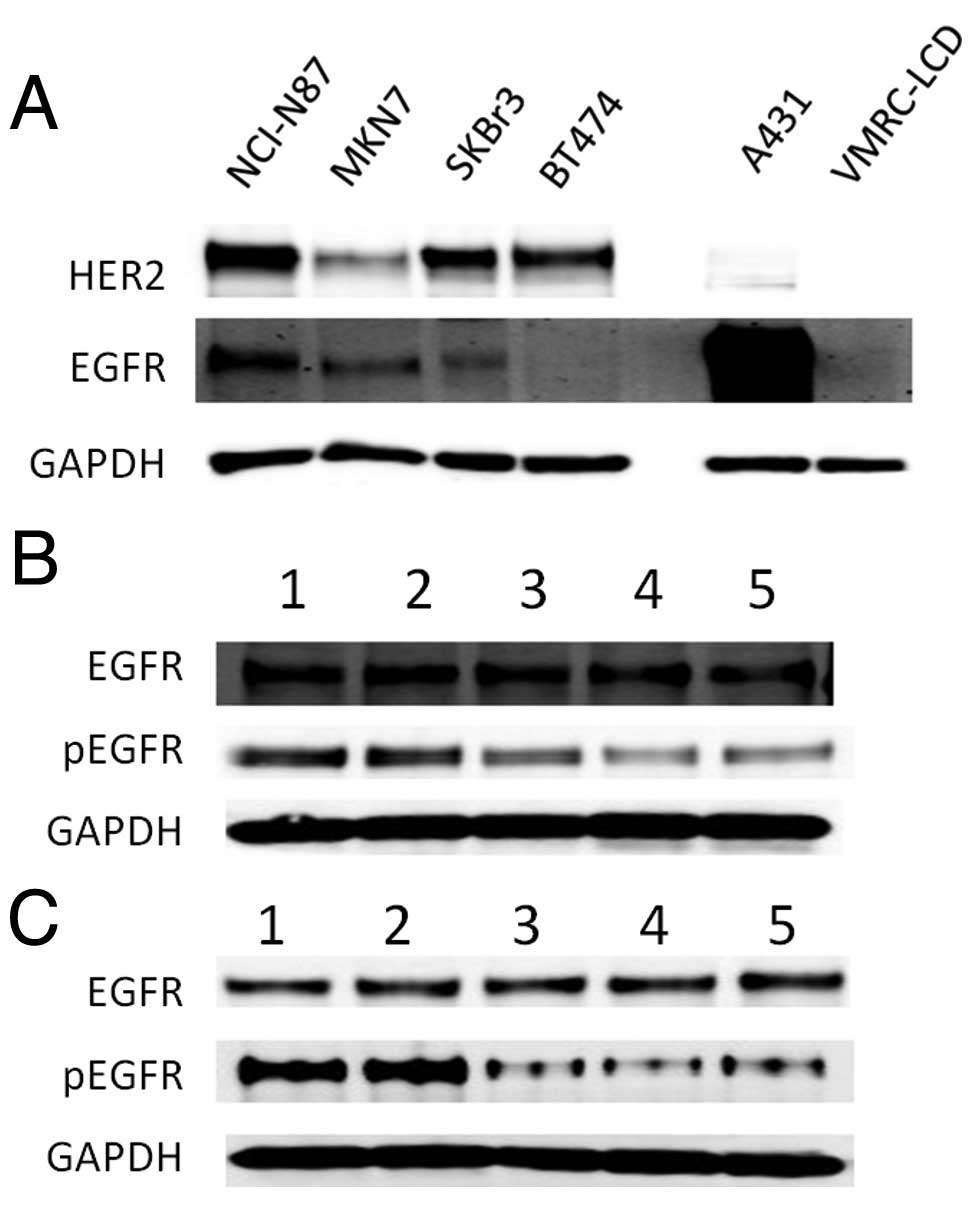
FUT1 knockdown downregulates EGFR
signaling in NCI-N87
To examine whether FUT1 suppression affects
EGFR signaling, first, western blotting for EGFR expression was
performed (Fig. 6A). EGFR
expression in each cell line was lower than that in the
EGFR-overexpressing A431 cell line, whereas EGFR expression in
NCI-N87 was higher than that in other HER2-overexpressing cell
lines. Then, we investigated EGFR signaling of NCI-N87 after
FUT1 suppression. The result showed that phosphorylation of
EGFR was downregulated in both normal cultural and EGF-stimulating
conditions. The total amount of EGFR did not change in either of
the conditions (Fig. 6B and C).
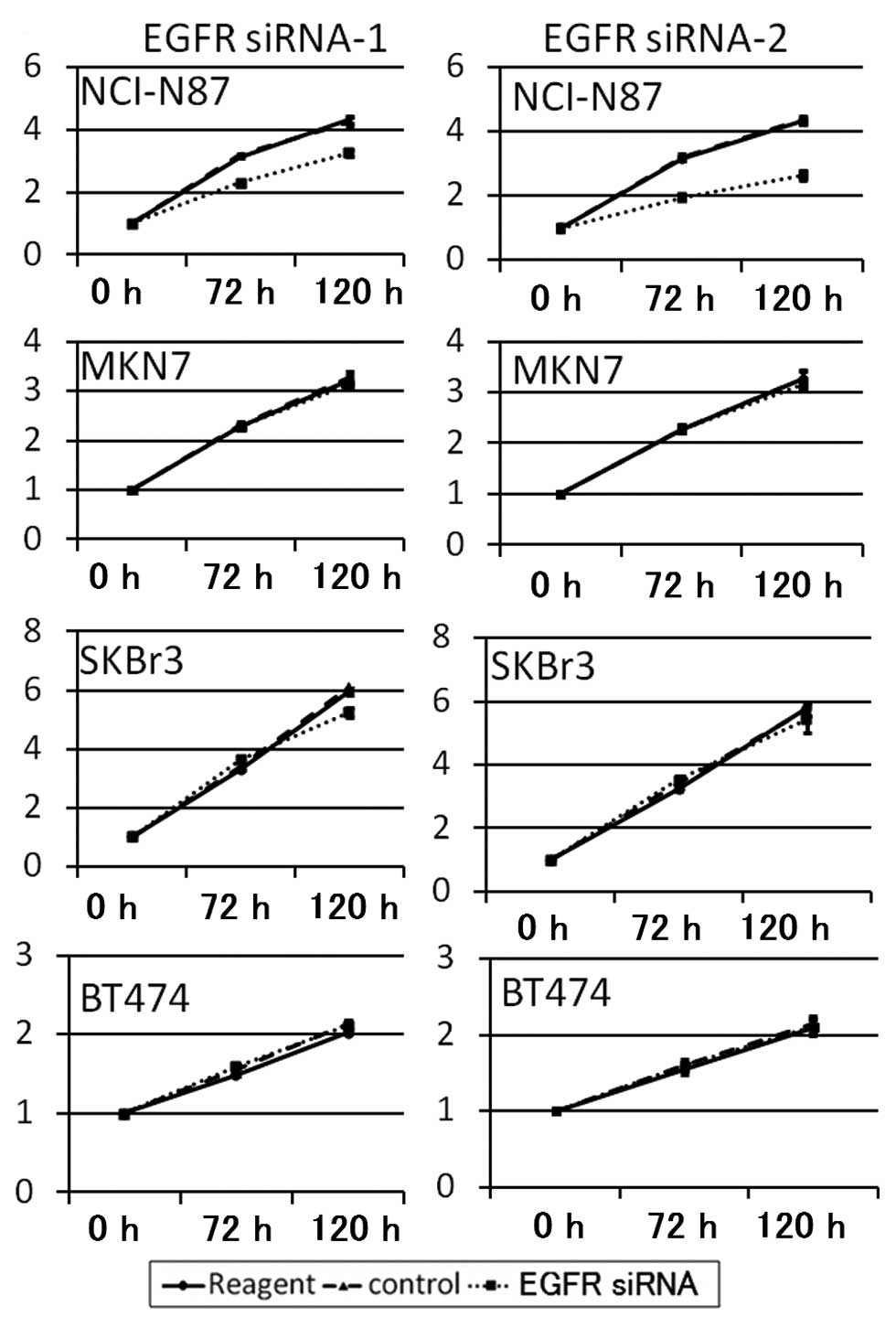
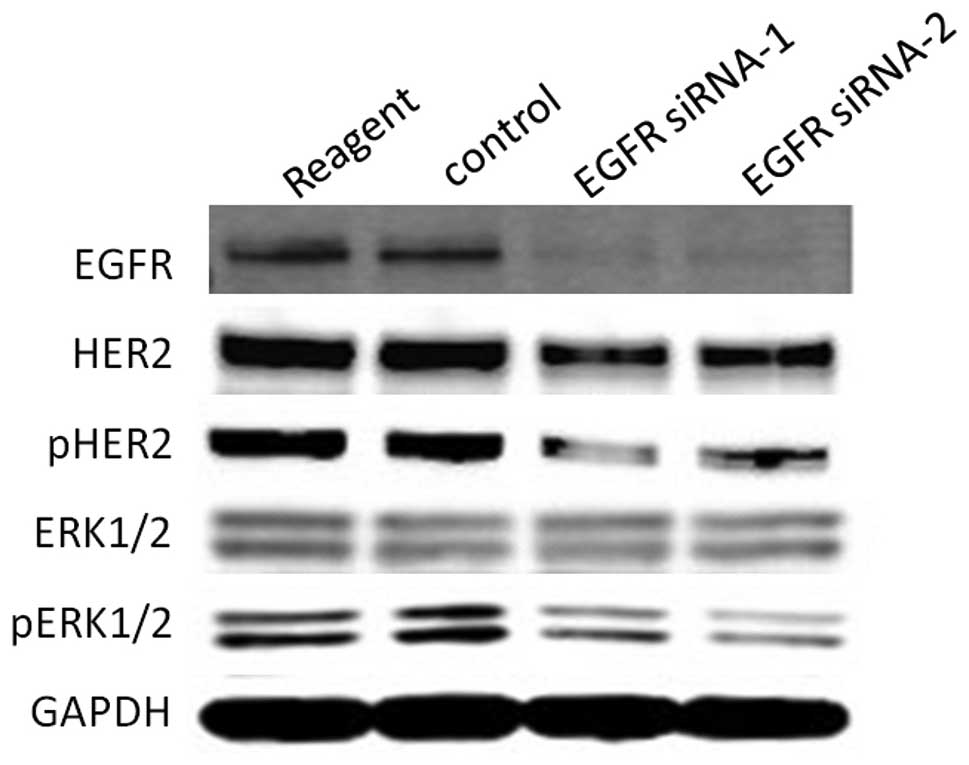
Suppression of EGFR signaling
downregulates HER2 signaling and proliferation of NCI-N87
cells
To confirm the proliferation dependence of EGFR
signaling the cell lines were transfected with EGFR siRNA. EGFR
siRNA-1 and EGFR siRNA-2 suppressed the proliferation of NCI-N87
cells by 23.6 and 44.7%, respectively. However the proliferation of
other cell lines were not changed by EGFR knockdown (Fig. 7). Next, to investigate whether EGFR
suppression affects HER2 signaling in NCI-N87 cells, western
blotting was performed. EGFR suppression downregulated HER2, pHER2
and pERK as well as FUT1 suppression in NCI-N87 cells
(Fig. 8). The results indicated
that the proliferation of NCI-N87 cells was also dependent on EGFR
signaling and EGFR suppression resulted in downregulation of HER2
signaling in this cell line.
Discussion
LeY antigen belongs to the histo-blood group
antigens and α1,2-fucosyltransferase is the key enzyme, which also
FUT1 and FUT2 encode. Previous studies suggested that
forced expression of α1,2-fucosyltransferase in RMG-I human ovarian
cancer cell line caused overexpression of LeY antigen and promoted
cell proliferation via activation of EGFR and HER2 (15). Furthermore, Palumberi et
al(20) indicated that
suppression of α1,2-fucosyltransferase inhibited the cell
proliferation of the EGFR-overexpressing cell line A431. In the
present study, we attempted to suppress FUT1 gene by its
specific siRNA and observe whether FUT1 knockdown affected
the cell proliferation of HER2-overexpressing cell lines.
Our results indicated that FUT1 siRNA
downregulated FUT1 mRNA and altered fucosylation; it was
shown by inhibition of LeY antigen expression, in four
HER2-overexpressing cell lines. However, the effects on cell
proliferation varied. In NCI-N87 cells, FUT1 suppression
decreased the total amount of HER2, pHER2 and pERK, and inhibited
cell proliferation. However, FUT1 suppression in MKN7, SKBr3
and BT474 did not alter HER2, pHER2 or pERK levels and did not
affect cell proliferation.
In a previous study, HER2 inhibition led to
suppression of cell proliferation in HER2-overexpressing cell lines
(22–24). This observation is similar to our
results and indicates that HER2 plays an important role in cell
proliferation in HER2-overexpressing cells.
In addition, our study suggested that EGFR signaling
was involved in FUT1-mediated inhibition of HER2 signaling
in NCI-N87 cells. The experiment of EGFR siRNA transfection
indicated that the proliferation of NCI-N87 cells was depentdent
not only on HER2 signaling but also on EGFR signaling and EGFR
suppression led to HER2 signaling inhibition. Previous studies
indicated that cetuximab, a monoclonal antibody against EGFR,
inhibits cell proliferation in NCI-N87 cells (25), but not in SKBr3 or BT474 cells
(24). These results suggest that
EGFR potently contributes to the proliferation of NCI-N87
cells.
We speculate that FUT1 suppression leads to
HER2 inhibition and cell proliferation via EGFR signaling
inhibition through one or both mechanisms described below.
First, downregulation of EGFR by FUT1
suppression may attenuate HER2 transcription. Liu et
al(15) reported that
FUT1-overexpression upregulated EGFR signaling and increased
mRNA expression and protein levels of HER2. In our study,
FUT1 knockdown decreased the total amount of HER2 in NCI-N87
cells. Therefore, FUT1 knockdown may have decreased HER2
levels by downregulating EGFR signaling. Since the level of
attenuation of the total amount of HER2 was similar to that of
pHER2, a reduction of the total amount of HER2 may cause
downregulation of pHER2 and pERK in normal culture condition.
Second, FUT1 suppression may attenuate EGFR
and HER2 heterodimer formation. HER2 forms homodimers or
heterodimers with other EGFR family proteins, undergoes
autophosphorylation at specific tyrosine residues of its
intracellular domain and mediates signal transduction (17). In addition, EGFR forms homodimers or
heterodimers with other EGFR family proteins following ligand
stimulation (25).
Following starvation and short-time EGF stimulation,
phosphorylation of HER2 and ERK1/2 was markedly reduced in
FUT1-suppressed NCI-N87 cells. Zhang et al(26) reported that the suppression of
FUT1 and FUT4 reduced LeY antigen, decreased binding of EGF
to EGFR and resulted in inhibition of cell proliferation. In
addition, some reports have shown that fucosylation on EGFR alters
the binding affinity of EGF to EGFR and affects EGFR dimerization
(2,27,28).
Hence, we propose that FUT1 suppression
caused an alteration of fucosylation and attenuated EGF-mediated
EGFR and HER2 heterodimerization.
Besides, our results indicated that apoptosis
occurred in FUT1-mediated growth inhibition in NCI-N87
cells. G2/M fraction also tended to increase but not significantly.
Previous studies revealed that HER2 inhibition by trastuzumab
caused apoptosis in some HER2-overexpressing cell lines, e.g. SKBr3
or Calu-3 (29). However, it did
not cause apoptosis in SKOV-3 which had HER2-overexpression
(30). Hence, it is possible that
HER2 suppression causes various effects on cell proliferation among
each cell line.
Lapatinib is a dual tyrosine kinase inhibitor for
EGFR and HER2 and is used to treat trastuzumab-resistant HER2
positive cancers. Redundant signaling from other EGFR family
members is one of the molecular mechanisms of drug resistance to
trastuzumab (31). Inhibition of
EGFR and HER2 signaling is one strategy for treating
trastuzumab-resistant HER2 positive cancers.
The role of fucosylation in cell proliferation is
not completely understood. However, our results demonstrate that
FUT1 knockdown results in the inhibition of cell
proliferation and reduction of HER2, pHER2 and pERK in NCI-N87
cells. The reduction of pHER2 and pERK seems to depend on the
reduction of EGFR signaling caused by inhibition of fucosylation.
Further studies are necessary to identify a biomarker to predict
which HER2-positive cancer cells are sensitive to FUT1
inhibition. The development of a fucosyltransferase inhibitor may
constitute a novel drug for trastuzumab-resistant HER2 positive
cancers.
Acknowledgements
The authors thank Satoko Aoki for her technical
assistance.
References
|
1
|
Roseman S: Reflections on glycobiology. J
Biol Chem. 276:41527–41542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu YC, Yen HY, Chen CY, et al:
Sialylation and fucosylation of epidermal growth factor receptor
suppress its dimerization and activation in lung cancer cells. Proc
Natl Acad Sci USA. 108:11332–11337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Javaud C, Dupuy F, Maftah A, et al: The
fucosyltransferase gene family: an amazing summary of the
underlying mechanisms of gene evolution. Genetica. 118:157–170.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matzhold EM, Helmberg W, Wagner T, et al:
Identification of 14 new alleles at the fucosyltransferase 1, 2,
and 3 loci in Styrian blood donors, Austria. Transfusion.
49:2097–2108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dettke M, Pálfi G and Loibner H:
Activation-dependent expression of the blood group-related Lewis Y
antigen on peripheral blood granulocytes. J Leukoc Biol.
68:511–514. 2000.PubMed/NCBI
|
|
6
|
Hokke CH, Neeleman AP, Koeleman CA and van
den Eijinden DH: Identification of an α3-fucosyltransferase and a
novel α2-fucosyltransferase activity in cercariae of the
schistosome Trichobilharzia ocellata: biosynthesis of the
Fucα1-->2Fucα1-->3[Gal(NAc)β1-->4] GlcNAc sequence.
Glycobiology. 8:393–406. 1998.
|
|
7
|
Nakagoe T, Fukushima K, Itoyanagi N, et
al: Expression of ABH/Lewis-related antigens as prognostic factors
in patients with breast cancer. J Cancer Res Clin Oncol.
128:257–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuboi K, Asao T, Ide M, et al:
α1,2-fucosylation is a superior predictor of postoperative
prognosis for colorectal cancer compared with blood group A, B, or
sialyl Lewis X antigen generated within colorectal tumor tissue.
Ann Surg Oncol. 14:1880–1889. 2007.
|
|
9
|
Madjd Z, Parsons T, Watson NF, Spendlove
I, Ellis I and Durrant LG: High expression of Lewis y/b antigens is
associated with decreased survival in lymph node negative breast
carcinomas. Breast Cancer Res. 7:R780–787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arai Y and Nishida M: Differential
diagnosis between normal endometrium and endometrial hyperplasia
with immunostaining cytology using anti-LeY monoclonal antibody.
Int J Gynecol Cancer. 13:42–46. 2003. View Article : Google Scholar
|
|
11
|
Kim YS, Yuan M, Itzkowitz SH, et al:
Expression of LeY and extended LeY blood group-related antigens in
human malignant, premalignant, and non-malignant colonic tissues.
Cancer Res. 46:5985–5992. 1986.PubMed/NCBI
|
|
12
|
Kitamura K, Stockert E, Garin-Chesa P, et
al: Specificity analysis of blood group Lewis-y (Le(y)) antibodies
generated against synthetic and natural Le(y) determinants. Proc
Natl Acad Sci USA. 91:12957–12961. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwamori M, Tanaka K, Kubushiro K, et al:
Alterations in the glycolipid composition and cellular properties
of ovarian carcinoma-derived RMG-1 cells on transfection of the α1,
2-fucosyltransferase gene. Cancer Sci. 96:26–30. 2005.PubMed/NCBI
|
|
14
|
Zhao Y, Lin B, Hao YY, et al: The effects
of Lewis (y) antigen content on drug resistance to carboplatin in
ovarian cancer line RMG-I. Prog Biochem Biophys. 35:1175–1182.
2008.
|
|
15
|
Liu JJ, Lin B, Hao YY, et al: Lewis(y)
antigen stimulates the growth of ovarian cancer cells via
regulation of the epidermal growth factor receptor pathway. Oncol
Rep. 23:833–841. 2010.PubMed/NCBI
|
|
16
|
Brennan PJ, Kumogai T, Berezov A, et al:
HER2/Neu: mechanisms of dimerization/oligomerization. Oncogene.
19:6093–6101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slamon DJ, Godolphin W and Jones LA:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gravalos C and Jimeno A: HER2 in Gastric
Cancer: A New Prognostic Factor and a Novel Therapeutic Target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Owens MA, Horten BC and Da Silva MM: HER2
amplification ratios by fluorescence in situ hybridization and
correlation with immunohistochemistry in a cohort of 6556 breast
cancer tissues. Clin Breast Cancer. 5:63–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palumberi D, Aldi S, Ermini L, et al:
RNA-mediated gene silencing of FUT1 and FUT2 influences
expression and activities of bovine and human fucosylated nucleolin
and inhibits cell adhesion and proliferation. J Cell Biochem.
111:229–238. 2010.PubMed/NCBI
|
|
21
|
Chang WW, Lee CH, Lee P, et al: Expression
of Globo H and SSEA3 in breast cancer stem cells and the
involvement of fucosyl transferases 1 and 2 in Globo H synthesis.
Proc Natl Acad Sci USA. 105:11667–11672. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mittendorf EA, Liu Y, Tucker SL, et al: A
novel interaction between HER2/neu and cyclin E in breast cancer.
Oncogene. 29:3896–3907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanner M, Hollmén M, Junttila TT, et al:
Amplification of HER-2 in gastric carcinoma: association with
Topoisomerase IIa gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brockhoff G, Heckel B, Schmidt-Bruecken E,
et al: Differential impact of Cetuximab, Pertuzumab and Trastuzumab
on BT474 and SK-BR-3 breast cancer cell proliferation. Cell Prolif.
40:488–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel D, Bassi R, Hooper A, et al:
Anti-epidermal growth factor receptor monoclonal antibody cetuximab
inhibits EGFR/HER-2 heterodimerization and activation. Cancer Sci.
99:1611–1617. 2008.PubMed/NCBI
|
|
26
|
Zhang Z, Sun P, Liu J, et al: Suppression
of FUT1/FUT4 expression by siRNA inhibits tumor growth.
Biochim Biophys Acta. 1783:287–296. 2008.
|
|
27
|
Miyoshi E, Moriwaki K and Nakagawa T:
Biological function of fucosylation in cancer biology. J Biochem.
143:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Gu J, Ihara H, et al: Core
fucosylation regulates epidermal growth factor receptor-mediated
intracellular signaling. J Biol Chem. 281:2572–2577. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dogan I, Cumaoglu A, Aricioglu A and
Ekmekci A: Inhibition of ErbB2 by herceptin reduces viability and
survival, induces apoptosis and oxidative stress in Calu-3 cell
line. Mol Cell Biochem. 347:41–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bijman MN, van Berkel MP, Kok M, Janmaat
ML and Boven E: Inhibition of functional HER family members
increases the sensitivity to docetaxel in human ovarian cancer cell
lines. Anticancer Drugs. 20:450–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kruser TJ and Wheeler DL: Mechanisms of
resistance to HER family targeting antibodies. Exp Cell Res.
316:1083–1100. 2010. View Article : Google Scholar : PubMed/NCBI
|