Introduction
Matrix metalloproteinases (MMPs) are a group of
zinc-dependent endopeptidases that are known to degrade
extracellular matrix (ECM). MMPs are secreted as inactive zymogens
from cancer cells or macrophages and activated by proteases such as
plasmin or trypsin (1,2). Biological activities of MMPs are
downregulated by α2-macroglobulin (α2M) or tissue inhibitors of
metalloproteinases (TIMPs) produced by macrophages, fibroblasts or
other types of cells (3–5). Therefore, a quantitative imbalance
between MMPs and their inhibitors such as α2M and TIMPs is thought
to be a causative factor in invasion and metastasis. It has also
been shown that MMP-2 degrades the ECM of atherosclerotic plaques
and has an important role in plaque vulnerability at
atherosclerotic regions (6,7). We previously reported that the
quantitative imbalance between proteases, such as prostate-specific
antigen (PSA) and MMP-2, and their inhibitors, including α2M and
α2-plasmin inhibitor, is a causative factor in invasion and
metastasis of prostate cancer (PCa) (8,9).
C-reactive protein (CRP) and serum amyloid A (SAA)
are widely used as acute inflammatory biomarkers in various
conditions such as infection, inflammation, malignancy and tissue
disturbance (10–12). CRP is most widely used as a
sensitive inflammatory biomarker in routine clinical examination.
In recent years, the determination of high sensitivity CRP (hs-CRP)
has been possible due to the wide use of low concentration range
measurements in routine clinical examination. It has been
demonstrated that hs-CRP reflects the degree of localized vascular
inflammation and is a useful prognostic marker of cardiovascular
events (13,14). On the other hand, serum SAA level is
generally increased in patients with viral infection or in
corticosteroid-treated patients in contrast to CRP (15,16).
The production of CRP and SAA in liver cells is regulated by
interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis
factor-α (TNF-α) secreted from macrophages in various conditions
(17,18). α2M is the most abundant proteinase
inhibitor in the blood, and it is also involved in the inflammatory
reaction through its function as a carrier protein of IL-6
(19). We previously demonstrated
that the serum levels of IL-6, CRP and SAA were affected by serum
α2M concentration in PCa patients with or without α2M deficiency,
and these markers are considered α2M-dependent acute inflammatory
biomarkers (20,21).
However, the relationship between the serum levels
of MMP-2 and acute inflammatory biomarkers in patients with NSCLC
progression has yet to be demonstrated. Therefore, we quantified
serum levels of MMP-2, CRP and SAA in localized and metastatic
NSCLC patients to establish the clinical significance and changes
of these biomarkers during NSCLC disease progression. Although this
study includes only a limited number of NSCLC patients, it is the
first report to investigate the clinical significance and changes
of MMP-2, CRP and SAA in patients with NSCLC in relation to disease
progression.
Materials and methods
Patients
Thirty-seven untreated adult men participated in
this study, of whom 13 were healthy controls (mean age 62.6 years,
range 53–72) and 24 were diagnosed with non-small cell lung cancer
(NSCLC) at the Kitasato University Hospital. The 24 cases included
12 localized NSCLC (6 adenocarcinomas and 6 squamous cell
carcinomas) (mean age 64.8 years, range 52–78) and 12 metastatic
NSCLC (6 adenocarcinomas and 6 squamous cell carcinomas) (mean age
67.6 years, range 54–79). NSCLC was clinically staged according to
the TNM classification (22). Serum
α2M levels in the 24 NSCLC patients and the 13 healthy controls
were within reference range. Serum samples were obtained from these
patients and stored at −80°C until use. Informed consent was
obtained from all subjects in this study.
Acute inflammatory biomarkers
The measurement of MMP-2 levels in serum was
determined by measuring pro-MMP-2 using a one-step sandwich enzyme
immunoassay (Fuji Chemical Industries, Toyama, Japan) (23). CRP and SAA levels in serum were
measured by latex nephelometry using the LX-M (Eiken Chemical Co.,
Tokyo, Japan).
Statistical analysis
The Wilcoxon signed-rank test and the Mann-Whitney U
test were used for statistical analyses, and p<0.05 was
considered to indicate statistically significant differences.
Ethics approval
This study was conducted in accordance with the
Declaration of Helsinki. This study had no impact on the management
of patients, and informed consent was obtained from all
subjects.
Results
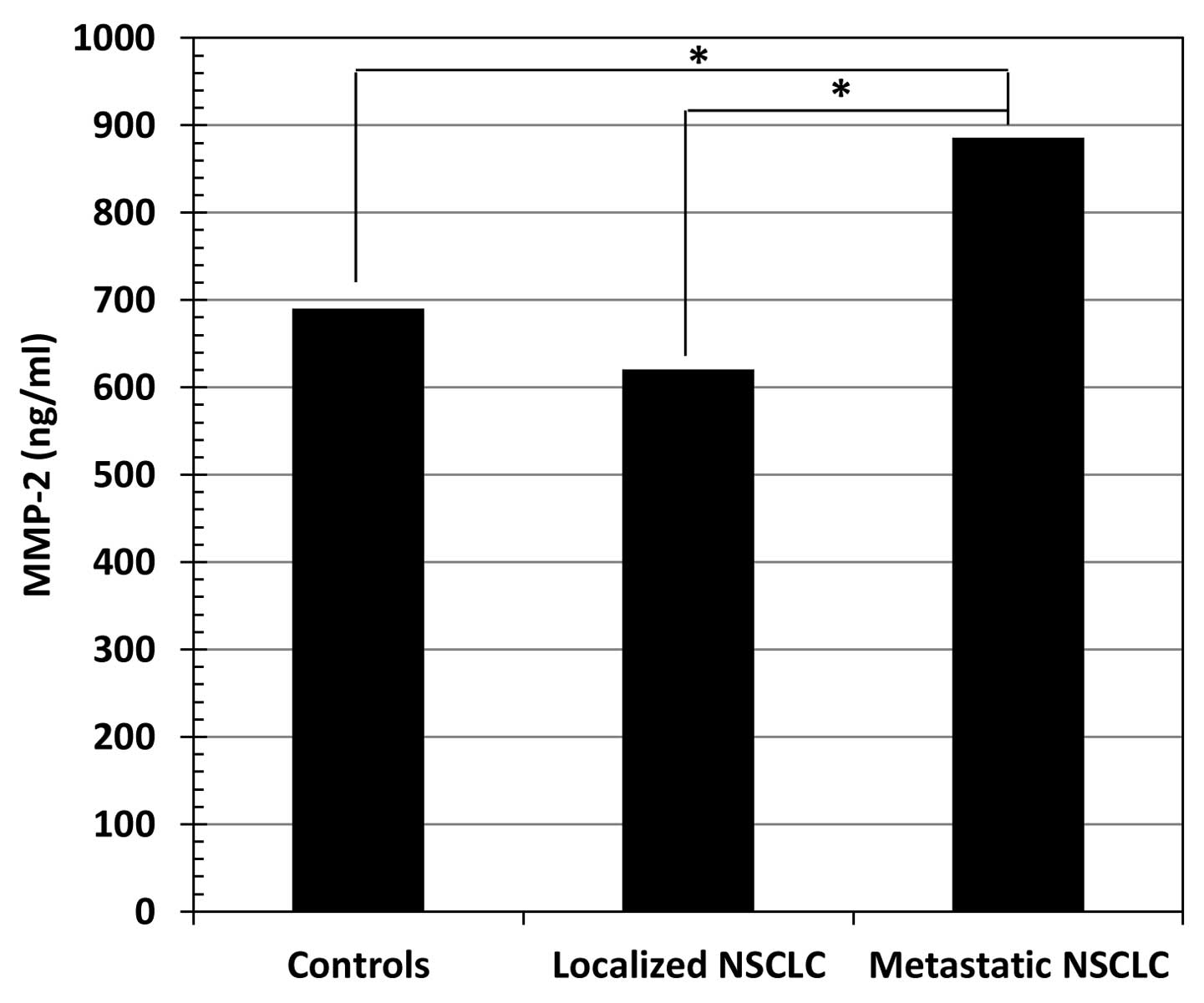
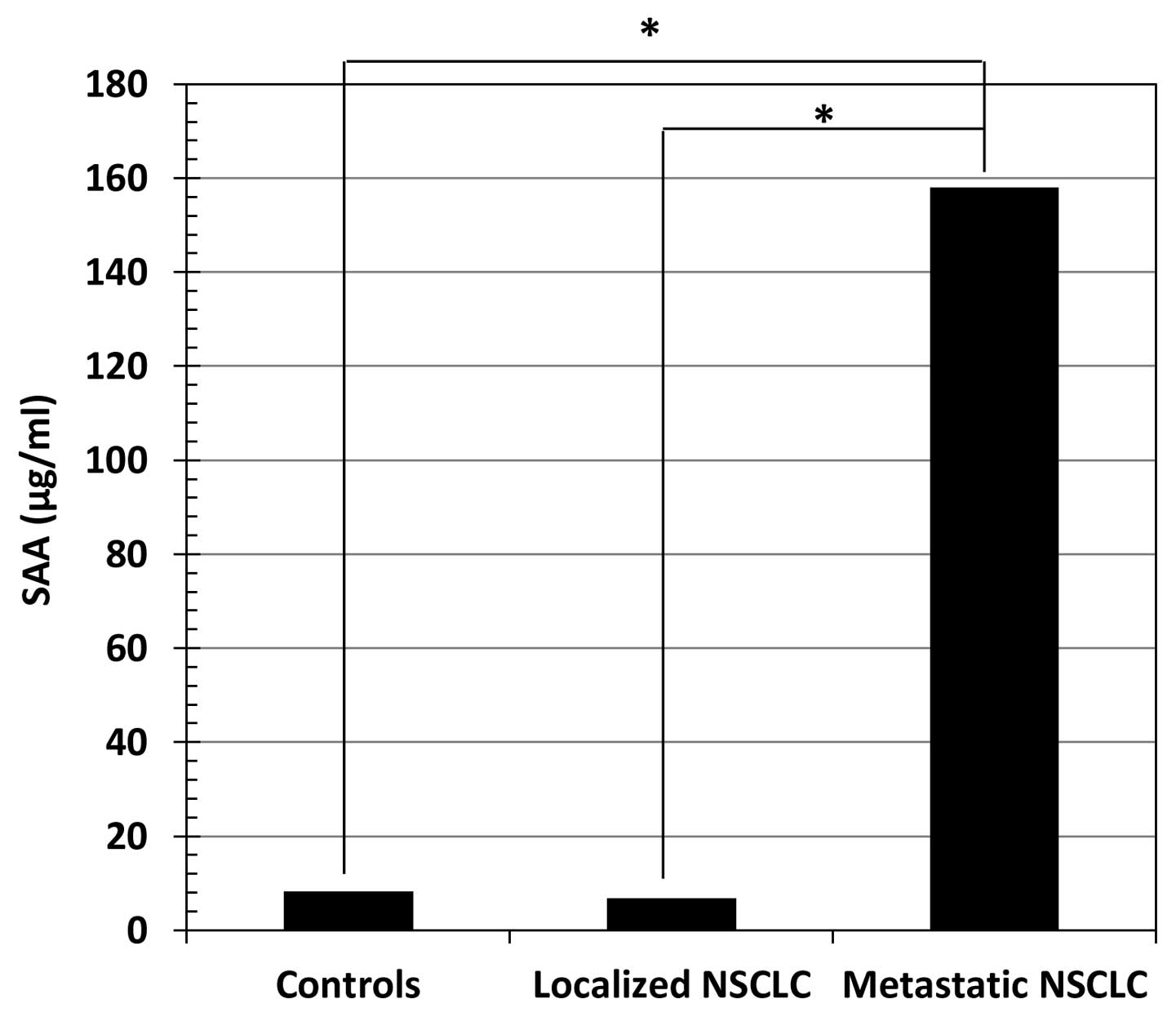
Concentrations of MMP-2, CRP and SAA in
serum
Table I shows the
concentrations (median value, range) of MMP-2, CRP and SAA in the
sera of healthy controls and NSCLC patients. The serum levels of
MMP-2 (Fig. 1), CRP (Fig. 2) and SAA (Fig. 3) in metastatic NSCLC patients were
significantly higher than in healthy controls (p<0.01 for all
three markers) and localized NSCLC patients (p<0.01 for all
three markers).
 | Table IConcentration (median value, range) of
MMP-2, CRP and SAA in healthy controls and NSCLC patients. |
Table I
Concentration (median value, range) of
MMP-2, CRP and SAA in healthy controls and NSCLC patients.
| Healthy controls
(n=13) | Localized NSCLC
(n=12) | Metastatic NSCLC
(n=12) | p-value of healthy
controls vs. localized NSCLC | p-value of healthy
controls vs. metastatic NSCLC | p-value of localized
vs. metastatic NSCLC |
|---|
| MMP-2 (ng/ml) | 690 (624–782) | 620 (347–844) | 886 (685–1,040) | NS | <0.01 | <0.01 |
| CRP (μg/dl) | 27.5 (8–401) | 207.6 (18–594.5) | 4,488
(224–9,940) | NS | <0.01 | <0.01 |
| SAA (μg/ml) | 8.2 (7.2–18.6) | 6.85 (2–337) | 158 (5–1,474) | NS | <0.01 | <0.01 |
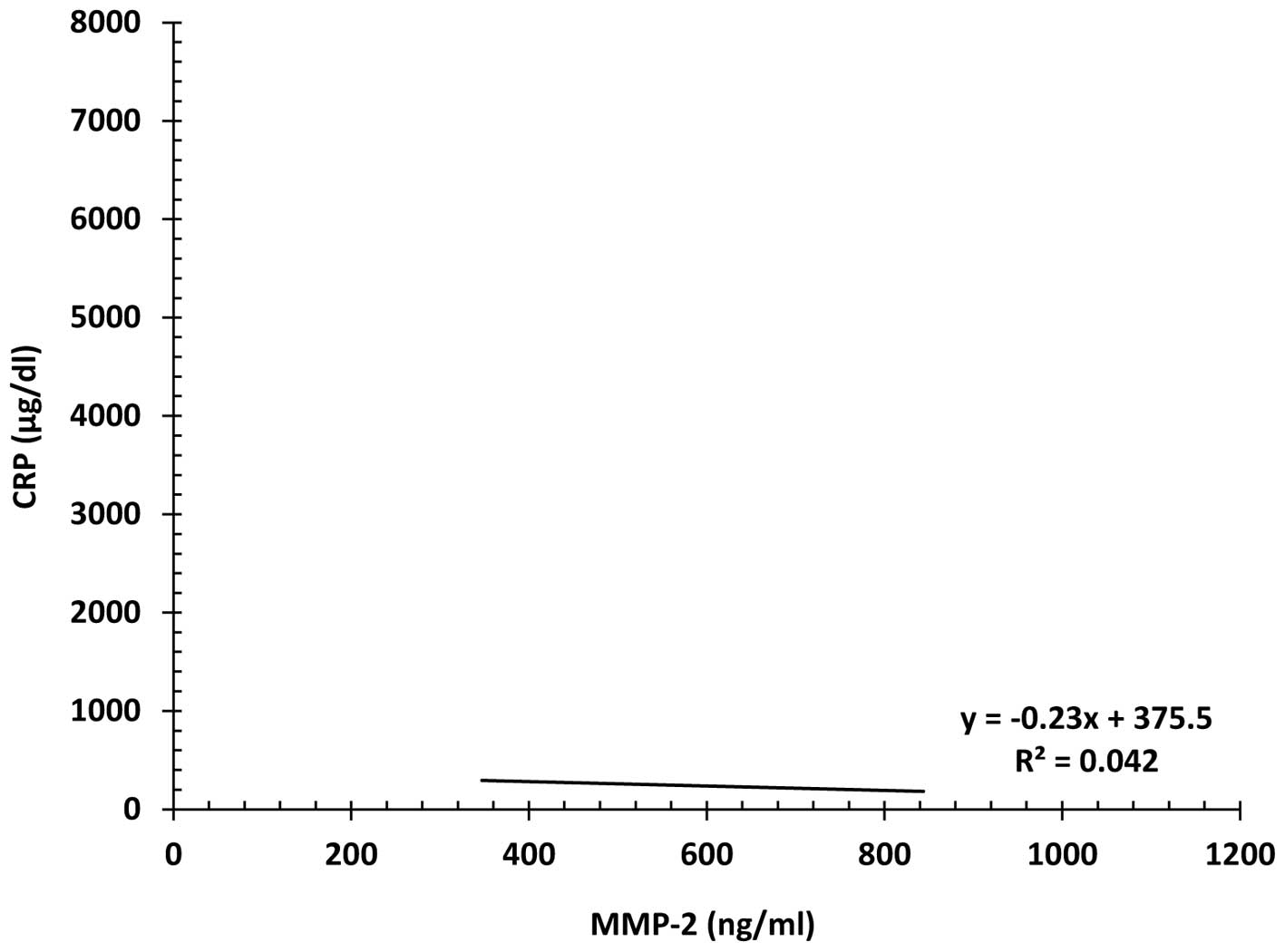
Correlation between serum MMP-2 and CRP
levels
There was a weak, but significant negative
correlation between serum MMP-2 and CRP levels in localized NSCLC
patients (r2=0.042) (p<0.05) (Fig. 4). On the other hand, there was a
weakly significant positive correlation between serum MMP-2 and CRP
levels in metastatic NSCLC patients (r2=0.051)
(p<0.01) (Fig. 5).
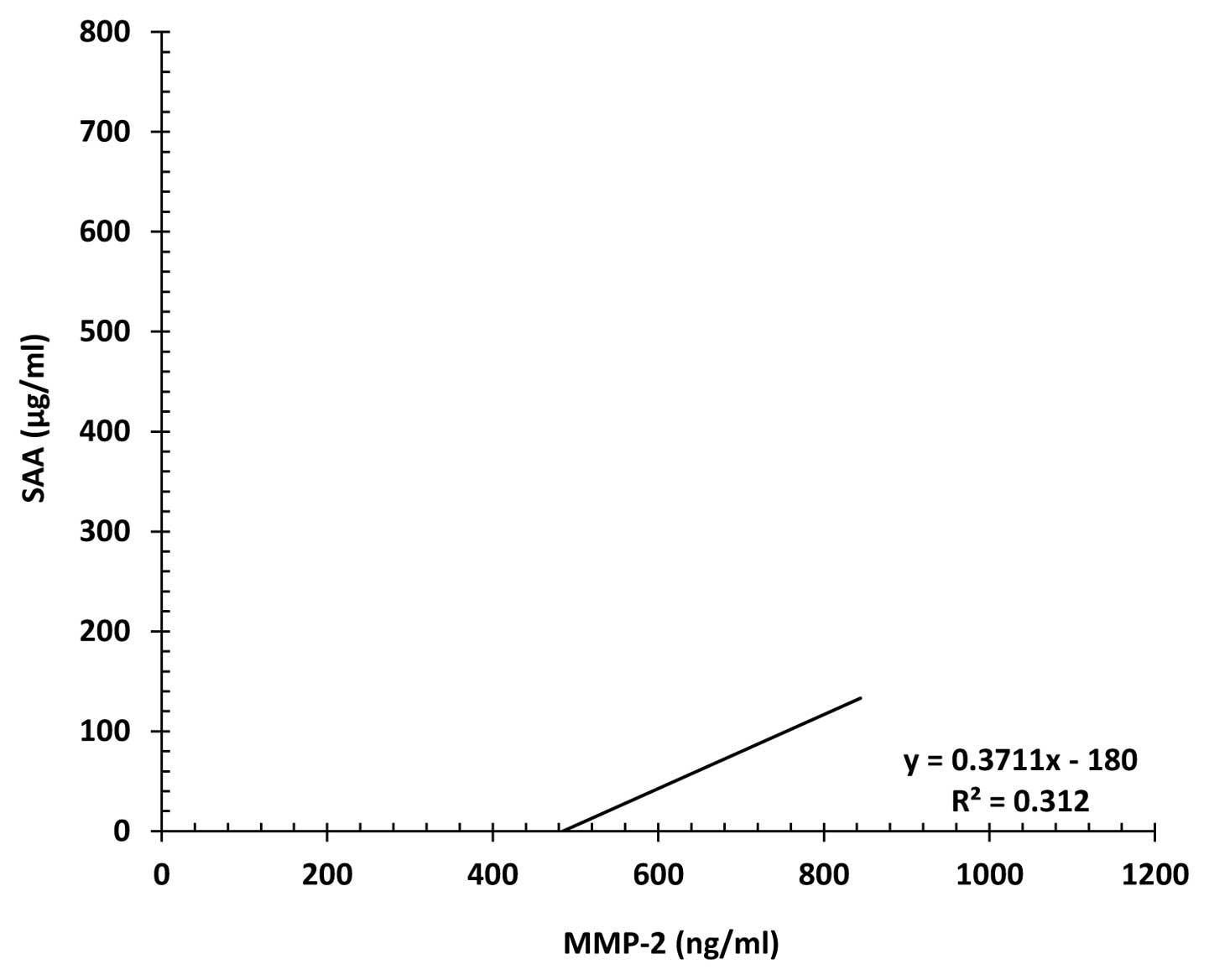
Correlation between serum MMP-2 and SAA
levels
There was a significant positive correlation between
serum MMP-2 and SAA levels in localized (r2=0.312)
(p<0.01) (Fig. 6) and metastatic
(r2=0.231) (p<0.01) (Fig.
7) NSCLC patients.
Discussion
MMPs are endopeptidases which degrade the ECM of the
cellular basement membrane. Among members of the MMP family, MMP-2
(gelatinase A) and MMP-9 (gelatinase B) have been shown to degrade
type IV collagen which is a major component of the cellular
basement membrane. Therefore, it is thought that MMP-2 and MMP-9
are associated with invasion and metastasis of cancer cells. Noh
et al showed that MMP-2 concentration in ascites might be a
prognostic marker in advanced gastric cancer patients with
disseminated metastasis (24). It
has also been reported that the expression of MMP-2 in body fluid
can be used as an additive diagnostic marker for metastatic breast
cancer patients (25). Safranek
et al demonstrated that mRNA expression of MMP-7 and MMP-9
in lung tissue of patients with NSCLC is higher than in the
surrounding tissue and in benign lung disease tissue, and these
findings support the important roles of these MMPs in the growth of
lung cancer (26). Furthermore, it
has also been shown that high expression of MMP-9 is associated
with poor prognosis in patients with NSCLC (27). In the present study, we demonstrated
that serum MMP-2 levels were markedly increased in metastatic NSCLC
patients as compared to localized NSCLC. Therefore, it is
hypothesized that MMP-2 is involved in the invasion and metastasis
of NSCLC, as is the case with other types of cancer, and high serum
MMP-2 levels in NSCLC patients can predict tumor progression.
CRP is a plasma protein produced by liver cells
following cytokine stimulation, mainly IL-6, but also IL-1β and
TNF-α (17,18). Serum CRP levels are increased in
various conditions including infection, inflammation and tissue
disturbance such as malignancy or myocardial infarction, but serum
CRP levels are rarely increased in viral infection, multiple
myeloma and non-active systemic lupus erythematosus (15,28,29).
It has been shown that elevated serum CRP levels are associated
with tumor progression and poor prognosis of esophageal cancer
(12). Chua et al
demonstrated that inflammatory and tumor markers in serum predict
survival in patients with epithelial appendiceal neoplasms
undergoing surgical cyto-reduction and intra-peritoneal
chemotherapy (30). It has also
been reported that elevated preoperative serum CRP levels predict
poor survival in patients undergoing resection for NSCLC (31).
SAA is a plasma protein produced by liver cells
following cytokine stimulation, mainly IL-1β, but also IL-6 and
TNF-α, and is generally increased in patients with viral infection
and corticosteroid treatment, a characteristic that differs from
CRP (17,18). The degree of change in serum SAA
levels is believed to be larger compared to CRP in various
conditions, and IL1-β stimulation of SAA production is hard to
suppress by corticosteroid treatment (16). It has been reported that serum SAA
levels are useful in predicting survival of patients with gastric
cancer (32). Cocco et al
demonstrated that SAA may be a novel biomarker to monitor disease
recurrence and response to therapy in patients with uterine serous
papillary cancer (33). It has also
been shown that elevated serum SAA levels may be used as a
potential biomarker for gastric cancer (34). In this study, serum CRP and SAA
levels in metastatic NSCLC patients were significantly higher than
in healthy controls and localized NSCLC patients, and there was a
significant positive correlation between serum MMP-2 and CRP levels
as well as SAA levels in metastatic NSCLC patients. Elevated serum
levels of CRP and SAA in metastatic NSCLC patients are considered
to reflect the tissue disturbance and inflammation that are
associated with invasion and metastasis of NSCLC, and high serum
CRP and SAA levels can predict tumor progression and poor prognosis
of NSCLC.
In conclusion, the present study demonstrated that
serum MMP-2 levels were notably increased in metastatic NSCLC
patients. Furthermore, serum levels of CRP and SAA were also
markedly increased with NSCLC disease progression, and there was a
significant positive correlation between serum MMP-2 and these
acute inflammatory biomarkers in metastatic NSCLC patients.
Therefore, the measurement of MMP-2, CRP and SAA in NSCLC patients
may be an auxiliary indicator to monitor tumor progression and poor
prognosis of NSCLC.
Acknowledgements
This study was supported by grants from the Ministry
of Education, Culture, Sports and Technology (A11771512) and the
Parents’ Association Grant of Kitasato University, School of
Medicine.
References
|
1
|
Baramova EN, Bajou K, Remacie A, L’Hoir C,
Krell HW, Weidle UH and Foidart JM: Involvement of PA/plasmin
system in the processing of pro-MMP-9 and in the second step of
pro-MMP-2 activation. FEBS Lett. 405:157–162. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monea S, Lehit K, Keski-Oja and Mignatii
P: Plasmin activates pro-matrix metalloproteinases-2 with a
membrane-type 1 matrix metalloproteinase-dependent mechanism. J
Cell Physiol. 192:160–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arbeláez LF, Bergmann U, Tuuttila A,
Shanbhag VP and Stigbrand T: Interaction of matrix
metalloproteinases-2 and -9 with pregnancy zone protein and
α2-macroglobulin. Arch Biochem Biophys. 347:62–68. 1997.PubMed/NCBI
|
|
4
|
Beekman B, Drijfhout JW, Ronday HK and
Tekoppele JM: Fluorogenic MMP activity assay for plasma including
MMPs complexed to α2-macroglobulin. Ann NY Acad Sci. 878:150–156.
1999.PubMed/NCBI
|
|
5
|
Chen WT and Wang JY: Specialized surface
protrusions of invasive cells, invadopodia and lamellipodia, have
differential MT1-MMP, MMP-2 and TIMP-2 localization. Ann NY Acad
Sci. 878:361–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding S, Zhang M, Zhao Y, Chen W, Yao G,
Zhang C, Zhang P and Zhang Y: The role of carotid plaque
vulnerability and inflammation in the pathogenesis of acute
ischemic stroke. Am J Med Sci. 336:27–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez B, Ruiz C, Chacon P, Alvarez-Sabin
J and Matas M: Serum values of metalloproteinase-2 and
metalloproteinase-9 as related to unstable plaque and inflammatory
cells in patients with greater than 70% carotid artery stenosis. J
Vasc Surg. 40:469–475. 2004.PubMed/NCBI
|
|
8
|
Kanoh Y, Akahoshi T, Ohara T, Ohtani N,
Mashiko T, Ohtani S, Egawa S and Baba S: Expression of matrix
metalloproteinase-2 and prostate-specific antigen in localized and
metastatic prostate cancer. Anticancer Res. 22:1813–1818.
2002.PubMed/NCBI
|
|
9
|
Kanoh Y, Ohtani H, Egawa S, Baba S and
Akahoshi T: Changes of proteases and proteinase inhibitors in
androgen-dependent advanced prostate cancer patients with
α2-macroglobulin deficiency. Clin Lab. 58:217–225. 2012.PubMed/NCBI
|
|
10
|
Kanoh Y and Ohtani H: Levels of
interleukin-6, CRP and α2-macrogloburin in cerebrospinal fluid
(CSF) and serum as indicator of blood-CSF barrier damage. Biochem
Mol Biol Int. 43:269–278. 1997.
|
|
11
|
dos Anjos BL and Grotto HZ: Evaluation of
C-reactive protein and serum amyloid A in the detection of
inflammatory and infectious disease in children. Clin Chem Lab Med.
48:493–499. 2010.PubMed/NCBI
|
|
12
|
Fujiwara H, Suchi K, Okamura H, Umehara S,
Toda M, Shiozaki A, Kubota T, Ichikawa D, Okamoto K, Ochiai T,
Kokuba Y, Sonoyama T and Otsuji E: Elevated serum CRP levels after
induction chemoradiotherapy reflect poor treatment response in
association with IL-6 in serum and local tumor site in patients
with advanced esophageal cancer. J Surg Oncol. 103:62–68. 2011.
View Article : Google Scholar
|
|
13
|
Imazio M, Brucato A, Maestroni S, Cumetti
D, Dominelli A, Natale G and Trinchero R: Prevalence of C-reactive
protein elevation and time course of normalization in acute
pericarditis: implication for the diagnosis, therapy, and prognosis
of pericarditis. Circulation. 213:1092–1097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kablak-Ziembicka A, Przewlocki T,
Sokolowski A, Tracz W and Podolec P: Carotid intima-media
thickness, hs-CRP and TNF-α are independently associated with
cardiovascular event risk in patients with atherosclerotic
occlusive disease. Atherosclerosis. 214:185–190. 2011.
|
|
15
|
Kanoh Y, Ohara T and Akahoshi T: Acute
inflammatory biomarkers in cerebrospinal fluid as indicators of
blood cerebrospinal fluid barrier damage in Japanese subjects with
infectious meningitis. Clin Lab. 57:37–46. 2011.
|
|
16
|
Smith JW, Colombo JL and McDonald TL:
Comparison of serum amyloid A and C-reactive protein as indicators
of lung inflammation in corticosteroid treated and
non-corticosteroid cystic fibrosis patients. J Clin Lab Anal.
6:219–224. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yap SH, Moshage HJ, Hazenberg BP, Roelofs
MH, Bijizet J, Limburg PC, Aarden LA and van Rijiswijk MH: Tumor
necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6
stimulated synthesis of C-reactive protein (CRP) and serum amyloid
A (SAA) in primary cultures of human hepatocytes. Biochim Biophys
Acta. 1091:405–408. 1991. View Article : Google Scholar
|
|
18
|
Smith JW and McDonald TL: Production of
serum amyloid A and C-reactive protein by HepG2 cells stimulated
with combinations of cytokines or monocyte conditioned media: the
effects of prednisolone. Clin Exp Immunol. 90:293–299. 1992.
View Article : Google Scholar
|
|
19
|
Matsuda T, Hirano T, Nagasawa S and
Kishimoto T: Identification of α2 macroglobulin as a carrier
protein for IL-6. J Immunol. 142:148–152. 1989.
|
|
20
|
Kanoh Y, Ohtani H, Egawa S, Baba S and
Akahoshi T: Levels of acute inflammatory biomarkers in advanced
prostate cancer patients with α2-macroglobulin deficiency. Int J
Oncol. 39:1553–1558. 2011.
|
|
21
|
Kanoh Y, Ohtani H, Egawa S, Baba S and
Akahoshi T: Clinicopathological characteristic of
androgen-dependent advanced prostate cancer patients with
α2-macroglobulin deficiency. Int J Oncol. 41:39–45. 2012.PubMed/NCBI
|
|
22
|
Mountain C: A new international staging
system for lung cancer. Chest. 89:S225–S233. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujimoto N, Mouri N, Iwata K, Ohuchi E,
Okada Y and Hayakawa T: A one-step sandwich enzyme immunoassay for
human matrix metalloproteinase 2 (72-kDa gelatinase/type IV
collagenase) using monoclonal antibodies. Clin Chim Acta.
221:91–103. 1993. View Article : Google Scholar
|
|
24
|
Noh S, Jung JJ, Jung M, Kim TS, Park CH,
Lim SJ, Jeung HC, Cheol H, Chung HC and Rha SY: MMP-2 as a putative
biomarker for carcinomatosis in gastric cancer.
Hepatogastroenterology. 58:2015–2019. 2011.PubMed/NCBI
|
|
25
|
Noh S, Jung JJ, Jung M, Kim TS, Park CH,
Lim SJ, Jeung HC, Cheol H, Chung HC and Rha SY: Body fluid MMP-2 as
a putative biomarker in metastatic breast cancer. Oncol Lett.
3:699–703. 2012.PubMed/NCBI
|
|
26
|
Safranek J, Pesta M, Holubec L, Kulda V,
Dreslerova J, Vrzalova J, Topolcan O, Pesek M, Finek J and Treska
V: Expression of MMP-7, MMP-9 TIMP-1 and TIMP-2 mRNA in lung tissue
of patients with non-small cell lung cancer (NSCLC) and benign
pulmonary disease. Anticancer Res. 29:2513–2517. 2009.PubMed/NCBI
|
|
27
|
Peng WJ, Zhang JQ, Wang BX, Pan HE, Lu MM
and Wang J: Prognostic value of matrix metalloproteinase 9
expression in patients with non-small cell lung cancer. Clin Chim
Acta. 413:1121–1126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bataille R, Boccadoro M, Klein B, Durie
and Pileri A: C-reactive protein and beta-2 microglobulin produce a
simple and powerful myeloma staging system. Blood. 80:733–737.
1992.PubMed/NCBI
|
|
29
|
Firooz N, Albert D, Wallace D, Ishimori M,
Berel D and Weisman M: High-sensitivity C-reactive protein and
erythrocyte sedimentation rate in systemic lupus erythematosus.
Lupus. 20:588–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chua TC, Chong CH, Liauw W, Zhao J and
Morris DL: Inflammation markers in blood and serum tumor markers
predict survival in patients with epithelial appendiceal neoplasms
undergoing surgical cytoreduction and intraperitoneal chemotherapy.
Ann Surg. 256:342–349. 2012. View Article : Google Scholar
|
|
31
|
O’Dowd C, McRae LA, McMillian DC, Kirk A
and Milroy R: Elevated preoperative C-reactive protein predicts
poor cancer specific survival in patients undergoing resection for
non-small cell lung cancer. J Thorac Oncol. 5:988–992.
2010.PubMed/NCBI
|
|
32
|
Chan DC, Chen CJ, Chu HC, Chang WK, Yu JC,
Chen YJ, Wen LL, Huang SC, Ku CH, Liu YC and Chen JH: Evaluation of
serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol.
14:84–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cocco E, Bellone S, El-Sahwi K,
Cargnelutti M, Casagrande F, Buza N, Tavassoli FA, Siegel ER,
Visintin I, Ratanr E, Silasi DA, Azodi M, Schwartz PE, Rutherford
TJ, Pecorelli S and Santin AD: Serum amyloid A (SAA): a novel
biomarker for uterine serous papillary cancer. Br J Cancer.
101:335–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C, Pan C, Shen J, Wang H and Yong L:
Identification of serum amyloid A in the serum of gastric cancer
patients by protein expression profiling. Oncol Lett. 3:1259–1262.
2012.PubMed/NCBI
|