Introduction
Cutaneous melanoma is one of the most aggressive
human tumors, which originate from melanocytes, primarily in the
skin, in the eye, the brain and mucosa. Melanoma affects patients
in all age ranges and its incidence has dramatically risen over the
past 50 years in industrialized countries. A particularly worrying
feature of the tumor is its increasing incidence and its capacity
for rapid metastatic spread. While very early stage melanoma
(localized, stage I) is >90% curable, disseminated stage IV
melanoma leads to a life expectancy of less than a year (1). Metastases may establish in various
organs, including skin, lungs, liver, brain and bone. Thus,
inhibiting invasion and metastasis is the key to extending the
survival time of these patients.
It is widely known that formation of metastasis
starts with dissemination of tumor cells from the primary tumor and
is a complex and multistep process. Degradation and remodeling of
the extracellular matrix (ECM) and basement membranes by
proteolytic enzymes are essential steps in these processes. Neutral
proteinases of the matrix metalloproteinase (MMP) family actively
participate in basement membrane proteolysis, acting mainly in the
pericellular environment. Among the increasing number of
ECM-degrading proteinases involved in cancer progression, most
attention has been focused on the MMP family, mainly on MMP-2 and
MMP-9. Increased expression of MMP-2 and MMP-9 was showed to
correlate with an invasive phenotype of cancer cells (2,3).
TGF-β1 is a multifunctional cytokine that
regulates cell growth, differentiation, migration, apoptosis and
extracellular remodeling. In later stages of tumorigenesis,
TGF-β1 may function to promote cell invasion and
metastasis through paracrine mechanisms, which could be involved in
the promotion of angiogenesis and extracellular matrix protein
synthesis (4). TGF-β1
transduces its signals from the cell membrane to the nucleus
through serine/threonine kinase receptors and their downstream
effectors, Smad molecules. Increased expression and secretion of
the different TGF-β isoforms in melanoma cell lines when compared
with normal melanocytes has been documented by several studies
(5–8).
Clinical experiments have shown that hyperthermia,
as part of multimodal regimens, is a tolerable and clinically
practical supplementary therapy for patients with advanced
malignant tumors, recurrent tumors and metastatic disease.
Recently, it has been reported that hyperthermia delays tumor
growth and inhibits lymph node metastasis in animal models. The
in vivo experimental study of Nagashima et al found
that local hyperthermia (heating at 43°C for 40 min) inhibited
lymph node metastasis of hamster oral squamous cell carcinoma when
the primary tumor responded histologically to hyperthermia
treatment (9). In vitro
research aimed at trying to explain the mechanism has been carried
out. One study demonstrated that a transient increase in
intracellular cAMP was a critical signal for heat shock to induce
tumor specific-suppression of MT1-MMP production and proMMP-2
activation in HT-1080 cells after heat shock at 42°C for 3 h
(10). However, Nathanson et
al(11) reported opposite
results. B16-F1 melanomas that survived 43.5°C heat in vitro
for 15 min and cultured for 10 days were able to bind significantly
increased amounts of the basement membrane protein laminin.
Motility of the heat-resistant B16-F1 cells in vitro toward
the chemoattractant laminin was significantly increased. Thus, the
increased expression levels of putative laminin receptors may be
associated with increased metastasis of melanomas after subcurative
hyperthermia.
Magnetic fluid hyperthermia (MFH) is a recent
development, and a new approach for local targeting hyperthermia or
thermoablation (12). In this
technique, energy is coupled magnetically to iron oxide
superparamagnetic nanoparticles in the target region. The magnetic
fluid is injected directly into the target region and subsequently
heated in an externally applied alternating magnetic field (AMF) to
achieve better selectively targeted hyperthermia to the tumor. The
excellent power absorption capabilities of magnetic fluids,
attributable to the large number and surface of the heating
elements, have been confirmed by in vitro experiments.
Moreover, magnetic nanoparticles can be taken up by cancer cells
and intracellularly heated (13–15).
To date, hyperthermia induced by direct injection of magnetic
nanoparticles has been successfully used in preclinical and even
phase III studies of various malignant tumors such as mammary
carcinoma, prostate cancer, malignant glioma and melanoma and has
resulted in an encouragingly marked inhibition of tumor growth
(16–23).
Our previous research showed that heating suppresses
the invasive potential of MCF-7 cells and downregulates the
expression of TGF-β1 and MMPs (24). Therefore, we concluded that
hyperthermia should be regarded as an important therapy for cancers
which may metastasize at early stages. However, the molecular
mechanisms are still unclear and no evidence exists in other types
of carcinoma.
In order to further study the mechanisms involved in
hyperthermia, we established an orthotopic mouse model of cutaneous
malignant melanoma. Mouse malignant melanoma B16F10 cells are
promising as an experimental model of invasion because of their
metastatic biologic characteristics. In this study, we heated
B16F10 cells in a water bath in vitro and by MFH in
vivo respectively. Then, changes in the invasive ability and
TGF-β1 expression in malignant melanoma cells were
analyzed.
Materials and methods
Cell culture
The murine B16F10 melanoma cell line was obtained
from the National Centre for Cell Sciences and was grown and
maintained in Dulbecco's modified Eagle's medium (DMEM;
Gibco/Invitrogen, Carlsbad, CA, USA), containing 10% FBS in a 5%
CO2 incubator at 37°C.
Cell treatment
Exponential growing cells were sealed using parafilm
in cell culture flasks (1×106 cells/flask) and immersed
in a thermostated water bath at the indicated temperature (43, 45
and 47°C) for 30 min. The control group was treated at 37°C for 30
min. The temperature of the medium increased quickly and reached
the set temperatures within 3 min. The temperature in the medium
was monitored with a fiber optic thermometer probe (FX-9020;
Anritsu Meter Co., Tokyo). After heating, the cells were
immediately incubated at 37°C in 5% CO2.
MTT assay
Cells were incubated for 4 h to restore stability
after heating, and then were harvested, counted, and inoculated (at
the appropriate concentrations in a volume of 100 μl) into 96-well
microtiter plates, 8 replicates were prepared for each group. After
different incubation times at 37°C in a humidified 5%
CO2 atmosphere, the MTT assay was performed. MTT (Sigma,
St. Louis, MO) was dissolved at a concentration of 5 mg/ml in
Hank's salt solution and filtered with a 0.45 μm filter (in order
to avoid MTT aggregates). MTT solution (10 ml) was added to each
well and also to the control wells without cells. After 4 h of
incubation, microtiter plates were centrifuged at 2,000 rpm for 10
min; medium was then removed, and 100 μl of DMSO was added to each
well. After thorough mixing with a mechanical plate mixer,
absorbance of the wells was read in a scanning well microculture
plate reader at test and reference wavelengths of 550 and 620 nm,
respectively. Absorbance values from all wells were corrected
against the control absorbance levels.
Cell migration assay
B16F10 cells heated at different temperatures by
water bath were allowed to form a monolayer on a fibronectin (5
μg/ml)-coated surface. A wound was made in the monolayer of cells
by scratching a line on the monolayer with a pipette tip. Cells
were then washed with PBS to remove cell debris and fed with fresh
culture medium. Cells were allowed to proliferate and migrate into
the wound during the next 24 h. Migration of cells into the wound
was observed using a microscope (Olympus BX51, Tokyo, Japan). Three
wounds were sampled for each treatment and experiments were carried
out in triplicate.
Matrigel invasion and metastasis
assays
The invasive activity of B16F10 cells after heating
at different temperatures was assayed in a Transwell cell culture
chamber (6.5 mm, 8 μm Costar, Corning). Polyvinylpyrrolidone-free
polycarbonate filters were smeared with 8.0 μg fibronectin
(Genscrip cat. no: RP10840) in a volume of 10 μl serum-free DMEM on
the reverse side, and dried at room temperature. Matrigel
(containing laminin, collagen type IV, heparan sulfate proteoglycan
and entactin) (BD Biosciences cat. no: 356230) was diluted to 500
μg/ml with cold phosphate-buffered saline (PBS), applied to the
upper surface of the filter (5 μg/filter), and dried at room
temperature. B16F10 cells which were heated by water bath for 30
min and then incubated for 2 h at 37°C in 5% CO2 were
harvested with 1 mM EDTA in PBS, washed twice and resuspended to
give a final concentration of 2.0×106/ml in serum-free
RPMI-1640 with 0.1% bovine serum albumin (BSA). Cell suspensions
(100 μl) were added to the upper compartment and the filter chamber
was incubated for 24 h at 37°C in a 5% CO2 atmosphere.
The cells on the upper surface of the filters were removed by
wiping them with a cotton swab. The filters were then stained with
crystal violet for 30 min. The cells that had invaded through
Matrigel and reached to the reverse side were counted under a
microscope in five predetermined fields at a magnification of ×400.
Each assay was performed in triplicate.
Gelatin zymography
B16F10 cells were heated by water bath for 30 min as
noted and then incubated for 24 h with serum-free DMEM at 37°C in
5% CO2. The culture supernatant was collected by
centrifugation and then concentrated by lyophilization. The extract
was then subjected to zymography on 7.5% SDS-PAGE (sodium dodecyl
sulfate polyacrylamide gel electrophoresis) co-polymerized with
0.1% gelatin. Gel was washed in 2.5% Triton X-100 for 30 min to
remove SDS and was then incubated overnight in a reaction buffer
(50 mM Tris-HCl pH 7.0, 4.5 mM CaCl2, 0.2 M NaCl). After
incubation, the gel was stained with 0.5% Coomassie Blue containing
30% methanol and 10% glacial acetic acid. The bands were visualized
by destaining the gel with the solution containing 30% methanol and
10% glacial acetic acid. The experiments were repeated three times
under similar conditions, and one experiment was selected for
representation.
Western blot experiments
Whole cell lysates were prepared by lysing the cells
on ice with a protein extraction kit (SBS, China) for 20 min. Equal
amounts of protein (30 μg/lane) were electrophoresed under
non-reducing conditions on 10% acrylamide gels. After SDS-PAGE,
proteins were transferred to a polyvinylidene difluoride membrane
(Bio-Rad, USA). To block non-specific binding, the membrane was
incubated in TBS with 0.1% Tween-20 (TBST) containing 5% nonfat
milk for 2 h. Subsequently, the membrane was incubated for 2 h with
antibodies against TGF-β1 and Smad4 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA, 1:1000), respectively, in TBST
with 5% non-fat milk. After washing in TBST, horseradish
peroxidase-conjugated anti-mouse or anti-rabbit IgG (Santa Cruz
Biotechnology) was used as a secondary antibody (1:2000 in TBST
with 2% BSA, incubated for 1 h). Each sample was also probed with
an anti-β-actin antibody (Santa Cruz Biotechnology) as a loading
control. Bands were scanned using a densitometer (GS-700, Bio-Rad),
and quantification was performed using Quantity One 4.6.2 software
(Bio-Rad).
RT-PCR analysis
The expression of TGF-β1 and Smad4 mRNA
was analyzed by semi-quantitative RT-PCR. B16F10 cells were heated
by water bath for 30 min as noted and then incubated for 24 h.
Total RNA was extracted from the cells using TRIzol reagent
(Invitrogen) respectively and 5 μg RNA was used to synthesize cDNA
using an RT-PCR kit (M-MLV) (KeyGEN, KGA1305) following the
manufacturer's protocol. The cDNA was used to amplify the
TGF-β1 and Smad4 mRNA fragment, while the housekeeping
gene β-actin was also amplified as an internal standard. The
corresponding primer sequences were: β-actin forward,
5′-ACCTTCTACAATGAGCTGCGT-3′ and reverse,
5′-ATAGCACAGCCTGGATAGCAA-3′ (157 bp); TGF-β1 forward,
5′-GGCCAGATCCTGTCCAAGC-3′ and reverse, 5′-GTGGGTTTCCACCATTAGCAC-3′
(201 bp); Smad4 forward, 5′-GTCTGAGCATTGTGCATAGTTTG-3′ and reverse,
5′-GACGGGCATAGATCACATGAG-3′ (246 bp).
The cycling program was performed as follows: 1
cycle of 94°C for 5 min; 33 cycles of 94°C for 30 sec, 54°C for 45
sec, 72°C for 60 sec; followed by a final elongation step at 72°C
for 10 min. Then RT-PCR products were electrophoresed through a
1.5% agarose gel with ethidium bromide. The experiments were
repeated three times under similar conditions, and one experiment
was selected for representation.
Tumor model
Specific pathogen-free female C57BL/6 mice were
purchased from Beijing Center for Disease Control and Prevention
(China) and were maintained in our animal facility for at least 2
weeks before use. The mice were housed under specific pathogen-free
conditions in a barrier facility with a 12-h light/dark cycle. The
mouse model of melanoma was established by transplanting a 0.1-ml
cultured B16F10 cell suspension at a concentration of
1×107 cells/ml into the subcutaneous tissue of the back
of each mouse. Forty mice were randomized and divided into four
groups: 45°C hyperthermia group (H1), 50°C hyperthermia group (H2),
magnetic fluid group (M) and control group (C). Orthotopic tumors
were staged for 7 days and reached ~0.5 cm3 in size. The
tumor-bearing mice of the M group, H1 group and H2 group were
locally injected with magnetic fluid in the tumor area, then
underwent radiation by an alternating magnetic field (AMF). The
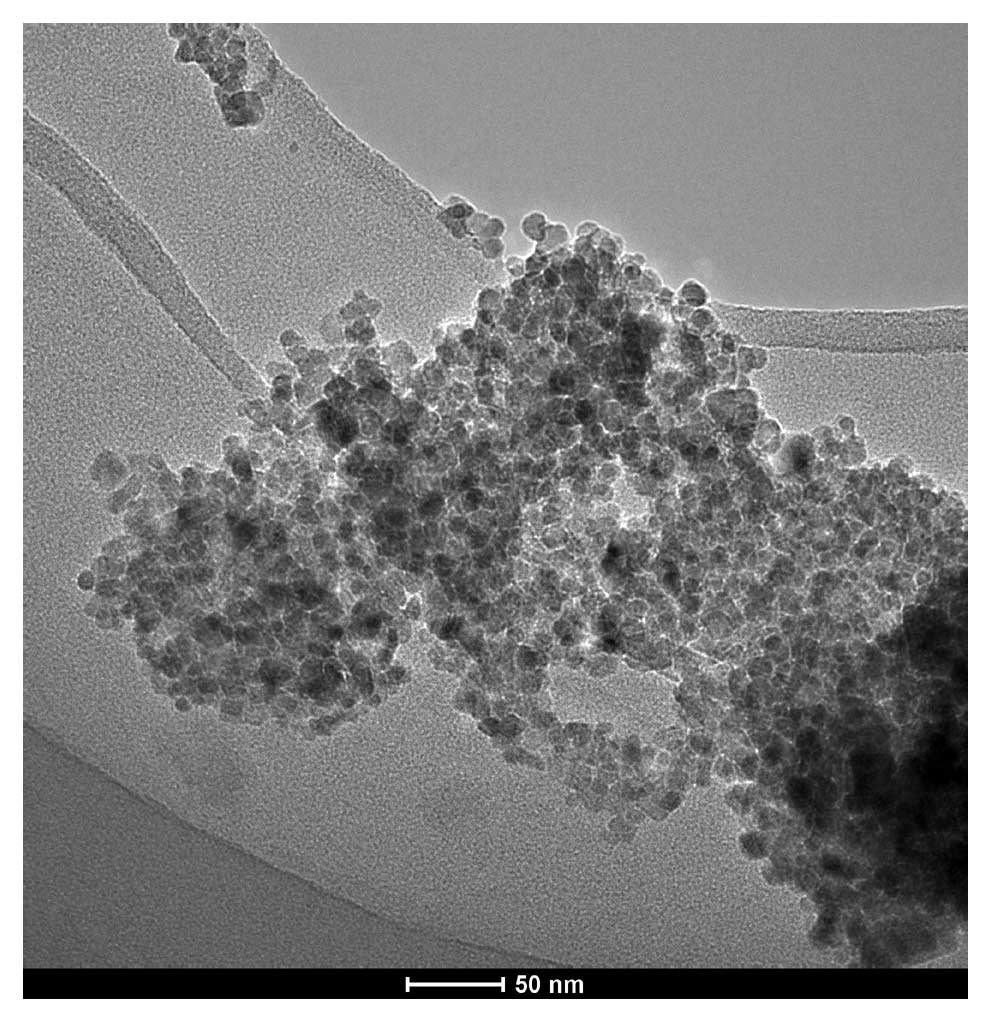
magnetic fluid (Fig. 1) was
purchased from Anhui Jinke Magnetic Liquids Ltd. and consisted of
uncoated and water-based particles of Fe3O4
~10 nm in diameter, and the concentration of the ferrites in
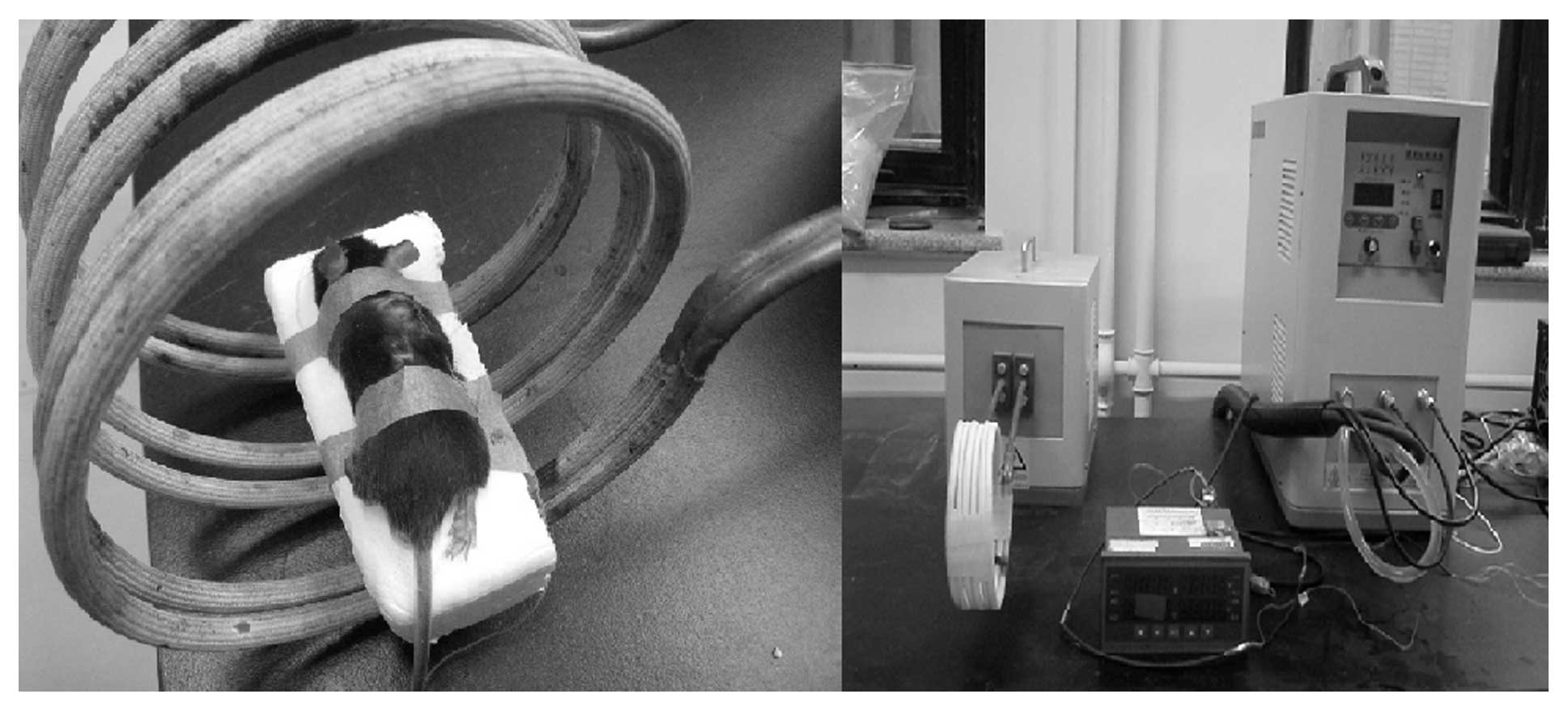
aqueous solution was 100 mg/ml. The magnetic field applicator
(Fig. 2) was produced by our
laboratory at the Department of Engineering Physics, Tsinghua
University, and the parameters of the AMF were carefully adjusted
until a local tumor temperature (45 or 50°C) was maintained for 30
and 10 min, respectively. After 24 h, 3 mice from each group were
sacrificed for anatomy to observe the magnetic fluid distribution
and to prepare for the following immunohistochemistry tests.
Tumor growth was monitored at a defined regular
interval (2 days) by measuring the diameters using a vernier
caliper. Tumor volume was determined based on the following
formula: Tumor volume = 0.5 × a × b2 (a and b represent
the maximal and minimal tumor diameter, respectively). Mice
survival times were determined. After a 90-day follow-up period,
all mice were sacrificed.
Immunohistochemistry
The paraffin-embedded tumor tissues were sectioned
into 5-μm slices, deparaffinized in xylene, rehydrated in graded
ethanol concentrations (100, 95, 70, and 50%), and finally
submerged in phosphate-buffered saline (PBS). Endogenous peroxidase
was blocked by incubation with 0.3% H2O2 for
30 min in the dark, and then sections were placed in an autoclave
with 0.01 M sodium citrate solution at 121°C for 5 min for antigen
retrieval. Immunohistochemical staining was performed using primary
antibodies for TGF-β1. The sections were incubated with
antibodies (1:100) overnight at 4°C and then incubated with
biotin-labeled anti-rabbit IgG (ZSGB-Bio, Beijing, China) at room
temperature for 30 min. The tissue sections were counterstained
with hematoxylin, dehydrated in graded ethanol concentrations (50,
70, 95, and 100%) and xylene, covered with glass coverslips, and
then observed using a bright field microscope.
Statistical analysis
Statistical analysis was performed using SPSS
(version 13.0). Data are expressed as means ± SD for n replicates
as indicated in figure legends. One-way analysis of variance
followed by an SNK test was used to assess significant differences
between the control and experimental groups.
Results
Hyperthermia inhibits proliferation
potential and mobility of B16F10 cells
B16F10 cells treated by water bath at 43, 45 and
47°C for 30 min were then tested by MTT assay and scratch-wounding
assays. As shown in Fig. 3, the
proliferative ability of the cells after being heated at 45 and
47°C was markedly higher than at 43°C (P<0.05). As shown in
Fig. 4, the mobility of B16F10
cells was obviously decreased by heating at 45 and 47°C.
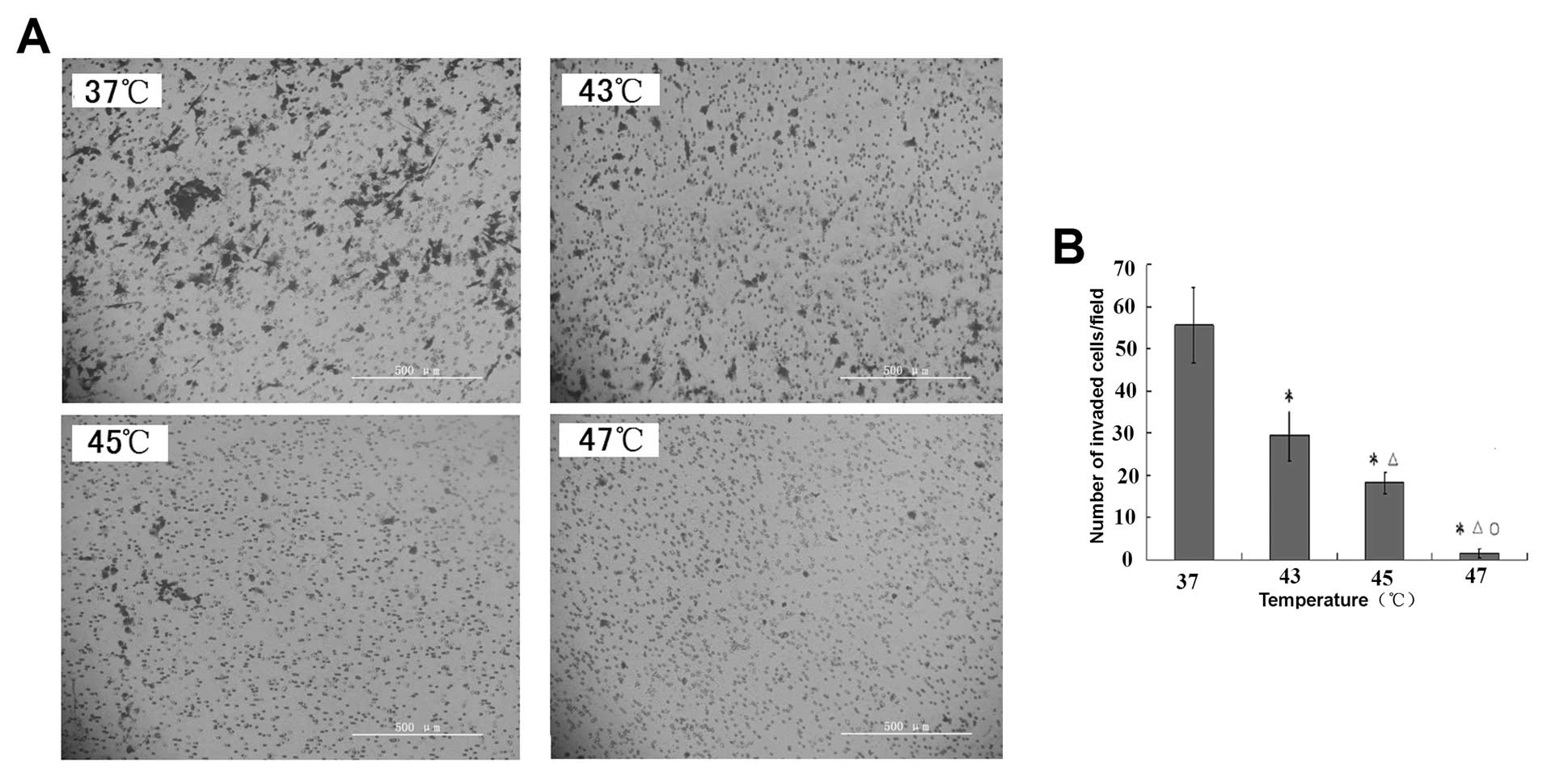
Hyperthermia inhibits invasive and
metastatic potential of B16F10 cells
We examined changes in the invasive and metastatic
potential of B16F10 cells after hyperthermia treatment using a
Matrigel invasion assay. Representative images of the Matrigel
invasion assay showed a decrease in the number of invasive B16F10
cells after hyperthermia (x400) (Fig.
5A). Column data showed that hyperthermia decreased the
invasiveness of B16F10 cells in a temperature-dependent manner
(Fig. 5B). Compared with the
control group (37°C), B16F10 cells heated at 43, 45 and 47°C showed
significantly lower numbers of invasive cells (P<0.01).
Downregulation of MMP-2 and MMP-9
activity
In order to ascertain whether the downregulation of
MMP-2 and MMP-9 in B16F10 cells is induced by hyperthermia, we
examined the effect of hyperthermia treatment at different
temperature points in the secretion and activity of MMP-2 and MMP-9
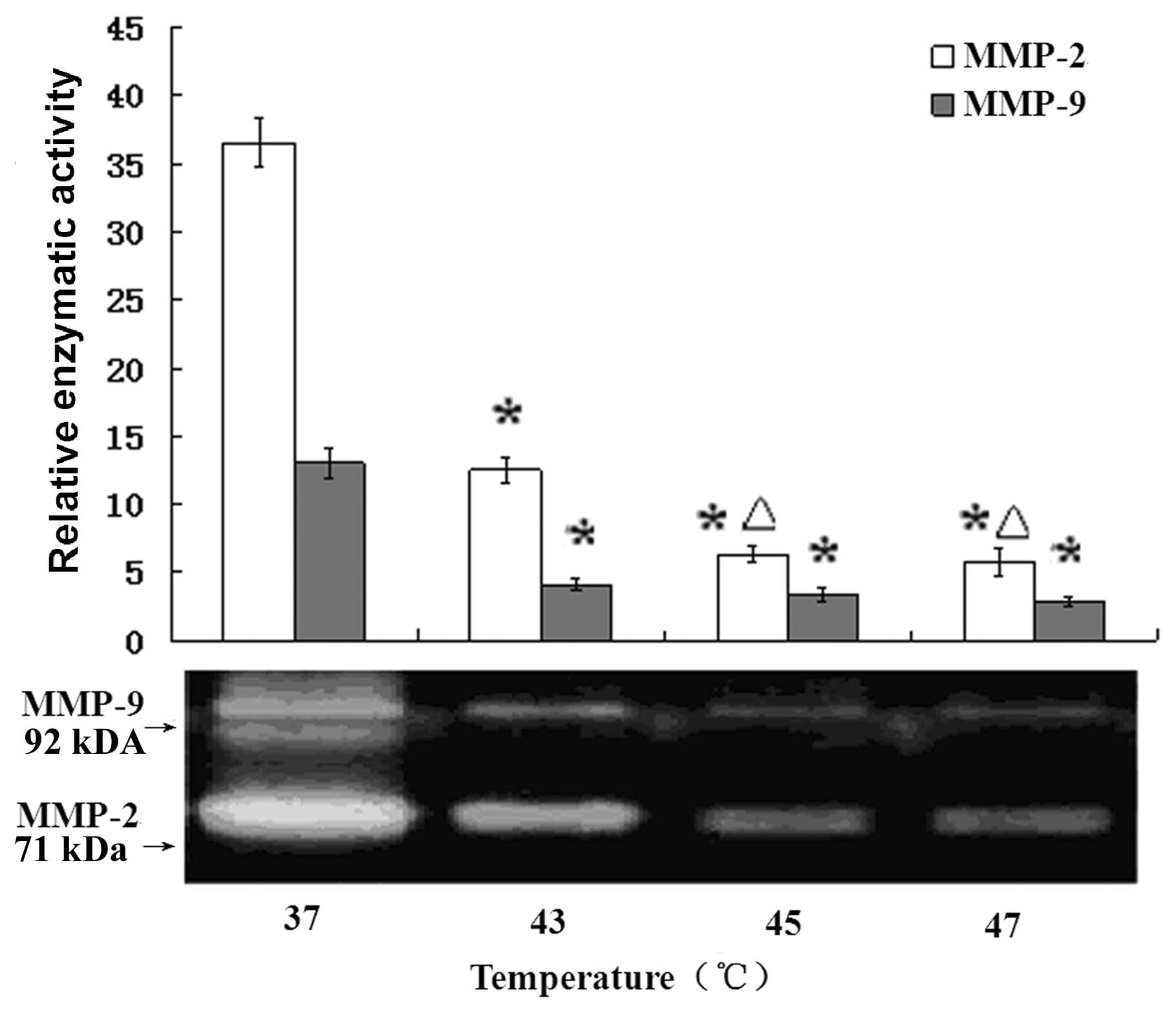
by gelatin zymographic analysis. As shown in Fig. 6, the white bands were areas degraded
by MMP-2 and MMP-9. The corresponding image represents the
quantitative analysis of the band intensities using a contour tool
by Quantity One-4.6.2 (Basic) which clearly showed that
hyperthermia treatment caused significant inhibition of the
secretion and activity of MMP-2 and MMP-9 in B16F10 cells
(P<0.01) and the decrease appeared to be
temperature-dependent.
Regulation of the protein expression of
TGF-β1 and Smad4 by hyperthermia
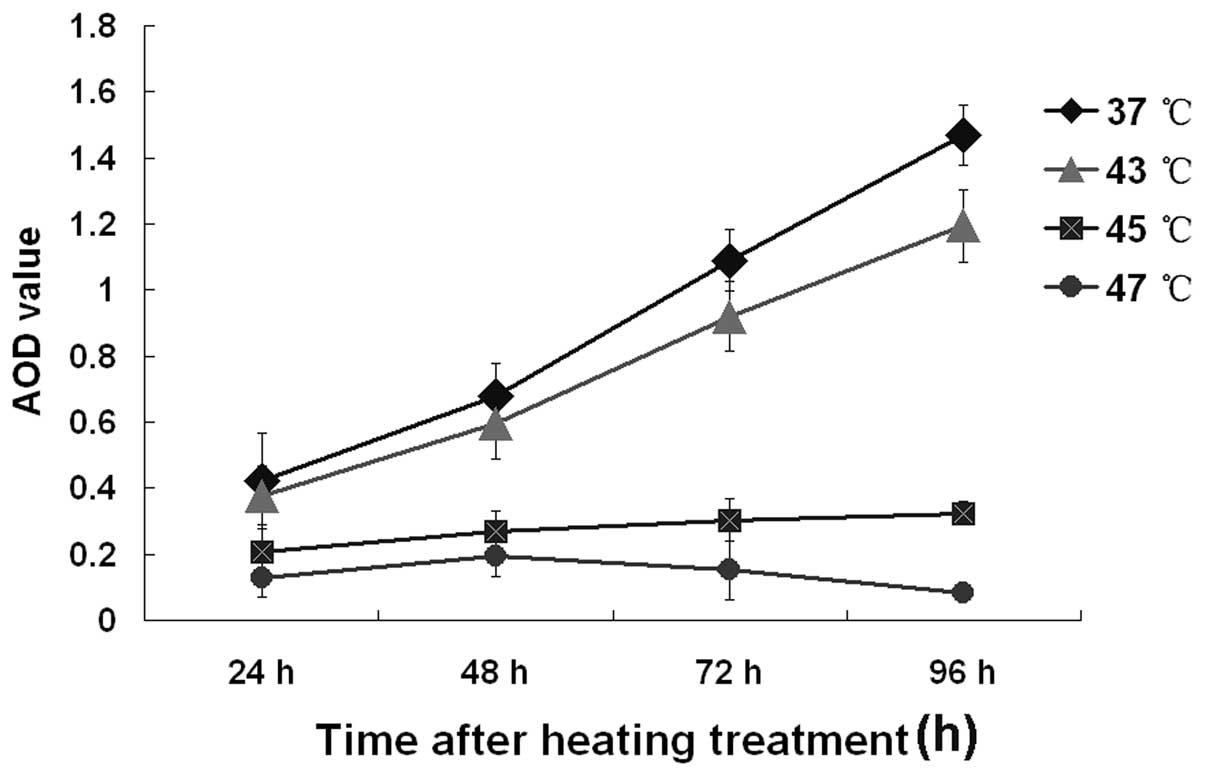
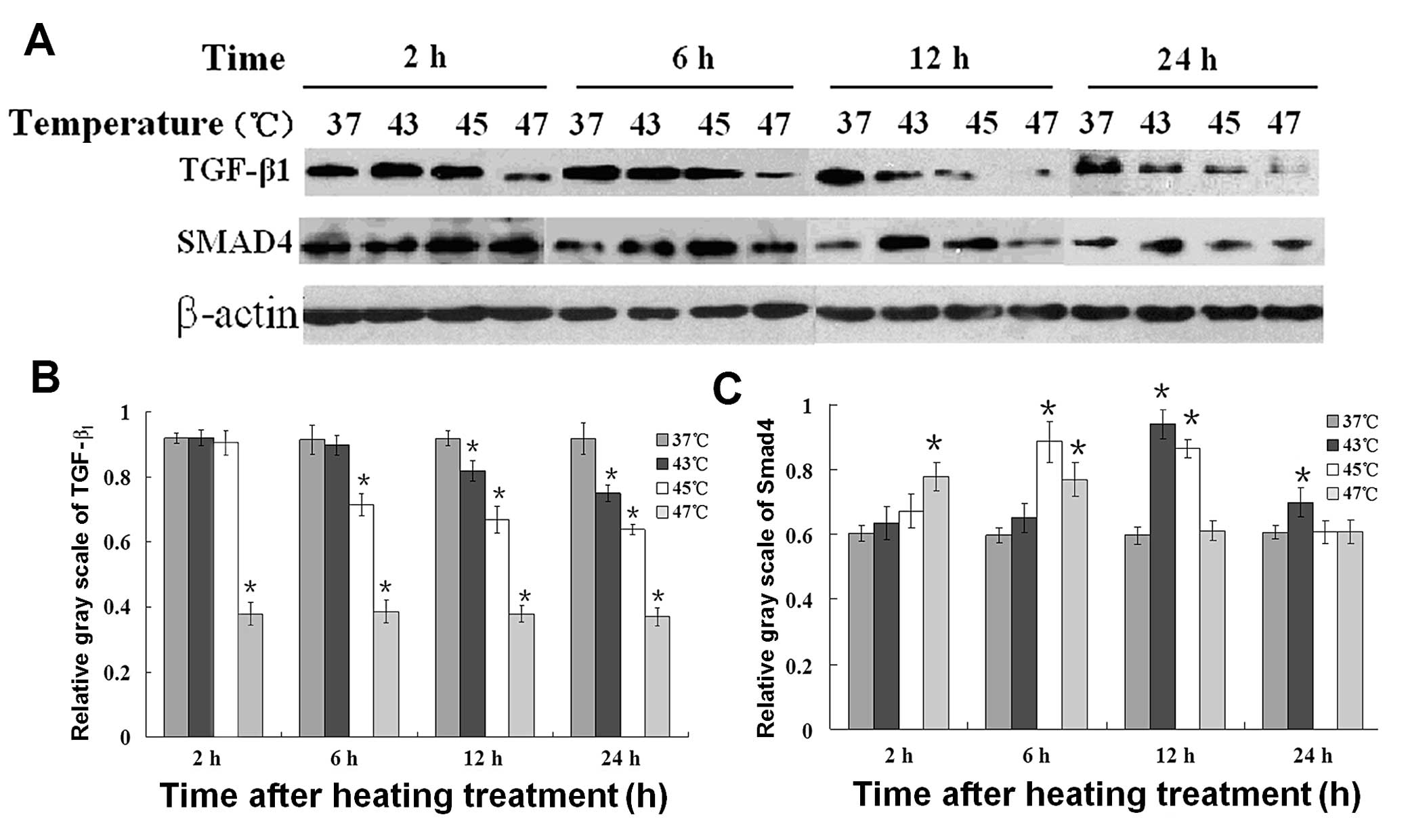
Western blot analysis (Fig. 7A) was performed to ascertain whether
the protein expression of TGF-β1/Smad4 in B16F10 cells
is affected by hyperthermia. Fig.
7B shows the corresponding average optical densities of
TGF-β1 and Smad4 after different temperature treatments.
The reduction showed temperature dependence for TGF-β1,
while the upregulation of Smad4 lasted for a short time at 43°C and
45°C but not at 47°C. Moreover, at the higher temperature, the
increase in Smad4 protein expression occurred earlier. The bar
graph represents the corresponding average optical densities using
Quantity One-4.6.2 (Basic).
Alteration of the mRNA expression of
TGF-β1, Smad 4 and MMPs
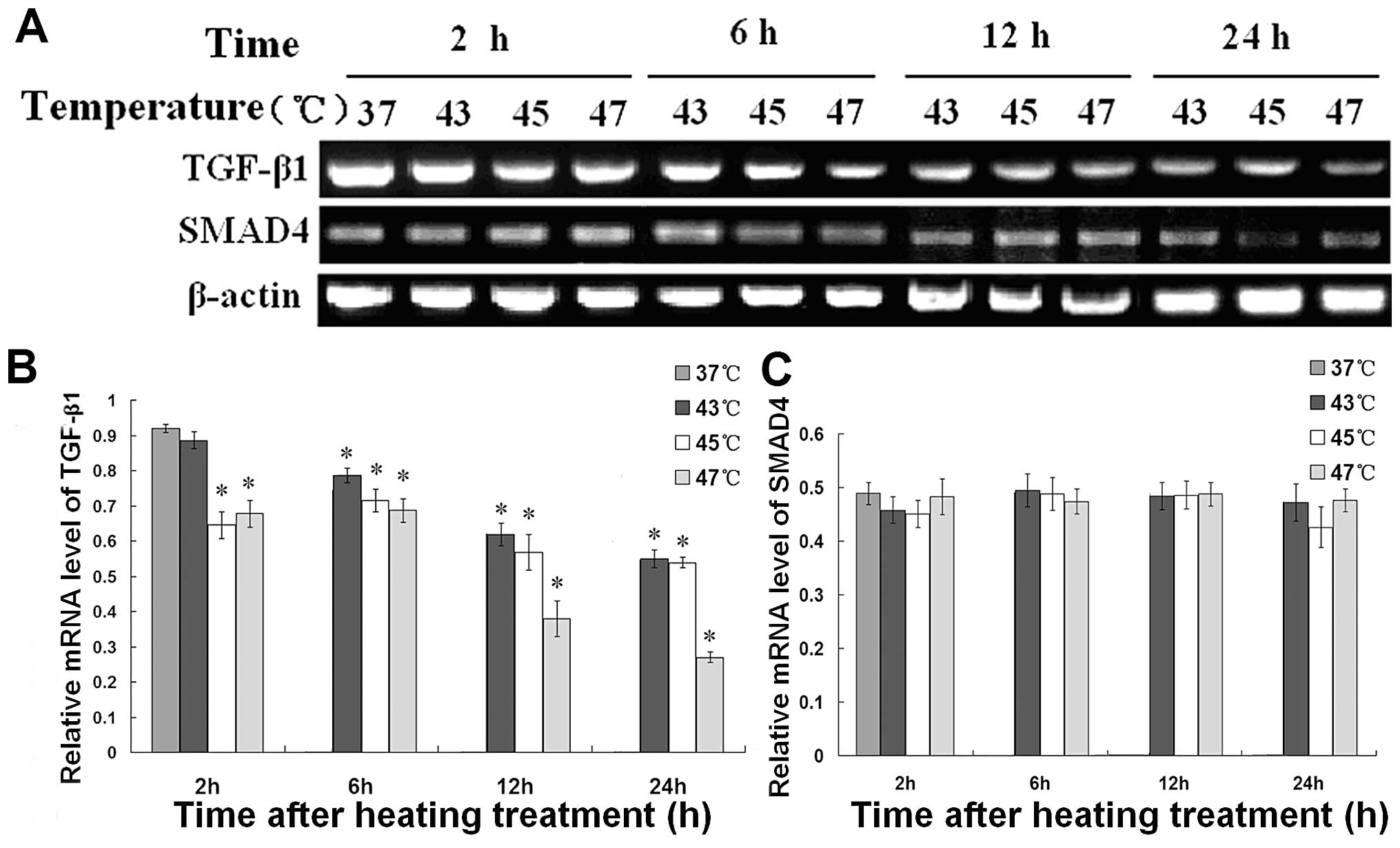
RT-PCR was performed to ascertain whether the mRNA
expression of TGF-β1 and Smad4 in B16F10 cells is
suppressed by hyperthermia. As shown in Fig. 8, the mRNA expression of
TGF-β1 was significantly reduced by hyperthermia when
the temperature was above 43°C, while the mRNA level of Smad4 did
not change significantly.
Regulation of TGF-β1 protein
expression by hyperthermia in vivo
MFH at 45°C (H1 group) and 50°C (H2 group) inhibited
tumor growth. Compared with 45°C MFH (H1 group), 50°C MFH (H2
group) had a greater inhibitive effect on tumor growth (P<0.05).
The survival times of the mice in the 50°C MFH H2 group and 47°C
MFH H1 group were longer than that of the control group
(P<0.05). Immunohistochemical assay showed that the expression
of TGF-β1 was markedly decreased in the 50°C MFH H2
group compared with the other groups (P<0.05) (Fig. 9).
Tumor growth inhibition and survival in
the mouse model
In the in vivo study, no mice died during the
MFH process as described above, and no infiltration of magnetic
fluid was found in the surrounding tissue and organs. As shown in
Fig. 10, the tumor growth rate of
the control group and magnetic fluid group was significantly lower
than H1 and H2 groups treated at 45 and 50°C, respectively
(P<0.05) and the survival period of the tumor-bearing mice which
was observed for 90 days after tumor implantation showed that
magnetic fluid hyperthermia (especially for H2 group at 50°C)
prolonged the life span of the mouse model significantly.
Discussion
Although melanomas represent only approximately 4%
of all cutaneous cancers, they are responsible for >80% of
deaths from skin-related cancers (25). In its early stages, malignant
melanoma can be cured by surgical resection, but once it has
progressed to the metastatic stage it is extremely difficult to
treat and does not respond to current therapeutic methods (such as
radiation therapy and chemotherapy). In the present study, we
explored hyperthermia as a prospective therapy for malignant
melanoma.
It is no doubt that with temperatures higher than
43°C, the cytotoxicity of hyperthermia becomes stronger. Our MTT
assay showed that higher temperature hyperthermia strengthened the
lethal effect. Moreover, the present study was the first attempt to
study the effect of local hyperthermia with a heating temperature
above 43°C on the invasive ability of malignant melanoma cells. Our
results showed that migratory and invasive ability of the remnant
carcinoma cells was suppressed after hyperthermia at 43, 45 and
47°C for 30 min, respectively.
It is known that degradation of the basal lamina and
extracellular matrix (ECM) is crucial for invasion and metastasis
of malignant cells. Among the currently known 24 types of human
MMPs, MMP-2 (or gelatinase A) is most frequently overexpressed in
cancer and is instrumental in cutting through basement membrane
barriers (26–29). It was reported that heat shock at
42°C suppressed the production of membrane-type 1 MMP (MT1-MMP),
which in turn inhibited MMP-2 activation and increased release of
tissue inhibitor of MMP-2 from the cell surface (30). Consistent with this finding, our
study revealed a similar effect on both MMP-2 and MMP-9 in
malignant melanoma B16F10 cells by heating at higher temperature
and for a shorter time.
Several investigators demonstrated that
TGF-β1 expression in a variety of malignancies was
associated with increased tumorigenesis (31–34).
The TGF-β1 pathway has been implicated in many of these
metastatic processes and it has been shown to dramatically impact
the ability of tumor cells to spread throughout the body (35). Previous experiments have reported
that TGF-β1 can augment the aggressiveness of carcinomas
that are resistant to its growth inhibiting effects (36), and promote breast carcinoma
metastasis to the bone (36,37).
Padua et al(38) discovered
that even transient exposure of breast cancer cells to the
signaling molecule TGF-β1 promoted their extravasation
from blood vessels and entry into the lung. These effects are
closely associated with MMP activities as mentioned above.
TGF-β1 binds to the type II TGF-β receptor (TβRII),
which, in turn, recruits a TGF-β type I receptor (TβRI), into a
heterotetrameric receptor complex, after that TβRI is
phosphorylated and activated. Activated TβRI phosphorylates the
receptor-specific Smad2/3, which enables them to oligomerize with
the common mediator Smad4, to translocate to the nucleus and
regulate target gene transcription through cooperation with other
DNA-binding factors and/or transcription factors (39).
Although normal melanocytes are extremely sensitive
to the antiproliferative effects of TGF-β, melanoma cells exhibit
increased resistance, proportional to the stage of tumor
progression. Melanoma cell proliferation is only moderately
inhibited by TGF-β in contrast to the strong antiproliferative
effect exerted on normal melanocytes. Also, it has been shown that
metastatic melanoma cell populations exhibit a further decreased
response to TGF-β-dependent growth inhibition than melanoma cells
originating from primary tumors (40). Yet, TGF-β is perfectly capable of
inducing Smad signaling and Smad-dependent transcription in
melanoma cells (41). Contrary to
other tumor types, no genetic alteration of TGF-β signaling
molecules has been identified in melanoma that could explain their
resistance to the growth inhibitory activity of TGF-β. For example,
it has been clearly established that a number of TGF-β target genes
are upregulated in melanoma cells exposed to TGF-β, in particular
those involved in invasion and metastasis (42,43).
We previously discussed the possible mechanism associated with
TGF-β1, the expression of which may be decreased after
hyperthermia in breast carcinoma MCF-7 cells. In the present study,
we further revealed that the same regulation of TGF-β1
occurred in mouse malignant melanoma B16F10 cells both in
vivo and in vitro.
Our results revealed that hyperthermia at 43, 45,
47°C for 30 min downregulated the TGF-β1 mRNA
transcription level and protein expression in B16F10 cells. Smad4
protein was upregulated for a short time after hyperthermia
treatment, while Smad4 mRNA showed an insignificant difference
between the hyperthermic-treated and control group. We infer that
the Smad4 mRNA transcription level should not be a key factor in
its increased protein expression. The results here imply that
expression of TGF-β1 in malignant melanoma might be used
to judge the curative effect and prognosis for hyperthermia.
In the in vivo test, we observed the mice of
all groups after treatment, and tried to find out the reason for
their death through anatomy. Although the survival period of the
tumor-burdened mice was significantly prolonged in the MFH-treated
groups (P<0.05), no metastatic areas were found in the treatment
and control groups, which could not be used to prove the
suppressive effect of hyperthermia on metastasis in vivo yet
it definitely resulted in a lower growth rate and less invasive
ability in the treatment group.
Although, intracavity microwave coagulation therapy
and radiofrequency ablation are the main methods of hyperthermia
used in the clinic at present, both methods fail to achieve the set
point of temperature intratumorally and achieve homogenous
distribution of temperature (44,45).
Magnetic fluid hyperthermia is a promising approach to cancer
therapy since it not only kills cancer cells directly, but can also
alter the character of tumor cells with high metastatic potential,
despite that the concrete mechanism needs to be further
researched.
From the present study, we confirmed that
hyperthermia induced by magnetic fluid can be used safely to
inhibit tumor growth in a mouse model without heat damage to
surrounding normal tissue. A well-homogenized distribution of high
intratumoral temperature can be achieved by image guidance which we
did not make use of in the present study. As dosage of radiotherapy
is limited by the tolerance of surrounding normal tissue, the
temperature of hyperthermia should also be limited. For safe
consideration, heating radiator can hardly be cut off between tumor
and normal tissues even by image guidance, so local hyperthermia
induced by magnetic fluid may be far from a radical treatment in
the clinic. However, this method can be used as an effective
cytoreductive treatment thus providing a greater chance of efficacy
of further treatment such as surgery.
In conclusion, our study suggests that hyperthermia
heating at 43°C or above can strongly reduce the proliferation and
invasive ability of B16F10 cells in vitro, and their
protease production can be decreased as well. In addition, magnetic
fluid hyperthermia at 45 and 50°C significantly inhibited the
growth of malignant melanoma in a mouse model and suppressed the
expression of TGF-β1 in tumor tissue. Therefore, our
study implies that the expression of TGF-β1, MMP-2 and
MMP-9 in malignant melanoma may be used to judge the curative
effect and prognosis for hyperthermia. Moreover, we can prospect
that, magnetic fluid hyperthermia may be an effective adjuvant
therapy, not destroying malignant melanoma cells directly, but
suppressing the ability of invasiveness and mobility of the remnant
carcinoma cells.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (nos. 30571779 and 10775085) and the
Yu-Yuan Medical Foundation of Tsinghua University.
References
|
1
|
Kim CJ, Reintgen DS and Balch CM: The new
melanoma staging system. Cancer Control. 9:9–15. 2002.
|
|
2
|
Hofmann UB, Westphal JR, Van Muijen GNP
and Ruiter DJ: Matrix metalloproteinases in human melanoma. J
Invest Dermatol. 115:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vihinen P and Kahari VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krasagakis K, Tholke D, Farthmann B,
Eberle J, Mansmann U and Orfanos CE: Elevated plasma levels of
transforming growth factor (TGF)-beta1 and TGF-beta2 in patients
with disseminated malignant melanoma. Br J Cancer. 77:1492–1494.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krasagakis K, Garbe C, Schrier PI and
Orfanos CE: Paracrine and autocrine regulation of human melanocyte
and melanoma cell growth by transforming growth factor beta in
vitro. Anticancer Res. 14:2565–2571. 1994.PubMed/NCBI
|
|
6
|
Rodeck U, Melber K, Kath R, Menssen HD,
Varello M, Atkinson B and Herlyn M: Constitutive expression of
multiple growth factor genes by melanoma cells but not normal
melanocytes. J Invest Dermatol. 97:20–26. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodeck U, Bossler A, Graeven U, Fox FE,
Nowell PC, Knabbe C and Kari C: Transforming growth factor beta
production and responsiveness in normal human melanocytes and
melanoma cells. Cancer Res. 54:575–581. 1994.PubMed/NCBI
|
|
8
|
Van Belle P, Rodeck U, Nuamah I, Halpern
AC and Elder DE: Melanoma-associated expression of transforming
growth factor-beta isoforms. Am J Pathol. 148:1887–1894.
1996.PubMed/NCBI
|
|
9
|
Nagashima K, Takagi R and Hoshina H:
Effect of local hyperthermia on metastases in oral squamous cell
carcinoma. Int J Oral Maxillofac Surg. 31:84–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato T, Sawaji Y, Matsui N, Sato H,
Motoharu S, Mori Y and Ito A: Heat shock suppresses membrane type
1-matrix metalloproteinase production and progelatinase A
activation in human fibrosarcoma HT-1080 cells and thereby inhibits
cellular invasion. Biochem Biophys Res Commun. 265:189–193. 1999.
View Article : Google Scholar
|
|
11
|
Nathanson SD, Cerra SD, Hetzel FW, et al:
Changes associated with metastasis in B16-F1 melanoma cells
surviving heat. Archives Sur. 125:216–219. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jordan A, Scholz R, Wust P, Fakhling H and
Felix R: Magnetic fluid hyperthermia (MFH): cancer treatment with
AC magnetic field induced excitation of biocompatible
superparamagnetic nanoparticles. J Magn Magn Mater. 201:413–419.
1999. View Article : Google Scholar
|
|
13
|
Du LH, Zhou JM, Wang XW, Sheng L, Wang GH,
Xie XX, Zhao LY, Liao YP and Tang JT: Effect of local hyperthermia
induced by nanometer magnetic fluid on the rabbit VX2 liver tumor
model. Prog Nat Sci. 19:1705–1712. 2009. View Article : Google Scholar
|
|
14
|
Wilhelm C, Fortin JP and Gazeau F: Tumour
cell toxicity of intracellular hyperthermia mediated by magnetic
nanoparticles. J Nanosci Nanotechnol. 7:2933–2937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao FP, Cai YY, Zhou J, Xie XX, Ouyang WW,
Zhang YH, Wang XF, Wang XW, Zhao LY and Tang JT: Pullulan acetate
coated magnetite nanoparticles for hyperthermia: preparation,
characterization and in vitro experiments. Nano Res. 3:23–31. 2010.
View Article : Google Scholar
|
|
16
|
Landeghem FK, Maier-Hauff K, Jordan A,
Hoffmann KT, Gneveckow U, Scholz R, Thiesen B, Buck W and Deimling
AV: Post-mortem studies in glioblastoma patients treated with
thermotherapy using magnetic nanoparticles. Biomaterials. 30:52–57.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki M, Shinkai M, Honda H and Kobayashi
T: Anticancer effect and immune induction by hyperthermia of
malignant melanoma using magnetite cationic liposomes. Melanoma
Res. 13:129–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hilger I, Hergt R and Kaiser WA: Use of
magnetic nanoparticle heating in the treatment of breast cancer.
IEE Proc Nanobiotechnol. 152:33–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johannsen M, Gneveckow U, Eckelt L,
Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA and
Jordon A: Clinical hyperthermia of prostate cancer using magnetic
nanoparticles: presentation of a new interstitial technique. Int J
Hyperthermia. 21:637–647. 2005. View Article : Google Scholar
|
|
20
|
Jordan A and Maier-Hauff K: Magnetic
nanoparticles for intracranial thermotherapy. J Nanosci
Nanotechnol. 7:4604–4606. 2007.PubMed/NCBI
|
|
21
|
Johannsen M, Thiesen B, Jordan A,
Taymoorian K, Gneveckow U, Waldofner N, Scholz R, Koch M, Lein M,
Jung K and Loening SA: Magnetic fluid hyperthermia (MFH) reduces
prostate cancer growth in the orthotopic Dunning R3327 rat model.
Prostate. 64:283–392. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito A, Tannka K, Kondo K, Shinkai M, Honda
H, Matsumoto K, Saida T and Kobayashi T: Tumor regression by
combined immunotherapy and hyperthermia using magnetic
nanoparticles in an experimental subcutaneous murine melanoma.
Cancer Sci. 94:308–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jordan A, Scholz R, Maier-Hauff K, van
Landeghem FK, Waldoefner N, Teichgraeber U, Pinkemelle J, Brugn H,
Neuman F, Thiesen B, et al: The effect of thermotherapy using
magnetic nanoparticles on rat malignant glioma. J Neurooncol.
78:7–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie XX, Shao XF, Gao FP, Jin HK, Zhou JM,
Du LH, Zhang YY, Ouyang WW, Wang XW, Zhao LY, et al: Effect of
hyperthermia on invasion ability and TGF-β1 expression of breast
carcinoma MCF-7 cells. Oncol Rep. 25:1573–1579. 2011.PubMed/NCBI
|
|
25
|
Houghton AN and Polsky D: Focus on
melanoma. Cancer Cell. 2:275–278. 2002. View Article : Google Scholar
|
|
26
|
Chiang WC, Wong YK, Lin SC, Chang KW and
Liu CJ: Increase of MMP-13 expression in multi-stage oral
carcinogenesis and epigallocatechin-3-gallate suppress MMP-13
expression. Oral Dis. 12:27–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puente XS, Sanchez LM, Overall CM and
Lopez-Otin C: Human and mouse proteases: a comparative genomic
approach. Nat Rev Genet. 4:544–558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
29
|
Ispanovic E and Haas TL: JNK and PI3K
differentially regulate MMP-2 and MT1-MMP mRNA and protein in
response to actin cytoskeleton reorganization in endothelial cells.
Am J Physiol Cell Physiol. 291:579–588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawaji Y, Sato T, Seiki M and Ito A: Heat
shock-mediated transient increase in intracellular 3′,5′-cyclic AMP
results in tumor specific suppression of membrane type 1-matrix
metalloproteinase production and progelatinase A activation. Clin
Exp Metastasis. 18:131–138. 2000.PubMed/NCBI
|
|
31
|
Welm AL: TGFbeta primes breast tumor cells
for metastasis. Cell. 133:27–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hasegawa Y, Takanashi S, Kanehira Y,
Tsushima T, Imai T and Okumura K: Transforming growth factor-beta1
level correlates with angiogenesis, tumor progression, and
prognosis in patients with nonsmall cell lung carcinoma. Cancer.
91:964–971. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito H, Tsujitani S, Oka S, Kondo A,
Ikeguchi M, Maeta M and Kaibara N: The expression of transforming
growth factor-beta1 is significantly correlated with the expression
of vascular endothelial growth factor and poor prognosis of
patients with advanced gastric carcinoma. Cancer. 86:1455–1462.
1999. View Article : Google Scholar
|
|
34
|
Tsushima H, Kawata S, Tamura S, Ito N,
Shirai Y, Kiso S, Imai Y, Shimomukai H, Nomura Y, Matsuda Y and
Matsuzawa Y: High levels of transforming growth factor beta 1 in
patients with colorectal cancer: association with disease
progression. Gastroenterology. 110:375–382. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oft M, Heider KH and Beug H: TGF-beta
signaling is necessary for carcinoma invasiveness and metastasis.
Curr Biol. 8:1243–1252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin JJ, Selander K, Chirgwin JM, Dallas M,
Grubbs BG, Wieser R, Massague J, Mundy GR and Guise TA: TGF-beta
signaling blockade inhibits PTHrP secretion by breast cancer cells
and bone metastases development. J Clin Invest. 103:197–206. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui W, Fowlis DJ, Bryson S, Duffie E,
Ireland H, Balmain A and Akhurst RJ: TGF beta1 inhibits the
formation of benign skin tumors, but enhances progression to
invasive spindle carcinomas in transgenic mice. Cell. 86:531–542.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Padua D, Zhang XHF, Wang QQ, Nadal C,
Gerald WL, Gomis RR and Massague J: TGFbeta primes breast tumors
for lung metastasis seeding through angiopoietin-like 4. Cell.
133:66–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krasagakis K, Kruger-Krasagakes S, Fimmel
S, Eberle J, Tholke D, von der Ohe M, Mansmann U and Orfanos CE:
Desensitization of melanoma cells to autocrine TGF-beta isoforms. J
Cell Physiol. 178:179–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Janji B, Melchior C, Gouon V, Vallar L and
Kieffer N: Autocrine TGF-beta-regulated expression of adhesion
receptors and integrin-linked kinase in HT-144 melanoma cells
correlates with their metastatic phenotype. Int J Cancer.
83:255–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miyoshi E, Nishikawa A, Ihara Y, Saito H,
Uozumi N, Hayashi N, Fusamoto H, Kamada T and Taniguchi N:
Transforming growth factor beta up-regulates expression of the
N-acetyl glucoseminyl transferase V gene in mouse melanoma cells. J
Biol Chem. 270:6216–6220. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park SS, Li L, Korn TS, Mitra MM and
Niederkorn JY: Effect of transforming growth factor-beta on
plasminogen activator production of cultured human uveal melanoma
cells. Curr Eye Res. 15:755–763. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rodeck U, Nishiyama T and Mauviel A:
Independent regulation of growth and SMAD-mediated transcription by
transforming growth factor beta in human melanoma cells. Cancer
Res. 59:547–550. 1999.PubMed/NCBI
|
|
44
|
Hilger I, Hiergeist R, Hergt R, Winnefeld
K, Schubert H and Kaiser W: Thermal ablation of tumors using
magnetic nanoparticles: an in vivo feasibility study. Invest
Radiol. 37:580–586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brusentsova NA, Nikitinb L, Brusentsovac
T, Brusentsava TN, Kuznetsov AA, Beyburtskiy FS, Shumakov LI and
Jurchenko NY: Magnetic fluid hyperthermia of the mouse experimental
tumor. J Magn Magn Mater. 252:378–380. 2002. View Article : Google Scholar
|