Introduction
Although cancer is currently one of the most leading
causes of mortality, prevention and therapy remains inadequate.
Traditional antitumor approaches including surgery, radiotherapy
and chemotherapy have various adverse effects derived from a lack
of specificity; they also induce severe damage or dysfunction of
normal cells (1). Immunotherapy
including cancer vaccines, which is designed to modulate immune
responses of patients to induce specific removal of cancer cells
has gained increasing attention (2). For efficacious function of cancer
vaccines without side effects, the selection of proper
tumor-specific antigens or tumor-associated antigens as a target is
important (3,4). To stimulate immune responses to
selected tumor antigens, antigenic sources such as peptides,
proteins, inactivated tumor cells and DNAs may be used (5). Among the antigens, peptides are the
easiest to produce and to monitor immune responses. Therefore, the
innovation of a peptide vaccine strategy with enhanced
immunostimulatory efficacy may greatly contribute to the
development of cancer vaccines (6).
CpG-DNA, a sequence containing unmethylated CpG
dinucleotides flanked by specific base sequences, has
immunostimulatory activities (7–9):
activation of antigen-presenting cells, induction of Th1-biased
immune responses and immunoglobulin (Ig) isotype switching
(10–12). Although a number of researchers use
phosphorothioate-modified CpG-DNA (PS-ODN) due to its nuclease
resistance and efficient uptake into cells, PS-ODN induces
PS-ODN-specific IgM production and backbone-related side effects
such as transient lymphadenopathy, lymphoid follicle destruction
and arthritis (13–16). Therefore, we isolated the natural
phosphodiester bond CpG-DNA [PO-ODN, MB-ODN 4531(O)] from
Mycobacterium bovis genomic DNA and confirmed its
stimulating activity to induce optimal innate immune responses
(17). The activities of CpG-DNA as
a potent adjuvant are enhanced through encapsulation in liposomes
(18,19). When the effect of the lipid
composition on the activity of the phosphodiester bond CpG-ODN was
investigated in human and mouse immune cells in vitro,
DOPE:CHEMS (1:1 ratio) proved to have a superior effect (20). Furthermore, encapsulation of the
phosphodiester bond CpG-DNA in a DOPE:CHEMS (1:1 ratio) complex
[Lipoplex(O)] enhanced adjuvant activity in mice when proteins were
used as antigens (21). Therefore,
we applied this formulation to peptides and discovered that
complexes of the B cell epitope peptide and Lipoplex(O)
significantly induced peptide-specific IgG production (20,22).
To validate the application of our novel strategy to
cancer vaccines, we used the peptide TM4SF5R2-3 targeting the
transmembrane 4 superfamily member 5 protein (TM4SF5) as a
tumor-specific antigen. TM4SF5 has been implicated in
hepatocellular carcinoma (HCC) (23). Immunization with the peptide vaccine
composed of TM4SF5R2-3 and Lipoplex(O) induced functional antitumor
effects in vivo; preventive and therapeutic effects against
tumor formation of implanted HCC cells in mice (24).
In the development of a cancer vaccine, induction of
a memory response by the vaccination is an essential prerequisite
for practical application (2). In
this study, we examined tumor formation in the mice immunized with
the TM4SF5R2-3 peptide vaccine after delayed challenge of HCC cells
and discovered that the peptide vaccine induced a functional memory
response.
Materials and methods
Synthesis of CpG-DNA and a B cell epitope
peptide
MB-ODN 4531(O), a natural phosphodiester bond
CpG-DNA, consists of 20 bases containing three CpG motifs
(underlined): AGCAGCGTTCGTGTCGGCCT (17). The B cell epitope peptide of human
TM4SF5 (TM4SF5R2-3, 138NRTLWDRCEAPPRV151) was
selected and produced as previously described (24).
Preparation of B cell epitope and CpG-DNA
co-encapsulated in DOPE:CHEMS complexes
Liposome complexes consisting of B cell epitope and
CpG-DNA [MB-ODN 4531(O)] co-encapsulated with DOPE:CHEMS were
prepared as previously reported (20). Briefly, DOPE and CHEMS were mixed in
10% ethanol at a molar ratio of 1:1, evaporated with nitrogen gas
to produce a solvent-free lipid film and resuspended in a mixture
containing equal volumes of water-soluble MB-ODN 4531(O) (50 μg)
and peptide (50 μg), followed by vigorous stirring at room
temperature for 30 min. After adjusting the pH to 7.0, the peptide
and Lipoplex(O) complex was sonicated lightly for 30 sec with a
sonicator (Sonifier 450; Branson Ultrasonics). After the complex
was filtered with a 0.22 μm filter, it was freeze-thawed 3 times
with liquid nitrogen.
Antigen-specific Ig ELISA assay
Mouse sera were achieved by orbital bleeding. To
determine the amounts and titers of total IgG, 96-well immunoplates
(Nalge Nunc International) were coated with 5 μg/ml of each peptide
and then blocked with 0.05% of Tween-20 in PBS (PBST) containing 1%
BSA. Total IgG levels were measured as previously described
(20).
FACS analysis
The mice were immunized on 3 occasions at 10-day
intervals and their splenocytes were obtained 10 days after the
last immunization. The expression of IgM, IgD, B220 and/or CD138
was analyzed with a FACS Aria II flow cytometer (BD Biosciences).
Splenocytes were washed with PBS containing 0.1% bovine serum
albumin and incubated for 20 min at 4°C with 10 μg/ml of
anti-FcγRII/III antibody (BD Biosciences) to block Fc receptors.
After blocking, the cells were incubated with the indicated
antibodies (IgM-FITC, IgD-PE, B220-Pacific Blue and CD138-APC; BD
Biosciences) for 1 h at 4°C. FACS data were analyzed with the aid
of WinMDI 2.8 FACS software.
Cell culture
The mouse hepatoma cell line BNL-HCC was obtained
from ATCC. BNL-HCC is a chemically transformed mouse liver cell
line derived from the normal BALB/c embryonic liver cell line BNL
CL2 (25). The cells were cultured
in a DMEM medium containing 10% FBS, 25 mM HEPES, 100 U/ml of
penicillin and 100 μg/ml of streptomycin at 37°C in an atmosphere
of 95% air and 5% CO2.
Hepatocellular carcinoma mouse model
We purchased four-week-old male BALB/c
(H-2b) mice from Central Lab. Animal, Inc. Mice were
maintained under specific-pathogen-free conditions in a controlled
environment (20–25°C, 32–37% humidity). All animal procedures
performed in this study were in accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Veterinary Research and Quarantine Service of Korea. The
protocol was approved by the Institutional Animal Care and Use
Committee of Hallym University (permit no. Hallym 2010-82). On 3
occasions at 10-day intervals, the mice were injected
intraperitoneally with 50 μg of TM4SF5R2-3 peptide supplemented
with 50 μg of MB-ODN4531(O) encapsulated in the DOPE:CHEMS complex
(200 μl/mouse). As a control, mice were injected with PBS or 50 μg
of TM4SF5R2-3 peptide encapsulated in the DOPE:CHEMS complex.
Seventy days after the third immunization, the mice were inoculated
subcutaneously in the right dorsal flank with PBS or
5×106 of BNL-HCC cells in a 50% Matrigel solution
(HBSS/Matrigel, 1:1 v/v; BD Biosciences) as previously described
(26). The size of the tumor was
measured at 5-day intervals with calipers in 3 dimensions, and
tumor volumes were calculated as width2 × length/2. The
mice were sacrificed on the indicated days after the tumor cell
implantation and the tumors were surgically excised and weighed.
The survival rate was recorded for 90 days after tumor cell
implantation. The mice were sacrificed under Zoletil 50 + Rompun
anesthesia and all efforts were made to minimize suffering. Mice
were sacrificed when the tumor size reached 2,000 mm3 or
the mice lost >20% of initial body weight to minimize suffering
from a large tumor burden.
Statistics
Results are expressed as the means ± standard
deviation. Statistical significance between the 2 samples was
performed using the Student's t-test. A P-value of <0.05 was
considered to indicate a statistically significant difference. A
survival analysis was performed using the Kaplan-Meier method and
compared with a log-rank test.
Results
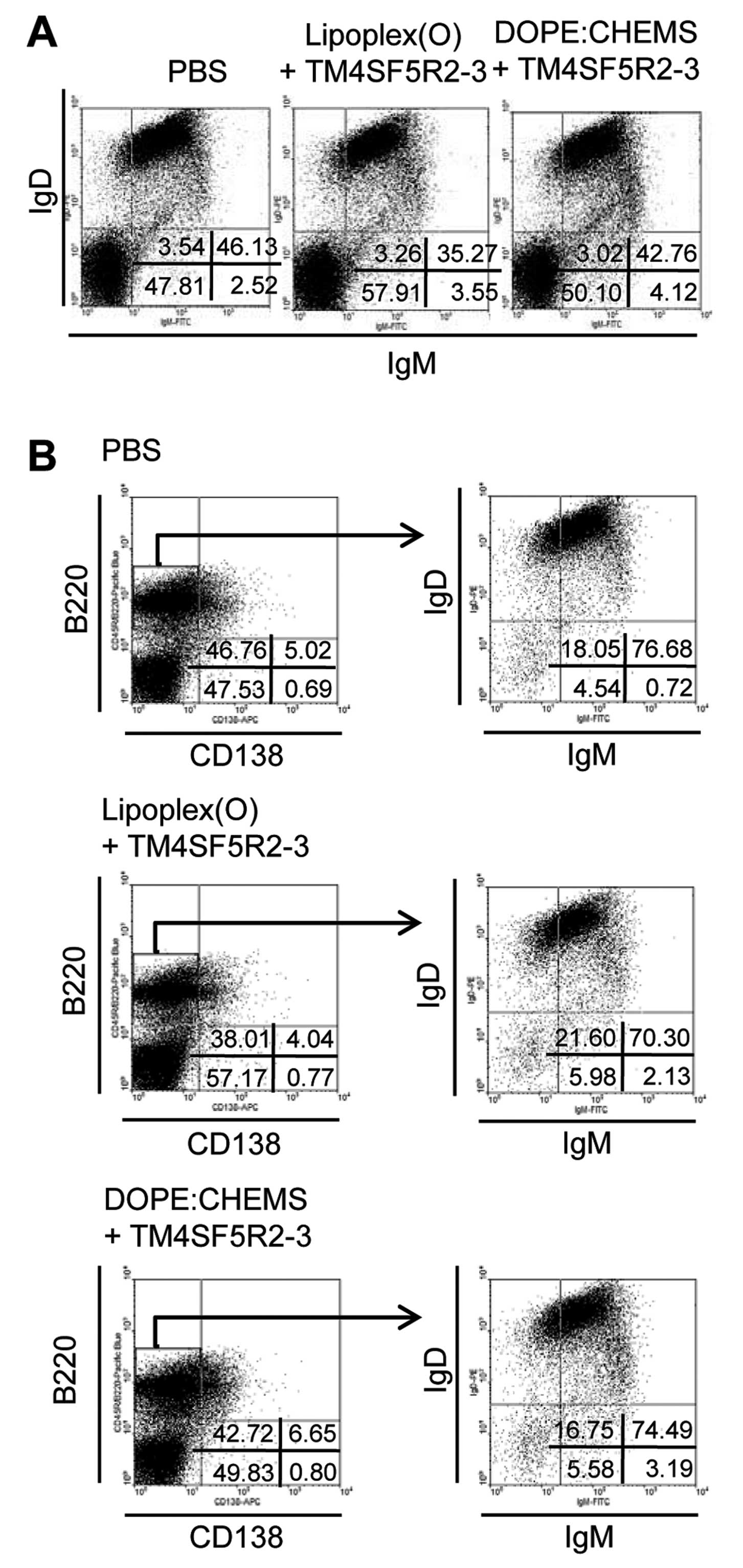
Increase in the putative memory B cell
population in the mice immunized with a complex of the TM4SF5R2-3
peptide and Lipoplex(O)
We previously confirmed the prophylactic effect of a
peptide vaccine composed of the TM4SF5R2-3 peptide and Lipoplex(O)
complex in an HCC model (23). In
this study, we immunized mice with the peptide vaccine 3 times at
10-day intervals and directly challenged the mice with
hTM4SF5-expressing BNL-HCC cells on Day 30. Immunological memory is
a critical feature of the adaptive immune response and memory
function of humoral immunity involves the development of memory B
cells. As memory B cells are considered a subpopulation of
isotype-switched B cells
(B220+IgD−IgM−CD138−)
(27–29), we analyzed the surface marker
expression of spleen cells from 3 different experimental groups of
mice (8 mice/group) 10 days after the last immunization. The
population of IgD−IgM− cells was larger in
the splenocytes from the mice immunized with a complex of
TM4SF5R2-3 peptide and Lipoplex(O) compared with the other 2
control groups (57.91 vs. 47.81 or 50.10) (Fig. 1A). For a detailed analysis, we gated
a B220+CD138− cell population and then
analyzed the expression of IgD and IgM (Fig. 1B). The
B220+CD138−IgD−IgM−
cell population was larger in the mice immunized with the
TM4SF5R2-3 peptide and Lipoplex(O) complex compared to the control
mouse group immunized with PBS or the TM4SF5R2-3 peptide and
DOPE:CHEMS (5.98 vs. 4.54 and 5.58). Therefore, we predicted a
memory response of our vaccine and performed further experiments to
confirm whether the long-lasting memory response protects mice from
delayed challenge of cancer cells.
Production of IgG by delayed implantation
of cancer cells in the mice immunized with the TM4SF5R2-3 peptide
and Lipoplex(O) complex
The entire experimental schedule is depicted in
Fig. 2A. To determine whether the
state of memory is induced by a re-encounter of the antigens, we
challenged the immunized mice with BNL-HCC cells 70 days after the
third immunization (on Day 90) and checked IgG production and tumor
formation (Fig. 2A). As a negative
control, two groups of mice were immunized with PBS or TM4SF5R2-3
peptide encapsulated in DOPE:CHEMS and then implanted with BNL-HCC
cells. The highest amount of TM4SF5R2-3-specific IgG in the
vaccinated mice was detected on Day 30 (Fig. 2B). The amount of IgG decreased
thereafter but remained significantly higher until Day 90 in the
immunized mice compared to the control mouse group. A significant
amount of antibodies was also observed in the mice immunized with
TM4SF5R2-3 peptide encapsulated in DOPE:CHEMS. However, when we
implanted BNL-HCC cells into the mice, the amount of IgG
significantly increased again only in the mice immunized with the
TM4SF5R2-3 peptide and Lipoplex(O) complex (Fig. 2B). This implies that CpG-DNA may be
essential for the induction of a memory response.
Prophylactic efficacy of the vaccine
against tumor formation of HCC cells implanted in mice
We examined the physical phonotype of mice after
challenging them with BNL-HCC cells for 60 days. Based on tumor
size (Fig. 3A), tumor volume
(Fig. 3B) and tumor weight
(Fig. 3C), tumor formation was
greatly inhibited in the mice immunized with the TM4SF5R2-3 peptide
and Lipoplex(O) complex. Immunization did not affect the body
weight of the mice during the experiment, suggesting that there was
no significant side effects (Fig.
3D). Slight decreases in the tumor volume and tumor weight were
detected in the mice immunized with the TM4SF5R2-3 peptide
encapsulated in DOPE:CHEMS without CpG-DNA suggesting a partial
tumor suppressive effect.
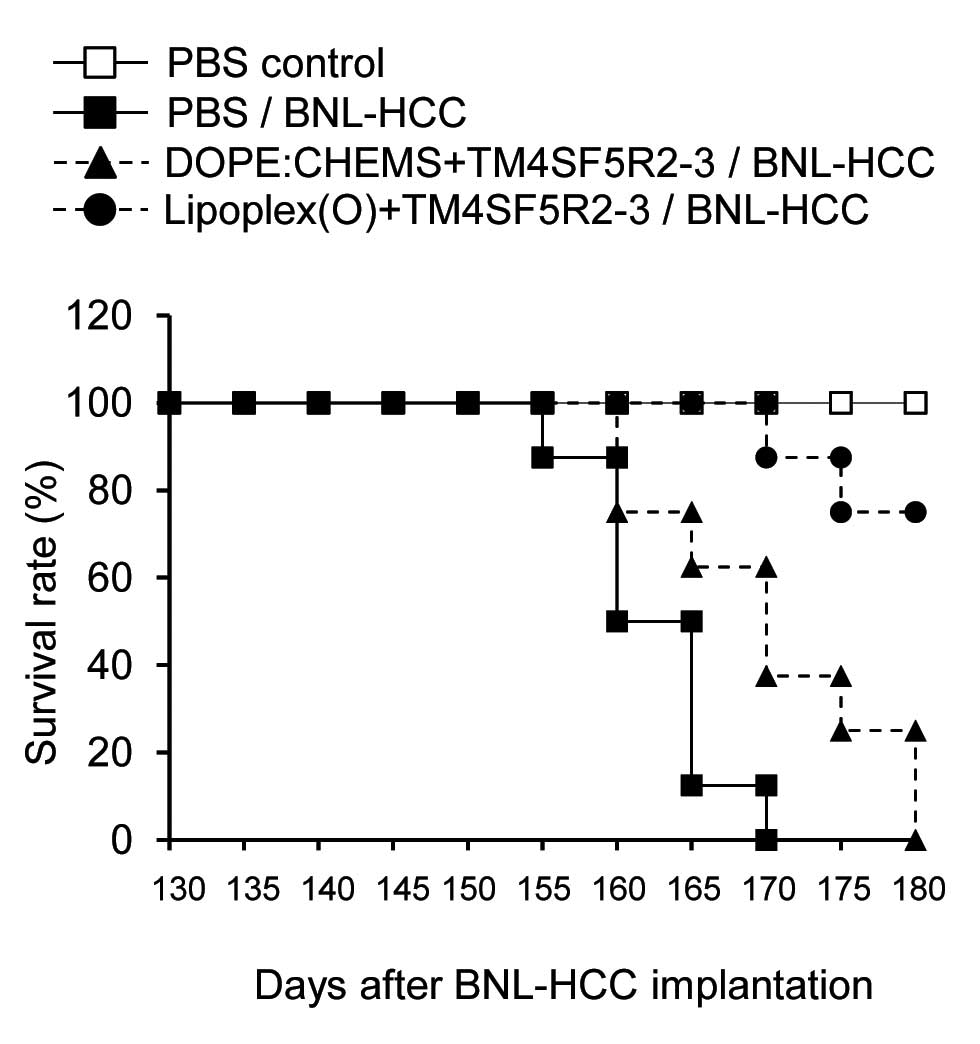
Enhanced survival of the immunized mice
in response to delayed challenge of HCC cells
To further evaluate the prophylactic efficacy of the
vaccine, we assessed the survival rate of the mice challenged with
BNL-HCC cells until Day 180 (3 months after implantation). The
unimmunized control mice challenged with BNL-HCC cells were
sacrificed by Day 170 (Fig. 4). In
contrast, ~75% of the mice immunized with TM4SF5R2-3 peptide and
Lipoplex(O) survived until Day 180. Survival of the mice immunized
with TM4SF5R2-3 peptide encapsulated with DOPE:CHEMS was also
enhanced, which is in accordance with the lower tumor growth
compared to the PBS control mice (Fig.
3); however all of the mice were sacrificed by day 180.
Therefore, only the vaccine composed of TM4SF5R2-3 peptide and
Lipoplex(O) demonstrated a prominent long-lasting prophylactic
effect when we performed a cancer cell challenge experiment ~2
months after the final immunization.
Discussion
The immune system in our body recognizes antigens
and coordinates specific immune responses against them through
multi-cellular cooperation mainly involving antigen-presenting, B
and T cells (1). When the antigens
are proteins, usage of synthetic peptides rather than the whole
proteins offers several advantages: They are easy to produce
promptly and their cost is much lower. Peptides bind the B cell
receptor and major histocompatibility (MHC) molecules as B- and
T-cell epitopes, by which they induce and regulate immune
responses. Therefore, peptide vaccines have the potential to be
widely used in cancers and other infectious diseases (6,30,31).
However, the weak immunogenicity of peptides limits their
application as a vaccine. Therefore, it is necessary to maximize
the efficacy of peptide-driven immune responses. Recently, we
developed a powerful strategy to induce the production of
epitope-specific antibodies using peptides as an antigen (20,21).
To magnify the immune responses, we utilized Lipoplex(O), comprised
of natural CpG-DNA and a specific liposome complex as an adjuvant.
We previously applied this strategy as a cancer vaccine (24) and we further evaluated the peptide
vaccine in the context of the memory function.
Cancer vaccines require the induction of active
tumor-specific immune responses without harming normal cells. The
tumor-specific immune responses may prevent tumor formation
(prophylactic vaccine) and/or treat established tumors in patients
(therapeutic vaccine). The expression of TM4SF5 at the mRNA level
has been reported in several types of human cancers including
pancreatic cancer, soft tissue sarcoma, gastric cancer, carcinoma
of the papilla vateri and colon cancer (32). The overexpression of TM4SF5 at the
protein level was found by immunohistochemical staining in 7 out of
9 HCC tissues, whereas normal liver tissues did not express TM4SF5
(23). Therefore, TM4SF5 may be an
effective target for an HCC vaccine as a tumor specific antigen
(33,34). Previously, we confirmed that the
monoclonal antibody produced using our strategy had a
growth-inhibitory effect on human and mouse HCC cell lines (Huh-7
and BNL-HCC) expressing the antigen and that the monoclonal
antibody may detect TM4SF5 in tumor tissues derived from an HCC
mouse model (20,23). Furthermore, the peptide vaccine
composed of TM4SF5R2-3 peptide and Lipoplex(O) induced prophylactic
and therapeutic effects against tumor formation of implanted HCC
cells in mice without prominent side effects (24).
In addition to the specificity of immune responses
against tumors, cancer vaccines should induce immunological memory
to suppress the recurrence of cancers (2). A number of vaccine approaches have
revealed efficacy in murine cancer models; however they are
ineffective or have limited efficacy in patients (2). In this study, we investigated whether
our peptide vaccine induces memory response in mice. While it is
difficult to define the exact events in vivo leading to the
enhancement of immune responses, the effect of vaccine-induced
protection against infection and disease appears to be correlated
with the ability to induce long-term memory B cells and the
production of specific antibodies (35). Therefore, we first performed a
population analysis on splenocytes from the mice and discovered a
slight increase in the isotype-switched B cell population
(B220+CD138−IgD−IgM−),
which presumably includes memory B cells (Fig. 1). We then analyzed the amounts of
epitope-specific antibodies for 150 days and observed that a
significant amount of epitope-specific antibodies remained on Day
90 (70 days after the last immunization) and the level increased
after the implantation of the HCC cells in the vaccinated mice
(Fig. 2). To further evaluate the
memory function, we examined the phenotypes of HCC-implanted mice
and observed that the tumor growth was inhibited in the vaccinated
mice, based on the tumor volume, tumor weight and survival of mice
(Figs. 3 and 4). Taken together, these results indicate
that our vaccine has an immunological memory response lasting at
least 70 days after the last immunization. To further investigate
the long-term memory response induced by this vaccine, the
examination period must be extended in future studies.
The mechanisms involved in the induction of a memory
response remain unclear. According to a recent influenza vaccine
study, early CD4+ T cells appear to be required for
long-term memory (36). However,
there are controversial reports suggesting that memory B cells
persist in mice deprived of a T cell population and that a unique
IgG memory B cell niche is important for memory (37,38).
Previously, we confirmed that specific CD4 T cells and MHC
molecules are necessary for antibody production induced by our
strategy despite that we used only B cell epitope without any
carrier (20). Therefore, it is
possible that our system requires T cells for the memory response.
Contribution of toll-like receptor (TLR) agonists to memory B cell
function has also been documented (27,39).
CpG-DNA, a TLR agonist, was recently reported as an effective
adjuvant for cancer vaccines inducing long-term antitumor activity
using irradiated tumor cells and proteins as an antigen (40,41).
Liposomes are known to enhance antibody production and cytotoxic T
lymphocyte (CTL) responses (42–44).
Furthermore, the activities of CpG-DNA are reportedly enhanced by
the encapsulation in liposomes (18,19).
Therefore, the cancer vaccine composed of the TM4SF5R2-3 peptide
and Lipoplex(O) used in this study may be powerful in inducing a
short-term immune response as well as a long-lasting memory
response.
Acknowledgements
This study was supported by a grant from the Hallym
Research Fund (HRF-201203-011) and grants from the National
Research Foundation (2012R1A2A2A01009887, 20110027774 and
20120006130) funded by the Ministry of Education, Science and
Technology of the Republic of Korea.
References
|
1
|
Abbas A, Lichtman A and Pillai S: Cellular
and Molecular Immunology. 6th edition. Saunders Elsevier;
Philadelphia, PA: 2007
|
|
2
|
Aly HA: Cancer therapy and vaccination. J
Immunol Methods. 382:1–23. 2012. View Article : Google Scholar
|
|
3
|
Hislop AD, Taylor GS, Sauce D and
Rickinson AB: Cellular responses to viral infection in humans:
lessons from Epstein-Barr virus. Annu Rev Immunol. 25:587–617.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finn OJ: Cancer immunology. N Engl J Med.
358:2704–2715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schreiber TH, Raez L, Rosenblatt JD and
Podack ER: Tumor immunogenicity and responsiveness to cancer
vaccine therapy: the state of the art. Semin Immunol. 22:105–112.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bijker MS, Melief CJ, Offringa R and van
der Burg SH: Design and development of synthetic peptide vaccines:
past, present and future. Expert Rev Vaccines. 6:591–603. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bode C, Zhao G, Steinhagen F, Kinjo T and
Klinman DM: CpG DNA as a vaccine adjuvant. Expert Rev Vaccines.
10:499–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klinman DM, Currie D, Gursel I and
Verthelyi D: Use of CpG oligodeoxynucleotides as immune adjuvants.
Immunol Rev. 199:201–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krieg AM: CpG motifs in bacterial DNA and
their immune effects. Annu Rev Immunol. 20:709–760. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu RS, Targoni OS, Krieg AM, Lehmann PV
and Harding CV: CpG oligodeoxynucleotides act as adjuvants that
switch on T helper 1 (Th1) immunity. J Exp Med. 186:1623–1631.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carson DA and Raz E: Oligonucleotide
adjuvants for T helper 1 (Th1)-specific vaccination. J Exp Med.
186:1621–1622. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis HL, Weeratna R, Waldschmidt TJ,
Tygrett L, Schorr J and Krieg AM: CpG-DNA is a potent enhancer of
specific immunity in mice immunized with recombinant hepatitis B
surface antigen. J Immunol. 160:870–876. 1998.PubMed/NCBI
|
|
13
|
Kim D, Rhee JW, Kwon S, Sohn WJ, Lee Y, et
al: Immunostimulation and anti-DNA antibody production by backbone
modified CpG-DNA. Biochem Biophys Res Commun. 379:362–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lipford GB, Sparwasser T, Zimmermann S,
Heeg K and Wagner H: CpG-DNA-mediated transient lymphadenopathy is
associated with a state of Th1 predisposition to antigen-driven
responses. J Immunol. 165:1228–1235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heikenwalder M, Polymenidou M, Junt T,
Sigurdson C, Wagner H, et al: Lymphoid follicle destruction and
immunosuppression after repeated CpG oligodeoxynucleotide
administration. Nat Med. 10:187–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng GM, Nilsson IM, Verdrengh M, Collins
LV and Tarkowski A: Intra-articularly localized bacterial DNA
containing CpG motifs induces arthritis. Nat Med. 5:702–705. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KW, Jung J, Lee Y, Kim TY, Choi SY, et
al: Immunostimulatory oligodeoxynucleotide isolated from genome
wide screening of Mycobacterium bovis chromosomal DNA. Mol
Immunol. 43:2107–2118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henriksen-Lacey M, Korsholm KS, Andersen
P, Perrie Y and Christensen D: Liposomal vaccine delivery systems.
Expert Opin Drug Deliv. 8:505–519. 2011. View Article : Google Scholar
|
|
19
|
Suzuki Y, Wakita D, Chamoto K, Narita Y,
Tsuji T, et al: Liposome-encapsulated CpG oligodeoxynucleotides as
a potent adjuvant for inducing type 1 innate immunity. Cancer Res.
64:8754–8760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim D, Kwon S, Rhee JW, Kim KD, Kim YE, et
al: Production of antibodies with peptide-CpG-DNA-liposome complex
without carriers. BMC Immunol. 12:292011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim D, Kwon S, Ahn CS, Lee Y, Choi SY,
Park J, Kwon HY and Kwon HJ: Adjuvant effect of
liposome-encapsulated natural phosphodiester CpG-DNA. BMB Rep.
44:758–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim D, Kwon HJ and Lee Y: Activation of
Toll-like receptor 9 and production of epitope specific antibody by
liposome-encapsulated CpG-DNA. BMB Rep. 44:607–612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SA, Lee SY, Cho IH, Oh MA, Kang ES, et
al: Tetraspanin TM4SF5 mediates loss of contact inhibition through
epithelial-mesenchymal transition in human hepatocarcinoma. J Clin
Invest. 118:1354–1366. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon S, Kim D, Park BK, Cho S, Kim KD, Kim
YE, Park CS, Ahn HJ, Seo JN, Choi KC, Kim DS, Lee Y and Kwon HJ:
Prevention and therapy of hepatocellular carcinoma by vaccination
with TM4SF5 epitope-CpG-DNA-liposome complex without carriers. PLoS
One. 7:e331212012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshiji H, Kuriyama S, Ways DK, Yoshii J
and Miyamoto Y: Protein kinase C lies on the signaling pathway for
vascular endothelial growth factor-mediated tumor development and
angiogenesis. Cancer Res. 59:4413–4418. 1999.PubMed/NCBI
|
|
26
|
Yoshiji H, Kuriyama S, Kawata M, Yoshii J,
Ikenaka Y, et al: The angiotensin-I-converting enzyme inhibitor
perindopril suppresses tumor growth and angiogenesis: possible role
of the vascular endothelial growth factor. Clin Cancer Res.
7:1073–1078. 2001.
|
|
27
|
Richard K, Pierce SK and Song W: The
agonists of TLR4 and 9 are sufficient to activate memory B cells to
differentiate into plasma cells in vitro but not in vivo. J
Immunol. 181:1746–1752. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McHeyzer-Williams LJ and McHeyzer-Williams
MG: Antigen-specific memory B cell development. Annu Rev Immunol.
23:487–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayakawa K, Ishii R, Yamasaki K, Kishimoto
T and Hardy RR: Isolation of high-affinity memory B cells:
phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad
Sci USA. 84:1379–1383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ben-Yedidia T and Arnon R: Epitope-based
vaccine against influenza. Expert Rev Vaccines. 6:939–948. 2007.
View Article : Google Scholar
|
|
31
|
Ben-Yedidia T and Arnon R: Design of
peptide and polypeptide vaccines. Curr Opin Biotechnol. 8:442–448.
1997. View Article : Google Scholar
|
|
32
|
Müller-Pillasch F, Wallrapp C, Lacher U,
Friess H, Büchler M, Adler G and Gress TM: Identification of a new
tumour-associated antigen TM4SF5 and its expression in human
cancer. Gene. 208:25–30. 1998.PubMed/NCBI
|
|
33
|
Lee SA, Ryu HW, Kim YM, Choi S, Lee MJ, et
al: Blockade of four-transmembrane L6 family member 5
(TM4SF5)-mediated tumorigenicity in hepatocytes by a synthetic
chalcone derivative. Hepatology. 49:1316–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lekishvili T, Fromm E, Mujoomdar M and
Berditchevski F: The tumour-associated antigen L6 (L6-Ag) is
recruited to the tetraspanin-enriched microdomains: implication for
tumor cell motility. J Cell Sci. 121:685–694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Plotkin SA: Vaccines: correlates of
vaccine-induced immunity. Clin Infect Dis. 47:401–409. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galli G, Medini D, Borgogni E, Zedda L,
Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert
AK, Brauer V, Banzhoff A, Rappuoli R, Del Giudice G and Castellino
F: Adjuvanted H5N1 vaccine induces early CD4+ T cell
response that predicts long-term persistence of protective antibody
levels. Proc Natl Acad Sci USA. 106:3877–3882. 2009.PubMed/NCBI
|
|
37
|
Vieira P and Rajewsky K: Persistence of
memory B cells in mice deprived of T cell help. Int Immunol.
2:487–494. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cancro MP: The persistence of memory: a
unique niche for IgG memory B cells. Proc Natl Acad Sci USA.
107:12737–12738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bernasconi NL, Onai N and Lanzavecchia A:
A role for Toll-like receptors in acquired immunity: up-regulation
of TLR9 by BCR triggering in naive B cells and constitutive
expression in memory B cells. Blood. 101:4500–4504. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiriva-Internati M, Yu Y, Mirandola L,
Jenkins MR, Chapman C, Cannon M, Cobos E and Kast WM: Cancer testis
antigen vaccination affords long-term protection in a murine model
of ovarian cancer. PLoS One. 5:e104712010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cerkovnik P, Novakovic BJ, Stegel V and
Novakovic S: Tumor vaccine composed of C-class CpG
oligodeoxynucleotides and irradiated tumor cells induces long-term
antitumor immunity. BMC Immunol. 11:452010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chikh G and Schutze-Redelmeier MP:
Liposomal delivery of CTL epitopes to dendritic cells. Biosci Rep.
22:339–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang JS, Choi MJ, Cheong HS and Kim K:
Development of Th1-mediated CD8+ effector T cells by
vaccination with epitope peptides encapsulated in pH-sensitive
liposomes. Vaccine. 19:3608–3614. 2001.PubMed/NCBI
|
|
44
|
Gursel I, Gursel M, Ishii KJ and Klinman
DM: Sterically stabilized cationic liposomes improve the uptake and
immunostimulatory activity of CpG oligonucleotides. J Immunol.
167:3324–3328. 2001. View Article : Google Scholar : PubMed/NCBI
|