Introduction
KISS1 was originally discovered as metastasis
suppressor gene in human melanoma and breast cancer cell lines
reducing metastasis in vivo(1–6). The
KISS1 gene encodes a peptide of 145 amino acids, which is cleaved
proteolytically into shorter peptides, the kisspeptins (KP). KP-54,
KP-14, KP-13 and KP-10 are active agonists binding to the KISS1
receptor, the Gq-protein coupled receptor GPR54. All kisspeptins
share a common C-terminal structure, which is necessary for
receptor activation showing the highest potency for KP-10 (7–9).
Based on clinical studies, importance of the
interaction of kisspeptin with GPR54 is discussed controversially
for the antimetastatic effects of KISS1. In healthy tissues, the
receptor is expressed mainly in brain, pancreas and placenta
(10). Upregulation of receptor
levels from normal to tumor tissue were detected in breast,
ovarian, small intestine and colon cancer (7). Others showed no change of GPR54
expression in breast cancer between background and tumor tissue.
However, elevated levels of KISS1 expression correlated with poor
patient prognosis and outcome (11). Further analysis resulted in
differences regarding breast cancer progression and histology.
GPR54 and KISS1 were upregulated in invasive tumors compared to
non-invasive forms leading to shorter relapse-free survival in
patients (12). In contrast,
matching of GPR54 and kisspeptin expression and overall survival in
ovarian cancer patients showed longer survival concomitant with
increased expression levels (13).
In pancreatic cancer, high levels of GPR54 and kisspeptin were
correlated (14) with a longer
overall patient survival (15).
Increased receptor and KISS1 levels were observed in hepatocellular
carcinoma compared to normal tissue (16). In renal cell carcinoma, GPR54 was
increased, but kisspeptin showed no changes in expression (17). In summary, there is no definite
correlation between GPR54 and KISS1 expression and cancer
progression.
Research with regard to the antimetastatic effects
in vitro indeed shows an interaction of the KISS1/GPR54
system and cellular motility mechanisms. The KISS1/GPR54 system is
involved in reduced migration and invasion, modified adhesion
processes, changes in cytoskeleton and chemotactic behavior
(7,8,14,17–24).
In this context, some studies indicate an antiproliferative action
of kisspeptin and induction of apoptosis. In contrast, there are
data in literature showing no effect on cell proliferation by the
KISS1/GPR54 system. One explanation for the controversial results
might be the use of different cell lines and especially differences
in their GPR54 expression. Studies, showing an antiproliferative
effect of kisspeptin, used cells overexpressing the receptor. Some
experiments were carried out by transfecting Chinese hamster ovary
CHO cells with GPR54 (8,18). Others used murine fibroblast NIH3T3
cells (19) and human breast cancer
MDA-MB-435s cells overexpressing GPR54 (25). Only one study used non-transfected
human umbilical vein endothelial cells (HUVECs), which showed an
endogenous receptor expression (20). In these studies, an
antiproliferative effect of kisspeptin was found. However, no
influence on proliferation was observed in pancreatic cancer cell
lines AsPC-1 and PANC-1 (14), in
renal cell carcinoma cell lines Caki-1 and ACHN (17), in HUVECs (26) and trophoblast cells (21). Low endogenous GPR54 expression was
detected in these cell lines. Further experiments in breast cancer
cell lines MDA-MB-231 and MCF-7 showed no effect on proliferation
as well, but no information of the receptor status was given
(22). No involvement of the
KISS1/GPR54 system in antiproliferative actions was detected by
transfection of the KISS1 gene into MDA-MB-231 breast cancer cells
(11), SKOV3 ovarian cancer cells
(23) and C8161 melanoma cells
(2). In summary, these results show
no antiproliferative action in cancer cells, which express GPR54
endogenously. The antiproliferative effect was only observed in
artificial cell models transfected to overexpress the receptor.
In the present study, the effect of the KISS1/GPR54
system on proliferation was studied in the breast cancer cell lines
T47D, ZR75-1, MDA-MB-231, MDA-MB-435s, MDA-MB-453, HCC 70, HCC
1806, HCC 1937 and MCF-7. This tumor was chosen based on different
information in literature, showing reduced proliferation (25) on the one hand and on the other hand
no changes in proliferation after treatment with kisspeptin
(11,22). GPR54 expression levels were measured
and the effect of kisspeptin on proliferation was compared to
neuronal cells transfected to overexpress the receptor. The aim of
this study was to investigate the relationship of antiproliferative
effects of kisspeptin and the nature of GPR54 expression.
Materials and methods
Cell lines and culture conditions
The human breast cancer cell lines T47D, ZR75-1,
MDA-MB-231, MDA-MB-435s, MDA-MB-453, HCC 70, HCC 1806, HCC 1937 and
MCF-7 were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA). In order to guarantee the identity of
the cell lines over the years, cells were expanded after purchase
and aliquots were stored in liquid nitrogen. Every year a new
frozen stock was opened and expanded to carry out the experiments.
Murine GPR54 stable transfected neuronal B35 cell clones (rat) were
kindly provided by Robert P. Millar (Edinburgh, UK). Cells were
cultured as monolayer in medium [MEM, Biochrom, Berlin, Germany;
containing insulin (0.05 IU/ml) and transferrin (1 μg/ml) for human
breast cancer cell lines; DMEM, Gibco®, Life
Technologies, Darmstadt, Germany; containing G418 (1 mg/ml) as
transfection media for B35 clones] supplemented with 10% fetal calf
serum (Biochrom), penicillin (100 U/ml) and streptomycin (100
μg/ml) (Gibco, Life Technologies) at 37°C in a humidified
atmosphere of 5% CO2 in air.
Chemicals
Kisspeptin-10 (KP-10;
Tyr-Asn-Trp-Asn-Ser-Phe-Gly-Leu-Arg-Phe) was synthesized by Peptide
Specialty Laboratories (Heidelberg, Germany). KP-10 was initially
dissolved in dimethyl sulfoxide (DMSO) and diluted in water for
injection. KP-10 solutions used for experiments contained
<0.006% DMSO with no influence on cell viability referring to
controls.
Immunocytochemistry
Cells were grown to ~70% confluence on Lab-Tek
two-well chamber slides (Nunc, Thermo Fisher Scientific,
Langenselbold, Germany). Before each treatment, cells were washed
with phosphate buffered saline (PBS). Cells were fixed in 4%
paraformaldehyde, treated with hydrogen peroxide (3%) and incubated
with blocking solution (Histostain® Bulk kit, Life
Technologies). As primary antibody, a polyclonal rabbit anti-human
GPR54 (SP4238P, Acris, Herford, Germany) was used diluted 1:250 in
PBS. Cells were incubated at 4°C overnight before they were again
treated with Histostain Bulk kit according to the manufacturer’s
description combined with detection reagent AEC substrate chromogen
3-amino-9-ethylcarbazole (Dako, Carpinteria, CA, USA). Controls
were performed by omission of the primary antibody.
Isolation of mRNA and cDNA synthesis
Total RNA was prepared by the RNeasy mini kit
protocol (Qiagen, Hilden, Germany). The concentration of RNA in
each sample was determined by photospectroscopy. First-strand cDNA
was generated by reverse transcription of 1 μg total RNA in a 40 μl
reaction volume containing DNase I, dNTPs, Primer
p(dT)15 primer (Roche Diagnostics, Mannheim, Germany),
RNase inhibitor (RNasin®, Promega, Madison, WI, USA), 5X
First-strand buffer, DTT and SuperscriptTM II reverse
transcriptase (Life Technologies). Samples were tested for
integrity by PCR analysis of the ribosomal housekeeping gene
L7.
PCR amplification
cDNA templates were amplified in a 15 μl reaction
volume containing 0.3 U KAPA2G™ Fast (2X ReadyMix with Dye; Peqlab,
Erlangen, Germany) and 0.5 μM of the appropriate primers (GPR54 in
breast cancer cell lines: sense primer 5′ CGA CTT CAT GTG CAA GTT
CGT C 3′, antisense primer 5′ CAC ACT CAT GGC GGT CAG AG 3′; GPR54
in transfected B35 cells: sense primer 5′ TGA CCG CCA TGA GTG TGG
AC 3′, antisense primer 5′ GCG GAG TGG CTG TAG GAC AT 3′; L7: sense
primer 5′ AGA TGT ACA GAA CTG AAA TTC 3′, antisense primer 5′ ATT
TAC CAA GAG ATC GAG CAA 3′) in a thermal cycler (T3000, Biometra,
Goettingen, Germany). PCR products were separated by gel
electrophoresis and visualized by ethidium bromide staining and UV
photometric detection. Bands were analyzed using the Biometra
BioDoc Analyze system (Biometra, Goettingen, Germany). For semi
quantitative analysis, amount of amplification product was
standardized to the amount of L7 transcripts of each sample using
L7 as ribosomal housekeeping gene.
For analysis of GPR54 mRNA, PCR was run up to 35
cycles to get a signal for some of the breast cancer samples,
whereas amplification product of transfected B35 clones was found
by <25 cycles. For reasons of comparison, cDNA samples of the
transfected B35 clones were diluted 1:100 and arranged together
with the undiluted breast cancer samples. According to this, the
amount of the housekeeping gene L7 used as standard is very low for
the clone in contrast to the breast cancer cells (Fig. 2).
Protein extraction and western blot
analysis
Cell pellets were washed with PBS and resuspended in
CelLytic™ buffer (Sigma, St. Louis, MO, USA) containing protease
inhibitor (Sigma). Equal amounts of protein per sample were diluted
with 4X LDS sample buffer supplemented with 10X sample reducing
agent (NuPAGE®, Life Technologies). After denaturation,
samples were separated on SDS-PAGE (ProSieve® 50 Gel
solution; Lonza, Rockland, ME, USA; 5% for concentrating and 10%
for separating) under reducing conditions. Gels were blotted on
PVDF membranes (Millipore, Billerica, MA, USA). Membranes were
blocked with 5% instant skimmed milk powder, spray-dried (Saliter
GmbH, Oberguenzburg, Germany) in TBST (137 mM NaCl, 2.7 mM KCl,
24.8 mM Tris, 0.1% Tween, pH 7.4) for 1 h, washed with TBST and
incubated at 4°C overnight with polyclonal rabbit anti-GPR54
(AKR-001, Alomone Labs, Jerusalem, Israel) in a 1:1000 dilution in
TBST. After washing, horseradish peroxidase-linked species-specific
whole anti-rabbit IgG (GE Healthcare Europe, Munich, Germany;
1:33000 in TBST) was put on the membranes for 1 h. Membranes were
washed and exposed to chemiluminescent HRP substrate (Immobilon™;
Millipore) for detection of specifically bound antibody by X-ray
film (Biomax MR, Kodak, Rochester, NY, USA). Monoclonal rabbit
antibody for actin (Epitomics, Burlingame, CA, USA) in a 1:1000
dilution in TBST was used for standardization.
Proliferation assay
Cell lines were grown (plating density:
1–2×104 cells/well in 96-well plates depending on their
metabolism) in phenol red-free medium (DMEM, Gibco, Life
Technologies) supplemented with 10% charcoal treated fetal calf
serum (PAN Biotech, Aidenbach, Germany), L-glutamine (2 μmol/ml)
(Biochrom), penicillin (100 U/ml) and streptomycin (100 μg/ml)
(Gibco, Life Technologies) at 37°C in a humidified atmosphere of 5%
CO2 in air overnight. KP-10 solutions and vehicle
(control) were added in final concentrations of 10−11 M
– 10−5 M every day up to 72 h. The experimental setting
included treatments with KP-10 once daily and twice daily.
Experiments were done in six replicates for each sample and
proliferation was determined by a colorimetric assay
(alamarBlue®, AbD Serotec, Oxford, UK). Changes in
viability were used as marker for proliferation. Optical density of
the reduced dye was measured at 570 nm vs. 630 nm by a microplate
reader (Synergy HT, BioTek, Vermont, USA).
Statistical analysis
All experiments were repeated at least three times
with different passages of the respective cell lines. Data were
tested for significant differences by one-way analysis of variance
followed by Dunnett’s multiple comparison test respectively by
Tukey’s multiple comparison test using GraphPad Prism software
(GraphPad Software Inc., La Jolla, CA, USA).
Results
GPR54 expression in breast cancer cell
lines
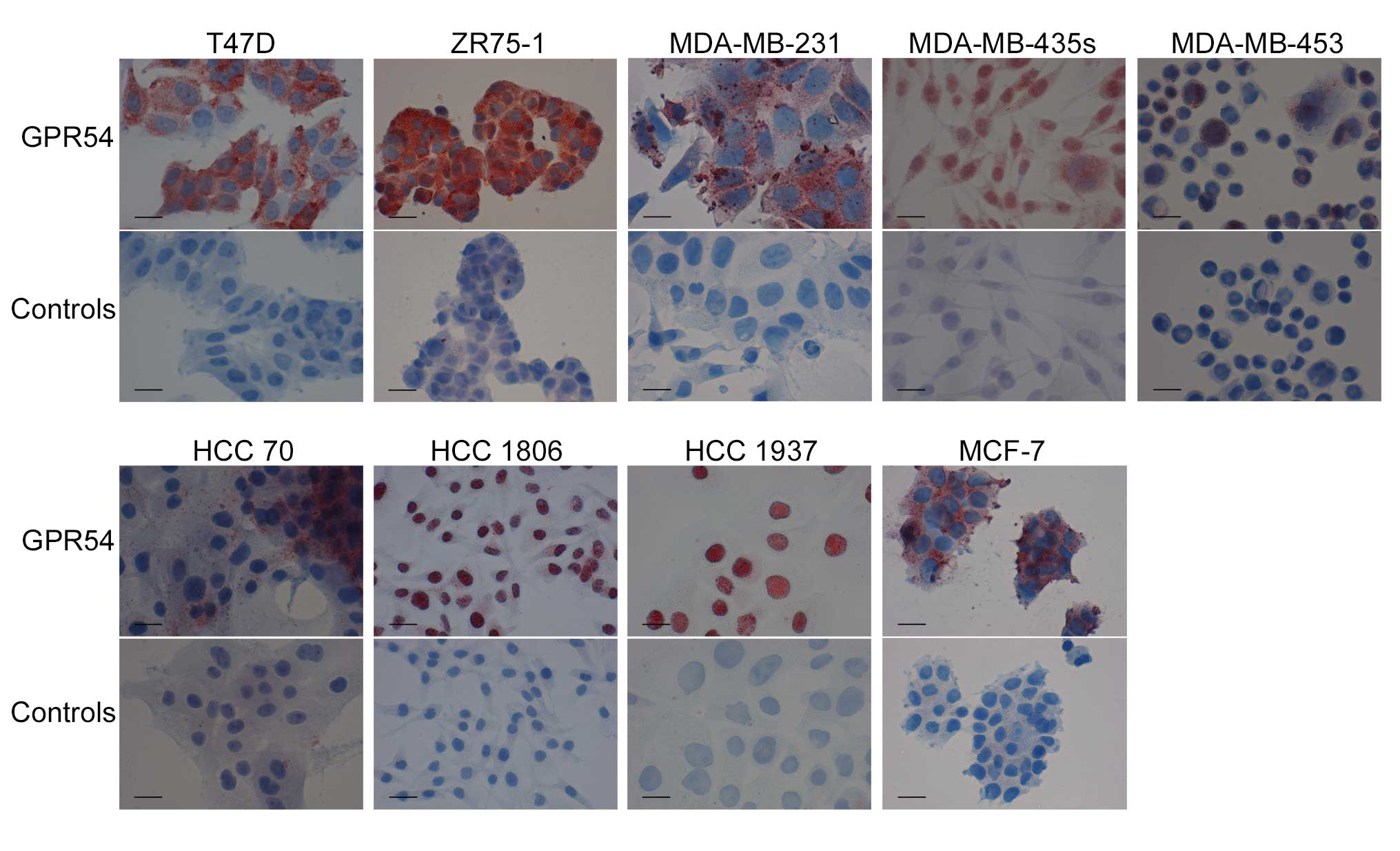
GPR54 expression was analyzed by immune cytochemical
staining in breast cancer cell lines T47D, ZR75-1, MDA-MB-231,
MDA-MB-435s, MDA-MB-453, HCC 70, HCC 1806, HCC 1937 and MCF-7
(Fig. 1). All cell lines expressed
GPR54, visualized by red staining with GPR54 antibody. Cell lines
were further investigated on mRNA and protein levels. mRNA analysis
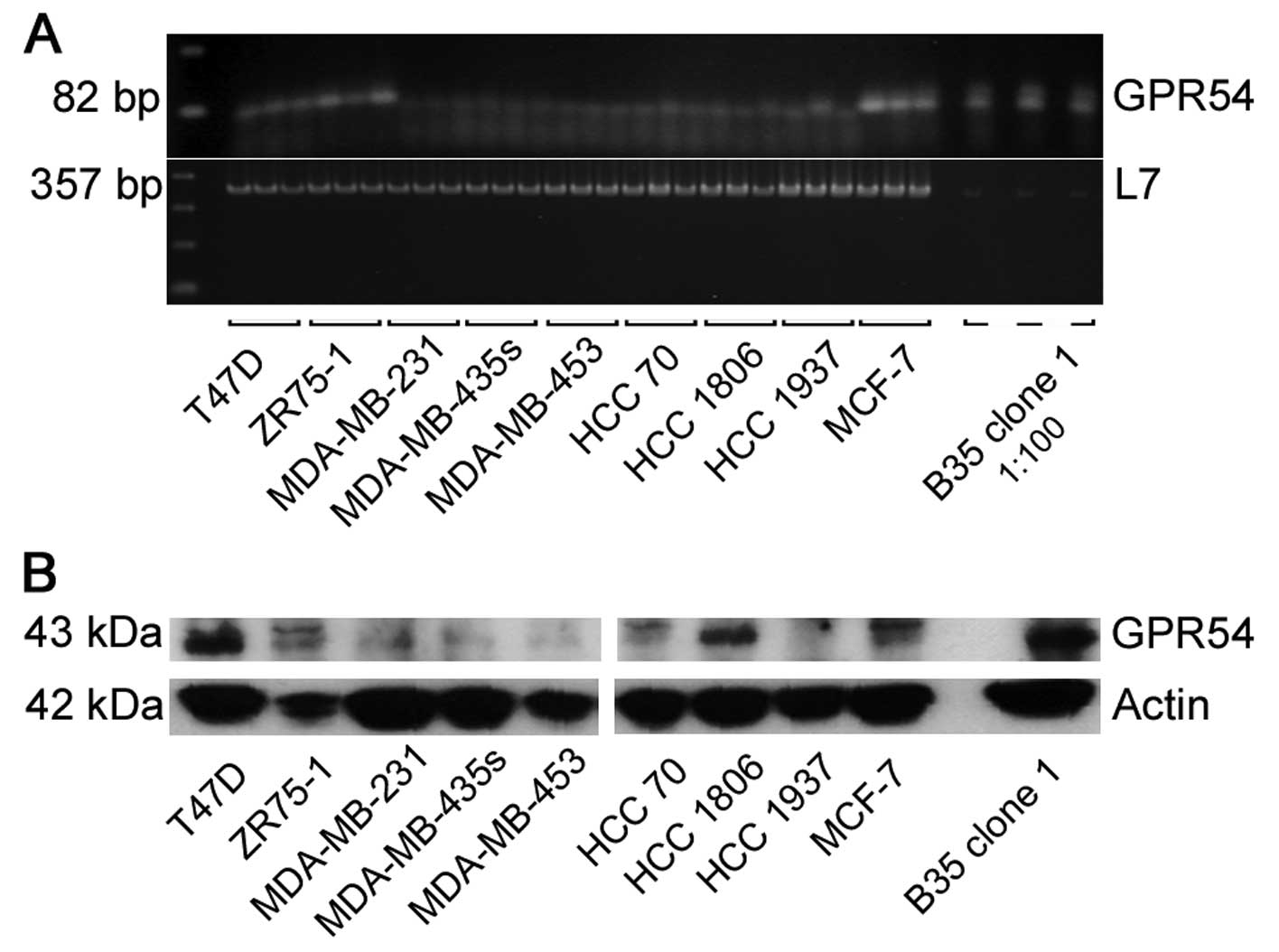
was done by RT-PCR for GPR54 (Fig.
2A). T47D, ZR75-1 and MCF-7 showed receptor mRNA expression. In
MDA-MB-231, MDA-MB-435s, MDA-MB-453, HCC 70, HCC 1806 and HCC 1937
no GPR54 mRNA was found. GPR54 protein levels were detected in
every breast cancer cell line with different quantities by western
blot analysis (Fig. 2B).
GPR54 expression in cells overexpressing
the receptor
As an artificial cell model overexpressing GPR54,
B35 neuronal rat cells stable transfected with murine GPR54 were
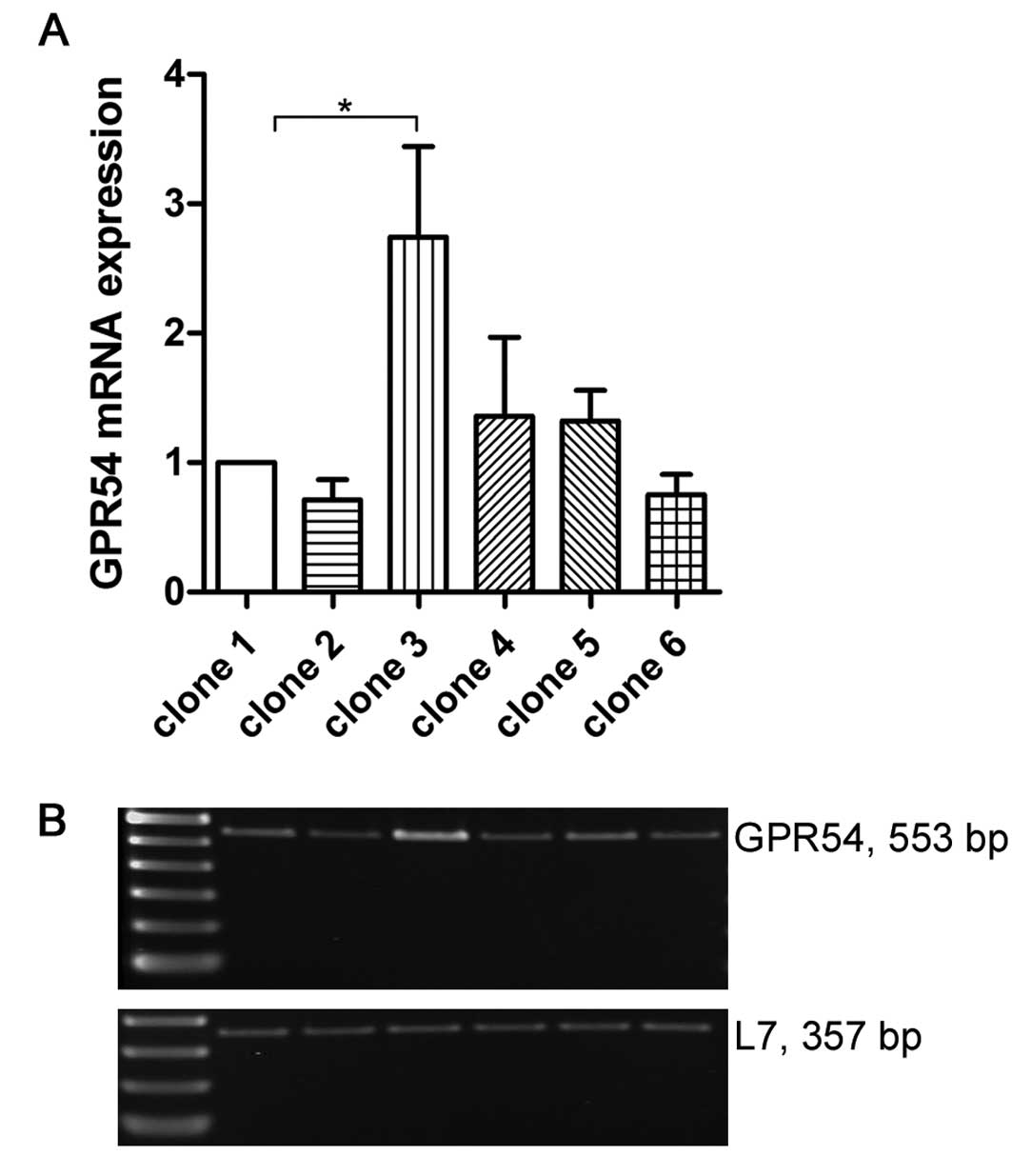
chosen. Different clones of transfected B35 cells were tested for
their GPR54 expression levels. mRNA of six clones was analyzed by
RT-PCR. Results are shown in relation to B35 clone 1 (Fig. 3). Clone 2, 4, 5 and 6 expressed
GPR54 in a similar quantity compared to clone 1. GPR54 expression
level in clone 3 was 2.5-fold higher than in clone 1 (p<0.01).
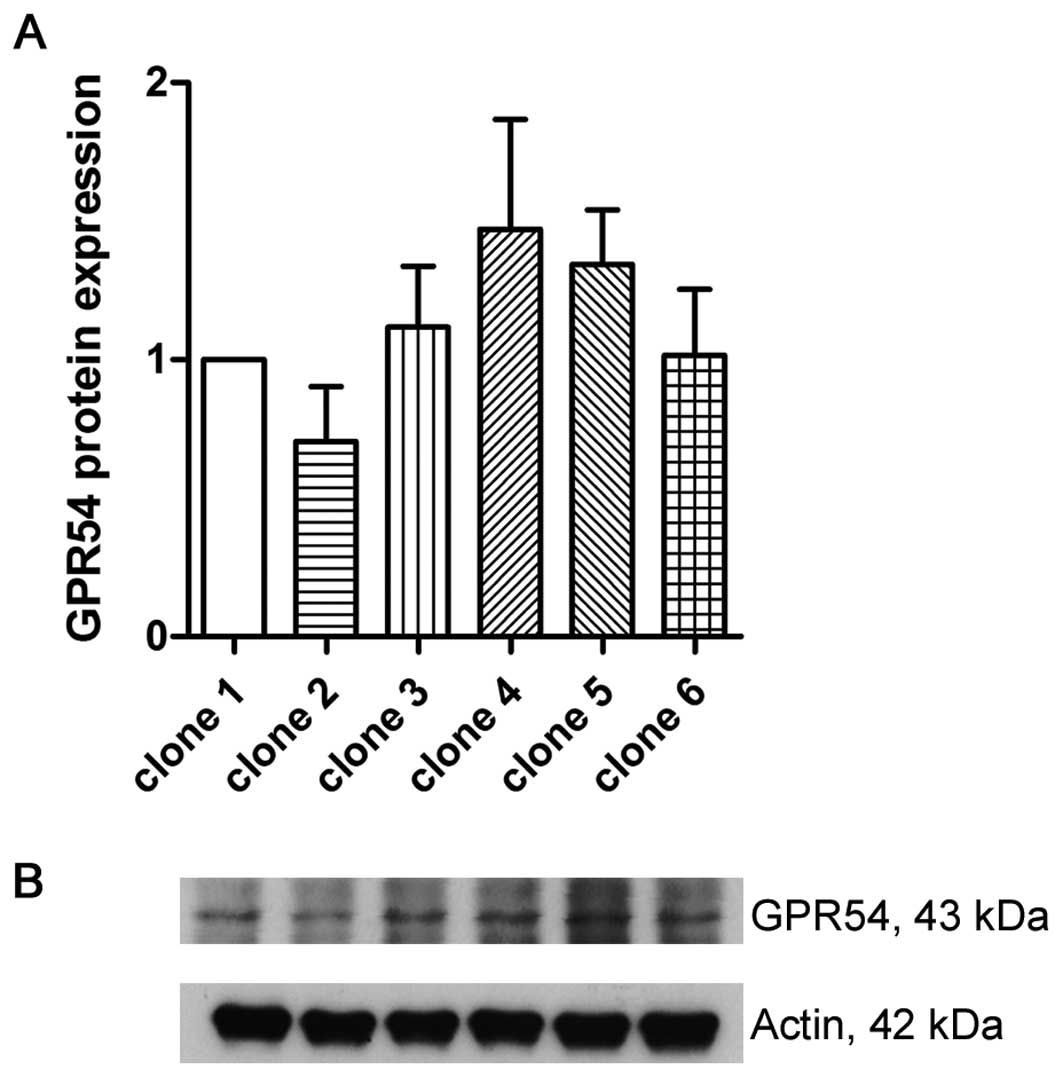
On protein levels, all of the clones showed no significant
difference of GPR54 expression (Fig.
4).
Endogenous and artificial GPR54
expression
mRNA analysis on GPR54 in B35 clone 1 and breast
cancer cell lines showed a highly different extent of expression
(Fig. 2A). In transfectants, GPR54
levels were extremely increased compared to the breast cancer cells
with regard to the used initial cDNA concentration (1:100 for B35
clone 1). This effect was not observed on protein levels for the
same amount of protein of each sample (Fig. 2B).
Kisspeptin-10 has no effect on
proliferation in breast cancer cells
For proliferation studies, four breast cancer cell
lines were chosen. MDA-MB-231, MDA-MB-435s, HCC 1806 and MCF-7
showed different GPR54 expression levels according to the results
on mRNA and protein analysis (Fig.
2). Proliferation was measured after treatment with KP-10 in
different concentrations. KP-10 was added once daily (Fig. 5A) or twice daily (data not shown) to
account for its rapid degradation (27–29).
Under both treatments, no effect on proliferation was detected in
all of the breast cancer cell lines.
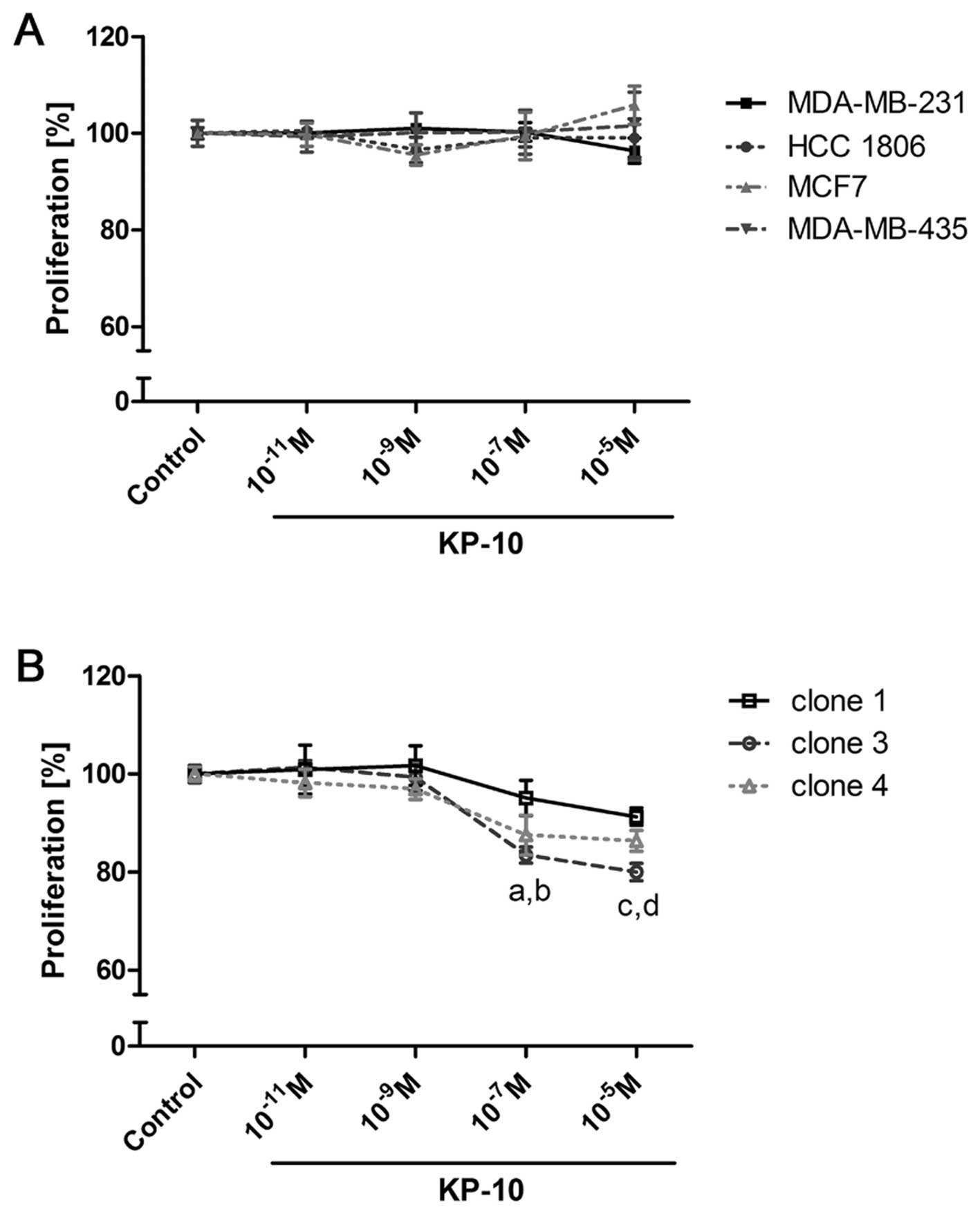 | Figure 5Proliferation of breast cancer cell
lines and B35 mGPR54 clones after KP-10 treatment. Proliferation
was measured after 72 h of daily KP-10 treatment with increasing
concentrations in MDA-MB-231, MDA-MB-435s, HCC 1806, MCF-7 (A) and
in B35 clone 1, 3 and 4 (B). Results in (A and B) are
representative for at least three experiments with different
passages of each cell line [n=6 respectively n=8 (mean ± SEM); a,
p<0.001, c3 vs. control; b, p<0.01, c4 vs. control; c,
p<0.001, c3 vs. control; d, p<0.001, c4 vs. control]. |
Kisspeptin-10 inhibits proliferation in
cells overexpressing GPR54
The effect of KP-10 on proliferation was studied in
cells stably transfected with GPR54. B35 cells (rat) overexpressing
murine GPR54 were used. Proliferation was analyzed in three B35
clones treated with KP-10 once daily (Fig. 5B) respectively, twice daily (data
not shown) in different concentrations. The two treatments showed
comparable results. Transfection was stable as controlled by RT-PCR
samples of cells growing in experimental or transfection media
(data not shown). KP-10 showed an antiproliferative effect in
transfected B35 cells. Proliferation of clone 1 was inhibited in
concentrations of 10−5 M KP-10 vs. control (91.3%; not
significant). The proliferation of clone 3 and clone 4 was
significantly inhibited at concentrations of 10−7 M vs.
control (83.5%; p<0.001, respectively 87.5%; p<0.01) and
10−5 M KP-10 vs. control (80.0% respectively 86.4%;
p<0.001).
Discussion
The antimetastatic effect of kisspeptin was
investigated and validated in a large number of studies showing
reduced migration and invasion, modified adhesion processes,
changes in cytoskeleton and chemotactic behavior (7,8,14,17–24).
However, the results on the influence of kisspeptin on cell
proliferation differed. In cell lines transfected with GPR54, an
antiproliferative effect of kisspeptin was shown, e.g. in Chinese
hamster ovary CHO cells (8,18), murine fibroblast NIH3T3 cells
(19) and human breast cancer
MDA-MB-435s cells (25). HUVECs
with endogenous receptor expression also showed reduced
proliferation by kisspeptin treatment (20). In contrast, another study in HUVECs
did not detect an influence of kisspeptin on proliferation
(26). No changes in proliferation
were shown in pancreatic cancer cell lines AsPC-1 and PANC-1
(14), in renal cell carcinoma
Caki-1 and ACHN cells (17) and
trophoblasts (21). These cell
lines showed low endogenous GPR54 expression. Similar results were
detected in MDA-MB-231 and MCF-7 breast cancer cells, but
information was not given on GPR54 receptor levels (22). No effect on proliferation was shown
in MDA-MB-231 breast cancer cells (11), SKOV3 ovarian cancer cells (23) and C8161 melanoma cells (2) transfected with the KISS1 gene. In
summary, no antiproliferative effects of kisspeptin were shown in
cancer cells expressing GPR54 endogenously. In contrast, cells
artificially overexpressing the receptor were reduced in their
proliferation by kisspeptin. The present study offers evidence for
a connection between the antiproliferative effect of kisspeptin and
the nature of GPR54 expression.
Breast cancer cell lines were used as experimental
model. Diverging results have been published using these cells.
Reduced proliferation of MDA-MB-435s breast cancer cells treated
with kisspeptin was shown (25),
while no changes in proliferation of MDA-MB-231 and MCF-7 breast
cancer cells were found (11,22).
GPR54 expression levels were assessed by immunocytochemistry, on
mRNA and protein levels because of controversial findings in
literature. T47D and ZR75-1 were described as GPR54-positive
(12). GPR54 was found in
MDA-MB-231 (11,30), but there are also studies showing no
receptor expression in this cell line (12). In MDA-MB-435s no GPR54 was detected
(12,25,31).
MCF-7 cells showed receptor expression (12). In the present study, all of the
tested breast cancer cell lines were assessed as GPR54-positive
based on the immune cytochemical and western blot results. Compared
to the findings by western blot analysis, mRNA of GPR54 was only
detected in cells with higher endogenous GPR54 protein levels.
Proliferation studies in breast cancer cell lines
with natural GPR54 expression showed no effect of kisspeptin.
However, in B35 neuronal rat cells transfected with murine GPR54,
inhibition of proliferation was recorded. Murine and human GPR54
proteins are homologous up to 82% within their amino acid structure
(10). Gene products of human KISS1
and murine KISS1 share related parts and are much conserved within
their active short forms (19).
Both, receptors and ligands, were used interchangeable with similar
effects (8,32). Regarding to this, experiments
carried out in breast cancer cell lines were compared to
experiments with transfected B35 cells. The breast cancer cell
lines represented cells with endogenous GPR54 expression and the
B35 clones were used as an artificial cell model for GPR54
transfected cells. Differences in the amount of cellular GPR54 were
observed by mRNA analysis. On protein levels, this trend could not
be confirmed. This may be due to differences in the antigen
structure and no commercially available antibody for parallel
detection of human and murine GPR54. The results showed an
antiproliferative effect of kisspeptin only in cells overexpressing
GPR54 artificially. This effect did not occur in cells with
spontaneous GPR54 expression. These findings are in agreement with
another study showing an antiproliferative effect in MDA-MB-435s
cells transfected with GPR54 (25).
As shown in the present study, no changes in proliferation were
detectable in these cells without transfection.
In studies with HUVECs endogenously expressing
GPR54, and transfected CHO cells, a dose-dependent receptor
activation was measured showing a ten times more sensitive reaction
in the overexpressing cells (26).
Thus, there is evidence for GPR54 mediated cellular mechanisms
involved in proliferation, that are only detectable in cells with
up regulated receptor expression. The results of the present study
showed a connection between the antiproliferative effect of
kisspeptin and the nature of GPR54 expression. All tested cell
lines with endogenous GPR54 expression showed no changes in
proliferation by kisspeptin. The effect was only detectable in
cells with artificial receptor expression. Based on this, the
antiproliferative action of kisspeptin seems to be not relevant in
the pathophysiological context.
Acknowledgements
This study was supported by a grant of the Deutsche
Krebshilfe, Dr Mildred Scheel Stiftung. We thank Robert P. Millar
and Kevin Morgan (Edinburgh, UK) for the gift of the transfected
cell lines. We thank Sonja Blume, Renate Dietrich and Matthias
Läsche for technical assistance.
References
|
1
|
Welch DR, Chen P, Miele ME, McGary CT,
Bower JM, Stanbridge EJ, et al: Microcell-mediated transfer of
chromosome 6 into metastatic human C8161 melanoma cells suppresses
metastasis but does not inhibit tumorigenicity. Oncogene.
9:255–262. 1994.
|
|
2
|
Lee JH, Miele ME, Hicks DJ, Phillips KK,
Trent JM, Weissman BE, et al: KiSS-1, a novel human malignant
melanoma metastasis-suppressor gene. J Natl Cancer Inst.
88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH and Welch DR: Identification of
highly expressed genes in metastasis-suppressed chromosome 6/human
malignant melanoma hybrid cells using subtractive hybridization and
differential display. Int J Cancer. 71:1035–1044. 1997. View Article : Google Scholar
|
|
4
|
Lee JH and Welch DR: Suppression of
metastasis in human breast carcinoma MDA-MB-435 cells after
transfection with the metastasis suppressor gene, KiSS-1. Cancer
Res. 57:2384–2387. 1997.PubMed/NCBI
|
|
5
|
Miele ME, Robertson G, Lee JH, Coleman A,
McGary CT, Fisher PB, et al: Metastasis suppressed, but
tumorigenicity and local invasiveness unaffected, in the human
melanoma cell line MelJuSo after introduction of human chromosomes
1 or 6. Mol Carcinog. 15:284–299. 1996. View Article : Google Scholar
|
|
6
|
Miele ME, Jewett MD, Goldberg SF, Hyatt
DL, Morelli C, Gualandi F, et al: A human melanoma
metastasis-suppressor locus maps to 6q16.3-q23. Int J Cancer.
86:524–528. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohtaki T, Shintani Y, Honda S, Matsumoto
H, Hori A, Kanehashi K, et al: Metastasis suppressor gene KiSS-1
encodes peptide ligand of a G-protein-coupled receptor. Nature.
411:613–617. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotani M, Detheux M, Vandenbogaerde A,
Communi D, Vanderwinden JM, Le Poul E, et al: The metastasis
suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of
the orphan G protein-coupled receptor GPR54. J Biol Chem.
276:34631–34636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muir AI, Chamberlain L, Elshourbagy NA,
Michalovich D, Moore DJ, Calamari A, et al: AXOR12, a novel human G
protein-coupled receptor, activated by the peptide KiSS-1. J Biol
Chem. 276:28969–28975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kirby HR, Maguire JJ, Colledge WH and
Davenport AP: International Union of Basic and Clinical
Pharmacology. LXXVII Kisspeptin receptor nomenclature,
distribution, and function. Pharmacol Rev. 62:565–578. 2010.
View Article : Google Scholar
|
|
11
|
Martin TA, Watkins G and Jiang WG: KiSS-1
expression in human breast cancer. Clin Exp Metastasis. 22:503–511.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marot D, Bieche I, Aumas C, Esselin S,
Bouquet C, Vacher S, et al: High tumoral levels of Kiss1 and
G-protein-coupled receptor 54 expression are correlated with poor
prognosis of estrogen receptor-positive breast tumors. Endocr Relat
Cancer. 14:691–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prentice LM, Klausen C, Kalloger S, Köbel
M, McKinney S, Santos JL, et al: Kisspeptin and GPR54
immunoreactivity in a cohort of 518 patients defines favourable
prognosis and clear cell subtype in ovarian carcinoma. BMC Med.
5:332007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masui T, Doi R, Mori T, Toyoda E, Koizumi
M, Kami K, et al: Metastin and its variant forms suppress migration
of pancreatic cancer cells. Biochem Biophys Res Commun. 315:85–92.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagai K, Doi R, Katagiri F, Ito T, Kida A,
Koizumi M, et al: Prognostic value of metastin expression in human
pancreatic cancer. J Exp Clin Cancer Res. 28:92009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikeguchi M, Hirooka Y and Kaibara N:
Quantitative reverse transcriptase polymerase chain reaction
analysis for KiSS-1 and orphan G-protein-coupled receptor
(hOT7T175) gene expression in hepatocellular carcinoma. J Cancer
Res Clin Oncol. 129:531–535. 2003. View Article : Google Scholar
|
|
17
|
Shoji S, Tang XY, Umemura S, Itoh J,
Takekoshi S, Shima M, et al: Metastin inhibits migration and
invasion of renal cell carcinoma with overexpression of metastin
receptor. Eur Urol. 55:441–9. 2009. View Article : Google Scholar
|
|
18
|
Hori A, Honda S, Asada M, Ohtaki T, Oda K,
Watanabe T, et al: Metastin suppresses the motility and growth of
CHO cells transfected with its receptor. Biochem Biophys Res
Commun. 286:958–963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stafford LJ, Xia C, Ma W, Cai Y and Liu M:
Identification and characterization of mouse metastasis-suppressor
KiSS1 and its G-protein-coupled receptor. Cancer Res. 62:5399–5404.
2002.PubMed/NCBI
|
|
20
|
Ramaesh T, Logie JJ, Roseweir AK, Millar
RP, Walker BR, Hadoke PWF, et al: Kisspeptin-10 inhibits
angiogenesis in human placental vessels ex vivo and endothelial
cells in vitro. Endocrinology. 151:5927–5934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bilban M, Ghaffari-Tabrizi N, Hintermann
E, Bauer S, Molzer S, Zoratti C, et al: Kisspeptin-10, a
KiSS-1/metastin-derived decapeptide, is a physiological invasion
inhibitor of primary human trophoblasts. J Cell Sci. 117:1319–1328.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho SG, Li D, Stafford LJ, Luo J,
Rodriguez-Villanueva M, Wang Y, et al: KiSS1 suppresses
TNFalpha-induced breast cancer cell invasion via an inhibition of
RhoA-Mediated NF-kappaB activation. J Cell Biochem. 107:1139–1149.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang Y, Berk M, Singh LS, Tan H, Yin L,
Powell CT, et al: KiSS1 suppresses metastasis in human ovarian
cancer via inhibition of protein kinase C alpha. Clin Exp
Metastasis. 22:369–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olbrich T, Ziegler E, Türk G, Schubert A,
Emons G and Gründker C: Kisspeptin-10 inhibits bone-directed
migration of GPR54-positive breast cancer cells: Evidence for a
dose-window effect. Gynecol Oncol. 119:571–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker JA, Mirjolet JF, Bernard J, Burgeon
E, Simons MJ, Vassart G, et al: Activation of GPR54 promotes cell
cycle arrest and apoptosis of human tumor cells through a specific
transcriptional program not shared by other Gq-coupled receptors.
Biochem Biophys Res Commun. 326:677–686. 2005. View Article : Google Scholar
|
|
26
|
Cho SG, Yi Z, Pang X, Yi T, Wang Y, Luo J,
et al: Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor
angiogenesis by suppressing Sp1-mediated VEGF expression and
FAK/Rho GTPase activation. Cancer Res. 69:7062–7070. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takino T, Koshikawa N, Miyamori H, Tanaka
M, Sasaki T, Okada Y, et al: Cleavage of metastasis suppressor gene
product KiSS-1 protein/metastin by matrix metalloproteinases.
Oncogene. 22:4617–4626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harms JF, Welch DR and Miele ME: KISS1
metastasis suppression and emergent pathways. Clin Exp Metastasis.
20:11–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomita K, Oishi S, Ohno H, Peiper SC and
Fujii N: Development of novel G-protein-coupled receptor 54
agonists with resistance to degradation by matrix
metalloproteinase. J Med Chem. 51:7645–7649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pampillo M, Camuso N, Taylor JE,
Szereszewski JM, Ahow MR, Zajac M, et al: Regulation of GPR54
signaling by GRK2 and beta-arrestin. Mol Endocrinol. 23:2060–2074.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nash KT, Phadke PA, Navenot JM, Hurst DR,
Accavitti-Loper MA, Sztul E, et al: Requirement of KISS1 secretion
for multiple organ metastasis suppression and maintenance of tumor
dormancy. J Natl Cancer Inst. 99:309–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mikkelsen JD, Bentsen AH, Ansel L,
Simonneaux V and Juul A: Comparison of the effects of peripherally
administered kisspeptins. Regul Pept. 152:95–100. 2009. View Article : Google Scholar : PubMed/NCBI
|