Introduction
Colorectal cancer is the fourth most common cancer
in the world (1,2). In spite of radical surgery and
chemotherapy, the overall 5-year survival rate remains poor
(3). Cancer recurrence and
metastasis after surgery have been recognized as the main causes of
the poor outcome of colorectal cancer patients. It has been
documented that more than 50% of patients with colorectal cancer
will develop liver metastases (2).
Therefore, properly understanding the mechanisms underlying
colorectal cancer metastasis as well as identifying new molecular
targets for therapeutic agents is critical for the prevention of
colorectal cancer metastasis.
Aquaporins (AQPs) are a family of small, integral
membrane proteins involved in the selective transport of water
across cell membranes (4,5). Accumulating evidence suggests that the
expression of AQP3 is tightly correlated with cell proliferation
and migration. Hara-Chikuma and Verkman (6) showed the knockout of AQP3 could affect
the proliferation and migration ability of keratinocyte and slow
the wound healing rate of mouse skin. However, when the expression
of AQP3 was upregulated in human keratinocytes by transfection with
human AQP3 DNA plasmid, the cell proliferation was increased
(7). In human gastric
adenocarcinoma cells, AQP3 knockdown inhibited hEGF-induced AQP3
expression and, thus, cell migration and proliferation (8). Inhibition of AQP3 in human esophageal
and oral squamous cell carcinoma also suppressed cancer cell
proliferation (9). Since the
migration of tumor cells is an important step in tumor invasion and
metastasis (10), the above
findings strongly suggest that inhibition of AQP3 might be useful
in the treatment of cancer.
Some studies have shown that AQP1, AQP3 and AQP8 are
also expressed in the normal colon (11–13);
however, in colorectal carcinoma, the expression of AQP1 and AQP3
is much higher than in normal tissue (11,14),
indicating AQPs may be involved in the development of colorectal
carcinoma. However, there are few studies concerning the role of
AQP3 in colorectal carcinoma thus far. Based on the above findings,
we hypothesized that AQP3 is involved in the migration of
colorectal carcinoma. Therefore, in the present study, we
investigated the relationship between AQP3 and the migration of
colorectal carcinoma cells in vitro and analyzed the
expression of AQP3 and the differentiation, lymph node and distant
metastasis of human colorectal carcinoma.
Materials and methods
Cell culture
The human colorectal carcinoma cell line HCT116 was
purchased from the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences, and was cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), 100 U/ml of
penicillin and 100 U/ml of streptomycin, at 37°C, 5% CO2
and 95% humidity.
Wound healing assay
Six-well plates were coated with polylysine (PL).
HCT116 cells were seeded in the coated plates at a density of
5×105/well and cultured to ~80% confluence. Wound
healing assay was performed as previously described (15). Briefly, a scratch wound was
generated by scratching with a 200 μl pipette tip across the center
of the well. After scratching, the well was gently washed twice
with medium to remove the detached cells and fresh medium was added
into the wells. Cells were cultured at 37°C for indicated times and
images were captured at different time points.
Western blot analysis
Total protein was extracted as previously described
(16). Briefly, the cells were
lysed with RIPA lysis buffer (Beyotime, Haimen, Jiangsu, China) and
then centrifuged at 12,000 × g for 15 min at 4°C. The supernatants
were collected for the western blot analysis. Protein concentration
was determined with the BCA method. Proteins were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to the polyvinylidene fluoride (PVDF) membrane
(Millipore, Billerica, MA, USA). The blot was then probed with
primary antibody followed by reaction with horseradish
peroxidase-conjugated secondary antibody. The signal was detected
using enhanced chemiluminescence and recorded on X-ray film. The
relative density of the target bands was quantified using Gel-Pro
analyzer version 3.0 (Media Cybernetics, Bethesda, MD, USA).
Patients
Between January 2006 and December 2007, 163 patients
(94 men and 69 women; range, 37–80 years, average 66.1±11.3 years)
with colorectal cancer were enrolled from Xuanwu Hospital of
Capital Medical University. Colorectal cancer was diagnosed by
colonoscopy and confirmed as adenocarcinoma by pathological
examination. There were 4 cases of grade I, 79 cases of grade II,
59 cases of grade III and 21 cases of grade IV according to the
American Joint Committee on Cancer (AJCC) staging guidelines (7th
edition). All patients received colorectal cancer surgery according
to schedule and were followed up for 3 years by telephone or as
outpatients. The study was approved by the ethics committee of the
Xuanwu Hospital of Capital Medical University.
Immunohistochemistry
The expression of AQP3 was examined by
immunohistochemical (IHC) staining. IHC was carried out using a
specific mouse polyclonal anti-AQP3 antibody (Bioworld Technology,
Minneapolis, MN, USA). The results were scored using the Fromowitz
method (17) by two independent
pathologists. Briefly, the images were first scored according to
the percentage of AQP3 positive cells in the total tumor cells:
≤5%, score 0; 6–25%, score 1; 26–50%, score 2; 51–75%, score 3;
>75%, score 4. The images were then scored according to the
staining depth: negative staining, score 0; faint yellow, score 1;
brown madder, score 2; dark brown, score 3. The scores of the same
slide were summed to produce a final score: 0–1 was considered
negative (−); 2–3, weakly positive (+); 4–5, moderately positive
(++); 6–7, strong positive (+++). Negative and weakly positive were
considered as low expression. Moderately positive and strong
positive were considered as high expression.
Statistical analysis
Data are expressed as the means ± SD. Statistical
significance was determined using PASW 18.0 for Windows. Student’s
t-test was used to compare means for two groups and one-way ANOVA
was performed for multiple comparisons followed by Newman-Keuls
test for multiple comparisons. Strength of IHC was analyzed by
Pearson’s Chi-squared test. P<0.05 indicated statistically
significant differences.
Results
hEGF upregulates AQP3 expression in
HCT116 cells
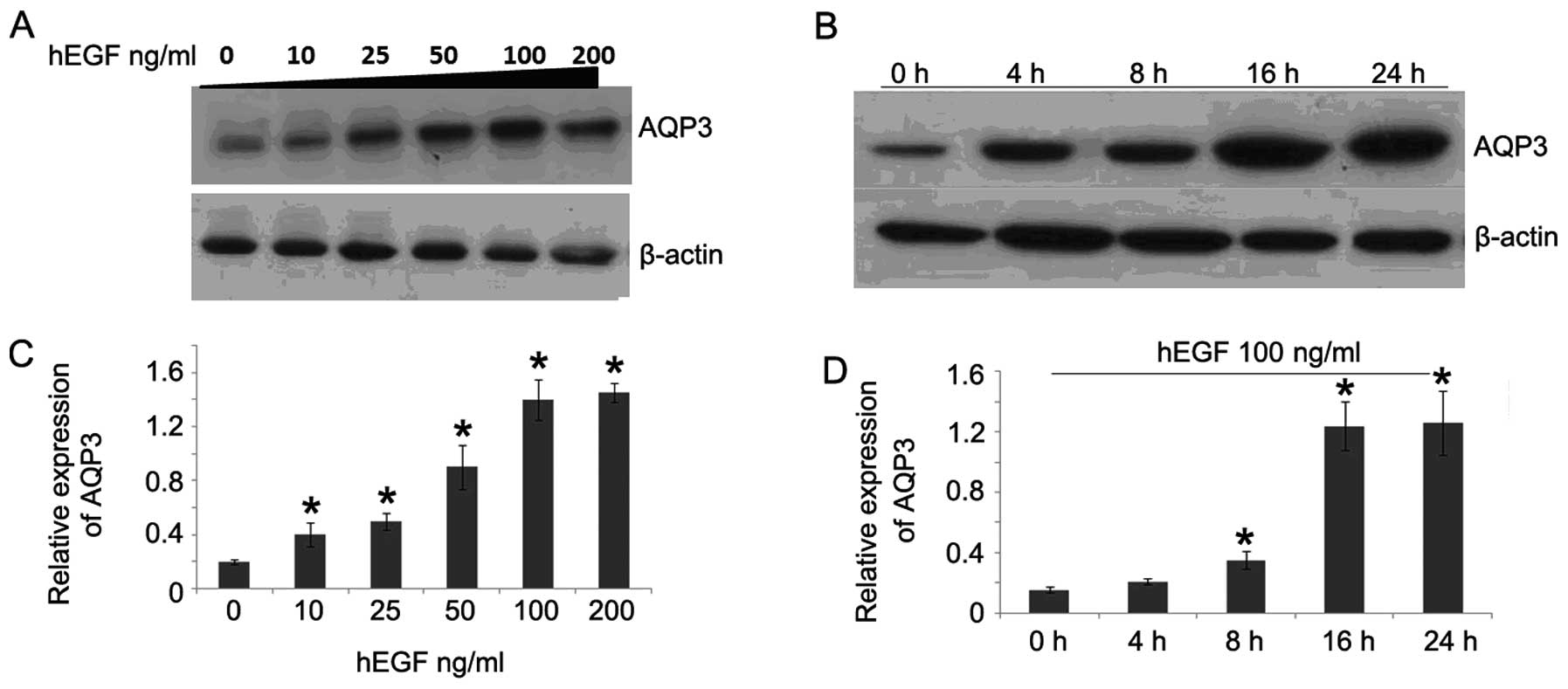
To study whether hEGF could stimulate AQP3
expression in human colorectal cancer cells, western blot analysis
was performed. As shown in Fig. 1A,
after hEGF treatment for 24 h, the expression of AQP3 was
significantly increased. The AQP3 expression was increased along
with the dose of hEGF, indicating hEGF may upregulate AQP3
expression in a dose-dependent manner. Then, we further
investigated the AQP3 expression in HCT116 cells after hEGF
treatment for different times. The results showed that AQP3
expression was also elevated along with the extension of
stimulating time (Fig. 1B and D).
These results suggest hEGF was able to upregulate AQP3 expression
in a dose- and time-dependent manner.
Upregulation of AQP3 enhances the
migration ability of HCT116 cells
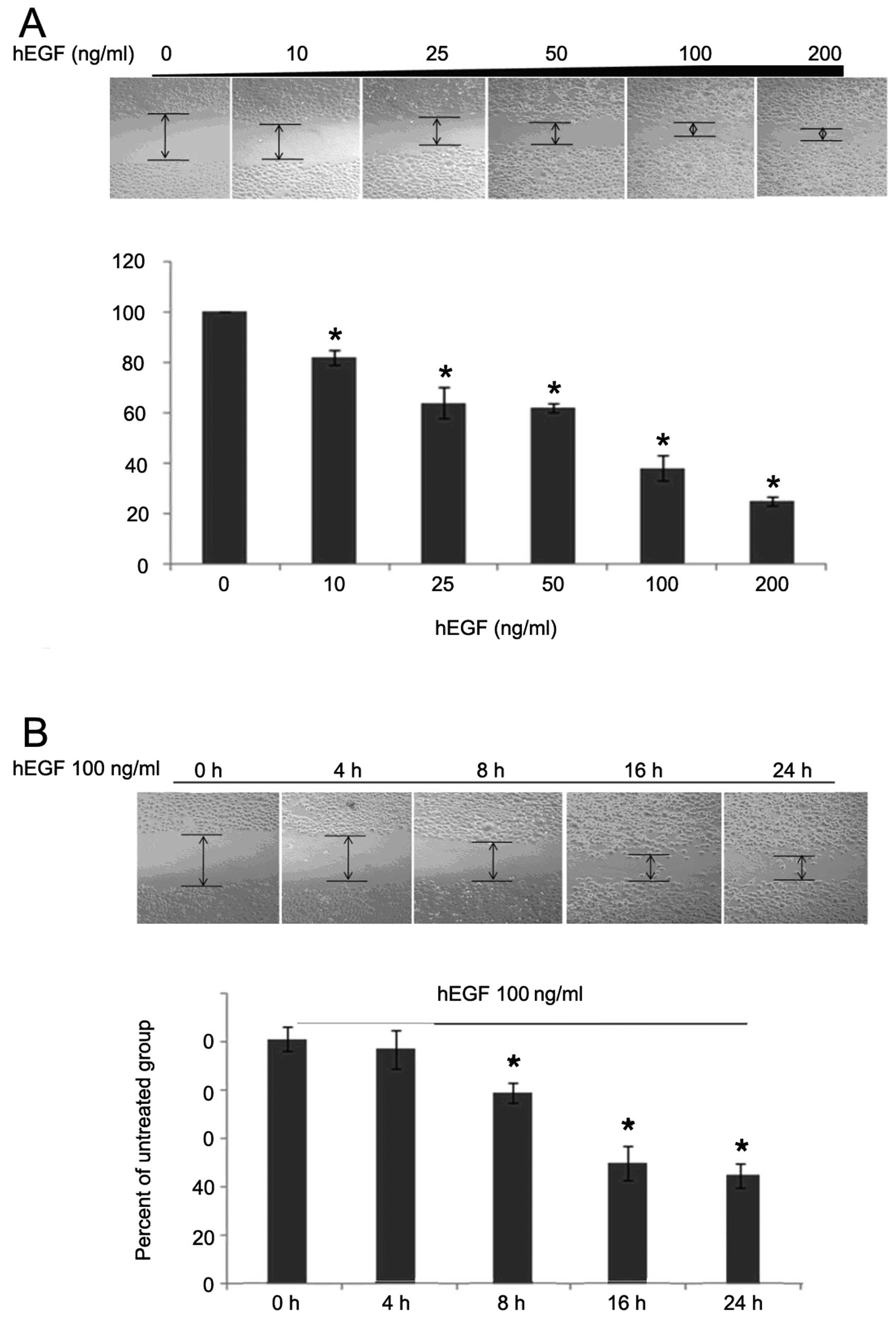
Since many studies have shown that overexpression of
AQP3 is able to promote cell migration, we considered whether AQP3
could also facilitate the migration of colorectal cancer cells.
Following treatment with hEGF, the wound gaps decreased
significantly in a time- and dose-dependent manner (Fig. 2). To further elucidate whether the
enhanced migration ability of HCT116 cells was due to the
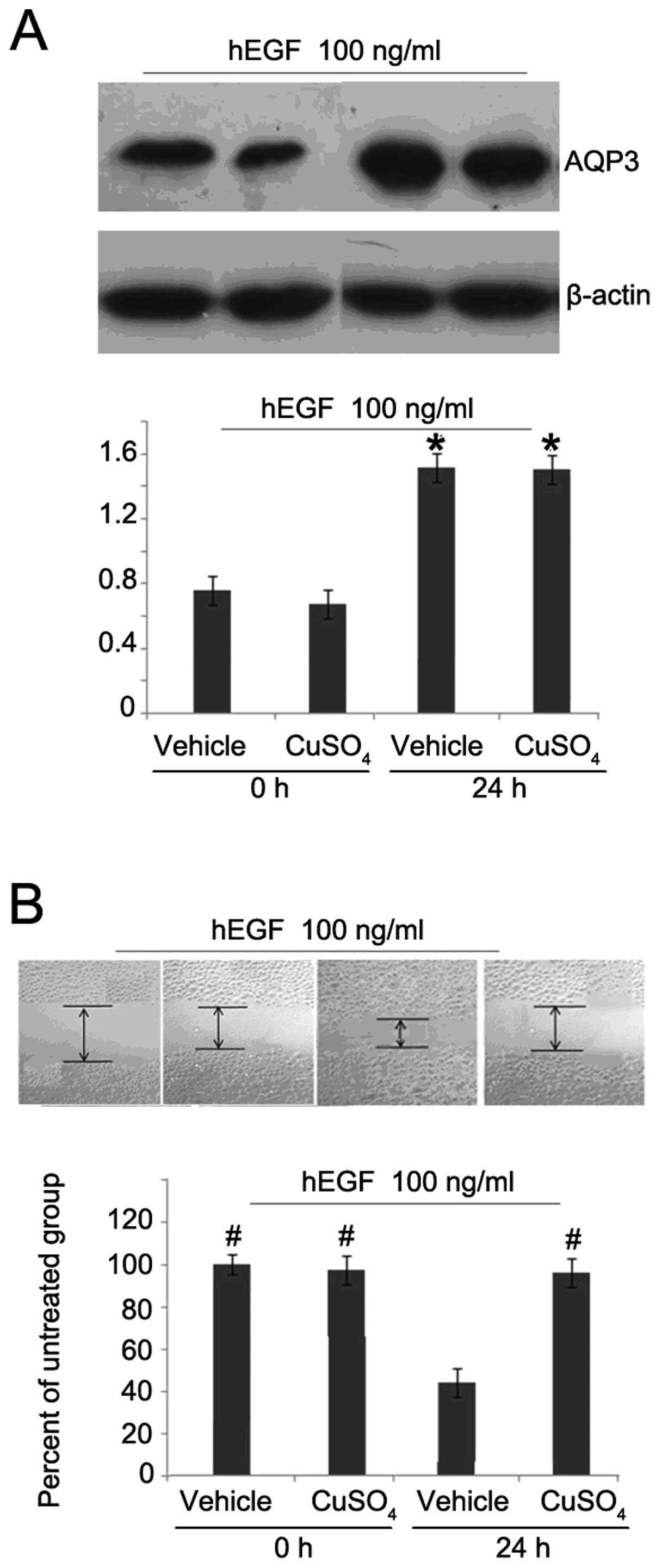
upregulation of AQP3 levels, we used a specific inhibitor of AQP3,
CuSO4, in the following experiment. After hEGF treatment
for 24 h, the expression of AQP3 was not altered by
CuSO4 (Fig. 3A).
However, the migration ability of HCT116 cells was obviously
decreased (Fig. 3B), suggesting
that AQP3 plays an important role in the migration of human
colorectal cancer cells.
hEGF upregulates AQP3 expression through
the PI3K/AKT pathway
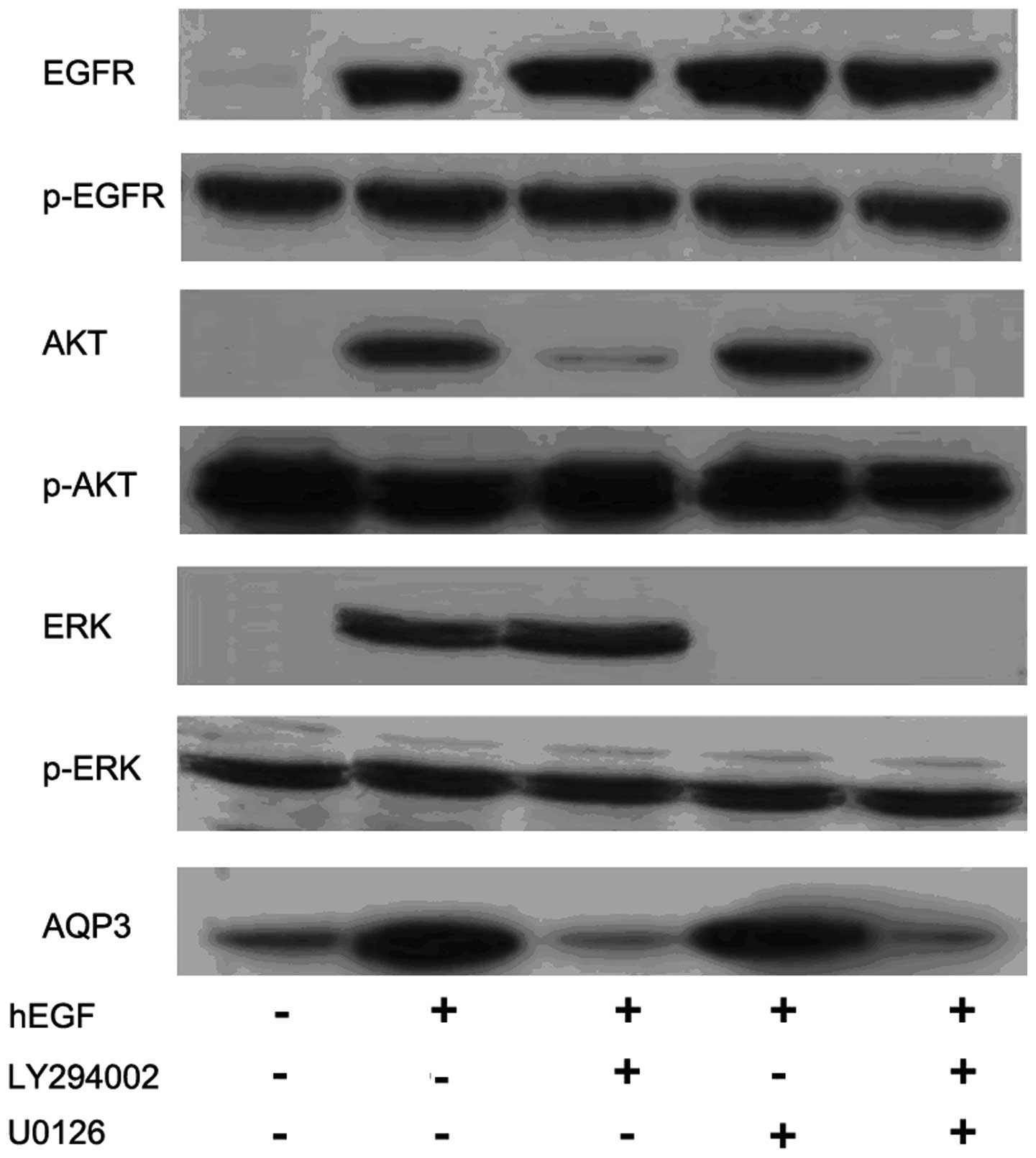
Additional experiments were conducted to further
investigate the cell signaling pathways involved in hEGF-induced
AQP3 expression in human colorectal cancer cells (Fig. 4). Western blot analysis showed that
pretreatment of the PI3K/AKT inhibitor LY294002 (20 μM) for 1.5 h
almost completely abolished hEGF (100 ng/ml)-induced AQP3
expression. However, pretreatment with the ERK inhibitor, U0126,
showed only a slight effect on hEGF-induced AQP3 expression.
LY294002 and U0126 did not affect the EGFR expression and
phosphorylation.
Expression of AQP3 in colorectal
carcinoma tissues and corresponding normal tissues
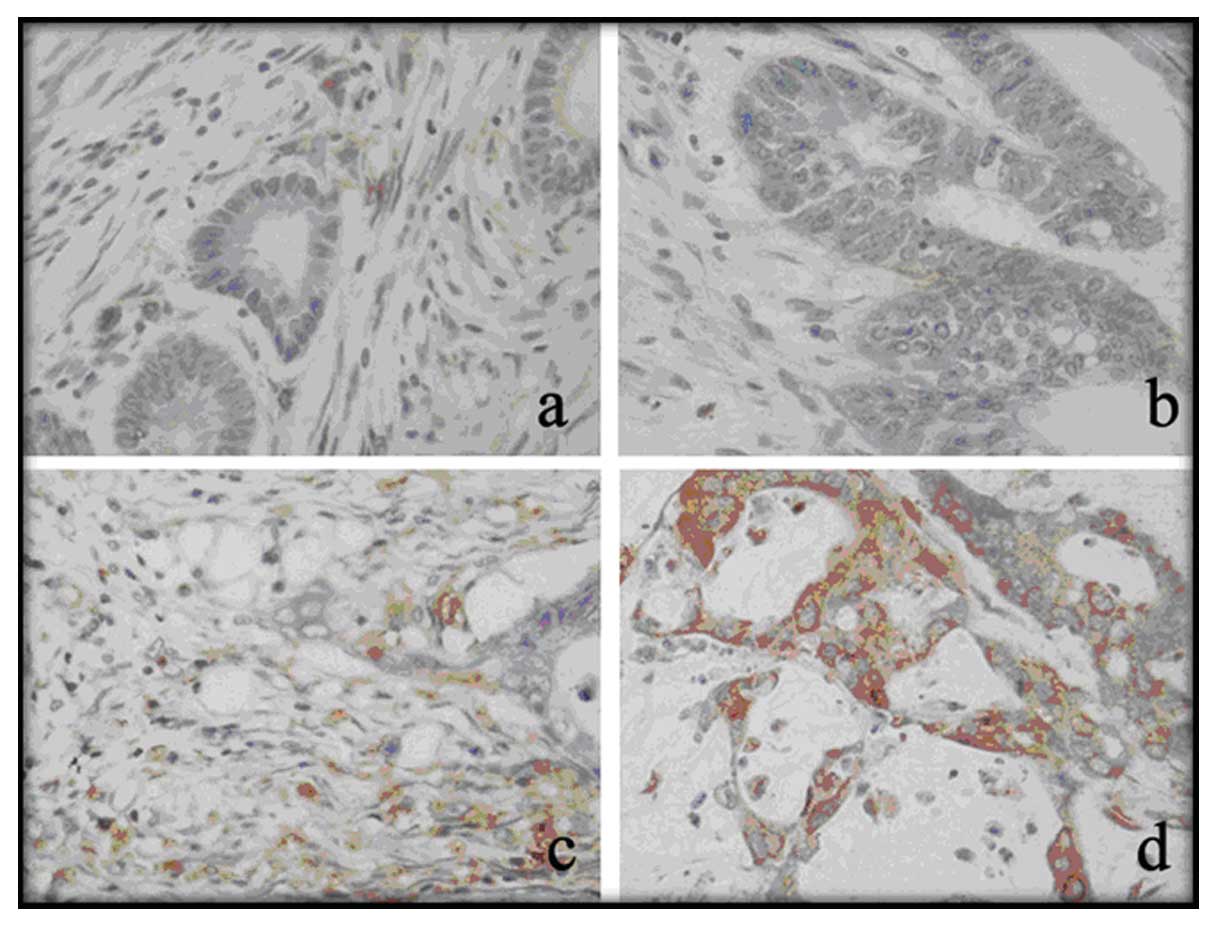
AQP3 expression was found in both normal colonic
epithelium and colorectal carcinoma tissue as shown by IHC staining
(Fig. 5). AQP3 protein was found in
the membranes and cytoplasm of colonic epithelial cells. In the
normal colonic tissues, 119 cases (73.0%) showed low expression and
44 cases (27.0%) showed high expression (Table I); whereas in colorectal carcinoma
tissues, 70 cases (42.9%) showed low expression and 93 cases
(57.1%) showed high expression. The difference was significant
between the carcinoma tissues and normal tissues.
 | Table IComparison of AQP3 in colorectal
carcinoma tissues and corresponding normal tissues. |
Table I
Comparison of AQP3 in colorectal
carcinoma tissues and corresponding normal tissues.
| AQP3 | |
|---|
|
| |
|---|
| Low expression
(%) | High expression
(%) | P-value |
|---|
| Normal tissues | 119 (73.0) | 44 (27.0) | 0.000 |
| Tumor tissues | 73 (44.8) | 90 (55.2) | |
AQP3 expression is associated with
differentiation, lymph node and distant metastasis in colorectal
carcinoma
Since we found the AQP3 was highly expressed in
colorectal carcinoma tissues, we sought to investigate whether
there was any clinical value in the AQP3 expression in colorectal
carcinoma. Patients were grouped according to the clinical
parameters, as shown in Table II.
We found that in the high/medium differentiation group, 50.7%
(69/136) of patients showed high expression of AQP3, whereas 77.8%
(21/27) of patients in the low differentiation group showed high
expression of AQP3. A significant difference was found between the
two groups. Patients with lymph node metastasis showed a higher
rate of AQP3 overexpression (52/80, 65.0%) than those without node
metastasis (38/83, 45.8%). Similarly, patients with distant
metastasis at initial diagnosis (20/21, 95.2%) also exhibited a
higher rate of AQP3 overexpression than those without (70/142,
49.3%). However, when the patients were grouped by gender, age and
adjacent organ invasion, no significant differences were
detected.
 | Table IICorrelation between AQP3 expression
and multiple clinical parameters. |
Table II
Correlation between AQP3 expression
and multiple clinical parameters.
| AQP3 | |
|---|
|
| |
|---|
| Low expression
(%) | High expression
(%) | P-value |
|---|
| Gender | | | |
| Male | 45 (46.9) | 51 (53.1) | 0.631 |
| Female | 28 (41.8) | 39 (58.2) | |
| Age (years) | | | |
| <60 | 18 (42.9) | 24 (57.1) | 0.366 |
| 60–69 | 26 (53.1) | 23 (46.9) | |
| ≥70 | 29 (40.3) | 43 (59.7) | |
| Differentiation | | | |
| Medium/high | 67 (49.3) | 69 (50.7) | 0.011 |
| Low | 6 (22.2) | 21 (77.8) | |
| Adjacent organ | | | |
| No invasion | 68 (45.3) | 82 (54.7) | 0.774 |
| Invasion | 5 (38.5) | 8 (61.5) | |
| Lymph node
metastasis | | | |
| No | 45 (54.2) | 38 (45.8) | 0.018 |
| Yes | 28 (35.0) | 52 (65.0) | |
| Distant metastasis
(Initial diagnosis) | | | |
| No | 72 (50.7) | 70 (49.3) | 0.000 |
| Yes | 1 (4.8) | 20 (95.2) | |
Discussion
In the present study, we first showed that hEGF was
able to upregulate AQP3 expression and subsequent migration ability
through the PI3K/Akt signaling pathway in human colorectal
carcinoma cells. CuSO4, an inhibitor for AQP3 signaling,
blocked hEGF-induced upregulation of colorectal carcinoma cell
migration, but displayed only a minor effect on AQP3 expression and
EGFR expression and activation. We also found the AQP3 expression
in colorectal carcinoma tissues was significantly higher than in
normal tissues by immunohistochemistry. Significantly, the
overexpression of AQP3 was associated with differentiation, lymph
node and distant metastasis in patients with colorectal
carcinoma.
EGF, which is a single-chain polypeptide separated
from male rat submandibular gland in 1962 (18), controls cell proliferation,
differentiation, apoptosis and migration through binding to its
receptor, EGFR. In normal tissues, there is very low expression of
EGF and EGFR, while in many malignant cells, they are usually
overexpressed (19,20). Previous studies have found that EGF
could induce AQP3 upregulation and cell migration in human ovarian
and gastric cancer cells (8,21).
Therefore, we assumed that EGF-induced cell migration is also
mediated by AQP3. In the present study, we showed that hEGF induced
AQP3 upregulation in human colorectal carcinoma cell lines in a
time- and dose-dependent manner and increased cell migration. In
addition, when the cells were pretreated with CuSO4, the
increase of cell migration induced by hEGF was abolished,
indicating AQP3 is directly involved in the cell migration.
Ciardiello and Tortora (22) reported that after the activation of
EGFR, EGF regulates cell proliferation, adhesion and movement
through the downstream PI3K/AKT or MAPK signaling pathways. In our
study, the results showed that pretreatment with the ERK inhibitor
did not alter the AQP3 expression in colorectal cancer cells,
whereas the PI3K/AKT inhibitor abolished hEGF-induced AQP3
expression in colorectal cancer cells, indicating hEGF-induced AQP3
expression in colorectal cancer cells is mediated by the PI3K/AKT,
but not the ERK pathway. Some studies showed AQP3 expression
induced by hEGF or HGF was regulated via the ERK signaling pathway
in human gastric carcinoma cells (9,23). In
another study regarding EGF-induced AQP3 upregulation and cell
migration in human ovarian cancer cells, it was indicated that the
PI3K and ERK pathways were both involved in EGF-induced cell
migration and AQP3 expression (21). The inconsistency may be due to the
difference in cancer type.
Tumor cell adhesion and migration play an important
role in tumor metastasis. Since overexpression of AQP3 could
enhance the migration ability of various tumor cells, it also
played a role in the clinical progression of tumors. A
retrospective analysis of 149 cases of lung cancer specimens showed
the level of AQP3 was correlated with the pathological type,
differentiation grade and clinical staging (24). Shen et al(25) found that the expression of AQP3 was
higher in undifferentiated gastric carcinoma than in
well-differentiated tumors, and was associated with lymph node
metastasis and lymphatic vessel invasion. Moon et
al(14) detected the expression
of AQP3 in 16 cases of colorectal carcinoma tissues and
corresponding adjacent normal colon tissue and showed high AQP3
expression in colorectal carcinoma. These results suggest the
expression of AQP3 in colorectal carcinoma could affect tumor
biological behavior and prognosis. In the present study, we found a
stronger AQP3 expression in colorectal carcinoma than in normal
tissues in accordance with a previous study (14). Additional analysis showed that AQP3
expression was associated with differentiation, lymph node and
distant metastasis in colorectal carcinoma. In the patients with a
low differentiation grade, lymph node or distant metastasis, high
level of AQP3 was often observed. These clinical data suggest high
expression of AQP3 predicts tumor metastasis and poor prognosis in
colorectal carcinoma.
In conclusion, our results suggest AQP3
overexpression in human colorectal carcinoma cells could facilitate
cell migration. The PI3K/AKT, but not the ERK, signaling pathway is
involved in hEGF-induced AQP3 expression. AQP3 was overexpressed in
colorectal carcinoma tissues compared with that in corresponding
normal tissues and correlated with the differentiation grade, lymph
node and distant metastasis of the tumor. Our results suggest an
important role of AQP3 in human colorectal carcinoma as a potential
indicator and therapeutic target for colon tumor metastasis and
prognosis. Our data also indicate hEGF may associate poor prognosis
of colorectal carcinoma and contribute to potential therapeutic
strategies for the prevention of colon tumor metastasis.
References
|
1
|
Remontet L, Esteve J, Bouvier AM, et al:
Cancer incidence and mortality in France over the period 1978–2000.
Rev Epidemiol Sante Publique. 51:3–30. 2003.
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3
|
Giuliani F, De Vita F, Colucci G and
Pisconti S: Maintenance therapy in colon cancer. Cancer Treat Rev.
36(Suppl 3): S42–S45. 2010. View Article : Google Scholar
|
|
4
|
Agre P: The aquaporin water channels. Proc
Am Thorac Soc. 3:5–13. 2006. View Article : Google Scholar
|
|
5
|
Magni F, Sarto C, Ticozzi D, et al:
Proteomic knowledge of human aquaporins. Proteomics. 6:5637–5649.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hara-Chikuma M and Verkman AS: Aquaporin-3
facilitates epidermal cell migration and proliferation during wound
healing. J Mol Med. 86:221–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakahigashi K, Kabashima K, Ikoma A,
Verkman AS, Miyachi Y and Hara-Chikuma M: Upregulation of
aquaporin-3 is involved in keratinocyte proliferation and epidermal
hyperplasia. J Invest Dermatol. 131:865–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Y, Zhu Z, Sun M, et al: Critical
role of aquaporin-3 in the human epidermal growth factor-induced
migration and proliferation in the human gastric adenocarcinoma
cells. Cancer Biol Ther. 9:1000–1007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kusayama M, Wada K, Nagata M, et al:
Critical role of aquaporin 3 on growth of human esophageal and oral
squamous cell carcinoma. Cancer Sci. 102:1128–1136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bogenrieder T and Herlyn M: Axis of evil:
molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fischer H, Stenling R, Rubio C and
Lindblom A: Differential expression of aquaporin 8 in human colonic
epithelial cells and colorectal tumors. BMC Physiol. 1:12001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishibashi K, Sasaki S, Saito F, Ikeuchi T
and Marumo F: Structure and chromosomal localization of a human
water channel (AQP3) gene. Genomics. 27:352–354. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasegawa H, Lian SC, Finkbeiner WE and
Verkman AS: Extrarenal tissue distribution of CHIP28 water channels
by in situ hybridization and antibody staining. Am J Physiol.
266:C893–C903. 1994.PubMed/NCBI
|
|
14
|
Moon C, Soria JC, Jang SJ, et al:
Involvement of aquaporins in colorectal carcinogenesis. Oncogene.
22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim M, Yoon S, Lee S, et al: Gremlin-1
induces BMP-independent tumor cell proliferation, migration, and
invasion. PLoS One. 7:e351002012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du J, Cheng B, Zhu X and Ling C:
Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant
origin, maintains glucocorticoid efficacy with reduced side
effects. J Immunol. 187:942–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fromowitz FB, Viola MV, Chao S, et al: ras
p21 expression in the progression of breast cancer. Hum Pathol.
18:1268–1275. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen S and Elliott GA: The stimulation of
epidermal keratinization by a protein isolated from the
submaxillary gland of the mouse. J Invest Dermatol. 40:1–5.
1963.PubMed/NCBI
|
|
19
|
Pryczynicz A, Guzinska-Ustymowicz K,
Kemona A and Czyzewska J: Expression of EGF and EGFR strongly
correlates with metastasis of pancreatic ductal carcinoma.
Anticancer Res. 28:1399–1404. 2008.PubMed/NCBI
|
|
20
|
Jin Y, Li JP, Tang LY, et al: Protein
expression and significance of VEGF, EGFR and MMP-9 in non-small
cell lung carcinomas. Asian Pac J Cancer Prev. 12:1473–1476.
2011.PubMed/NCBI
|
|
21
|
Ji C, Cao C, Lu S, et al: Curcumin
attenuates EGF-induced AQP3 up-regulation and cell migration in
human ovarian cancer cells. Cancer Chemother Pharmacol. 62:857–865.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ciardiello F and Tortora G: A novel
approach in the treatment of cancer: targeting the epidermal growth
factor receptor. Clin Cancer Res. 7:2958–2970. 2001.PubMed/NCBI
|
|
23
|
Wang J, Gui Z, Deng L, et al: c-Met
upregulates aquaporin 3 expression in human gastric carcinoma cells
via the ERK signalling pathway. Cancer Lett. 319:109–117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu YL, Matsuzaki T, Nakazawa T, et al:
Expression of aquaporin 3 (AQP3) in normal and neoplastic lung
tissues. Hum Pathol. 38:171–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen L, Zhu Z, Huang Y, et al: Expression
profile of multiple aquaporins in human gastric carcinoma and its
clinical significance. Biomed Pharmacother. 64:313–318. 2010.
View Article : Google Scholar : PubMed/NCBI
|