Introduction
Many tumors express tumor-associated antigens
(TAAs). Antigen-presenting cells (APCs), such as dendritic cells
(DCs) process and present TAAs to T cells, thereby eliciting a
tumor-specific immune response (1).
However, since TAAs are considered autologous, many tumor cells
often escape antitumor immunosurveillance. Various factors
affecting this immune escape include the limited expression of the
major histocompatibility complex (MHC) antigens and costimulatory
molecules, the production of immune inhibitory cytokines (2) and tumorigenic viruses that inhibit the
development of DCs (3). Therefore,
recruitment of professional APCs to the tumor site, upregulating
the expression of the MHC and associated costimulatory molecules,
and enhancing the T cell-primed capability of APCs may be essential
for generating specific antitumor immune responses. DCs are the
most functional of the professional APCs, with a unique ability to
capture and process antigens in peripheral blood and tissues. To
achieve this, DCs migrate to draining lymphoid organs, where they
select rare antigen-specific T cells in order to initiate immune
responses. DCs also present antigens to CD4+ T-helper
cells, which in turn regulate antigen-specific CD8+
cytotoxic T cells and B cells, as well as non-antigen-specific
macrophages, eosinophils (4) and
natural killer (NK) cells (5).
The ability of DCs to capture, process and present
antigens to lymphocytes, and to induce and sustain primary immune
responses makes them optimal candidates for vaccination protocols
in cancer (6–8). Advancements in the isolation and
propagation of DCs in vitro include the use of DCs as
adjuvants to stimulate antigen-specific T cell activation and
anticancer immunity (9–11). In immunotherapeutics, DCs are pulsed
with i) defined peptides of known sequences, ii) undefined
acid-eluted peptides from autologous tumors, iii) apoptotic tumor
cells, iv) whole tumor lysates, v) retroviral and adenoviral
vectors, vi) tumor cell-derived RNA, vii) fusion of DCs with tumor
cells, and viii) exosomes derived from DCs pulsed with tumor
peptides (12–18). Adenovirus infection can induce DC
differentiation, which can cause prolonged survival and resistance
to spontaneous and Fas-mediated cell death. Adenoviral-transfected
DCs can also augment T-cell and NK cell activation relevant to DC
secretion of IL-12, resulting in protective antitumor immunity
(19–21). In addition, intratumoral
administration of adenoviral vectors expressing cytokine
gene-modified DCs can enhance local and systemic antitumor effects
and achieve tumor eradication (22,23).
Chemokines comprise a family of proteins that cause
leukocyte chemotaxis and activation, and are associated with the
regulation of angiogenesis (24).
The secondary lymphoid tissue chemokine (SLC, also named 6Ckine,
Exodus-2 and TCA4) is a CC chemokine expressed in high endothelial
venules, and in the T cell zones of the spleen and lymph nodes.
These molecules strongly attract T cells and mature DCs. SLC
recruits both Th1 cells and antigen-stimulated DCs into the T cell
zones of secondary lymphoid organs, resulting in T cell activation
(25–30). Gunn et al(31) reported that the homing of T cells
and DCs to secondary lymphoid organs was significantly decreased in
plt−/plt− (paucity of lymphoid node T cell)
mice, which lack the SLC gene. In addition to its immunotherapeutic
ability, SLC reportedly has a potent anti-angiogenesis effect when
bound to the CXCR3 receptor (32).
This evidence adds additional support for the use of SLC in cancer
therapeutics.
The anticancer abilities of SLC provide the
rationale to evaluate this chemokine in cancer immunotherapy.
Intratumoral injections of recombinant SLC in mouse lung cancer
models have demonstrated potent antitumor responses, and have led
to complete tumor eradication in 40% of treated mice (33). In the spontaneous murine
bronchoalveolar cell carcinoma model, a recombinant SLC protein
injected into the auxiliary lymph node region led to a marked
reduction in tumor burden, extensively accompanied by lymphocyte
and DC infiltration of the tumors and enhanced organism survival
(34).
Based on these previous results, we constructed and
utilized herein an adenoviral vector expressing the human SLC
protein (Ad-SLC) for transfecting human monocyte-derived DCs
(Ad-SLC-DCs). We show that the Ad-SLC-DCs produce a SLC protein
with SLC-dependent biologic activity, as evidenced by the capacity
of this protein to attract T cells, and to mediate an anti-gastric
cancer immune response.
Materials and methods
Construction of an adenoviral vector
expressing the human SLC protein
The adenoviral construct (Ad-SLC) is an E1,
E3-deleted, replication-deficient adenoviral serotype 5 vector,
carrying the SLC cDNA. The SLC gene was amplified by RT-PCR
(Reverse Transcription System; Promega, USA) from human lymph
nodes, using the following primer pairs: forward,
5′-TTTAGATCTATGGCTCAGTCACTGGCTCTGAG-3′ and reverse,
5′-CCCGTCGACCTATGGCCCTTTAGGGGTCTGTG-3′. The SLC cDNA amplicon was
digested with BglII (Promega) and SalI, and was
cloned into the BglII-SalI sites of the pDC316 vector
(Microbix Biosystems, Inc., Canada) to generate pDC316-SLC. This
recombinant plasmid was verified by restriction enzyme analyses and
sequencing (data not shown). The recombinant pDC316-SLC plasmid was
co-transfected with pBHGloxΔE1,3Cre (plasmid containing the E1,
E3-deleted adenovirus genome; Microbix Biosystems, Inc.) into
HEK293 cells [American Type Culture Collection (ATCC), Manassas,
VA] using Lipofectamine™ 2000 (Invitrogen, USA). This produced the
recombinant E1, E3-deleted adenovirus, Ad-SLC, following
intracellular homologous recombination. Recombinant adenoviruses
encoding β-galactosidase (Ad-LacZ) (BD Biosciences Clontech, USA),
without the SLC cDNA inserts, were used as negative controls. The
Ad-SLC clone was obtained when a typical cytopathic effect (CPE)
was induced in the HEK293 cells. This clone was confirmed by PCR,
RT-PCR, immunofluorescence and western blot assays. We obtained the
viral stock by amplifying in HEK293 cells, and purifying with CsCl
gradient centrifugation and dialysis. The clone was stored as
glycerol stocks (10% v/v) at −80°C. The titers (infectious units,
ifu) of each viral stock (including Ad-LacZ) were quantitated
following the Adeno-X™ Rapid Titer kit user manual guidelines (BD
Biosciences Clontech). We assayed for wild-type adenovirus
contamination in each viral stock by PCR amplification of a
fragment on the E1 region of the adenovirus genome, and plaque
assaying in hepatocellular carcinoma HepG2 cells.
Generation of DCs and T cells
All healthy donors signed informed consent, and this
study was approved by the Institutional Review Board of Chengdu
Army General Hospital. The peripheral blood mononuclear cell (PBMC)
samples were acquired from donor leukocyte-enriched buffy coats
using gradient centrifugation with Ficoll-Paque™ Plus (Amersham
Biosciences, Uppsala, Sweden). From these, we recovered the light
density fraction from ~45% of the interface. The CD14+
PBMCs were purified by positive selection using
CD14+-labelled microbeads and MACS columns (Miltenyi
Biotec, Germany). The CD14+ cells were resuspended at
2×106 cells/ml, and cultured in 6-well cell culture
plates (BD Falcon, USA) in RPMI-1640 (Invitrogen) supplemented with
5% human AB serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2
mmol/l glutamine (Invitrogen), 30 ng/ml recombinant human
interleukin-4 (IL-4, specific activity >5×106 U/mg;
Peprotech, Inc., USA) and 100 ng/ml recombinant human
granulocyte-macrophage colony stimulating factor (GM-CSF, specific
activity >1×107 U/mg; Peprotech, Inc.). Three days
following incubation, the cells were fed with cytokines and
incubated for another 3 days. After 6 days of culture, the adherent
and non-adherent cells were harvested and washed three times with
phosphate-buffered saline (PBS) and were used in subsequent
experiments.
The T cells were purified from the CD14+
monocyte-depleted PBMCs using positive selection on a nylon-wool
column, and were cultured in RPMI-1640 complete medium,
supplemented with 20 U/ml IL-2. The purity of the resulting T cells
was roughly 92–97%, as assessed by fluorescence-activated cell
sorter (FACS) analysis.
Adenoviral transfection and tumor antigen
pulse of human DCs
Based on our recent study to determine the
multiplicity of infection (MOI) and the transfection method, the
optimal transfection efficiencies and cell viabilities were ensured
using a centrifugation method at an MOI of 50 (data not shown).
These optimal conditions were utilized throughout, for
transfections with all of the recombinant adenoviruses (35,36).
Briefly, on Day 6 of the culture period, DCs were resuspended at
2×106 cells/ml in serum-free RPMI-1640 medium and the
recombinant adenoviruses were suspended in the same medium and
adjusted to 1×108 ifu/ml. DC samples (500 μl) were mixed
with 500 μl of adenovirus (at an MOI of 50) in 1.5 ml polypropylene
tubes, and centrifuged (Eppendorf 5415D in a 37°C incubator,
Germany) at 2000 × g at 37°C for 2 h. Meanwhile, 500 μl of the DCs
was mixed with 500 μl of virus-free medium that served as the
non-transfected control (NTDCs). After the transfections, the
Ad-SLC-DCs, Ad-LacZ-DCs and NTDCs were washed three times with PBS,
resuspended at 5×105 cells/ml in complete medium,
containing 30 ng/ml IL-4 and 100 ng/ml GM-CSF, and incubated in
12-well cell culture plates (BD Falcon). All cell cultures were
performed at 37°C in a humidified incubator with 5% CO2.
After 24 h, the DCs (except for those designated for the
phagocytosis assay and phenotype analyses) were pulsed with 100
μg/ml of whole SGC7901 cell lysates (from a gastric cancer cell
line), and incubated at 37°C for another 24 h.
Flow cytometric analysis of the phenotype
of DCs
Ad-SLC-DCs Ad-LacZ-DCs and NTDCs were harvested, and
flow cytometry was performed using the following monoclonal
antibodies: fluorescein isothiocyanate (FITC)-CD86, FITC-CD83,
FITC-HLA-DR, FITC-CD14, phycoerythrin (PE)-CD80, PE-CD1a, PE-CD11c
and PE-CCR7 (BD Biosciences Pharmingen, USA). The cells were
analyzed with a FACS, using an EPICS® Elite flow
cytometer (Beckman Coulter, USA). These data were analyzed with
WinMDI2.8 software.
SLC, RANTES, IL-12p70 and IL-10
assays
Two days after the transfections, the levels of SLC
and RANTES in the cell supernatants were assayed, using the
Quantikines® Human SLC/CCL21 immunoassay kit (R&D
Systems, USA) and the Human RANTES ELISA kit (Pierce Biotechnology,
Inc., USA), respectively, according to the kit instructions. The
sensitivities of these assays were 9.9 and 2 pg/ml, respectively.
Meanwhile, the cytokines IL-12p70 and IL-10 in the supernatants
were detected with LiquidChip assay, using the Human Hcyto-60k-03
LINCOplex Multiplex kit (Linco Research, Inc., USA), according to
the manufacturer’s protocol. The data were analyzed using
integrated system (IS) software (Qiagen, Germany). The LiquidChip
kit detected the IL-12p70 and IL-10 at 2–10 pg/ml.
Phagocytosis assay
After 24 h of transfection (without being antigen
pulsed), the DCs were harvested and placed in 24-well cell culture
plates at a density of 5×105 cells/well, in triplicate.
To each well 40,000 MW FITC-dextran (Sigma Chemical Co., USA) was
added to a final concentration of 1 mg/ml. The plates were
incubated at 37°C for 2 h, then the cells were washed three times
with PBS, fixed with 2% paraformaldehyde and assayed for the
fluorescence intensity and the percentage of FITC-dextran uptake
using FACS analysis.
Chemotaxis and chemotaxis blocking
assay
The chemotaxis function of the supernatant derived
from the Ad-SLC-DCs, Ad-LacZ-DCs and NTDCs was assessed using
Transwells® filter apparatus (Corning Costar Corp.,
USA). Briefly, we loaded 600 μl of culture supernatants from Day 8
DCs in 24-well plates (lower chamber) in duplicate, then placed
6.5-mm Transwells® filter inserts with 3.0-μm pore size
polycarbonate membranes in each well, and added 100 μl of T cells
(2×106 cells/ml) to the filter inserts (upper chamber).
RPMI-1640 containing 5% human AB serum and 600 μl of the
recombinant human SLC protein (0.1 ng/μl; R&D Systems) were
used as negative and positive controls, respectively. For the
chemotaxis blocking assay, we pre-incubated the Ad-SLC-DC-derived
supernatants with neutralizing concentrations of recombinant human
anti-SLC monoclonal antibody (1:10 final concentration; R&D
Systems) for 1 h at room temperature. These pre-incubated samples
were added to the lower chamber of the apparatus, replacing the
supernatant in the chemotaxis assay. The plates were incubated at
37°C in 5% CO2 for 4 h. The filter inserts were removed
from the wells, and 1×104/50 μl of 15-μm polystyrene
beads were added to each well. The contents of each well were
thoroughly mixed, harvested, washed three times in PBS, fixed with
2% paraformaldehyde and analyzed using FACS analysis. The total
number of migrating cells per sample was determined using the
following formula: (Number of counted cells/number of counted
beads) × 10,000.
T-cell stimulation
For the mixed leukocyte reaction (MLR), the Day 8
Ad-SLC-DCs, Ad-LacZ-DCs and the NTDCs were incubated with 25 μg/ml
mitomycin A at 37°C. After 30 min, the DCs were harvested and
washed three times with PBS (containing 1% human AB serum), and
then combined with autologous T cells, at a dendritic cell:T cell
(DC:T) ratio of 1:20 in 200 μl RPMI-1640 complete medium, in
round-bottom 96-well cell culture plates (Nunc, Denmark) in
triplicate. The plates were incubated at 37°C in 5% CO2
for 96 h, followed by pulsation with 1 μCi/well of
[3H]thymidine (3H-TdR, from AERE, Shanghai,
China). The labeled cells were harvested onto a glass fiber filter
paper after 16 h. The amount of incorporated 3H-TdR
[described as counts/min (cpm)] was detected using a LS6500 liquid
scintillation counter (Beckman Coulter), and the stimulating index
(SI) was calculated using the following formula: Experimental cpm -
blank cpm/control cpm - blank cpm.
IL-2 and T-bet assay
On Day 8 of culture, each DC group was co-cultured
with autologous, nylon-wool-purified T cells at a DC:T ratio of
1:20 (1×104 DCs plus 2×105 T cells) in 200 μl
RPMI-1640 complete medium in round-bottom 96-well cell culture
plates (Nunc) in triplicate. The plates were incubated in 5%
CO2 at 37°C. After 24 h, the supernatants were
collected, and the IL-2 concentrations were measured by ELISA,
according to the manufacturer’s protocol (BioSource, USA). The
sensitivity of the IL-2 assay was 15 pg/ml.
Meanwhile, each DC group (1×105 cells)
was incubated with mitomycin A at 37°C for 30 min, and then
co-cultured with autologous T cells (2×106 cells) at
37°C in 5% CO2 for 96 h. Following this, the T cells
were harvested and the total RNA was extracted using
TRIzol® reagent (Invitrogen), according to the
manufacturer’s protocol. The level of T-bet mRNA expression was
assessed by semi-quantitative RT-PCR (Reverse Transcription System;
Promega), using the following primer pairs: forward,
5′-CCCAGATGATTGTGCTCCAG-3′ and reverse, 5′-TCATGCTGACTGCTCGAAAC-3′,
with an expected PCR product of 450 bp. The β-actin PCR product
(245 bp) was used as a baseline control. The T-bet and β-actin
amplicons were visualized with electrophoresis on a 1% agarose gel
containing 0.5 μg/ml ethidium bromide. The amplicon densities were
analyzed with the Genetools Analysis Software, version 3.0 (SynGene
Lab). The results were expressed as a ratio of the quantified T-bet
product over the quantitated β-actin product.
Cytotoxicity assay
The SGC7901 cells were used as targets for detecting
the cytotoxic function of the T cells which were stimulated
separately with Ad-SLC-DCs, Ad-LacZ-DCs and NTDCs, using the
CytoTox 96® Non-Radioactive Cytotoxicity Assay kit
(Promega). The autologous nylon-wool-purified T cells were
co-cultured with the DCs at a DC:T ratio of 1:20 on Day 8 of the DC
culture period. After 96 h, the T cells were harvested and
incubated with tumor cells in round-bottom 96-well culture plates
at an effector:target (E:T) ratio of 20:1 and 50:1, in triplicate.
The controls including effector cell spontaneous lactate
dehydrogenase (LDH) release, target cell spontaneous LDH release,
target cell maximum LDH release, volume correction control and
culture medium background were also set up in triplicate. The plate
was centrifuged at 250 × g for 4 min, and incubated in a humidified
chamber at 37°C with 5% CO2 for 4 h. For the target cell
maximum LDH release control, 20 μl of lysis solution (10X) was
added to the appropriate wells 45 min prior to harvesting the
supernatants. After incubation, the plate was centrifuged at 250 ×
g for an additional 4 min. Supernatants (50 μl) from all wells were
transferred into the wells of a fresh flat-bottom 96-well assay
plate. Reconstituted substrate mix (50 μl) was added to this new
assay plate. The plate was incubated in the dark at room
temperature for 30 min, and the absorbance (A) was measured at 490
nm, following the addition of 50 μl of stop solution. The percent
cytotoxicity was computed using the following formula:
(Experimental A - culture medium background A) − (effector cell
spontaneous A - culture medium background A) − (target cell
spontaneous A - culture medium background A)/(target cell maximum A
− volume correction control A) − (target cell spontaneous A -
culture medium background A) × 100.
The same cytotoxicity assay was performed using LoVo
cell lysate (from a colon cancer cell line, as a different antigen
control) pulsed DC-mediated T cells to kill SGC7901 cells.
Statistical analyses
One-way analysis of variance (ANOVA) test was used
to compare differences among the Ad-SLC-DCs, Ad-LacZ-DCs and NTDC
groups, using SPSS 17.0 statistical software. Statistical
significance was determined at a P-value of 0.05 (P<0.05).
Results
Recombinant human Ad-SLC was successfully
constructed and expressed SLC protein in eukaryocytes
The adenovirus vector containing the human SLC gene
(Ad-SLC) was constructed successfully, as determined by PCR (data
not shown). The titer of each viral stock (containing Ad-LacZ) was
1×1010–1.2×1010 ifu/ml, as determined by
detecting the signal of the anti-hexon antibody. Any wild-type
adenoviral contamination was not detected by PCR and in the SGC7901
cells. SLC mRNAs were detected in the transfected HepG2 cells. SLC
proteins (green fluorescence) were also found in the cytoplasm and
membranes of the transfected cells. Western blot analysis with the
anti-SLC antibody showed a immunoreactive band of the predicted
size (14 kDa) in the cells transfected with Ad-SLC. The cells
transfected with the Ad-LacZ empty vector did not react in any of
the parallel experiments (Fig.
1).
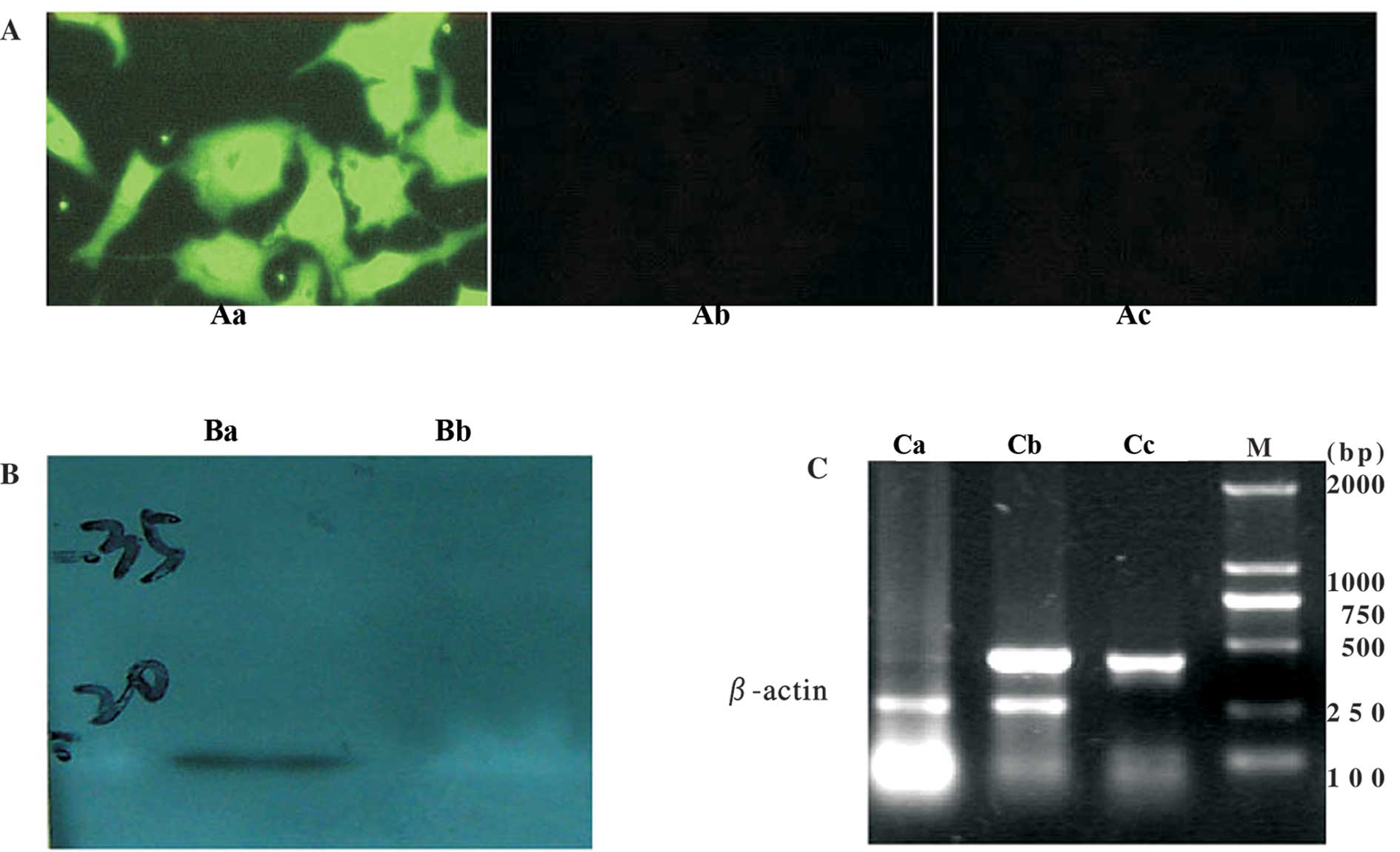 | Figure 1SLC mRNA and protein expression in
HepG2 cells. (A) Immunofluorescence staining in HepG2 cells
transfected with adenovirus vectors. Aa, Ad-SLC-transfected HepG2
cells (x200); Ab, Ad-LacZ-transfected HepG2 cells (x200); Ac,
non-transfected HepG2 cells (×200). (B) Western blot analysis. Ba,
Ad-SLC-transfected HepG2 cells, a 14-kDa band is observed; Bb,
Ad-LacZ-transfected HepG2 cells. (C) SLC mRNA expression measured
by RT-PCR. Lane Ca, Ad-LacZ-transfected HepG2 cells; lane, Cb,
Ad-SLC-transfected HepG2 cells; lane Cc, SLC cDNA PCR amplicon as
the positive control; lane M, DL2000 DNA marker. |
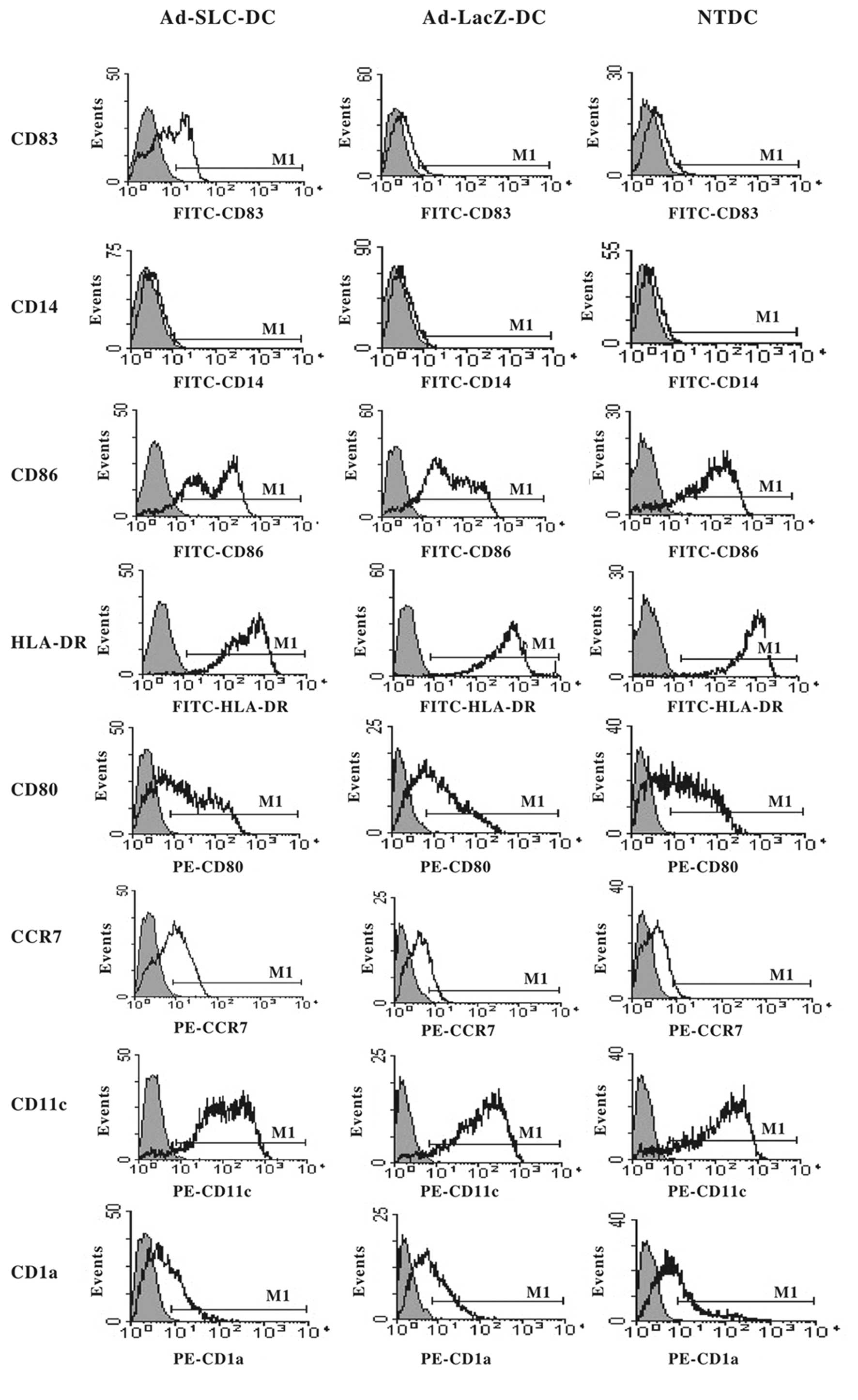
The phenotype of mature DCs is
upregulated after transfection with Ad-SLC
The phenotypes of the Ad-SLC-DCs, Ad-LacZ-DCs and
NTDCs were characterized by flow cytometry 48 h after transfection.
The expression of the DC surface markers was not significantly
altered by transfection with recombinant adenovirus, with the
exception of CD83, and CCR7. The immature DC phenotypes
(CD83low, CCR7low) were preserved in
Ad-LacZ-DCs and NTDCs, however, CD83 and CCR7 were significantly
augmented in Ad-SLC-DCs when compared with the other groups. The
costimulatory molecules CD80 and CD86, as well as the MHC HLA-DR
molecules maintained high expression levels, not only in the
adenovirus-transfected DCs, but also in the NTDCs (>90%). This
finding was identical as that for CD11c. The expression of CD1a and
CD14 was maintained at a low level in all groups (Fig. 2; P<0.05).
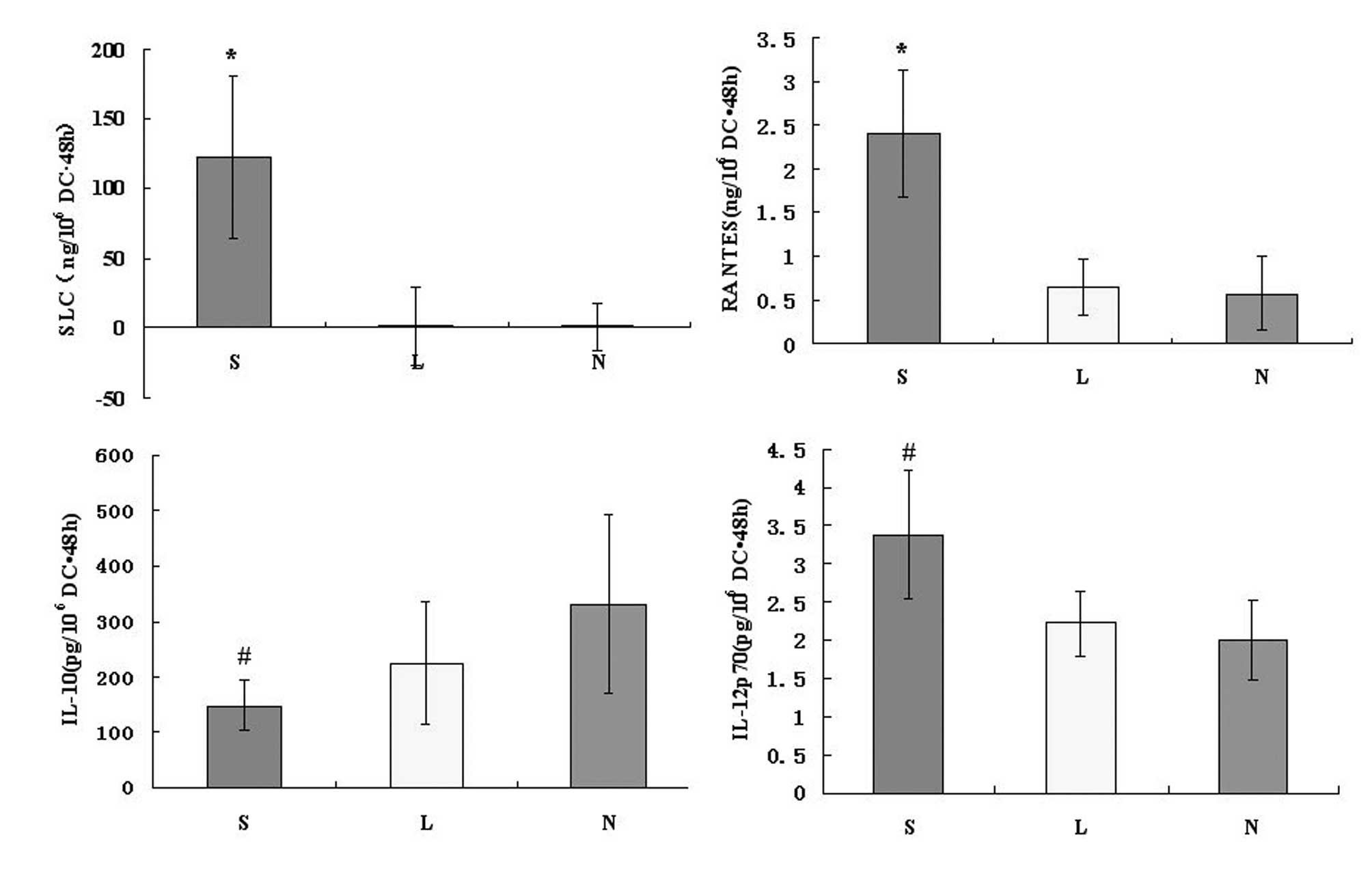
SLC protein is produced by
Ad-SLC-DCs
On culture Day 6, the immature human
monocyte-derived DCs were transfected with Ad-SLC and Ad-LacZ.
After 48 h, we assessed the SLC protein product using the anti-SLC
antibody by ELISA. As a result, the amount of SLC protein from the
Ad-SLC-DCs was 122.63±58.12 ng/106 cells · 48 h, while
the Ad-LacZ-DCs and NTDCs produced 1.43±28.23 and 1.00±17.09
ng/106 cells · 48 h of SLC protein, respectively.
Statistically, the amount of SLC proteins produced by Ad-SLC-DCs
were significantly higher than those measured in any of the other
two groups (Fig. 3; P<0.05).
The chemokine RANTES is secreted by
recombinant Ad-SLC-DCs
After 48 h of exposure to the transfecting agents,
the DCs were assayed for the presence of the chemokine RANTES and
the cytokines IL-10 and IL-12p70 using specific ELISA and
LiquidChip tests. The production of RANTES from the Ad-SLC-DCs was
increased significantly above that of the Ad-LacZ-DCs and NTDCs
(Fig. 3; F=25.56, P<0.05).
However, the amounts of IL-12p70 and IL-10 proteins secreted from
the Ad-SLC-DCs demonstrated no differences compared with the
protein levels in the Ad-LacZ-DCs and NTDCs (Fig. 3; F=2.63, P>0.05).
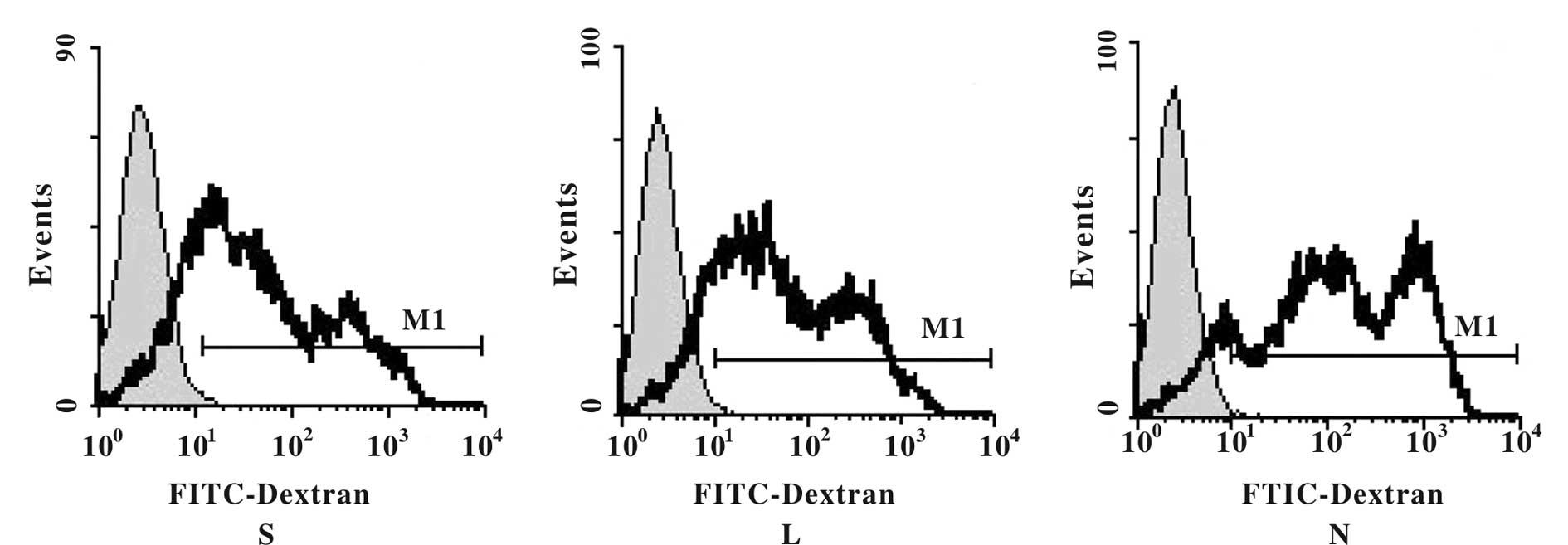
The phagocytic capability of DCs is
maintained throughout 24 h of transfection
To determine whether or not the phagocytic function
of the DCs was negatively impacted by adenoviral transfection, we
measured FITC fluorescence in the infected DCs. We found that each
DC group incorporated the FITC-dextran particles, as evidenced by
high fluorescence intensities; however, we observed no significant
impact on the percentage of FITC-uptake in cells that had been
transfected with the recombinant adenovirus. The phagocytic
capability of the DCs remained intact within 24 h of adenoviral
infection (Fig. 4; F=2.31,
P>0.05).
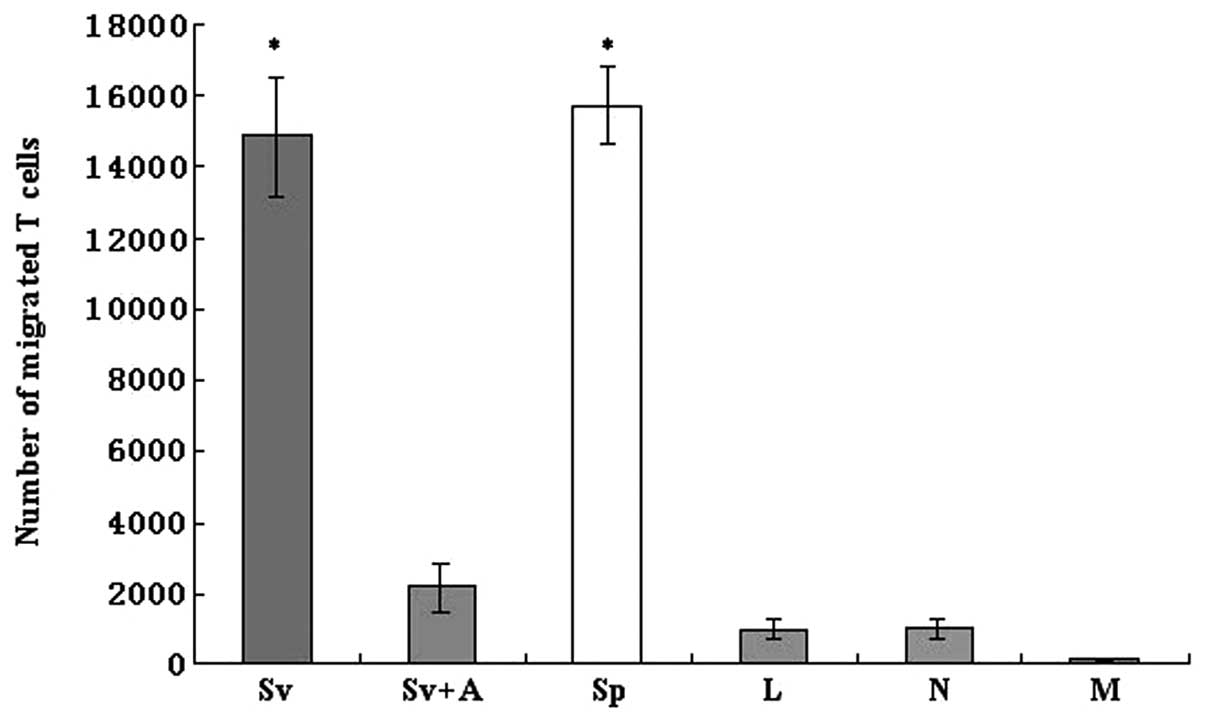
Ad-SLC transfection augments the
chemotaxis ability of DCs and this capacity is partially blocked by
the anti-SLC monoclonal antibody
The migration of naïve T cells to the Ad-SLC-DCs was
assessed using a Transwell chemotaxis assay apparatus. Our results
showed that the Ad-SLC-DC supernatants, as well as the SLC protein,
evoked enhanced T cell chemotaxis events, when compared to the
Ad-LacZ-DC and NTDC supernatants alone or to the control medium (5%
AB serum) (Fig. 5; F=186.68,
P<0.05). Yet, the enhanced attractive effects on T cells of the
Ad-SLC-DC supernatants were inhibited significantly by the anti-SLC
monoclonal antibody (Fig. 5;
P<0.05). Notably, our data showed that only a partial
chemoattractive activity of the Ad-SLC-DCs was reversed using the
anti-SLC antibody. These results indicate that other proteins with
attractant capabilities, such as RANTES, may exist in the DC
supernatants, and that these auxiliary molecules likely account for
the incomplete inhibition of T cell migration in the presence of
the anti-SLC antibody.
Ad-SLC-DCs stimulate naïve T cell
proliferation and induce Th1 differentiation
We investigated whether the Ad-SLC-DCs possess the
ability to prime naïve T cells in vitro, using mitomycin
A-treated DCs and 3H-TdR (cpm). Our results showed that
the Ad-SLC-DCs were able to prime naïve T cells, as compared to the
Ad-LacZ-DCs and the NTDCs (Fig. 6;
P<0.05). We did not detect any significant differences in the
T-cell priming capabilities of the Ad-LacZ-DCs and the NTDCs
(Fig. 6; P>0.05).
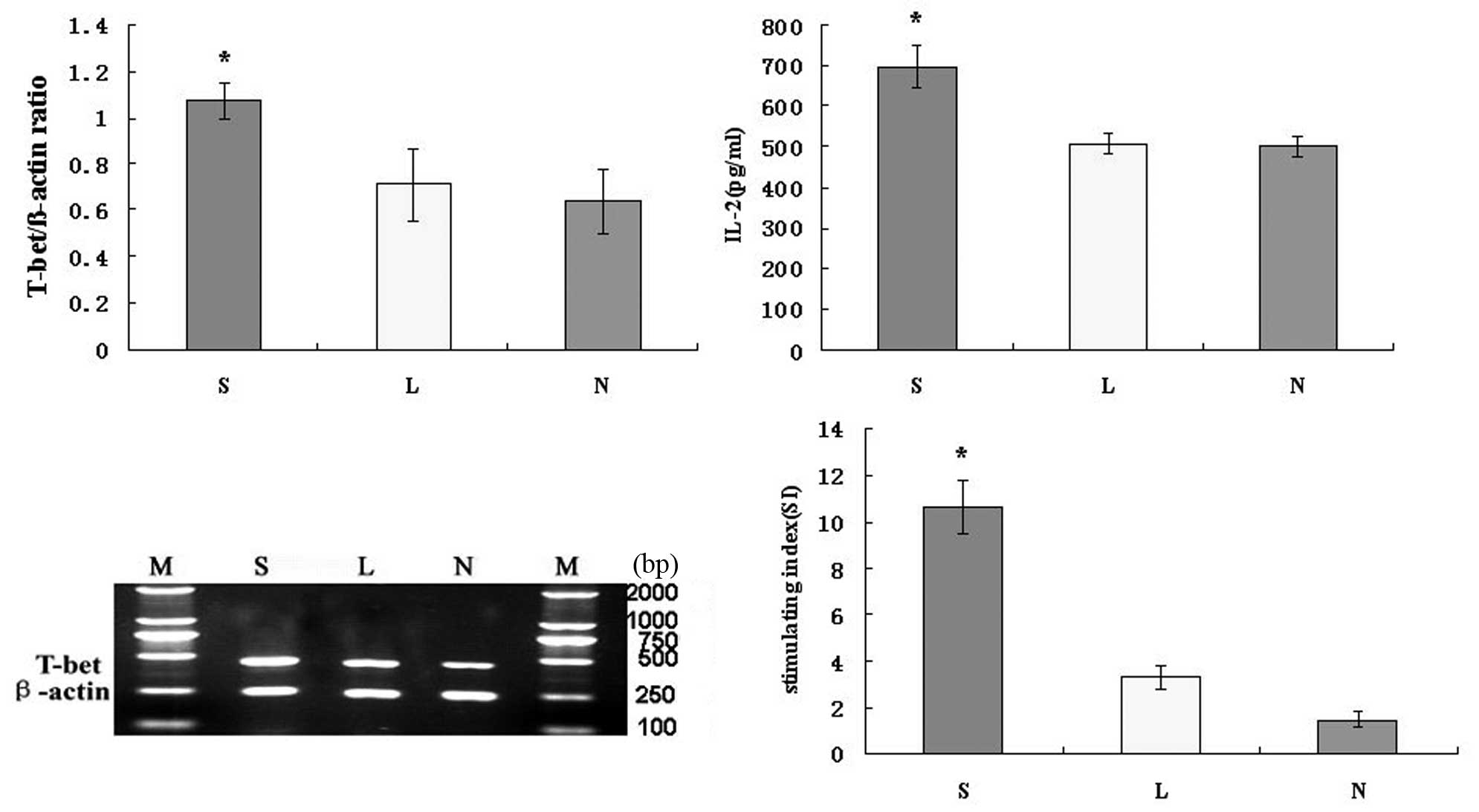
To determine whether the Ad-SLC-DCs induce Th1
differentiation, IL-2 protein and T-bet mRNA expression in
Ad-SLC-DC-transfected T cells was measured with ELISA or RT-PCR,
respectively. The IL-2 protein secretion from the T cells
co-cultured with the Ad-SLC-DCs was higher than those co-cultured
with the Ad-LacZ-DCs and NTDCs (Fig.
6; P<0.05). There was no significant difference in IL-2
secretion between the Ad-LacZ-DC and NTDC groups (Fig. 6; P>0.05). The level of T-bet mRNA
expressed by the T cells co-cultured with the Ad-SLC-DCs was
significantly higher than that by T cells exposed to the
Ad-LacZ-DCs and NTDCs (Fig. 6;
P<0.05). There was no significant difference in T-bet expression
levels between the Ad-LacZ-DC and NTDC groups (Fig. 6; P>0.05).
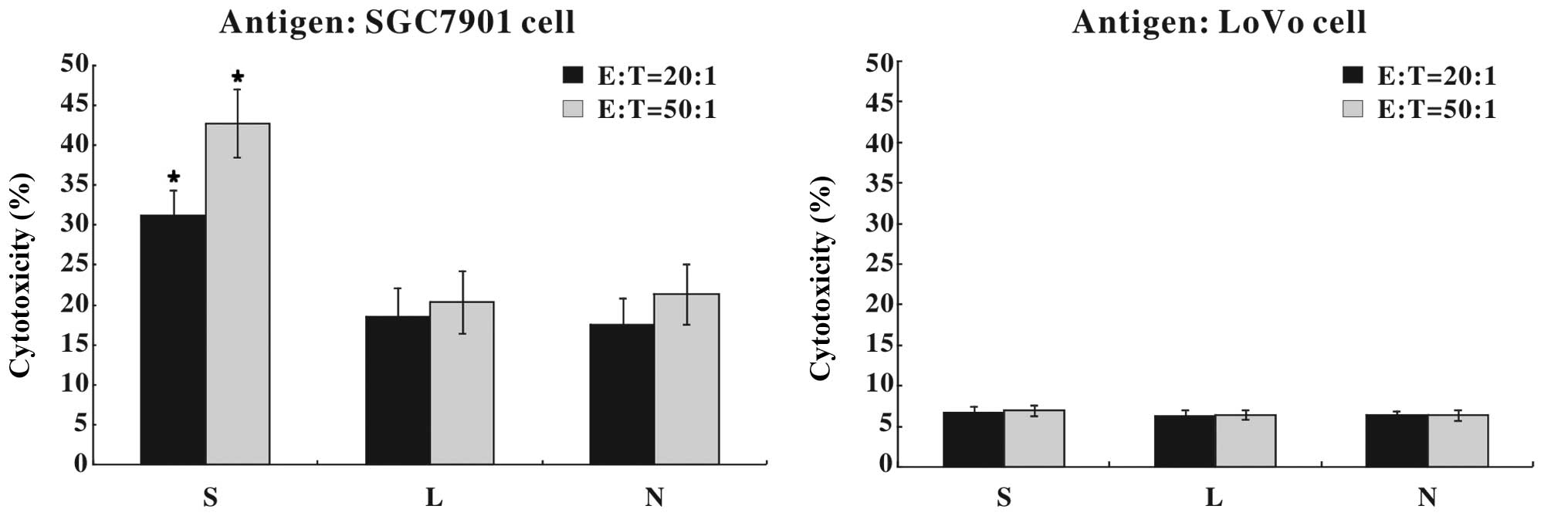
Ad-SLC-DCs induce specific anti-gastric
cancer immunity
Following 24 h of recombinant adenoviral infection,
the DCs were pulsed with whole SGC7901 or LoVo cell lysates as
tumor antigens, co-cultured with autologous T cells and assayed for
antitumor immunity. As shown in Fig.
7, 31.25±3.12% (E:T=20:1) or 42.78±4.25% (E:T=50:1) of the
SGC7901 cells were killed by the cytotoxic T cells that had been
primed with SGC7901 antigen-pulsed Ad-SLC-DCs, which was
significantly higher than that in the other two DC groups
(P<0.05). However, T cells primed with LoVo antigen-pulsed DCs
showed little cytotoxicity against SGC7901 cells.
Discussion
Herein, we report the successful construction of an
adenovirus vector expressing a chemokine protein (SLC), The protein
is expressed in transfected eukaryocytes at the mRNA and protein
level. It is a peptide with a molecular weight of about 14 kDa.
The ability of DCs to capture, process and present
antigens to and subsequently prime CD4+ and
CD8+ T cells makes them candidate adjuvants in
anticancer immunotherapy. Previous studies using animal models have
demonstrated that cytokine gene modification in DCs can enhance
their antitumor capacity (15,22,23).
DCs are notoriously difficult to transfect using conventional
methodologies and non-viral vectors, such as plasmid DNA/cationic
liposome complexes, electroporation or CaPO4
coprecipitation (37). However,
they are amenable to engineering with viral vectors such as the
adenovirus, despite their lack of the cell surface Coxsackie B and
adenovirus receptor (CAR). The suboptimal attachment of the
adenovirus to DC surfaces leads to low gene transfer efficiency.
This can be circumvented by increasing the contact between the
adenovirus and the DC cell membrane by centrifugation or utilizing
small volumes for transfection (35,36,38) or
by adding cationic liposomes to mediate the infection (39,40).
In this study, we used centrifugation to optimize the adenoviral
transfection efficiency of the SLC gene into human monocyte-derived
DCs.
Commonly, immature DCs are generated from monocytes
that have been cultured for 5–7 days with GM-CSF and IL-4. Mature
DCs are harvested after exposure to microbial, proinflammatory or T
cell-derived stimuli such as TNFα, IL-1β, IFNα, IFNγ and PGE2 for
2–3 days. Our findings indicated that in addition to the high level
expression of CD80, CD86, CD11c and HLA-DR, the transfected DCs
required 2 additional days in culture to express sufficient levels
of the mature markers CD83 and CCR7 on the surfaces of the
Ad-SLC-DCs. This latter finding indicates that the secreted SLC
protein in the tranduced DCs can partially auto-promote their
phenotypic maturation. Moreover, it demonstrated enhanced DC
antigen-presentation and subsequent T cell activation. Overall, our
data suggest that DCs transfected with Ad-SLC can upregulate the
expression of costimulatory molecules and the MHC complex, and
promote DC maturation. This latter observation is profound,
considering that the phagocytic capability, and thus the
antigen-capture capacity of the DCs was maintained even after 24 h
of transfection. Thus, we determined that we can pulse DCs with
tumor antigens within 24 h of adenovirus-mediated gene
modification, and it will not decrease the ability of DCs to uptake
these antigens.
In this study, we also found that the cultural
supernatants of the Ad-SLC-DCs augmented the chemoattraction of DCs
to nylon-wool-purified T cells. This function was partially blocked
by the anti-SLC monoclonal antibody. We did not find this effect in
the Ad-LacZ-DC or the NTDC supernatants. This result demonstrates
that Ad-SLC imbued the transfected DCs with an attractant(s) to
naïve T cells. Thus, intratumoral administration of Ad-SLC-modified
DCs appears to be an effective strategy for cancer immunotherapy by
attracting T cells to tumor sites, and then presenting the tumor
antigens to the T cells.
SLC can recruit both T cells and matured DCs into
the T cell zones of secondary lymphoid organs via CCR7, a chemokine
receptor belonging to the subfamily of G protein-coupled
seven-transmembrane receptors (41–43).
This recruitment converges the immune response elements, including
T cells and mature antigen-loaded DCs to the sites of SLC
production, resulting in T cell activation and an antitumor
response (26,33). Our study showed that Ad-SLC
augmented the CCR7 expression in the transfected DCs, and that the
Ad-SLC-DCs produced the SLC protein to attract T cells. Thus, the
Ad-SLC-modified DCs created a microenvironment, inhabited by
co-localized T cells and mature DCs.
In contrast to immunization with purified peptide
antigens such as α fetoprotein (AFP) or carcinoembryonic antigen
(CEA), whole tumor cell lysate immunizations can provide DCs with
the entire repertoire of available tumor antigens. This may
increase the likelihood of antitumor immune responses and reduce
the potential of a phenotypic-modulated tumor resistance. In the
study presented herein, we transfected DCs with Ad-SLC, and then
pulsed the transfectants with whole-cell SGC7901 lysates. Although
the secretion of cytokines IL-12p70 and IL-10 from DCs transfected
with Ad-SLC was not altered, we found that Ad-SLC significantly
augmented the production of immunoenhancing chemokine, RANTES, in
the transfected DCs, as compared to that in the Ad-LacZ-DCs and in
the NTDCs. RANTES can stimulate T cell activation through the T
cell receptor (TCR), characterized by increased secretion of IL-2
and IL-5, upregulation of IL-2 receptors and enhanced cellular
proliferation. Moreover, RANTES activates antigen-specific
cytotoxic T cells at high concentrations through self-aggregation
on the cell surface (44–47). Therefore, our findings that the
RANTES production was increased in the Ad-SLC-modified DCs predict
that the Ad-SLC-modified DCs may polarize the immune response to
the Th1 phenotype, and increase the cytolytic activity of T cells,
subsequently inducing a potent antitumor immunity as an
adjuvant.
Subsequent studies revealed that T cell
proliferation was significantly stimulated by the Ad-SLC-DCs. This
enhancement might have been produced by the SLC-induced DC
maturation and by the DC secretion of RANTES and SLC. SLC can
stimulate the proliferation of CD4+ and CD8+
T cells and induces Th1 polarization (48,49).
We also determined that the Ad-SLC-DCs induced Th1 polarization,
which is evidenced by the enhanced expression of IL-2 and T-bet
mRNA in the T cells. Interestingly, our findings demonstrated a
hitherto unknown capability of the Ad-SLC-DCs to increase T cell
abundance and hence proliferation and Th1 polarization evidenced by
upregulation of IL-2 and T-bet expression in T cells.
Finally, our investigations revealed that the
survival of the gastric cancer cells, SGC7901, was significantly
reduced after they were mixed with T cells primed with the
Ad-SLC-modified SGC7901 cell antigen-loaded DCs. The
β-galactosidase-modified DCs and non-transfected DCs did not
possess the anti-gastric cancer adjuvant function of the
Ad-SLC-DCs. Presumably, this may be because the SLC chemokine is
required for a full adjuvant effect.
In summary, the Ad-SLC-modified DCs maintained their
capacity of phagocytosis within 24 h of transfection, indicating
that they also uptake antigens. After an additional 24 h, the
Ad-SLC vector upregulated the expression of the MHC class II, CCR7,
CD83 and costimulators on the DC surfaces. These DCs attracted
naïve T cells and underwent intense maturation. After being pulsed
by tumor antigens, the Ad-SLC-DCs secreted high levels of
immunoenhancement chemokine SLC and RANTES, induced T cell
proliferation and Th1 differentiation, leading to antitumor
cytotoxic responses. In short, the Ad-SLC promotes DC maturation,
enhances antigen presentation and T cell stimulation, and induces
strong antitumor immunities. Our study indicates that intratumoral
administration of Ad-SLC-modified DCs may promote colocalization of
T cells and DCs in targeted tumor sites, and my eradicate tumors
through a T cell-dependent mechanism.
Acknowledgements
This study was supported by the Elite Training Fund
of Chengdu Army General Hospital.
Abbreviations:
|
DCs
|
dendritic cells
|
|
Ad-SLC
|
recombinant adenovirus carrying the
SLC gene
|
|
Ad-LacZ
|
recombinant adenovirus encoding
β-galactosidase
|
|
TAA
|
tumor-associated antigen
|
|
Ad-SLC-DCs
|
DCs transfected with adenovirus vector
encoding the SLC gene
|
|
NTDCs
|
non-transfected DCs
|
|
Ad-LacZ-DCs
|
DCs transfected with the adenovirus
vector encoding β-galactosidase
|
|
ifu
|
infectious units
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Huang AY, Golumbek P, Ahmadzadeh M, Jaffee
E, Pardoll D and Levitsky H: Role of bone marrow-derived cells in
presenting MHC class I-restricted tumor antigens. Science.
264:961–965. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Restifo NP, Esquivel F, Kawakami Y,
Yewdell JW, Mulé JJ, Rosenberg SA and Bennink JR: Identification of
human cancers deficient in antigen processing. J Exp Med.
177:265–272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li L, Liu D, Hutt-Fletcher L, Morgan A,
Masucci MG and Levitsky V: Epstein-Barr virus inhibits the
development of dendritic cells by promoting apoptosis of their
monocyte precursors in the presence of granulocyte
macrophage-colony-stimulating factor and interleukin-4. Blood.
99:3725–3734. 2000. View Article : Google Scholar
|
|
4
|
Lambrecht BN, Salomon B, Klatzmann D and
Pauwels RA: Dendritic cells are required for the development of
chronic eosinophilic airway inflammation in response to inhaled
antigen in sensitized mice. J Immunol. 160:4090–4097.
1998.PubMed/NCBI
|
|
5
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar
|
|
6
|
Steinman RM, Pack M and Inaba K: Dendritic
cells in the T-cell areas of lymphoid organs. Immunol Rev.
156:25–37. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan MC, Mommaas AM, Drijfhout JW, Jordens
R, Onderwater JJ, Verwoerd D, Mulder AA, van der Heiden AN,
Scheidegger D, Oomen LC, Ottenhoff TH, Tulp A, Neefjes JJ and
Koning F: Mannose receptor mediated uptake of antigens strongly
enhances HLA class II-restricted antigen presentation by cultured
dendritic cells. Eur J Immunol. 27:2426–2435. 1997. View Article : Google Scholar
|
|
8
|
Nair SK, Hull S, Coleman D, Gilboa E,
Lyerly HK and Morse MA: Induction of carcinoembryonic antigen
(CEA)-specific cytotoxic T-lymphocyte responses in vitro using
autologous dendritic cells loaded with CEA peptide or CEA RNA in
patients with metastatic malignancies expressing CEA. Int J Cancer.
82:121–124. 1999. View Article : Google Scholar
|
|
9
|
Timmerman JM and Levy R: Dendritic cell
vaccines for cancer immunotherapy. Annu Rev Med. 50:507–529. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lodge PA, Jones LA, Bader RA, Murphy GP
and Salgaller ML: Dendritic cell-based immunotherapy of prostate
cancer: immune monitoring of a phase II clinical trial. Cancer Res.
60:829–833. 2000.PubMed/NCBI
|
|
11
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bell D, Young JW and Banchereau J:
Dendritic cells. Adv Immunol. 72:255–324. 1999. View Article : Google Scholar
|
|
13
|
Henderson RA, Nimgaonkar MT, Watkins SC,
Robbins PD, Ball ED and Finn OJ: Human dendritic cells genetically
engineered to express high levels of the human epithelial tumor
antigen mucin (MUC-1). Cancer Res. 56:3763–3770. 1996.PubMed/NCBI
|
|
14
|
Ribas A, Bui LA, Butterfield LH, Vollmer
CM, Jilani SM, Dissette VB, Glaspy JA, McBride WH and Economou JS:
Antitumor protection using murine dendritic cells pulsed with
acid-eluted peptides from in vivo grown tumors of different
immunogenicities. Anticancer Res. 19:1165–1170. 1999.
|
|
15
|
Miller PW, Sharma S, Stolina M,
Butterfield LH, Luo J, Lin Y, Dohadwala M, Batra RK, Wu L, Economou
JS and Dubinett SM: Intratumoral administration of adenoviral
interleukin 7 gene-modified dendritic cells augments specific
antitumor immunity and achieves tumor eradication. Hum Gene Ther.
11:53–65. 2000. View Article : Google Scholar
|
|
16
|
Cao X, Zhang W, He L, Xie Z, Ma S, Tao Q,
Yu Y, Hamada H and Wang J: Lymphotactin gene-modified bone marrow
dendritic cells act as more potent adjuvants for peptide delivery
to induce specific antitumor immunity. J Immunol. 161:6238–6244.
1998.PubMed/NCBI
|
|
17
|
Celluzzi CM and Falo LD Jr: Physical
interaction between dendritic cells and tumor cells results in an
immunogen that induces protective and therapeutic tumor rejection.
J Immunol. 160:3081–3085. 1998.
|
|
18
|
Ribas A, Butterfield LH, McBride WH,
Jilani SM, Bui LA, Vollmer CM, Lau R, Dissette VB, Hu B, Chen AY,
Glaspy JA and Economou JS: Genetic immunization for the melanoma
antigen MART-1/Melan-A using recombinant adenovirus-transfected
murine dendritic cells. Cancer Res. 57:2865–2869. 1997.
|
|
19
|
Lyakh LA, Koski GK, Young HA, Spence SE,
Cohen PA and Rice NR: Adenovirus type 5 vector induces dendritic
cell differentiation in human CD14+ monocytes cultured
under serum-free conditions. Blood. 99:600–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lundqvist A, Choudhury A, Nagata T,
Andersson T, Quinn G, Fong T, Maitland N, Pettersson S, Paulie S
and Pisa P: Recombinant adenovirus vector activates and protects
human monocyte derived dendritic cells from apoptosis. Hum Gene
Ther. 13:1541–1549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller G, Lahrs S, Pillarisetty VG, Shah
AB and DeMatteo RP: Adenovirus infection enhances dendritic cell
immunostimulatory properties and induces natural killer and
T-cell-mediated tumor protection. Cancer Res. 62:5260–5266.
2002.
|
|
22
|
Kirk CJ, Hartigan-O’Connor D and Mulé JJ:
The dynamics of the T cell antitumor response: chemokine-secreting
dendritic cells can prime tumor-reactive T cells extranodally.
Cancer Res. 61:8794–8802. 2001.PubMed/NCBI
|
|
23
|
Kirk CJ, Hartigan-O’Connor D, Nickoloff
BJ, Chamberlain JS, Giedlin M, Aukerman L and Mule JJ: T
cell-dependent antitumor immunity mediated by secondary lymphoid
tissue chemokine: augmentation of dendritic cell-based
immunotherapy. Cancer Res. 61:2062–2070. 2001.PubMed/NCBI
|
|
24
|
Baggiolini M, Dewald B and Moser B: Human
chemokines: an update. Annu Rev Immunol. 15:675–705. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gunn MD, Tangemann K, Tam C, Cyster JG,
Rosen SD and Williams LT: A chemokine expressed in lymphoid high
endothelial venules promotes the adhesion and chemotaxis of naïve T
lymphocytes. Proc Natl Acad Sci USA. 95:258–263. 1998.PubMed/NCBI
|
|
26
|
Cyster JG: Chemokines and the homing of
dendritic cells to the T cell areas of lymphoid organs. J Exp Med.
189:447–450. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan VW, Kothakota S, Rohan MC,
Panganiban-Lustan L, Gardner JP, Wachowicz MS, Winter JA and
Williams LT: Secondary lymphoid-tissue chemokine (SLC) is
chemotactic for mature dendritic cells. Blood. 93:3610–3616.
1999.PubMed/NCBI
|
|
28
|
Hromas R, Kim CH, Klemsz M, Krathwohl M,
Fife K, Cooper S, Schnizlein-Bick C and Broxmeyer HE: Isolation and
characterization of Exodus-2, a novel CC chemokine with a unique 37
amino acid carboxyl-terminal extension. J Immunol. 159:2554–2558.
1997.PubMed/NCBI
|
|
29
|
Willimann K, Legler DF, Loetscher M, Roos
RS, Delgado MB, Clark-Lewis I, Baggiolini M and Moser B: The
chemokine SLC is expressed in T cell areas of lymph nodes and
mucosal lymphoid tissues and attracts activated T cells via CCR7.
Eur J Immunol. 28:2025–2034. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagira M, Imai T, Hieshima K, Kusuda J,
Ridanpää M, Takagi S, Nishimura M, Kakizaki M, Nomiyama H and
Yoshie O: Molecular cloning of a novel human CC chemokine secondary
lymphoid-tissue chemokine that is a potent chemoattractant for
lymphocytes and mapped to chromosome 9p13. J Biol Chem.
272:19518–19524. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gunn MD, Kyuwa S, Tam C, Kakiuchi T,
Matsuzawa A, Williams LT and Nakano H: Mice lacking expression of
secondary lymphoid organ chemokine have defects in lymphocyte
homing and dendritic cell localization. J Exp Med. 189:451–460.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soto H, Wang W, Strieter RM, Copeland NG,
Gilbert DJ, Jenkins NA, Hedrick J and Zlotnik A: The CC chemokine
SLC binds the CXC chemokine receptor CXCR3. Proc Natl Acad Sci USA.
95:8205–8210. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma S, Stolina M, Luo J, Strieter RM,
Burdick M, Zhu LX, Batra RK and Dubinett SM: Secondary lymphoid
tissue chemokine mediates T cell-dependent antitumor responses in
vivo. J Immunol. 164:4558–4563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma S, Stolina M, Zhu L, Lin Y, Batra
R, Huang M, Strieter R and Dubinett SM: Secondary lymphoid organ
chemokine reduces pulmonary tumor burden in spontaneous murine
bronchoalveolar cell carcinoma. Cancer Res. 61:6406–6412.
2001.PubMed/NCBI
|
|
35
|
Nishimura N, Nishioka Y, Shinohara T,
Ogawa H, Yamamoto S, Tani K and Sone S: Novel centrifugal method
for simple and highly efficient adenovirus-mediated green
fluorescence protein gene transfection into human monocyte-derived
dendritic cells. J Immunol Methods. 253:113–124. 2001. View Article : Google Scholar
|
|
36
|
Nishimura N, Nishioka Y, Shinohara T and
Sone S: Enhanced efficiency by centrifugal manipulation of
adenovirus-mediated interleukin 12 gene transduction into human
monocyte-derived dendritic cells. Hum Gene Ther. 12:333–346. 2001.
View Article : Google Scholar
|
|
37
|
Arthur JF, Butterfield LH, Roth MD, Bui
LA, Kiertscher SM, Lau R, Dubinett S, Glaspy J, McBride WH and
Economou JS: A comparison of gene transfer methods in human
dendritic cells. Cancer Gene Ther. 4:17–25. 1997.PubMed/NCBI
|
|
38
|
Zhong L, Granelli-Piperno A, Choi Y and
Steinman RM: Recombinant adenovirus is an efficient and
non-perturbing genetic vector for human dendritic cells. Eur J
Immunol. 29:964–972. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fasbender A, Zabner J, Chillón M, Moninger
TO, Puga AP, Davidson BL and Welsh MJ: Complexes of adenovirus with
polycationic polymers and cationic lipids increase the efficiency
of gene transfer in vitro and in vivo. J Biol Chem. 272:6479–6489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dietz AB and Vuk-Pavlović S: High
efficiency adenovirus-mediated gene transfer to human dendritic
cells. Blood. 91:392–398. 1998.PubMed/NCBI
|
|
41
|
Christopherson KW II, Campbell JJ and
Hromas RA: Transgenic overexpression of the CC chemokine CCL21
disrupts T-cell migration. Blood. 98:3562–3568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Förster R, Schubel A, Breitfeld D, Kremmer
E, Renner-Müller I, Wolf E and Lipp M: CCR7 coordinates the primary
immune response by establishing functional microenvironments in
secondary lymphoid organs. Cell. 99:23–33. 1999.PubMed/NCBI
|
|
43
|
Bromley SK, Thomas SY and Luster AD:
Chemokine receptor CCR7 guides T cell exit from peripheral tissues
and entry into afferent lymphatics. Nat Immunol. 6:895–901. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bacon KB, Premack BA, Gardner P and Schall
TJ: Activation of dual T cell signaling pathways by the chemokine
RANTES. Science. 269:1727–1730. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dairaghi DJ, Soo KS, Oldham ER, Premack
BA, Kitamura T, Bacon KB and Schall TJ: RANTES-induced T cell
activation correlates with CD3 expression. J Immunol. 160:426–433.
1998.PubMed/NCBI
|
|
46
|
Appay V, Dunbar PR, Cerundolo V, McMichael
A, Czaplewski L and Rowland-Jones S: RANTES activates
antigen-specific cytotoxic T lymphocytes in a mitogen-like manner
through cell surface aggregation. Int Immunol. 12:1173–1182. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo Z, Zhang M, Tang H and Cao X: Fas
signal links innate and adaptive immunity by promoting
dendritic-cell secretion of CC and CXC chemokines. Blood.
106:2033–2041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Flanagan K, Moroziewicz D, Kwak H, Hörig H
and Kaufman HL: The lymphoid chemokine CCL21 costimulates naive T
cell expansion and Th1 polarization of non-regulatory
CD4+ T cells. Cell Immunol. 231:75–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Marsland BJ, Bättig P, Bauer M, Ruedl C,
Lässing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L,
Nakano H, Nembrini C, Saudan P, Kopf M and Bachmann MF: CCL19 and
CCL21 induce a potent proinflammatory differentiation program in
licensed dendritic cells. Immunity. 22:493–505. 2005. View Article : Google Scholar : PubMed/NCBI
|