Introduction
Claudin family consists of 24 subtypes of essential
tight junction (TJ) integral membrane proteins that have molecular
weights of 20–27 kDa and contain four transmembrane domains
(1–3). Tight junctions are responsible for the
formation and maintenance of the permeability barrier in polarized
epithelial cells. The gene has subsequently been localized to
epithelial cells in a variety of tissues, including oral squamous
epithelium, and its protein shows tissue-specific distribution
patterns (4–6). It has become clear that, in addition
to having tissue- and cell-specific features, modification or loss
of these dynamic structures contributes to cancerization (7–10).
However, how the role of claudin-7 contributes to cancerization in
squamous cell carcinoma remains to be elucidated, and the patterns
of claudin-7 expression in carcinoma vary as shown below (3,11–18).
Loss of claudin-7 has been reported to correlate with a poor
prognosis in esophageal, colorectal, and nasopharyngeal cancers
(3,11–14).
On other hand, upregulation of claudin-7 has been reported to
correlate with poor prognosis of carcinogenesis in ovarian, breast,
and gastric carcinomas (15–18).
The TNM classification which was proposed by the
Union Internationale Contre le Cancer (UICC) (19) is a good system for describing the
condition of cancer patients. However, this system cannot predict
the biological characteristics of tumor cells. It is there
therefore important to look for new objective prognostic factors
that provide additional information on the biological
characteristics of tumors. It is believed that invasion and
metastasis are the most crucial characteristics of malignant
tumors. Thus, mode of invasion is used as a histopathological
classification category in oral squamous cell carcinoma (OSCC), as
described by Yamamoto et al(20), and this classification is frequently
used to predict progression, metastasis and prognosis (20–25)
(Table I). To provide proper
treatment, it is also important to examine the characteristics of
cancer cells at the invasive front of OSCC.
 | Table IYamamoto-Kohama classification. |
Table I
Yamamoto-Kohama classification.
| Grade | Histologic
grading |
|---|
| 1 | Well-defined
borderline |
| 2 | Cords, less marked
borderline |
| 3 | Groups of cells, no
distinct borderline |
| 4C | Diffuse invasion,
Cord-like type |
| 4D | Diffuse invasion,
Widespread type |
We examined immunohistochemically the expression of
claudin-7 in vivo and compared its expression in cell lines
derived from invasive OSCC in vitro to investigate the
interrelationship between clinicopathological factors including
criteria on mode of invasion and claudin-7 expression in OSCC.
Materials and methods
Specimens
Sixty-seven biopsy specimens of primary OSCC were
obtained from patients undergoing surgical resection at the
Department of Oral and Maxillofacial Surgery, Kanazawa University
Hospital between 1989 and 2009. The patients (38 male and 29 female
subjects) ranged in age from 32 to 91 years (mean age: 60 years).
Informed consent for experimental use of the samples was obtained
from the patients according to the hospital’s ethical
guidelines.
Staining methods
Immunohistochemical staining was performed by the
labeled streptavidin-biotin (LSAB) method after deparaffinization
and rehydration as described by Nozaki et al(25). The sections were reacted with the
following primary antibodies: anti-claudin-7 monoclonal antibody
(Invitrogen Corp., Camarillo, CA, USA) diluted 200-fold with PBS at
4°C overnight. Sections were then reacted with a secondary
antibody, biotin-labeled goat anti-rabbit immunoglobulin polyclonal
antibody (Dako Japan, Kyoto, Japan) at RT for 60 min. A section of
normal oral epithelium previously identified as strong staining was
used as a positive control with each batch. As a negative control,
PBS treated sections instead of claudin-7 antibody was used.
Cell culture and cell lines
All cell lines were maintained at 37°C in a
humidified incubator containing 5% CO2. The OSCC cell
lines HSC-4, OSC-20, OSC-19, OTC-04, HOC313 and TSU were maintained
in minimal essential medium (MEM; Sigma-Aldrich, Ayrshire, UK)
supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin. The cell lines were derived from OSCC with
the following grades of invasiveness, according to the
Yamamoto-Kohama criteria (20):
HSC-4 and OSC-20 cells from grade 3 as described for the low
invasive type; OSC-19 and OTC-04 from grade 4C, as described for
the mild invasive type; HOC313 and TSU from 4D as described for the
high invasive types.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
RT-PCR analysis was performed using a modified
method by Conboy et al(26).
RNA was extracted from cultured cells using an RNeasy kit (Qiagen,
Hilden, Germany). A 1-μg sample in 10 μl of RNase free water was
incubated for 5 min at 60°C and then quickly chilled on ice for 5
min. The RNA samples were reversed-transcribed into first-strand
cDNA at 40°C for 40 min in RT solution from the RNeasy kit. The
cDNA samples were amplified following addition of the PCR mixture
solution and the following primers for claudin-7, 5′-aat gta cga
ctc ggt gct cg-3′ (forward) and 5′-att ccc agg aca gga aca gg-3′
(reverse); for E-cadherin, 5′-agc cat ggg ccc ttg gag-3′ (forward)
and 5′-cca gag gct ctg tca cct tc-3′ (reverse); for Snail, 5′-acc
act atg ccg cgc tct ttc ctc g-3′ (forward) and 5′-gac agg aga agg
gct tct cgc cag t-3′ (reverse) and for β-actin, 5′-gaa aat ctg gca
cca cac ctt-3′ (forward) and 5′-ttg aag gta gtt tcg tgg at-3′
(reverse). PCRs were carried out under the following conditions: 3
min at 94°C, followed by cycles (30 for claudin-7, 30 for
E-cadherin, 30 for Snail and 20 for β-actin) of 1 min at 94°C, 1
min at 58°C, and 1 min at 72°C. All reactions were completed with a
final incubation at 72°C for 10 min. The lengths for the amplified
fragments for claudin-7, E-cadherin, Snail and β-actin genes were
288, 544, 637 and 592 bp, respectively. PCR products were detected
by 3.0% agarose gel electrophoresis and staining with ethidium
bromide.
Western blot analysis
Cultured cells on 80% confluent plates were used for
protein samples. The protein samples (30 μg) that were extracted
from the whole cellular structure using M-PER (Mammalian protein
extraction reagent) (Pierce, Rockford, IL, USA) were heated at 95°C
for 5 min before electrophoresis and then subjected to 10%
SDS-PAGE. After electrophoresis, the samples were transferred onto
PVDF membranes (ATTO Co., Tokyo, Japan) and incubated for 1 h with
200-fold diluted polyclonal anti-rabbit antibody against claudin-7
(Invitrogen, Carlsbad, CA, USA), a 2,000-fold diluted polyclonal
anti-mouse antibody against E-cadherin (BD Biosciences, San Jose,
CA, USA), a polyclonal anti-rabbit antibody against Snail (Abgent,
San-Diego, CA, USA) and 5000-fold diluted polyclonal anti-mouse
antibody β-actin (Sigma, St. Louis, MO, USA) respectively. The
membrane was washed three times with PBS and then incubated for 1 h
with 2000-fold diluted horseradish peroxidase-conjugated
anti-rabbit IgG (Amersham, Buckinghamshire, UK) to detect claudin-7
and Snail, 2000-fold diluted horseradish peroxidase-conjugated
anti-mouse IgG (Amersham) to detect E-cadherin and β-actin,
respectively. The blots were revealed by enhanced chemiluminescent
detection carried out according to the manufacturer’s
recommendations.
Assessment of immunohistochemical
staining of claudin-7 proteins
Statistical analysis was performed with the SPSS for
window version 16.0 (SPSS Inc., Chicago, IL, USA). The expression
of claudin-7 in tumor cells was evaluated as present or absent.
Only cases in which at least 25% of the tumor cells were
immunoreactive were scored as positive. The Mann-Whitney’s U test
and χ2 test were used to analyze the association of
claudin-7 expression with clinicopathological factors. Survival
rates of claudin-7 -positive and -negative patients were calculated
by the Kaplan-Meyer method, and examined for statistical
significance using the log-rank test. Differences were considered
significant at p-values of <0.05. Uni- and multi-variate
analyses for the 5-year overall survival of individual parameters
were performed.
Results
Immunohistochemistry and evaluation
The relationship between the clinicopathological
parameters and expression of claudin-7 is summarized in Table II. Claudin-7 immunostaining was
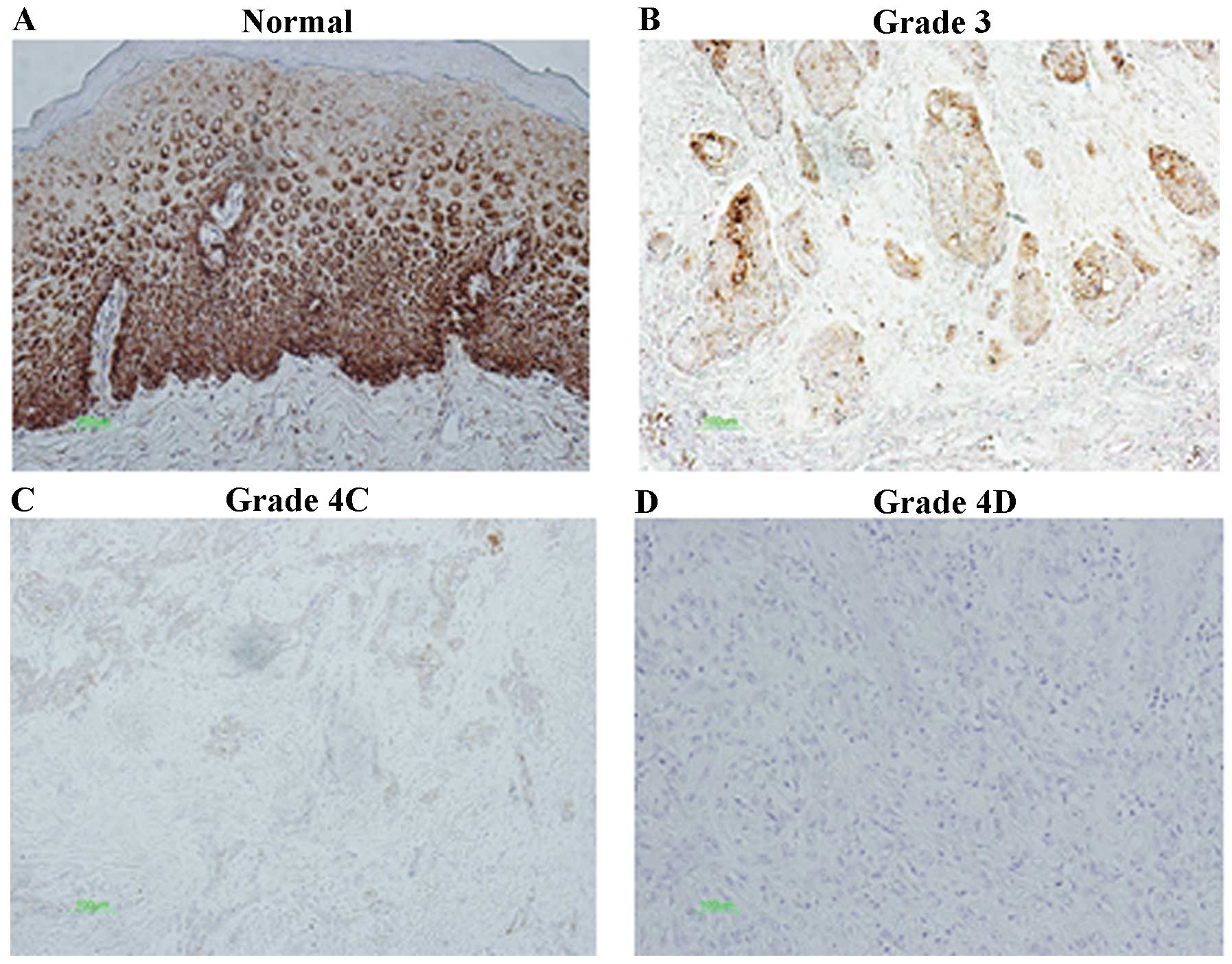
observed especially in membranes including the cytoplasm and
nucleus of tumor cells (Fig. 1).
Immunohistochemical staining showed that 35 specimens (52.2%) were
positive for claudin-7. There was a significant negative
correlation between claudin-7 and T-category (p<0.05), Lymph
node metastasis (p<0.01) and mode of invasion (p<0.001); the
number of claudin-7 positive cases were 11 (91.7%) for grade 1, 12
(75.0%) for grade 2, 9 (52.9%) for grade 3, 3 (18.8%) for grade 4C,
and 0 (0%) for grade 4D. Moreover, the number of claudin-7-positive
cases was 5 (26.3%) with lymph node metastasis and 30 (62.5%)
without lymph node metastasis. Therefore, claudin-7 expression
showed a negative correlation with lymph node metastasis
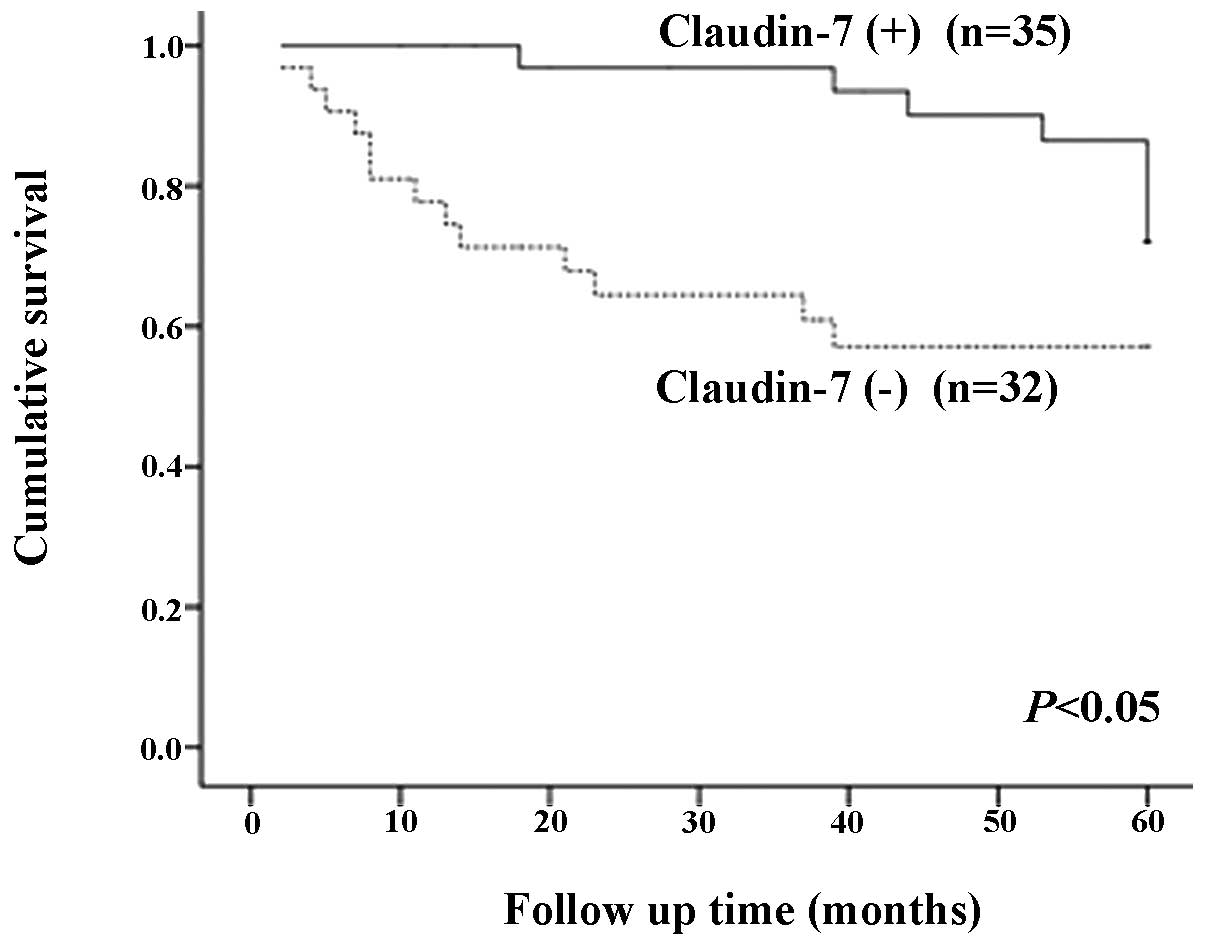
(p<0.01). The overall 5 year-survival rate was 77.1% in patients
showing claudin-7 expression and 59.4% in patients without
claudin-7 expression (P<0.05) (Fig.
2). Table III summarizes the
univariate and multivariate analyses of the correlation between the
clinicopathological and immunohistochemical variables, with respect
to overall survival. Multivariate analysis revealed that only the
3-4D mode of invasion was significant and independent variables
with relative risks of 7.44, although univariate analysis revealed
that T-category, N-category, Cell differentiation, mode of
invasion, expression of claudin-7 were also significant
variables.
 | Table IIClinicopathological parameters in
relation to claudin-7 expression. |
Table II
Clinicopathological parameters in
relation to claudin-7 expression.
| Parameter | Positive (%) | Negative (%) | Total |
|---|
| Age, years |
| ≥65 | 11 (47.8) | 12 (52.2) | 23 |
| <65 | 24 (54.5) | 20 (45.5) | 44 |
| Gender |
| Male | 20 (52.6) | 18 (47.4) | 38 |
| Female | 15 (51.7) | 14 (48.3) | 29 |
| Tumor site |
| Tongue | 20 (51.3) | 19 (49.7) | 39 |
| Gingiva | 6 (40.0) | 9 (60.0) | 15 |
| Oral floor | 4 (80.0) | 1 (20.0) | 5 |
| Buccal | 5 (71.4) | 2 (28.6) | 7 |
| Lip | 0 (0.0) | 1 (100.0) | 1 |
| T-category |
| T1 | 15 (75.0) | 5 (25.0) | 20a |
| T2 | 16 (48.5) | 17 (51.5) | 33a |
| T3 | 1 (25.0) | 4 (75.0) | 5a |
| T4 | 3 (33.3) | 6 (66.7) | 9a |
| Lymph node
metastasis |
| Negative | 30 (62.5) | 18 (37.5) | 48b |
| Positive | 5 (26.3) | 14 (73.7) | 19b |
| Differential
type |
| Well | 25 (58.1) | 18 (41.9) | 43 |
| Moderately | 9 (50.0) | 9 (50.0) | 18 |
| Poorly | 1 (16.7) | 5 (83.3) | 6 |
| Mode of
invasion |
| 1 | 11 (91.7) | 1 (8.3) | 12c |
| 2 | 12 (75.0) | 4 (25.0) | 16c |
| 3 | 9 (52.9) | 8 (47.1) | 17c |
| 4C | 3 (18.8) | 13 (81.2) | 16c |
| 4D | 0 (0.0) | 6 (100.0) | 6c |
| Total | 35 (52.2) | 32 (47.8) | 67 |
 | Table IIIUnivariate and multivariate analyses
for clinical parameters, claudin-7 expression in relation to
overall survival of 67 patients with oral squamous cell
carcinoma. |
Table III
Univariate and multivariate analyses
for clinical parameters, claudin-7 expression in relation to
overall survival of 67 patients with oral squamous cell
carcinoma.
| Variables | Clinical
groups | Survivors | Non-survivors | Log rank | Cox regression | Risk ratio (95%
CI) |
|---|
| |
| |
|---|
| n=43 | n=24 | χ2 | p-value | p-value |
|---|
| T category | T3,4/T1,2 | 5/38 | 9/15 | 10.48 | 0.0012 | NS | NS |
| N category |
N+/N0 | 8/35 | 11/13 | 8.79 | 0.0030 | NS | NS |
| Cell
differentiation | Mod-poor/Well | 12/31 | 12/12 | 5.63 | 0.018 | NS | NS |
| Mode of
invasion | 3-4D/1–2 | 18/25 | 21/3 | 14.46 | 0.0001 | 0.0012 | 7.44
(2.221–25.06) |
| Claudin-7 | +/− | 27/16 | 8/16 | 6.67 | 0.0098 | NS | NS |
Analysis of claudin-7 mRNA, Snail and
E-cadherin levels in OSCC cell lines by RT-PCR
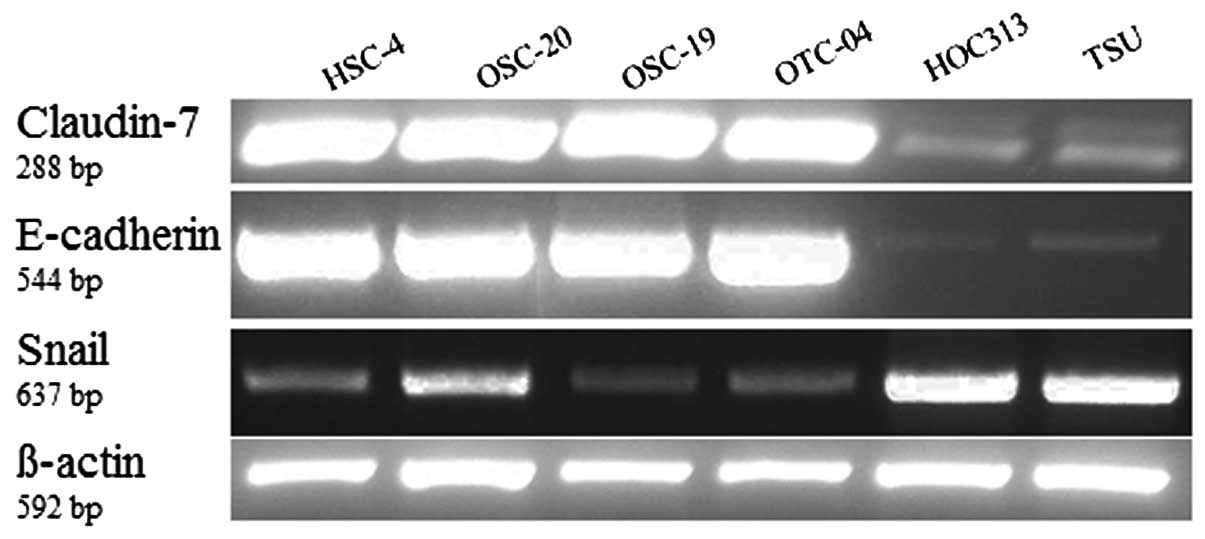
We further examined the levels of claudin-7 mRNAs,
E-cadherin and Snail in six cell lines by RT-PCR. Expressions of
claudin-7 and E-cadherin were significantly lower in the HOC313
cells and TSU cells (grade 4D) while expression of Snail was higher
in grade 4D than in the other cell lines (Fig. 3).
Analysis of claudin-7, Snail, E-cadherin
protein levels in OSCC cell lines by western blotting
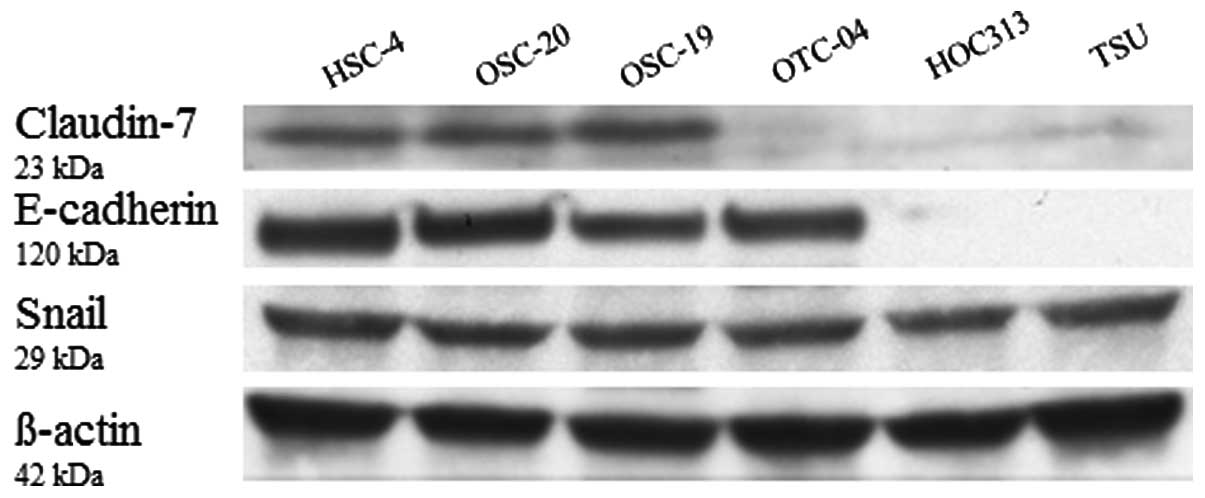
The expression of claudin-7 and E-cadherin protein
was significantly lower in the HOC313 and TSU cell lines (grade 4D)
while the expression of Snail was approximately consistent with the
other cell lines (Fig. 4).
Discussion
Oral squamous cell carcinoma is characterized by a
high degree of invasion into local tissue, as well as high
incidence of lymph node metastasis. However, there have been few
reports on the association between the expression of claudin-7 and
the invasive potential in OSCC. In this study, multivariate
analysis revealed that only mode of invasion in 3-4D was
significant. In addition, independent variables with relative risks
of 7.44 were significant variables. However, univariate analysis
revealed that T-category, N-category, cell differentiation, mode of
invasion, and expression of claudin-7 were also significant. Even
if limited to head and neck squamous cell carcinoma, how the
pattern of claudin-7 expression correlates with invasion and
metastasis is controversial. For example, Lourenço et
al(1) reported that loss of
claudin-7 is associated with the pathogenesis and a poor prognosis.
On the other hand Bello et al(27) stated that medium immunoreactivity of
claudin-7 tends to be associated with improved survival compared
with strong and low immunoreactivity. This study showed that as the
invasiveness of OSCC increased, the expression of claudin-7 became
weaker, while studies on Snail, which reported on the suppression
of E-cadherin and claudin, showed stronger expression in the most
invasive mode type 4D, which has the characteristics of EMT such as
spindly shape and decreased expression of E-cadherin (23,28).
Epithelial-mesenchymal transition (EMT) is one of
the mechanisms by which epithelial cells acquire the motile
properties required for invasion. Acquisition of the mesenchymal
state, with fibroblastic phenotype, is accompanied by E-cadherin
downregulation and upregulation of mesenchymal markers such as
Snail, enabling cells to dissociate from the epithelial tissue and
migrate (29). An inverse
correlation between Snail and E-cadherin expression has been
reported in many types of cancers including squamous cell carcinoma
(30). Snail binds to E-boxes
present in the E-cadherin promoter, consequently repressing
E-cadherin transcription (28–31).
In addition, induction of Snail expression causes loss of TJ
integral membrane proteins such as claudin by a similar mechanism
(6,31). Accordingly, Snail may functions as
an effector for EMT, and it enhances the invasive capacity of
squamous cell carcinoma through the regulation of proteolytic
enzymes, including claudin-7 in the course of EMT.
It is difficult to determine the difference between
the grade 4C type and the grade 4D type. As such, the diagnostic
criteria are based solely on histopathological findings and this
has created unevenness in judgments between institutions in Japan.
Diagnosis may be facilitated by applying the expression of adhesion
targets such as claudin-7 and E-cadherin, and the expression of
Snail to discriminate grade 4C from grade 4D. Moreover, new
identification criteria of grade 4D that include evaluation of the
property of adhesion and EMT may enhance the precision of the YK
criteria as a prognosis marker and contribute beneficially to the
development of strategies for OSCC treatment.
In conclusion, claudin-7 may be a useful marker to
identify the potential for progression with a central focus on
invasion in OSCC. It is necessary to clarify the mechanism between
claudin-7 expression and the process of malignant progression of
OSCC though continued research and its clinical application.
Acknowledgements
We would like to thank all the members of our
department for their helpful suggestions and support. This study
was supported by a Grant-in-Aid for Scientific Research (no.
24792194) from the Ministry of Education, Science, Sports and
Culture of Japan.
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
TJ
|
tight junction
|
|
UICC
|
Union International Contre le
Cancer
|
|
LSAB
|
labeled streptavidin-biotin
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Lourenço SV, Coutinho-Camillo CM, Buim ME,
et al: Claudin-7 down-regulation is an important feature in oral
squamous cell carcinoma. Histopathology. 57:689–698.
2010.PubMed/NCBI
|
|
2
|
Lu Z, Ding L, Hong H, Hoggard J, Lu Q and
Chen YH: Claudin-7 inhibits human lung cancer cell migration and
invasion through ERK/MAPK signaling pathway. Exp Cell Res.
317:1935–1946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lioni M, Brafford P, Andl C, et al:
Dysregulation of claudin-7 leads to loss of E-cadherin expression
and the increased invasion of esophageal squamous cell carcinoma
cells. Am J Pathol. 170:709–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turksen K and Troy TC: Junctions gone bad:
claudins and loss of the barrier in cancer. Biochim Biophys Acta.
1816:73–79. 2011.PubMed/NCBI
|
|
5
|
Cereijido M, Contreras RG, Shoshani L,
Flores-Benitez D and Larre I: Tight junction and polarity
interaction in the transporting epithelial phenotype. Biochim
Biophys Acta. 1778:770–793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsukita S, Yamazaki Y, Katsuno T and
Tamura A: Tight junction-based epithelial microenvironment and cell
proliferation. Oncogene. 27:6930–6938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marchiando AM, Graham WV and Turner JR:
Epithelial barriers in homeostasis and disease. Annu Rev Pathol.
5:119–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin TA and Jiang WG: Tight junctions
and their role in cancer metastasis. Histol Histopathol.
16:1183–1195. 2001.PubMed/NCBI
|
|
10
|
Mullin JM, Agostino N, Rendon-Huerta E and
Thornton JJ: Keynote review: epithelial and endothelial barriers in
human disease. Drug Discov Today. 10:395–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Usami Y, Chiba H, Nakayama F, et al:
Reduced expression of claudin-7 correlates with invasion and
metastasis in squamous cell carcinoma of the esophagus. Hum Pathol.
37:569–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bornholdt J, Friis S, Godiksen S, et al:
The level of claudin-7 is reduced as an early event in colorectal
carcinogenesis. BMC Cancer. 11:652011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsueh C, Chang YS, Tseng NM, et al:
Expression pattern and prognostic significance of claudins 1, 4,
and 7 in nasopharyngeal carcinoma. Hum Pathol. 41:944–950. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oshima T, Kunisaki C, Yoshihara K, et al:
Reduced expression of the claudin-7 gene correlates with venous
invasion and liver metastasis in colorectal cancer. Oncol Rep.
19:953–959. 2008.PubMed/NCBI
|
|
15
|
Kim CJ, Lee JW, Choi JJ, et al: High
claudin-7 expression is associated with a poor response to
platinum-based chemotherapy in epithelial ovarian carcinoma. Eur J
Cancer. 47:918–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernardi MA, Logullo AF, Pasini FS, et al:
Prognostic significance of CD24 and claudin-7 immunoexpression in
ductal invasive breast cancer. Oncol Rep. 27:28–38. 2012.PubMed/NCBI
|
|
17
|
Park JY, Park KH, Oh TY, et al:
Up-regulated claudin 7 expression in intestinal-type gastric
carcinoma. Oncol Rep. 18:377–382. 2007.PubMed/NCBI
|
|
18
|
Dahiya N, Becker KG, Wood WH, Zhang Y and
Morin PJ: Claudin-7 is frequently overexpressed in ovarian cancer
and promotes invasion. PLoS One. 6:e221192011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cancer UICl. TNM classification of
malignant tumors. 5th edition. Wiley-Less; New York: 1997
|
|
20
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshizawa K, Nozaki S, Kitahara H, et al:
Copper efflux transporter (ATP7B) contributes to the acquisition of
cisplatin-resistance in human oral squamous cell lines. Oncol Rep.
18:987–991. 2007.PubMed/NCBI
|
|
22
|
Yoshizawa K, Nozaki S, Okamune A, et al:
Loss of maspin is a negative prognostic factor for invasion and
metastasis in oral squamous cell carcinoma. J Oral Pathol Med.
38:535–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taki M, Kamata N, Yokoyama K, Fujimoto R,
Tsutsumi S and Nagayama M: Down-regulation of Wnt-4 and
up-regulation of Wnt-5a expression by epithelial-mesenchymal
transition in human squamous carcinoma cells. Cancer Sci.
94:593–597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawashiri S, Kumagai S, Kojima K, Harada H
and Yamamoto E: Development of a new invasion and metastasis model
of human oral squamous cell carcinomas. Eur J Cancer B Oral Oncol.
31B:216–221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nozaki S, Endo Y, Kawashiri S, et al:
Immunohistochemical localization of a urokinase-type plasminogen
activator system in squamous cell carcinoma of the oral cavity:
association with mode of invasion and lymph node metastasis. Oral
Oncol. 34:58–62. 1998. View Article : Google Scholar
|
|
26
|
Conboy JG, Chan J, Mohandas N and Kan YW:
Multiple protein 4.1 isoforms produced by alternative splicing in
human erythroid cells. Proc Natl Acad Sci USA. 85:9062–9065. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bello IO, Vilen ST, Niinimaa A, Kantola S,
Soini Y and Salo T: Expression of claudins 1, 4, 5, and 7 and
occludin, and relationship with prognosis in squamous cell
carcinoma of the tongue. Hum Pathol. 39:1212–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu LF, Hu Y, Yang CC, et al: Snail
overexpression induces an epithelial to mesenchymal transition and
cancer stem cell-like properties in SCC9 cells. Lab Invest.
92:744–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mikami S, Katsube K, Oya M, et al:
Expression of Snail and Slug in renal cell carcinoma: E-cadherin
repressor Snail is associated with cancer invasion and prognosis.
Lab Invest. 91:1443–1458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Usami Y, Satake S, Nakayama F, et al:
Snail-associated epithelial-mesenchymal transition promotes
oesophageal squamous cell carcinoma motility and progression. J
Pathol. 215:330–339. 2008. View Article : Google Scholar
|