Introduction
Hepatocellular carcinoma (HCC) is one of the most
common gastrointestinal malignancies and the leading cause of
cancer-related death in East Asia and South Africa (1). At present, the first-line treatment
for HCC is liver transplantation or surgical resection (2,3).
However, the overall survival rate after curative therapy is not
satisfactory due to the highly chemoresistant nature of this tumor
and frequent intrahepatic recurrence. Identification of the genes
responsible for the development and progression of HCC and
comprehension of the clinical significance of these genes are
critical for adequate treatment of HCC.
Glutathione (GSH) is a tripeptide thiol consisting
of glutamate, cysteine and glycine, and it plays an important role
in cellular defenses against oxidative stress and toxic compounds
(4). Sustenance of GSH levels in
cancer cells is essential for DNA synthesis, growth, multidrug
resistance, maintenance of redox status and tumor survival
(5–8). System xc-,
consisting of cystine/glutamic acid transporter (xCT)/SLC7A11 and
its chaperone CD98/4F2hc, functions as an exchange system for
cysteine/glutamate (9). Since
glutamate present in the extracellular medium can regulate cell
signaling through its receptor, upregulation of
xc- in tumor cells may also be associated
with increased glutamate signaling in the tumor cells themselves or
in adjacent cells (10,11). In contrast to normal cells, tumor
cells are characterized by rapid growth and proliferation (5). This is partly due to the fact that
tumor cells have cellular defenses against oxidative stress,
facilitating the cell cycle and resistance to apoptosis. xCT is
strongly associated with these systems and is therefore a potential
therapeutic target (6,10). We identified a CD44 variant that
regulates redox status by stabilizing xCT as a pivotal marker of
gastric cancer (12), yet the
clinical relevance of xCT in HCC has not yet been clarified.
We subsequently investigated the clinical importance
of the xCT gene by analyzing 130 consecutive patients with HCC as
well as several HCC cell lines. We suggest that xCT expression is a
candidate marker of HCC prognosis.
Materials and methods
Clinical tissue samples
One hundred and thirty patients (106 men and 24
women) with HCC were enrolled and underwent curative first-line
surgery at the Department of Gastroenterological Surgery, Kumamoto
University Hospital, between 2005 and 2010. Specimens of primary
HCC and adjacent normal liver tissues were procured from the
patients after written informed consent was obtained. This study
was approved by the Human Ethics Review Committee of the Graduate
School of Life Sciences, Kumamoto University (Kumamoto, Japan).
Cell lines
The Li-7 cell line was purchased from Riken
BioResource Center (Osaka, Japan) and was cultured in RPMI1640
medium (Wako, Osaka, Japan). The HepG2, PLC/PRF/5, HuH1, HuH-7, HLE
and HLF cell lines were purchased from the Japanese Collection of
Research Bioresources (Osaka, Japan) and the cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM). All media were
supplemented with 10% fetal bovine serum (FBS) with 100 units/ml
penicillin and 100 μg/ml streptomycin. All cultures were maintained
in a 5% CO2/95% air humidified atmosphere at 37°C.
RNA extraction and quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was obtained from the frozen tissue
samples and cell lines using a mirVana microRNA isolation kit
(Ambion) in accordance with the manufacturer’s instructions.
Reverse transcription was performed with 1.0 μg of total RNA as
previously described (13). qRT-PCR
was performed on a LightCycler 480 II using 2X PCR Master Mix and
Universal Probe Library (all were from Roche Diagnostic, Tokyo,
Japan). Primers were designed using the Roche webpage (http://app.roche-biochem.jp/) and the Universal Probe
Library in accordance with the manufacturer’s recommendations. The
primers used were as follows: xCT forward,
5′-CCATGAACGGTGGTGTGTT-3′ and reverse, 5′-GACCCT CTCGAGACGCAAC-3′
and universal probe #80; HPRT forward,
5′-TGACCTTGATTTATTTTGCATACC-3′ and reverse,
5′-CGAGCAAGACGTTCAGTCCT-3′ and universal probe #73. HPRT, 18S
ribosomal RNA, and GAPDH were tested as internal controls (14); HPRT proved to be the most suitable
reference gene. For amplification, an initial denaturation at 95°C
for 10 min was followed by 15 sec at 95°C, 15 sec at 60°C and 13
sec at 72°C. All experiments were performed twice to confirm
reproducibility.
Western blotting
Cells were lysed in a cell lysis buffer containing
25 mM Tris (pH 7.4), 100 mM NaCl, and 1% Tween-20. Equal amounts of
protein were loaded onto 10% gels and separated by SDS-PAGE.
Resolved proteins were transferred to polyvinylidene fluoride
(PVDF) membranes (Bio-Rad), blocked with 5% low-fat dry milk in
TBS-T (25 mM Tris pH 7.4, 125 mM NaCl, 0.4% Tween-20) for 1 h at
room temperature and incubated with the primary antibody overnight
at 4°C. The primary antibody, mouse monoclonal xCT antibody (KE021;
TransGene Inc., Hyogo, Japan), was used at a dilution of 1:1,000.
Blots were extensively washed with TBS-T and incubated for 1 h at
room temperature with HRP-conjugated secondary antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) diluted 1:2,000 in TBS-T. The
membranes were washed and visualized using a chemiluminescence
detection reagent kit (ECL Plus; GE Healthcare Corp.).
Immunofluorescence staining
Approximately 6-μm cryostat sections of HCC were
fixed for 10 min in 4°C acetone and air-dried for 1 h. Incubated
cells were washed with PBS twice and then fixed for 10 min in 4%
paraformaldehyde. Subsequently, the sections/cells were washed
twice in TBS and once in TBS/Tween-20 (0.05%) and then incubated
with 3% bovine serum albumin (BSA) (Sigma, Japan) for 15 min to
block nonspecific protein binding sites. They were subsequently
incubated for 1 h at room temperature with the primary antibody in
TBS containing 1% BSA. The primary antibody, mouse monoclonal xCT
antibody, was used at a dilution of 1:25. Samples were incubated
for 1 h at room temperature in the dark with the secondary
antibody, goat anti-mouse IgG labeled with HiLyte Fluor™ 555
(Anaspec Inc., San Jose, CA, USA), in TBS containing 1% BSA. The
mounting reagent was applied using ProLong Gold including
4′,6-diamidino-2-phenylindole (Invitrogen, Japan). For negative
controls, a mouse IgG (Dako Co., Japan) was used instead of the
primary antibody. Images were obtained with a FV300 fluorescence
microscope (Olympus, Japan).
Statistical analysis
Statistical analyses were performed using JMP ver.
8.0 (SAS Institute, Cary, NC, USA). Values are expressed as the
means ± SD. Differences between groups were calculated by the
Wilcoxon test. P<0.05 was defined as indicative of a
statistically significant difference.
Results
Expression of xCT in clinical tissue
specimens and clinicopathological characteristics
We performed a qRT-PCR analysis with the primary HCC
specimens. xCT expression was calculated by xCT/hypoxanthine
phosphoribosyltransferase 1 (HPRT1) expression. For the
clinicopathological evaluation, patients were divided into 2 groups
based on expression status. The expression value of xCT was
detectable in 34 (26.1%) tumor tissues. Clinicopathological factors
related to the xCT expression status of the 130 patients are
summarized in Table I. xCT
expression was not correlated with any of the clinicopathological
factors.
 | Table IxCT expression and clinicopathological
characteristics of the HCC patients. |
Table I
xCT expression and clinicopathological
characteristics of the HCC patients.
| | xCT expression | |
|---|
| |
| |
|---|
| Clinicopathological
factors | n | High | Low | P-value |
|---|
| Age (years) |
| <66 | 62 | 12 | 50 | 0.0921 |
| ≥66 | 68 | 22 | 46 | |
| Gender |
| Male | 106 | 28 | 78 | 0.8867 |
| Female | 24 | 6 | 18 | |
| AFPb |
| <20 | 74 | 20 | 54 | 0.7945 |
| ≥20 | 56 | 14 | 42 | |
| PIVKAIIa |
| <69 | 61 | 12 | 49 | 0.1138 |
| ≥69 | 69 | 22 | 47 | |
| Tumor diameter
(mm)a |
| <38 | 63 | 12 | 51 | 0.0738 |
| ≥38 | 67 | 22 | 45 | |
| Tumor number |
| Solitary | 97 | 25 | 72 | 0.8655 |
| Multiple | 33 | 9 | 24 | |
| Differentiation |
| Well/Mod | 107 | 26 | 81 | 0.2993 |
| Poor | 23 | 8 | 15 | |
| Vascular
invasionc |
| Negative | 72 | 17 | 55 | 0.4623 |
| Positive | 58 | 17 | 41 | |
| HCV-Ab |
| Negative | 72 | 21 | 51 | 0.3838 |
| Positive | 58 | 13 | 45 | |
| HBs-Ag |
| Negative | 91 | 25 | 66 | 0.6013 |
| Positive | 39 | 9 | 30 | |
Relationship between xCT expression and
prognosis
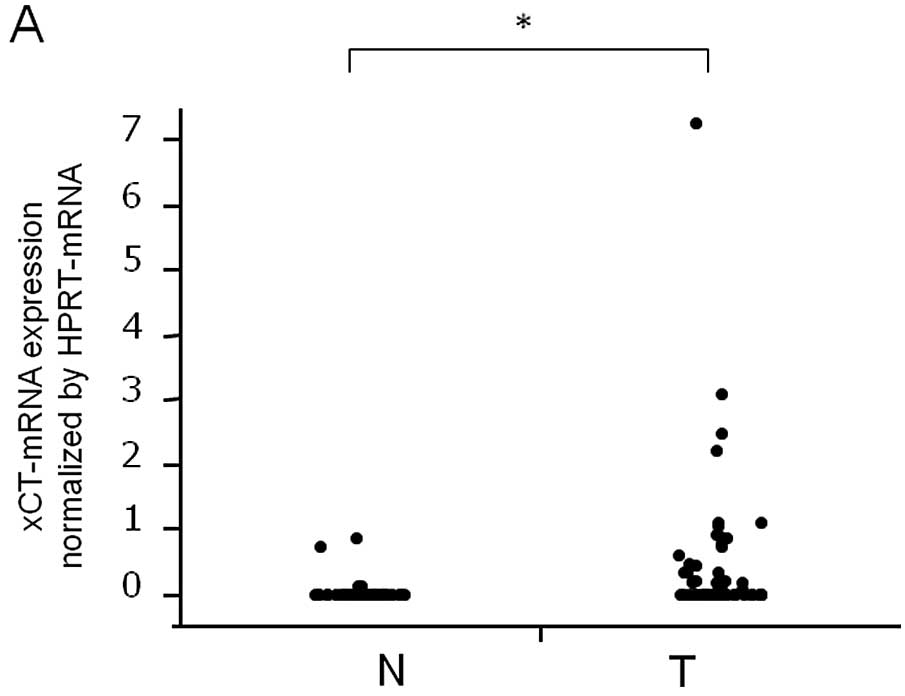
xCT mRNA expression was higher in HCC tissues than
in the corresponding normal tissues according to qRT-PCR analysis
(P<0.0001, Fig. 1A). The
relationship between each of the clinicopathological factors and
prognosis was analyzed by univariate analyses (Table II). The data indicated that poor
prognosis in HCC patients was correlated with a tumor diameter of
>38 mm, multiple tumors, positive vascular invasion and positive
xCT expression. Patients in the xCT mRNA present group had poorer
overall and disease-free survival than did those in the xCT mRNA
absent group (P=0.0130 and 0.0416, respectively) (Fig. 1B and C). The presence of xCT mRNA
expression proved to be the only poor prognostic factor in a
multivariate analysis of overall survival (Table III).
 | Table IIUnivariate analysis of the
clinicopathological factors for overall survival in HCC
patients. |
Table II
Univariate analysis of the
clinicopathological factors for overall survival in HCC
patients.
| Clinicopathological
factors | n | Median survival
(months) | P-value |
|---|
| Agea (years) |
| <66 | 62 | 43.0 | 0.2836 |
| ≥66 | 68 | 37.3 | |
| Gender |
| Male | 106 | 40.1 | 0.4509 |
| Female | 24 | 44.1 | |
| AFPb |
| <20 | 69 | 38.7 | 0.3001 |
| ≥20 | 63 | 41.1 | |
| PIVKAIIa |
| <69 | 61 | 42.2 | 0.1658 |
| ≥69 | 69 | 38.2 | |
| Tumor
diametera (mm) |
| <38 | 63 | 45.2 | <0.0001 |
| ≥38 | 67 | 33.1 | |
| Tumor number |
| Solitary | 97 | 41.2 | 0.0014 |
| Multiple | 33 | 36.6 | |
|
Differentiation |
| Well/Mod | 107 | 41.7 | 0.0913 |
| Poor | 23 | 37.3 | |
| Vascular
invasionc |
| Negative | 72 | 42.7 | 0.0108 |
| Positive | 58 | 38.1 | |
| HCV-Ab |
| Negative | 72 | 37.0 | 0.5459 |
| Positive | 58 | 43.8 | |
| HBs-Ag |
| Negative | 91 | 42.3 | 0.3656 |
| Positive | 39 | 36.6 | |
| xCT-mRNA
expression |
| Negative | 96 | 41.1 | 0.0130 |
| Positive | 39 | 37.0 | |
 | Table IIICox proportional hazards model for
overall survival of the HCC patients. |
Table III
Cox proportional hazards model for
overall survival of the HCC patients.
| Clinicopathological
factor | P-value | Risk ratio (95%
CI) |
|---|
| High xCT
expression | 0.0390 | 1.684
(1.026–2.915) |
| Multiple
tumors | 0.1034 | 1.558
(0.917–2.807) |
| Tumor diameter ≥38
mm | 0.2398 | 1.303
(0.839–2.048) |
| Vascular invasion,
positive | 0.9867 | 1.004
(0.641–1.553) |
Expression of xCT protein
All of the cell lines expressed xCT mRNA (Fig. 2A), which corresponded with the
expression of xCT protein (Fig.
2B). The representative immunohistochemical xCT staining
patterns are shown in Fig. 2;
membranous expression of xCT was confirmed in both the cell lines
(Fig. 2C) and the tissues from HCC
patients (Fig. 2D and E). One of
the 8 randomly selected patients had xCT protein staining of the
tumor cell membrane but not in the non-tumor tissue. The remaining
7 patients had no xCT expression in normal or cancerous
tissues.
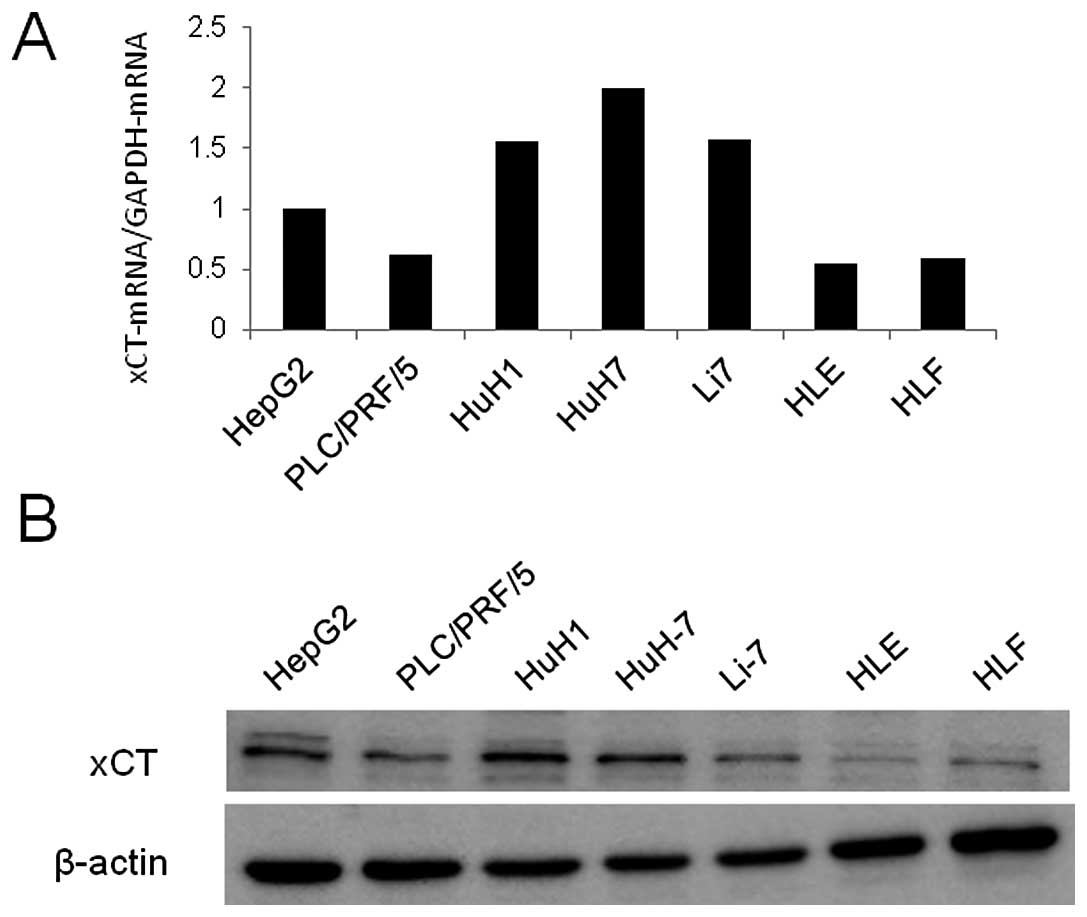 | Figure 2xCT protein expression in HCC cell
lines and human HCC tissue samples. (A) xCT mRNA expression in 7
HCC cell lines (HepG2, PLC/PRF/5, HuH1, HuH-7, Li7, HLE and HLF)
analyzed by quantitative RT-PCR. (B) xCT protein expression in the
7 HCC cell lines analyzed by western blotting. xCT expression in
(C) HuH1 cells and in (D and E) human tissue specimens (sample no.
165) assessed by immunofluorescence staining. T, tumor tissue; N,
normal tissue. Magnification, ×200 in D, magnification, ×600 in
E. |
Discussion
The qRT-PCR results and multivariate analysis
confirmed that the presence of xCT mRNA expression is an
independent predictive factor for poor prognosis in HCC patients.
Previous reports have shown that xCT expression plays a functional
role in tumor progression. Inhibition of the transporter function
with compounds such as sulfasalazine or (S)-4-carboxyphenylglycine
suppresses tumor cell growth and invasion in glioma and HCC
(5,11,15).
Overexpression of xCT, which results in increased
xc- activity, increases the levels and
activity of the transcription factor AP-1 and promotes the cell
cycle (16). In addition, xCT plays
an important role in drug resistance in several types of tumors
in vitro(17,18).
xCT mRNA expression was significantly higher in
tumor tissues than in normal tissues in the HCC patients. Although
xCT expression is not specific to tumor cells and has also been
observed in normal cell types such as fibroblasts (19), monocytes (20) and macrophages (21), our results indicate that functional
demand for xCT was higher in tumor tissues than in normal tissues.
However, we did not find any correlation between xCT mRNA
expression and clinicopathological factors. Further studies with
human subjects are required with the aim of determining the
relevance of xCT expression to functional aspects of tumors, such
as reactive oxygen species (ROS) level, expression of other amino
transporters and chemosensitivity.
We attempted to confirm the localization of xCT in
the tumor cell membrane since xCT is believed to function only in
the form of a membranous protein and since few reports have found
xCT expression on the tumor cell membrane (10,12).
We confirmed the localization of the xCT protein using frozen HCC
human tissues as well as HCC cell lines. Positive xCT expression
was much higher in the HCC cell lines than in the human tissue
samples as the cell lines were incubated with cysteine and not
cystine, resulting in xCT upregulation (22).
Sulfasalazine is a potent xCT inhibitor and has been
used for the clinical treatment of inflammatory bowel disease and
rheumatoid arthritis (23); it has
been shown to arrest growth via cystine starvation in various types
of cancer cells, including lymphoma, prostate cancer, HCC and
breast cancer (6,15,24,25).
Guo et al(15) demonstrated
that xCT dysfunction increased intracellular ROS levels, resulting
in the autophagic death of HCC cells. Only a few of our patients
with HCC showed high xCT expression in this study and these
patients could be candidates for xCT-targeted therapy.
In conclusion, the present study suggests that xCT
is useful as a predictive marker for patient prognosis and may be a
novel therapeutic target for HCC. We expect that the results of
this study will aid in the selection of patients and in customizing
therapy targeting xCT.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
xCT
|
transporter responsible for amino acid
transport system xc-
|
|
HPRT1
|
hypoxanthine phosphoribosyltransferase
1
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 62:10–29. 2012.
|
|
2
|
Carr BI: Hepatocellular carcinoma: current
management and future trends. Gastroenterology. 127(Suppl 1):
S218–S224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kassahun WT, Fangmann J, Harms J, et al:
Liver resection and transplantation in the management of
hepatocellular carcinoma: a review. Exp Clin Transplant. 4:549–558.
2006.PubMed/NCBI
|
|
4
|
Griffith OW: Biologic and pharmacologic
regulation of mammalian glutathione synthesis. Free Radic Biol Med.
27:922–935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung WJ, Lyons SA, Nelson GM, et al:
Inhibition of cystine uptake disrupts the growth of primary brain
tumors. J Neurosci. 25:7101–7110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doxsee DW, Gout PW, Kurita T, et al:
Sulfasalazine-induced cystine starvation: potential use for
prostate cancer therapy. Prostate. 67:162–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gout PW, Kang YJ, Buckley DJ, et al:
Increased cystine uptake capability associated with malignant
progression of Nb2 lymphoma cells. Leukemia. 11:1329–1337. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bannai S and Tateishi N: Role of membrane
transport in metabolism and function of glutathione in mammals. J
Membr Biol. 89:1–8. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verrey F, Closs EI, Wagner CA, et al: CATs
and HATs: the SLC7 family of amino acid transporters. Pflugers
Arch. 447:532–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo M, Wang YZ and Gout PW: The
xc- cystine/glutamate antiporter: a potential
target for therapy of cancer and other diseases. J Cell Physiol.
215:593–602. 2008.
|
|
11
|
Lyons SA, Chung WJ, Weaver AK, et al:
Autocrine glutamate signaling promotes glioma cell invasion. Cancer
Res. 67:9463–9471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishimoto T, Nagano O, Yae T, et al: CD44
variant regulates redox status in cancer cells by stabilizing the
xCT subunit of system xc- and thereby
promotes tumor growth. Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okabe H, Beppu T, Ueda M, et al:
Identification of CXCL5/ENA-78 as a factor involved in the
interaction between cholangiocarcinoma cells and cancer-associated
fibroblasts. Int J Cancer. February 15–2012.(Epub ahead of
print).
|
|
14
|
Fu LY, Jia HL, Dong QZ, et al: Suitable
reference genes for real-time PCR in human HBV-related
hepatocellular carcinoma with different clinical prognoses. BMC
Cancer. 9:492009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo W, Zhao Y, Zhang Z, et al: Disruption
of xCT inhibits cell growth via the ROS/autophagy pathway in
hepatocellular carcinoma. Cancer Lett. 312:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lastro M, Kourtidis A, Farley K and
Conklin DS: xCT expression reduces the early cell cycle requirement
for calcium signaling. Cell Signal. 20:390–399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Dai Z, Barbacioru C and Sadee W:
Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity
and chemoresistance. Cancer Res. 65:7446–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuno S, Sato H, Kuriyama-Matsumura K, et
al: Role of cystine transport in intracellular glutathione level
and cisplatin resistance in human ovarian cancer cell lines. Br J
Cancer. 88:951–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bannai S: Exchange of cystine and
glutamate across plasma membrane of human fibroblasts. J Biol Chem.
261:2256–2263. 1986.PubMed/NCBI
|
|
20
|
Eck HP and Droge W: Influence of the
extracellular glutamate concentration on the intracellular
cyst(e)ine concentration in macrophages and on the capacity to
release cysteine. Biol Chem Hoppe Seyler. 370:109–113. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rimaniol AC, Mialocq P, Clayette P, et al:
Role of glutamate transporters in the regulation of glutathione
levels in human macrophages. Am J Physiol Cell Physiol.
281:C1964–C1970. 2001.PubMed/NCBI
|
|
22
|
Ganapathy V, Thangaraju M and Prasad PD:
Nutrient transporters in cancer: relevance to Warburg hypothesis
and beyond. Pharmacol Ther. 121:29–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Capell HA, Madhok R, Porter DR, et al:
Combination therapy with sulfasalazine and methotrexate is more
effective than either drug alone in patients with rheumatoid
arthritis with a suboptimal response to sulfasalazine: results from
the double-blind placebo-controlled MASCOT study. Ann Rheum Dis.
66:235–241. 2007. View Article : Google Scholar
|
|
24
|
Gout PW, Buckley AR, Simms CR and
Bruchovsky N: Sulfasalazine, a potent suppressor of lymphoma growth
by inhibition of the x(c)-cystine transporter: a new action for an
old drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Narang VS, Pauletti GM, Gout PW, et al:
Sulfasalazine-induced reduction of glutathione levels in breast
cancer cells: enhancement of growth-inhibitory activity of
doxorubicin. Chemotherapy. 53:210–217. 2007. View Article : Google Scholar
|