Introduction
Prostate cancer, whose incidence and mortality
varies broadly, is a common cancer among men in developed countries
(1). Although hormonotherapy and
radiotherapy (RT) represent valid approaches for prostate cancer
treatment, there is a need for novel therapies that are based on
mechanisms which do not overlap with standard therapies and that
can improve patient survival (2,3).
Indeed, novel immunological approaches have been explored for
prostate cancer treatment, relying on the fact that a significant
number of prostate cancer patients have antibodies against
different tumor antigens [prostate-specific antigen (PSA), ErbB2,
prostatic acid phosphatase (PAP), prostasome-derived proteins] and
other structural cellular antigens (ribosomal proteins,
cytoskeleton and nuclear associated proteins) and that the presence
of a significant tumor lymphocyte infiltrate is associated with a
better prognosis in prostate cancer patients (4–11). The
development of clinical trials employing cancer vaccines targeting
PSA, PAP and prostate membrane antigen has clearly indicated that
immune response to tumor antigens can be boosted and, in some
cases, that vaccine administration can improve patient survival
(12–15). On the other hand, it has been
observed that immune response to tumor antigens can also be
enhanced after standard therapies in prostate cancer patients
(16). Significant evidence
indicates that RT exerts its effect not only by the elimination of
the RT vulnerable cancer cells but also by modification of the
tumor microenvironment inducing oxidative stress and inflammation
(16–20). Although the evidence that radiation
injury perturbs innate and adaptive immunity, immune response to
radiation tissue damage is not predictable and remains incompletely
understood. However, it has been reported that ionizing radiations
induce upregulation of heat shock proteins (HSPs) and growth factor
receptors in fibroblasts and extracellular matrix (ECM) remodeling
in fibroblasts and mammary gland (21–24).
In addition, it has been observed that RT induces reoxygenation of
hypoxic tumors and this phenomenon triggers the production of
reactive oxygen species (ROS) which alter molecule structure
(25–27). Reoxygenation of HeLa cancer cells
after hypoxia was shown to increase expression of several proteins
including the ribosomal P0 protein (26).
ECM is a composite and dynamic macromolecular
network with both structural and regulatory functions. ECM
components belong to four major types of macromolecules: the
collagens, elastin, proteoglycans and non-collagenous glycoproteins
(laminin, fibronectin, tenascin). A high incidence of
autoantibodies to ECM components during inflammation and cancer has
been reported (28–35). HSPs are overexpressed in several
tumors and are implicated in cancer cell proliferation, metastasis
and immune response (36).
Overexpression of HSPs or autoantibodies to HSPs were associated
with either a poor or good prognosis in cancer patients (36,37).
The ribosomal P proteins (P0, 38 kDa; P1, 19 kDa; and P2, 17 kDa)
constitute a pentameric complex forming the ribosomal stalk of the
60 S ribosomal subunit in the eukaryotic cells (38). P0 exists as a free protein in the
cytoplasm and on the surface of cancer cells (39). The presence of autoantibodies to
ribosomal P proteins in sera of patients with systemic lupus
erythematosus (LES) has been demonstrated (40,41).
We recently demonstrated the presence of autoantibodies to
ribosomal P proteins in head and neck as well as breast cancer
patients (42,43). Autoantibodies to P0 protein have
shown to mediate cell apoptosis (44).
In this study, we determined the occurrence of
antibodies to ECM components, HSPs, ribosomal P0 protein, EGFR,
ErbB2 and PSA in prostate cancer patients before and after local RT
and hormonotherapy. This study provides insight into the
immunobiological behavior of prostate cancer patients following
standard treatment.
Materials and methods
Study population
The study protocol was approved by the Ethics
Committee of the University of Rome ‘Tor Vergata’. Each patient was
given a detailed description of the procedure and was required to
sign an informed consent prior to participation in this study.
Thirty-five consecutive patients with localized prostate cancer
underwent CT-planned radical 3D conformal RT at the Radiation
Oncology Therapy Unit of the University of Rome ‘Tor Vergata’. The
median age at the time of treatment was 72 years (range 46–78
years); 21 patients were classified as T1c clinical stage, 8 as
T2a, 3 as T2c, 2 as T1a and 1 patient was T3b (TNM, American Joint
Committee on Cancer 2002). The Gleason score was 6 (3+3) in 20
patients, 7 (3+4) in 5 cases and 7 (4+3) in 3 cases, 8 (4+4) in 3
cases and 4 patients had a Gleason score of, respectively, 5 (3+2),
8 (5+3), 9 (4+5) and 10 (5+5); 9/35 cases were treated with
hormone-releasing hormone agonist in association with RT. The
median PSA value before RT was 7.9 ng/ml (range 0.53–72.3
ng/ml).
The serum of patients was collected prior to and 8
months following the last RT treatment. Sera from blood donors
(n=29) collected from the University of Rome ‘Sapienza’ transfusion
center were used as control. Patient and control sera were stored
at −20°C until use.
Radiation therapy
Bowel preparation was obtained suggesting a diet in
combination with a daily mild laxative to reduce intestinal gas and
obtain a reproducible bowel volume during CT and MRI acquisition
and treatment sessions. For bladder preparation, patients were
asked to empty their bladder for better daily prostate
localization.
CT scanning was performed with a GE
LightSpeed® Scanner (GE Healthcare Diagnostic Imaging,
Slough, UK). The scan was to start at the level of the iliac crests
and continue down through the perineum, with a 2.5-mm slice. CT
images were transferred to Precise Plan treatment planning system
(Elekta Oncology Systems, Crawley, UK).
For each patient, the clinical target volume (CTV)
and organs at risk (OARs) were outlined by the same radiation
oncologist. The target volume irradiated to 66 Gy (CTV1)
consisted of prostate and seminal vesicles; the boost irradiated to
76 Gy (CTV2) was the prostate only. Planning target
volumes (PTV1 and PTV2) were generated by an
asymmetric expansion of CTVs (6 mm in all directions except at the
posterior margin, where a 5-mm expansion was used). The rectum was
contoured on as solid organ from the 8th slice (2 cm) above the
anal verge to the rectosigmoid junction; the bladder was contoured
in its entirety; the penile bulb was defined as a pear-shaped
structure comprising the proximal part of the corpus spongiosum;
the femurs were defined too. Three-dimension conformal RT treatment
planning, with a six-field arrangement, was obtained. For an
adequate PTV coverage, it was accepted that the 95% of PTV volume
was covered by 95% of the prescribed dose and that the maximum dose
did not exceed 107% of the prescribed dose. Daily fractions of 2 Gy
(5 days a week) were delivered with conformal shaped treatment
fields (15 MV) using the multi-leaf collimator (MLC; 1 cm leaf
width) of an Elekta Precise linear accelerator (Elekta Precise
Treatment System Plus™). Two orthogonal portal images were used in
order to check set-up alignment. Digitally reconstructed
radiographs (DRRs), obtained from the CT localization scans, were
used as reference images. A matching software was applied to
quantify set-up errors between DRRs and portal images.
For biochemical failure definition we referred to
the Phoenix definition, revised by ASTRO and RTOG in Phoenix, as a
rise in PSA by 2 ng/ml or more above the nadir PSA (defined as the
lowest PSA achieved).
Acute rectal toxicity (within 90 days from the start
of RT) and late rectal toxicity were scored by the radiation
oncologist, according to the RTOG/EORTC toxicity scale.
Purified ECM molecules, HSPs, ribosomal
P0 protein, PSA, LTR-EGFR and LTR-ErbB2 transfectants and
antibodies
Purified human type I, III, IV and V collagens (CI,
CIII, CIV and CV), fibronectin (FN) and PSA were obtained from
Chemicon International (Temecula, CA, USA). Purified laminin (LM)
was obtained from Sigma-Aldrich (St. Louis, MO, USA). Purified HSPs
included HSP27 and HSP90α (human recombinant, StressMarq™
Biosciences Inc., Victoria, BC, Canada) and HSP65 (M. Tuberculosis
recombinant, LIONEX Diagnostics and Therapeutics GmbH, Germany).
The purity of antigens was >95% by SDS-PAGE and Coomassie Blue
staining. Mouse monoclonal antibodies anti-human CI, CIII, CIV and
CV were obtained from Chemicon International. Mouse monoclonal
anti-human FN and rabbit polyclonal anti-human LM, as well as
peroxidase-conjugated antibodies anti-human IgG and IgM or
anti-mouse and anti-rabbit IgG, were obtained from Sigma-Aldrich.
Antibodies to HSPs were purchased from StressMarq™ Biosciences Inc.
or Santa Cruz Biotech., Inc. (Santa Cruz, CA, USA). Anti-PSA
antibody was purchased from Dako (Carpinteria, CA, USA). LTR-EGFR
and LTR-ErbB2 transfectants and anti-EGFR and anti-ErbB2 antibodies
were previously described (45–47).
Ribosomal P0 protein generation was previously described (42,43).
Enzyme-linked immunosorbent assay
(ELISA)
Sera were assayed for the presence of antibodies
directed toward native ECM and HSP antigens by ELISA as previously
described (33,34,48).
Briefly, ECM and HSP antigens, as well as bovine serum albumin
(BSA), were diluted at 1–2 μg/ml. One hundred microliters of each
mixture were incubated overnight at 37°C in polyvinyl chloride
microtiter plates (Dynatech, Chantilly, VA, USA). Antigen-coated
wells were then blocked with 5% non-fat dry milk in PBS for 1 h at
37°C and incubated with human sera. Sera were initially assayed at
1:25, 1:50 and 1:100 dilutions. The 1:100 dilution was chosen for
further experiments since it was the highest serum concentration
that lacked background reactivity. Each serum was assayed in
duplicate for reactivity to ECM and HSP antigens or BSA. Anti-ECM
and anti-HSP antibodies diluted at 1 μg/ml were used as positive
controls. After an overnight incubation at 4°C, the plates were
washed 5 times with 1% non-fat dry milk in PBS and goat anti-human
IgG or goat anti-mouse or anti-rabbit IgG peroxidase-conjugated
antibodies were added and incubated for 1 h at 37°C. The plates
were washed and the wells were layered with a solution containing
o-phenylenediamine dihydrochloride in the presence of
H2O2. The reaction was blocked with 50 μl of
H2SO4(4N) and the absorbance of the samples
was read at 492 nm (33,34).
Western blotting
Electrophoresis of purified recombinant P0 protein
or PSA (0.2–0.5 μg/lane) or NIH3T3 and NIH-LTR-EGFR and LTR-ErbB2
cells (100 μg/lane) was carried out in denaturing 10–12% SDS
polyacrylamide gels. Following electrophoresis, the proteins were
transferred to nitrocellulose membranes at 40 V for 1 h (49–51).
The membranes were blocked for 6 h in a washing solution (0.1%
Tween-20 in Tris-buffered saline, pH 7.6) containing 5% non-fat dry
milk and subsequently incubated overnight at 4°C with either human
sera or specific monoclonal and polyclonal antibodies. Human sera
were initially titrated at 1:25, 1:50 and 1:100 dilutions. The
1:100 dilution was chosen for further experiments since it was the
highest serum concentration lacking background reactivity. After
extensive washings, the membranes were incubated with goat
anti-human IgG or goat anti-mouse or anti-rabbit IgG
peroxidase-conjugated antibodies. The immunocomplexes were finally
visualized by means of the Supersignal West Pico chemiluminescence
kit (Pierce, Rockford, IL, USA) (52). Criteria of serum positivity toward a
given antigen consisted in the appearance of an immunoreactive band
co-migrating with that detected by the positive control antibody
(45,53).
The intensity of coloring of immunoreactive P0 band
was expressed as densitometric unit(s) (DU) and was obtained using
the NIH Pro-Image 1.5 software after blot scanning by a UMAX VISTA
SUPER SPEEDY scanner (53). Values
obtained were used for statistical analysis to determine the
significance of antibody variances to P0 before and after
therapy.
Statistical analysis
Since several distributions were skew departing from
normality and sample size was limited a non-parametric approach was
preferred in the analysis. Continuous variables were described by
median, first and third quartiles and range, categorical variables
as frequencies. In order to show the distributions of humoral
immune responses, Box and Whisker plots were created. The bottom
and top edges of the box are located at the 25th and 75th
percentiles of the sample and, within the box, the median is
displayed as a line and the mean as a diamond. Outliers are
observations that are more extreme than the upper and lower fences
located at ±1.5 × interquartile range. Horizontal lines identify
the largest and the smallest value within these fences.
Wilcoxon sum rank test and Wilcoxon signed-rank test
were utilized as location tests in case of independent samples and
paired observations, respectively. A non-parametric analysis of
variance was used for assessing the effect of clinical pathological
variables and type of therapy on humoral immune responses. For this
purpose two classes were defined for both Gleason score (≤6 vs.
>6 ) and PSA before therapy (≤10 vs. >10).
The association between categorical variables was
assessed by the χ2 test; Fisher's exact test was
preferred in case of sparse tables. Difference in proportions for
paired observations was evaluated by the McNemar test.
Results
Humoral immune response to ECM molecules
before and after therapy
Sera from 35 patients with prostate cancer and 29
healthy donors were analyzed for the presence of autoantibodies to
purified ECM proteins including different types of collagen (CI,
CIII, CIV and V) and glycoproteins such as LM and FN. In order to
reveal the reactivity of patient immunoglobulin G to conformational
epitopes of the ECM, serum samples were analyzed by ELISA. The
level of IgG of both healthy donors and prostate cancer patients to
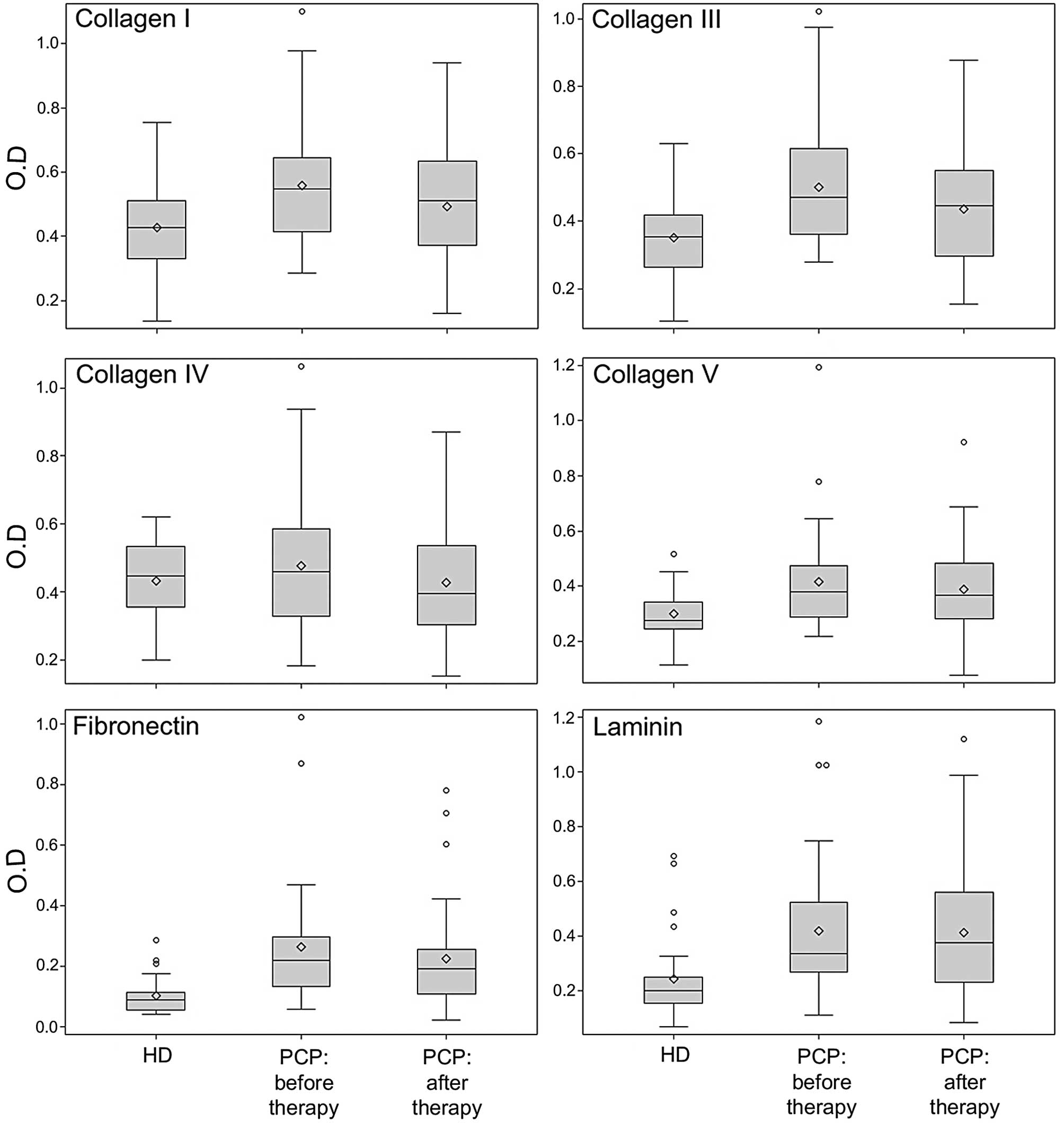
different ECM molecules is represented in Fig. 1.
Significant differences between healthy donors and
untreated patients were detected for the IgG serum levels to CI
(median value 0.43 vs. 0.55, p=0.0046), CIII (0.35 vs. 0.47,
p=0.0006), CV (0.28 vs. 0.38, p=0.0028), FN (0.09 vs. 0.22,
p<0.0001) and LM (0.20 vs. 0.33, p=0.0004). The level of
autoantibodies to CIV in untreated patients was not significant
compared to that of healthy donors. The increased autoantibody
level to CI, CIII, CV, FN and LM in prostate cancer patients was
not associated with PSA level and Gleason score.
It is important to note that 8 months after therapy
the IgG serum levels to CI (median value 0.55 vs. 0.51, p=0.0009),
CIII (median value 0.47 vs. 0.44, p=0.0003) and FN (median value
0.22 vs. 0.19, p=0.0003) significantly decreased. Conversely, after
treatment, no significant difference was observed in the level of
IgG to CIV, CV and LM. The decrease of autoantibody level to ECM
molecules was not associated with clinical pathological variables
or type of therapy.
Humoral immune response to HSPs before
and after therapy
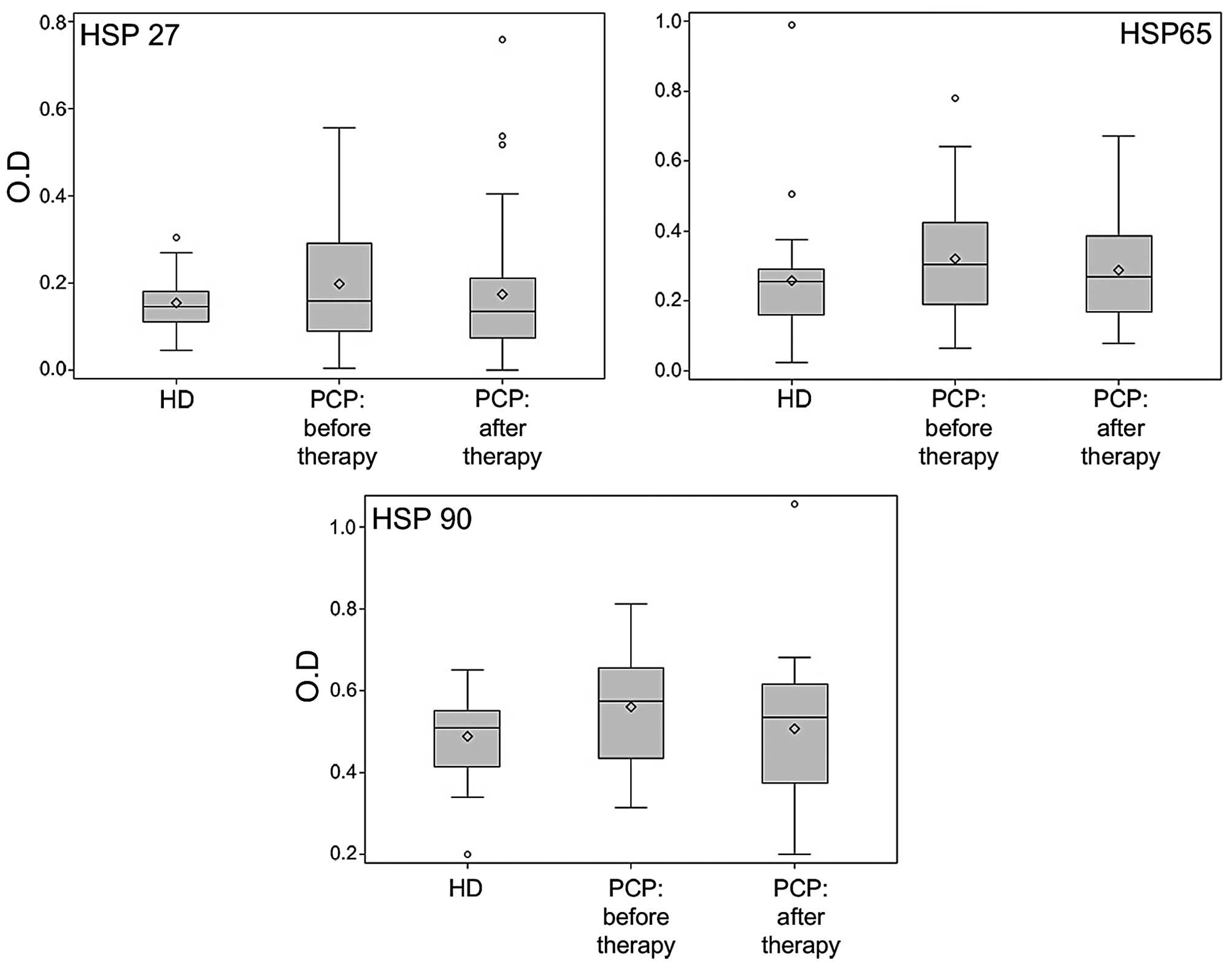
The IgG level of both healthy donors and prostate
cancer patients to HSPs is shown in Fig. 2. The patient serum IgG level to
HSP90 was significantly higher than that of healthy donors (0.51
vs. 0.58, p=0.0406). Conversely, the levels of HSP27 and HSP65 IgG,
although higher in patients with prostate cancer, were not
significant compared to those of healthy donors. The increased
level of autoantibodies to HSP90 was not associated with clinical
pathological variables. However, after therapy, the IgG levels to
HSP27 (median value 0.16 vs. 0.14, p=0.0205), HSP65 (median value
0.31 vs. 0.27, p=0.0098) and HSP90 (median value 0.58 vs. 0.54,
p=0.0012) significantly decreased. For the IgG levels to HSP27,
this decrease was associated with levels of PSA before therapy: for
patients with PSA ≤10 the median decrease was 0.045 while for
patients with PSA >10 a slight median increase of 0.005 was
observed (p=0.0394). For the IgG levels to HSP90, this decrease was
associated with levels of Gleason before RT: for patients with
Gleason ≤6 the median decrease was 0.075 while for patients with
Gleason >6 a slight median increase of 0.015 was observed
(p=0.0483). No significant effect of hormonotherapy was
detected.
Humoral immune response to ribosomal P0
protein, EGFR/ErbB2 and PSA before and after therapy
Employing the GST-P0 ribosomal protein, NIH3T3-EGFR
or -ErbB2 transfectant cell lines or a commercial source of human
PSA, serum from patients and normal donors was subjected to
qualitative immunoblot analysis. The GST protein, NIH3T3 cells and
BSA were used as control of specific immunoreactivity of serum with
GST-P0, LTR-transfectant cell lines and PSA, respectively. Criteria
of serum positivity toward a given antigen consisted in the
appearance of a specific immunoreactive band in the antigen source
sample, co-migrating with those detected by the positive control
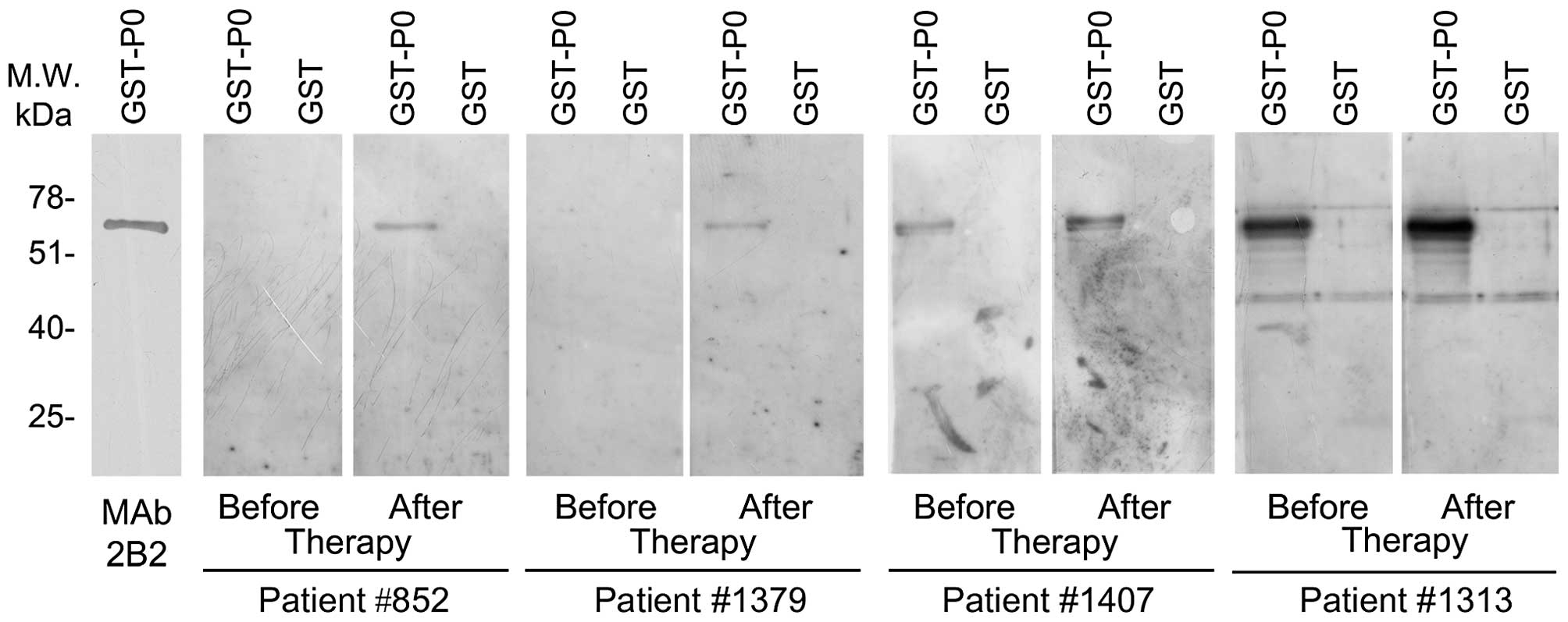
antibody. Representative experiments are illustrated in Fig. 3.
None of the healthy donor sera showed reactivity to
P0 protein, EGFR, ErbB2 or PSA. Conversely, 6 out of the 35
prostate cancer patient sera reacted to P0 protein. Immunity to P0
protein was associated with malignancy (p=0.0279). No patient serum
showed antibodies to EGFR, while 2 and 1 patients showed reactivity
to ErbB2 and PSA, respectively.
Of note, 8 months after therapy the number of
patients displaying antibodies to P0 increased (6/35 before therapy
vs. 11/35 after therapy). We quantitatively evaluated the
reactivity of the serum to the P0 protein by measuring the
intensity of the immunoreactive P0 band. Our results indicated that
reactivity to P0 also increased in 5 of the 6 patients with
pre-existing autoantibodies to P0 before therapy (median level 3.22
vs. 6.41). Overall, the level of autoantibodies to P0 increased
after therapy in 10 patients (median level before 0.87 and after
therapy 3.02, p=0.0020). It is noteworthy that 50% of the 10
patients with increased levels of autoantibodies to P0 were treated
with hormonotherapy and RT while in the remaining 25 patients with
stable or slightly decreased levels of autoantibodies to P0 only
16% received the combined treatment (p=0.0814). In addition, 10
patients with increased levels of autoantibodies to P0 showed PSA
mean level lower than the remaining 25 patients at 18 months (0.29
vs. 0.69, p=0.0274) and at 24 months after therapy (0.29 vs. 0.81,
p=0.0813). Moreover, treatment of patients did not alter the level
of antibodies against EGFR, ErbB2 and PSA.
Discussion
Several studies have demonstrated the existence of
antibodies to self-molecules in cancer patients, thus suggesting
that the human immune system may recognize self-antigens (35,42,43,45,53–57).
Antigen tissue overexpression and exposure of sequestered antigens
may explain tolerance circumvention (35). In this study we demonstrated that
immunity to P0 protein (p=0.0279), ECM molecules (CI, p=0.0046;
CIII, p=0.0006; CV, p=0.0028; FN, p<0.0001; and LM p=0.0004) and
to HSP90 (p=0.0406) was associated with malignancy in untreated
prostate cancer patients. No patient serum showed antibodies to
EGFR, while 2 and 1 patients showed reactivity to ErbB2 and PSA,
respectively. In addition, we demonstrated that 8 months after
therapy the IgG serum levels to CI (p=0.0009), CIII (p=0.0003), FN
(p=0.0003) and HSP90 (p=0.0012) significantly decreased.
Conversely, the level of autoantibodies to P0 increased after
therapy in 10 patients (p=0.0020), 50% of the 10 patients with
increased levels of autoantibodies to P0 were treated with
hormonotherapy and radiotherapy (RT). Treatment of patients did not
alter the level of antibodies against EGFR, ErbB2 and PSA.
The decrease of the levels of autoantibodies to ECM
molecules and HSP90 after RT might not be surprising. It has been
shown that ionizing radiation of mammary gland induced
rapid-remodeling of the stromal ECM and modified the integrity of
the epithelial basement membrane. Hence, it cannot be excluded that
the increase or remodeling of ECM molecules at tumor levels could
have increased the capture of anti-ECM antibodies in the irradiated
area, thus determining their decreased concentration in the blood.
Indeed, Collagen III was induced in the adipose stromal tissue of
the mammary gland within 1 day after irradiation (24). Fibrosis, which represents excessive
accumulation of collagen and other ECM components following
unbalanced ECM synthesis and degradation, is a dose-limiting
complication of RT at numerous primary anatomical sites (18). The increase of ECM at the site of
irradiation could have promoted the uptake of anti-ECM antibodies
in irradiated tissues, thereby determining their decline in the
blood.
Prostasomes are secretory granules synthesized,
stored and secreted by normal and neoplastic human prostate
epithelial cells (58). It has been
suggested that prostasomes in prostate cancer can be released into
the blood circulation (58) and
that the most frequently occurring prostasomal proteins are heat
shock proteins (HSPs) (6).
Radiation therapy has been demonstrated to increase the release of
HSP72 in the blood of prostate cancer patients (59). Accordingly, autoantibodies to HSPs
can bind circulating HSPs and form immunocomplexes which can be
eliminated by phagocytosis (60).
In addition, the elimination of prostate cancer cells after RT
could have decreased immune system stimulation (61). Alternatively, the presence of
immunocomplexes could have interfered with the detection of
anti-HSP autoantibodies by ELISA. The decrease of IgG levels to
HSP90 was associated with levels of Gleason prior to RT.
The strong in vivo immunogenicity of
ribosomal P0 protein before or after therapy is not surprising.
Different features of a given self-antigen concur to determine
whether such antigen is apt to induce an autoreactive immune
response (35). According to Plotz,
these features encompass: a) the antigen structure; b) its
immunological and pro-inflammatory properties; c) its expression
level, and (d) its catabolism and fate after cell death (62). In view of that, ribosomal P proteins
have long runs or clusters of charged residues and form large
complexes. Indeed, serum of some patients with systemic lupus
erythematosus (LES) contains autoantibodies to ribosomal P proteins
(40,41). Moreover, tissue inflammation induced
by RT could have resulted in increased exposure of cryptic and/or
modified ribosomal P0 epitopes and consequently in the stimulation
of a specific immune response to the antigen.
The presence of anti-P0 autoantibodies following RT
may have clinical implications. Monoclonal antibodies against human
ribosomal P proteins were able to penetrate into cultured cells and
cause apoptosis (44). In addition,
the ribosomal P0 protein has been shown to be present in the cell
membrane (42,43). The increased expression of the C-22
epitope of P0 on the surface of pharynx cancer cells following
cellular stress in vitro may indicate that anti-P0
autoantibodies could directly mediate apoptosis in this tumor
(42). Indeed, 10 patients with
increased levels of autoantibodies to P0 showed PSA mean level
lower than the remaining 25 patients at 18 months (0.29 vs. 0.69,
p=0.0274) and at 24 months after therapy (0.29 vs. 0.81, p=0.0813).
Overall, our study shows for the first time that the therapy in
patients with prostate cancer, while resulting in increased levels
of serum antibodies to P0 protein, reduces their levels against ECM
molecules or some HSPs. This phenomenon may be due to the different
structure and properties of the antigens tested. Antibody to
ribosomal P0 protein might have biological functions. In addition,
the levels of autoantibodies to ECM components, HSPs and P0 protein
might be an indicator of response to therapy in prostate cancer
patients or could be used in patient monitoring and may contribute
to the better understanding of the immunobiological behavior of
tumor tissue following therapy.
Acknowledgements
This study was supported by Ministero
dell'Istruzione, dell'Università e della Ricerca (MIUR, PRIN) (to
A.M and R.B). LTR-EGFR and LTR-ErbB2 cells were provided by Dr
Matthias Kraus. The authors thank Barbara Bulgarini for her
editorial assistance in the preparation of the manuscript. We wish
to thank nurse Franca Pietrasanta for assisting with the management
of patients.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
D'Amico AV: Radiation and hormonal therapy
for locally advanced and clinically localized prostate cancer.
Urology. 60(Suppl 1): 32–37. 2002. View Article : Google Scholar
|
|
3
|
Palumbo C, Bei R, Procopio A and Modesti
A: Molecular targets and targeted therapies for malignant
mesothelioma. Curr Med Chem. 15:855–867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNeel DG, Nguyen LD, Storer BE, Vessella
R, Lange PH and Disis ML: Antibody immunity to prostate cancer
associated antigens can be detected in the serum of patients with
prostate cancer. J Urol. 164:1825–1829. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olson BM and McNeel DG: Antibody and
T-cell responses specific for the androgen receptor in patients
with prostate cancer. Prostate. 67:1729–1739. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ronquist KG, Carlsson L, Ronquist G,
Nilsson S and Larsson A: Prostasome-derived proteins capable of
eliciting an immune response in prostate cancer patients. Int J
Cancer. 119:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Massoner P, Lueking A, Goehler H, et al:
Serum-autoantibodies for discovery of prostate cancer specific
biomarkers. Prostate. 72:427–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agarwal N, Padmanabh S and Vogelzang NJ:
Development of novel immune interventions for prostate cancer. Clin
Genitourin Cancer. 10:84–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mercader M, Bodner BK, Moser MT, et al: T
cell infiltration of the prostate induced by androgen withdrawal in
patients with prostate cancer. Proc Natl Acad Sci USA.
98:14565–14570. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimura S, Yang G, Ebara S, Wheeler TM,
Frolov A and Thompson TC: Reduced infiltration of tumor-associated
macrophages in human prostate cancer: association with cancer
progression. Cancer Res. 60:5857–5861. 2000.PubMed/NCBI
|
|
11
|
Vesalainen S, Lipponen P, Talja M and
Syrjänen K: Histological grade, perineural infiltration,
tumour-infiltrating lymphocytes and apoptosis as determinants of
long-term prognosis in prostatic adenocarcinoma. Eur J Cancer.
30A:1797–1803. 1994. View Article : Google Scholar
|
|
12
|
Gulley JL and Drake CG: Immunotherapy for
prostate cancer: recent advances, lessons learned, and areas for
further research. Clin Cancer Res. 17:3884–3891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nesslinger NJ, Ng A, Tsang KY, Ferrara T,
Schlom J, Gulley JL and Nelson BH: A viral vaccine encoding
prostate-specific antigen induces antigen spreading to a common set
of self-proteins in prostate cancer patients. Clin Cancer Res.
16:4046–4056. 2010. View Article : Google Scholar
|
|
14
|
Becker JT, Olson BM, Johnson LE, Davies
JG, Dunphy EJ and McNeel DG: DNA vaccine encoding prostatic acid
phosphatase (PAP) elicits long-term T-cell responses in patients
with recurrent prostate cancer. J Immunother. 33:639–647. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kantoff PW, Schuetz TJ, Blumenstein BA, et
al: Overall survival analysis of a phase II randomized controlled
trial of a Poxviral-based PSA-targeted immunotherapy in metastatic
castration-resistant prostate cancer. J Clin Oncol. 28:1099–1105.
2010. View Article : Google Scholar
|
|
16
|
Nesslinger NJ, Sahota RA, Stone B, et al:
Standard treatments induce antigen-specific immune responses in
prostate cancer. Clin Cancer Res. 13:1493–1502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koukourakis MI: Radiation damage and
radioprotectants: new concepts in the era of molecular medicine. Br
J Radiol. 85:313–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yarnold J and Brotons MC: Pathogenetic
mechanisms in radiation fibrosis. Radiother Oncol. 97:149–161.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mothersill CE, Moriarty MJ and Seymour CB:
Radiotherapy and the potential exploitation of bystander effects.
Int J Radiat Oncol Biol Phys. 58:575–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marzocchella L, Fantini M, Benvenuto M,
Masuelli L, Tresoldi I, Modesti A and Bei R: Dietary flavonoids:
molecular mechanisms of action as anti-inflammatory agents. Recent
Pat Inflamm Allergy Drug Discov. 5:200–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmid TE and Multhoff G:
Radiation-induced stress proteins-the role of heat shock proteins
(HSP) in anti-tumor responses. Curr Med Chem. 19:1765–1770. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boerma M, van der Wees CG, Vrieling H, et
al: Microarray analysis of gene expression profiles of cardiac
myocytes and fibroblasts after mechanical stress, ionising or
ultraviolet radiation. BMC Genomics. 6:62005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niehoff P, Wiltfang J, Springer IN,
Weppner N, Kimmig B and Acil Y: Increased excretion of collagen
crosslinks in irradiated patients indicates destruction of
collagen. Int J Radiat Biol. 82:503–509. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ehrhart EJ, Gillette EL and Barcellos-Hoff
MH: Immunohistochemical evidence of rapid extracellular matrix
remodeling after iron-particle irradiation of mouse mammary gland.
Radiat Res. 145:157–162. 1996. View
Article : Google Scholar
|
|
25
|
Harriss W, Bezak E, Yeoh E and Hermans M:
Measurement of reoxygenation during fractionated radiotherapy in
head and neck squamous cell carcinoma xenografts. Australas Phys
Eng Sci Med. 33:251–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magagnin MG, Sergeant K, van den Beucken
T, et al: Proteomic analysis of gene expression following hypoxia
and reoxygenation reveals proteins involved in the recovery from
endoplasmic reticulum and oxidative stress. Radiother Oncol.
83:340–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Izzi V, Masuelli L, Tresoldi I, Foti C,
Modesti A and Bei R: Immunity and malignant mesothelioma: from
mesothelial cell damage to tumor development and immune
response-based therapies. Cancer Lett. 322:18–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fernandez-Madrid F, Karvonen RL, Kraut MJ,
Czelusniak B and Ager JW: Autoimmunity to collagen in human lung
cancer. Cancer Res. 56:121–126. 1996.PubMed/NCBI
|
|
29
|
Kalluri R, Petrides S, Wilson CB, et al:
Anti-alpha1(IV) collagen autoantibodies associated with lung
adenocarcinoma presenting as the Goodpasture syndrome. Ann Intern
Med. 124:651–653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong YQ, Zhang ZJ, Liu B, et al:
Autoantibodies as potential biomarkers for nasopharyngeal
carcinoma. Proteomics. 8:3185–3193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Young AL, Bailey EE, Colaço SM, Engler DE
and Grossman ME: Anti-laminin-332 mucous membrane pemphigoid
associated with recurrent metastatic prostate carcinoma: hypothesis
for a paraneoplastic phenomenon. Eur J Dermatol. 21:401–404.
2011.
|
|
32
|
Bei R, Masuelli L, Palumbo C, Tresoldi I,
Scardino A and Modesti A: Long-lasting tissue inflammatory
processes trigger autoimmune responses to extracellular matrix
molecules. Int Rev Immunol. 27:137–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bei R, Mentuccia D, Trono P, et al:
Immunity to extracellular matrix antigens is associated with
ultrastructural alterations of the stroma and stratified epithelium
basement membrane in the skin of Hashimotos thyroiditis patients.
Int J Immunopathol Pharmacol. 19:661–674. 2006.
|
|
34
|
Masuelli L, Pompa G, Fabrizi M, et al:
Patients with periimplantitis, unlike those with a healthy
peri-implant microenvironment, display antibodies to more than one
heat shock protein (HSP 27, HSP 65 and HSP 90) linear epitope. Eur
J Inflammation. 9:257–268. 2011.
|
|
35
|
Bei R, Masuelli L, Palumbo C, Modesti M
and Modesti A: A common repertoire of autoantibodies is shared by
cancer and autoimmune disease patients: inflammation in their
induction and impact on tumor growth. Cancer Lett. 281:8–23. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jego G, Hazoumé A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
Nov 13–2010.(Epub ahead of print).
|
|
37
|
Suzuki H, Sugimura H and Hashimoto K:
Overexpression of heat shock protein 27 is associated with good
prognosis in the patient with oral squamous cell carcinoma. Br J
Oral Maxillofac Surg. 45:123–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wahl MC and Moller W: Structure and
function of the acidic ribosomal stalk proteins. Curr Protein Pept
Sci. 3:93–106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koscec M, Koren E, Wolfson-Reichlin M, et
al: Autoantibodies to ribosomal P proteins penetrate into live
hepatocytes and cause cellular dysfunction in culture. J Immunol.
159:2033–2041. 1997.PubMed/NCBI
|
|
40
|
Mahler M, Kessenbrock K, Szmyrka M, et al:
International multicenter evaluation of autoantibodies to ribosomal
P proteins. Clin Vaccine Immunol. 13:77–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gerli R and Caponi L: Anti-ribosomal P
protein antibodies. Autoimmunity. 38:85–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bei R, Masuelli L, Trono P, et al: The
ribosomal P0 protein induces a spontaneous immune response in
patients with head and neck advanced stage carcinoma that is not
dependent on its overexpression in carcinomas. Int J Oncol.
31:1301–1308. 2007.PubMed/NCBI
|
|
43
|
Marzocchella L, Sini V, Buonomo O, et al:
Spontaneous immunogenicity of ribosomal P0 protein in patients with
benign and malignant breast lesions and delay of mammary tumor
growth in P0-vaccinated mice. Cancer Sci. 102:509–515. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun KH, Tang SJ, Lin ML, Wang YS, Sun GH
and Liu WT: Monoclonal antibodies against human ribosomal P
proteins penetrate into living cells and cause apoptosis of Jurkat
T cells in culture. Rheumatology. 40:750–756. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bei R, Masuelli L, Moriconi E, Visco V,
Moretti A, Kraus MH and Muraro R: Immune responses to all ErbB
family receptors detectable in serum of cancer patients. Oncogene.
18:1267–1275. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Masuelli L, Focaccetti C, Cereda V, et al:
Gene-specific inhibition of breast carcinoma in BALB-neuT mice by
active immunization with rat Neu or human ErbB receptors. Int J
Oncol. 30:381–392. 2007.PubMed/NCBI
|
|
47
|
Bei R, Pompa G, Vitolo D, et al:
Co-localization of multiple ErbB receptors in stratified epithelium
of oral squamous cell carcinoma. J Pathol. 195:343–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Masuelli L, Marzocchella L, Focaccetti C,
et al: Local delivery of recombinant vaccinia virus encoding for
neu counteracts growth of mammary tumors more efficiently than
systemic delivery in neu transgenic mice. Cancer Immunol
Immunother. 59:1247–1258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Masuelli L, Marzocchella L, Quaranta A, et
al: Apigenin induces apoptosis and impairs head and neck carcinomas
EGFR/ErbB2 signaling. Front Biosci. 16:1060–1068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Masuelli L, Bei R, Sacchetti P, et al:
Beta-catenin accumulates in intercalated disks of hypertrophic
cardiomyopathic hearts. Cardiovasc Res. 60:376–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Masuelli L, Marzocchella L, Focaccetti C,
et al: Resveratrol and diallyl disulfide enhance curcumin-induced
sarcoma cell apoptosis. Front Biosci. 17:498–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Masuelli L, Budillon A, Marzocchella L, et
al: Caveolin-1 overexpression is associated with simultaneous
abnormal expression of the E-cadherin/α-β catenins complex and
multiple ErbB receptors and with lymph nodes metastasis in head and
neck squamous cell carcinomas. J Cell Physiol. 227:3344–3353.
2012.PubMed/NCBI
|
|
53
|
Bei R, Budillon A, Reale MG, et al:
Cryptic epitopes on alpha-fetoprotein induce spontaneous immune
responses in hepatocellular carcinoma, liver cirrhosis, and chronic
hepatitis patients. Cancer Res. 59:5471–5474. 1999.
|
|
54
|
Turriziani M, Fantini M, Benvenuto M, et
al: Carcinoembryonic antigen (CEA)-based cancer vaccines: recent
patents and antitumor effects from experimental models to clinical
trials. Recent Pat Anticancer Drug Discov. 7:265–296. 2012.
View Article : Google Scholar
|
|
55
|
Bei R and Mizejewski GJ: Alpha fetoprotein
is more than a hepatocellular cancer biomarker: from spontaneous
immune response in cancer patients to the development of an
AFP-based cancer vaccine. Curr Mol Med. 11:564–581. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bei R, Budillon A, Masuelli L, et al:
Frequent overexpression of multiple ErbB receptors by head and neck
squamous cell carcinoma contrasts with rare antibody immunity in
patients. J Pathol. 204:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Scardino A, Alimandi M, Correale P, et al:
A polyepitope DNA vaccine targeted to Her-2/ErbB-2 elicits a broad
range of human and murine CTL effectors to protect against tumor
challenge. Cancer Res. 67:7028–7036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Larsson A, Ronquist G, Wülfing C, et al:
Antiprostasome antibodies: possible serum markers for prostate
cancer metastasizing liability. Urol Oncol. 24:195–200. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hurwitz MD, Kaur P, Nagaraja GM, Bausero
MA, Manola J and Asea A: Radiation therapy induces circulating
serum Hsp72 in patients with prostate cancer. Radiother Oncol.
95:350–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Knobloch V and Plundrová D:
Immunocomplexes in pregnancy. II. Phagocytosed complexes in the
peripheral blood. Cesk Gynekol. 51:611–616. 1986.(In Czech).
|
|
61
|
Kossenkov AV, Vachani A, Chang C, et al:
Resection of non-small cell lung cancers reverses tumor-induced
gene expression changes in the peripheral immune system. Clin
Cancer Res. 17:5867–5877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Plotz PH: The autoantibody repertoire:
searching for order. Nat Rev Immunol. 3:73–78. 2003. View Article : Google Scholar : PubMed/NCBI
|