Introduction
The incidence and mortality of esophageal cancer
(EC) ranks eighth and sixth among all cancers and affects more
males than females (1). Esophageal
squamous cell carcinoma (ESCC) and adenocarcinoma (EAC) are two
main subtypes of EC in regards to their pathological
characteristics. ESCC remains the dominant subtype of EC. However,
ESCC is usually diagnosed locally at an advanced stage or with
lymph node metastases. The characteristics dictating the potential
for invasion and metastasis of esophageal carcinoma cells are
important prognostic factors. The overall 5-year survival rate of
ESCC patients is extemely poor despite advanced treatment (1,2). To
develop new treatment strategies and diagnostic methods, a better
understanding of the biological behavior of ESCC is needed.
The Lim and SH3 domain protein (LASP1) is an
actin-binding protein. It was initially identified from a cDNA
library of breast cancer metastases. The gene was mapped to human
chromosome 17q21 a region that is altered in 20–30% of human breast
cancers (3,4). LASP1 encodes a membrane-bound protein
of 261 amino acids containing an N-terminal LIM domain, followed by
two actin-binding domains in the core of the LASP1 protein
mediating an interaction between LASP1 and the actin cytoskeleton
at cell membrane extensions (5–9). The
exact functions of LASP1 are still not clear, yet it appears to be
involved in the dynamic actin assembly, such as focal contacts,
focal adhesions, lamellipodia, membrane ruffles and pseudopodia
(3,6,10–12).
Recently, high LASP1 expression has been observed in many types of
human cancers, including breast, ovarian, colorectal, liver and
bladder (13–17). Furthermore, silencing of LASP1 by
siRNA was found to suppress cell proliferation and migration of
breast cancer cells in vitro(13), arrest ovarian cancer cells at the
G2/M phase, reduce cell proliferation and affect zyxin localization
(14). In conclusion, previous
studies suggest that LASP1 is involved in cell migration, invasion
and proliferation and may play an important role in carcinogenesis
and cancer progression. However, to our knowledge, expression of
LASP1 in ESCC and its role in the progression of this disease have
not yet been reported.
In the present study, to investigate the roles of
LASP1 in ESCC, we used quantitative real-time polymerase chain
reaction (qRT-PCR) to evaluate the expression of LASP1, and found
that LASP1 was overexpressed in ESCC tissues and ESCC cell lines
when compared with adjacent normal esophageal tissues. This result
was confirmed by western blot analysis and immunohistochemistry
(IHC). We also studied the potential roles of LASP1 in ESCC cell
growth and migration by siRNA transfection. It was found that the
LASP1 gene may mediate cell proliferation, migration and invasion
of ESCC cells.
Materials and methods
Tissue samples and cell culture
Pairs of primary ESCC and matching adjacent normal
esophageal tissues (5 cm above the upper margin of the ESCC) were
obtained from 89 patients after esophagectomy at The Second Xiangya
Hospital of Central South University between 2008 and 2010. The
patients who underwent adjuvant chemotherapy or radiotherapy
preoperatively were excluded in this study. Tissue samples were
immediately snap-frozen in liquid nitrogen and then stored at
−80°C. Serial paraffin-wax sections from the specimens were stained
with hematoxylin and eosin, and the slides of all the cases were
reviewed by two pathologists to confirm the diagnosis based on UJCC
criteria. All information regarding the clinical and
histopathological data was collected. The Ethics Commitee of The
Second Xiangya Hospital approved this study, and written consent
was obtained from each patient.
Two human esophageal squamous cell lines, ECA109 and
KYSE510, were obtained from the American Type Culture Collection
(ATCC) and the German Collection of Microorganisms and Cell
Cultures (DSMZ), respectively, and were grown in HyClone RPMI-1640
medium (Thermo Scientific, Beijing, China) supplemented with 10%
fetal bovine serum (FBS), 100 U/ml of penicillin sodium, and 100
mg/ml of streptomycin sulfate and cultured at 37°C in a humidified
air atmosphere containing 5% carbon dioxide.
RNA extraction and qRT-PCR
Total RNA was extracted from 56 pairs of frozen
tissue (ESCC and matching adjacent normal tissues) and two cell
lines using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
following the manufacturer’s instructions. The final elution volume
was 30–50 μl, and all RNA samples were quantified using the DU 800
UV spectrophotometer (Beckman Coulter, Japan). All samples had an
OD value of 1.8–2.0 and an RNA integrity number >C 5.0.
To determine the expression of LASP1 in ESCC tissues
and ESCC cell lines, SYBR-Green qRT-PCR assay was used. In brief,
total RNA was polyadenylated by poly(A) polymerase and reverse
transcribed to cDNA using the OneStep PrimeScript® cDNA
Synthesis kit (Takara, Dalian, China) according to the
manufacturer’s instructions. Primer sets for LASP1 were sense,
5′-GGTGCGGCAAGATCGTGTA-3′ and antisense, 5′-TGCAGGTCTCGCAATGGAA-3′.
Real-time quantitative polymerase chain reaction (RQ-PCR) was
performed using the SYBR® PrimeScript™ RT-PCR II kit
(Takara) in the ABI PRISM StepOnePlus real-time PCR system (Applied
Biosystems, Foster City, CA, USA). Each amplification reaction was
performed in a final volume of 20 μl containing 40 ng of cDNA, 0.8
μl of each primer and 1X SYBR-Green PCR Master Mix. LASP1
expression data were normalized to β-actin, and the experiments
were performed in triplicate. Data are expressed as the means ± SD.
Relative quantification of LASP1 expression was calculated using
the 2−ΔΔCt method.
Western blotting
For western blotting, proteins were extracted from
13 pairs of frozen tissue (ESCC and matching adjacent normal
tissues) and two cell line lysates. Equal amounts of protein were
resolved by 12% SDS-PAGE. After transferring the protein to a
nitrocellulose membrane and blocking with 3% non-fat dry milk in 10
mM Tris, pH 7.5, 10 mM NaCl, 0.1% (w/v) Tween-20, the membrane was
first incubated with the antibody raised against LASP1 (1:4,000)
followed by incubation with HRP goat anti-rabbit IgG (Proteintech,
Chicago, IL, USA), which were diluted to 1:3,00. Visualization was
carried out using ECL (Thermo Scientific). Quantification of ECL
signals was carried out by densitometry using the Image-Pro Plus
software 6.0 (Media Cybernetics, Inc., Silver Spring, MD, USA). The
LASP1 protein was normalized to β-actin.
Immunohistochemistry (IHC)
IHC was performed to investigate the expression of
LASP1 protein in 20 pairs of human ESCC and matching adjacent
normal tissues. Sections (4 μm) were dewaxed in xylene and
rehydrated in graded ethanol. Sections were subjected to heat
pretreatment by boiling in 0.01 M of sodium citrate buffer (pH 6.0)
for 3 min in a pressure cooker for antigen retrieval. Endogenous
peroxidase was blocked by incubation in 0.1% hydrogen peroxide in
PBS for 10 min, and then sections were incubated with primary
antibodies against LASP-1 (1:400) (BT, USA) overnight at 4°C.
Aliphatic alcohol-polyoxyethylene ether carboxylic acid sodium salt
was used as the chromogen, and sections were counterstained with
hematoxylin.
siRNA transient transfection
The day before transfection, cells in the
exponential phase of growth were seeded into 24-well plates at a
density of 2–8×104 cells per well in 0.5 ml RPMI-1640
medium, grown for 24 h at 50–80% cell confluence and then
transiently transfected with 75 ng (10 nM) of siRNA-LASP1 (cat. no.
SIO2654855; Qiagen, Germany) (sense strand:
5′-GGUUCUUGCCUUUCUUAATT-3′; antisense, 5′-AUUA
AGAAAGGCAAGAACCTG-3′) or negative control siRNA (AllStars negative
nontrol siRNA, cat. no. SI03650318; Qiagen, Germany) using
HiPerFect transfection reagent (1.5 μ) (Qiagen) according to the
manufacturer’s protocol. Cells were incubated with the transfection
complexes under their normal growth conditions and gene silencing
was monitored after an appropriate time.
Cell proliferation assay
To evaluated the viability of ESCC cells after
transfection with siRNA-LASP1, cell proliferation was determined
using the MTT assay. In brief, the day before transfection, cells
were seeded into 96-well plates at a density of 5×103
cells/well. siRNA-LASP1 (75 ng) and negative-control siRNA were
transfected using 0.75 μl HiPerFect transfection reagent. At 24, 48
and 72 h after transfected with siRNA-LASP1, 10 μl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Roche Diagnostics GmbH, Mannheim, Germany) was added to each well
and incubation was carried out at 37°C for 4 h. The medium and MTT
were removed carefully with a 1-ml inoculator, and 100 μl DMSO was
added to each well. Cell proliferation was determined at 490 nm
according to the manufacturer’s protocol. Five wells were detected
for cell viability in each experimental group.
Cell migration and invasion assays
To investgated the motility of ESCC cells after
tansfection with siRNA-LASP1, cell migration assay was carried out
using 24-well Millicell hanging cell culture insert chambers. Upper
and lower culture compartments were separated by polycarbonate
membranes with 8-μm-sized pores (BD, USA) according to the
manufacturer’s instructions. In brief, 72 h after transfection, the
siRNA-LASP1-transfected, negative-control siRNA-transfected and
non-transfected cells were seeded into the upper chamber at
3×105 cells/well in 100 μl RPMI-1640 medium without
serum, and 600 μl RPMI-1640 medium was placed into the lower
chamber and incubation was carried out for 6 h at 37°C in 5%
CO2 humidified air. The cells at the upper surface of
the membrane were removed with a wet cotton swab, and the cells
that adhered to the lower surface of the membrane were fixed using
methanol. Cells were then stained with 0.1% crystal violet for
visualization. Migration was evaluated by the number of cells which
penetrated the membrane per field. Five wells were assayed for cell
migration in each experimental group.
The cell invasion assay was performed using 24-well
Millicell hanging cell culture inserts chambers. The upper and
lower culture compartments were separated by polycarbonate
membranes with 8-μm-sized pores coated with MaxGel ECM (Sigma,
USA). Seventy-two hours after transfection with siRNA-LASP1 or
negative-control siRNA, 100 μl of medium without serum containing
3×104 cells was seeded into the upper chamber per well.
RPMI-1640 medium (600 μl) was placed in the lower chamber, and
cells were incubated for 24 h at 37°C in 5% CO2
humidified air. The remaining steps were the same as for the
migration assay. Five wells were assayed for cell invasion in each
experimental group.
Statistical analysis
Data are presented as the means ± SD and were
analyzed using the software package SPSS 17.0 for Windows. Unless
otherwise noted, the differences between groups were analyzed using
the independent-samples t-test. To assess the significant
differences in LASP1 mRNA between ESCC and adjacent normal tissues,
a paired-samples t-test was used. P-value <0.05 was considered
to indicate a statistically significant difference.
Results
LASP1 is overexpressed in ESCC tissues
and cell lines as determined by qRT-PCR
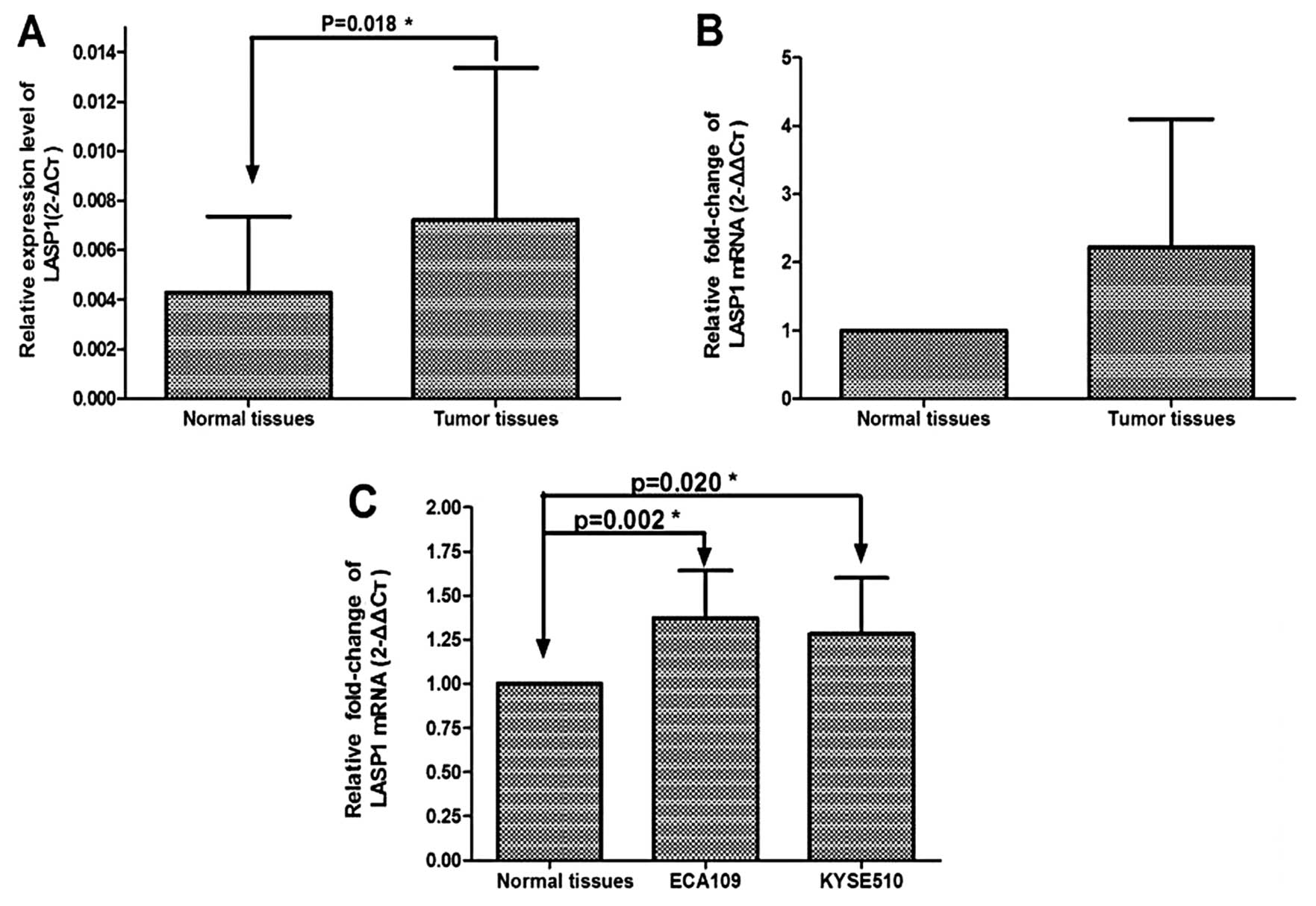
To evaluated the role of LASP-1 in ESCC, the mRNA
expression levels of LASP1 were measured in 56 pairs of tumor
tissues of ESCC and adjacent normal esophageal tissues using
qRT-PCR. The relative expression of LASP1 mRNA was significantly
higher in the tumor tissues compared to the adjacent normal tissues
(P=0.018) (Fig. 1A). LASP1
expression was 2.66-fold upregulated in ESCC compared to that of
the adjacent normal tissues (Fig.
1B). The expression of LASP1 mRNA was also evaluated in cell
lines. Consistent with ESCC tissues, the expression of LASP1 in the
ECA109 and KYSE510 cell lines was also relatively higher than that
in the adjacent normal esophageal tissues, and was 1.37- and
1.28-fold upregulated in esophageal cancer cell lines compared to
normal esophageal tissues, respectively (P=0.002, P=0.020)
(Fig. 1C).
Verification of the differential
expression of LASP1 by western blot analysis
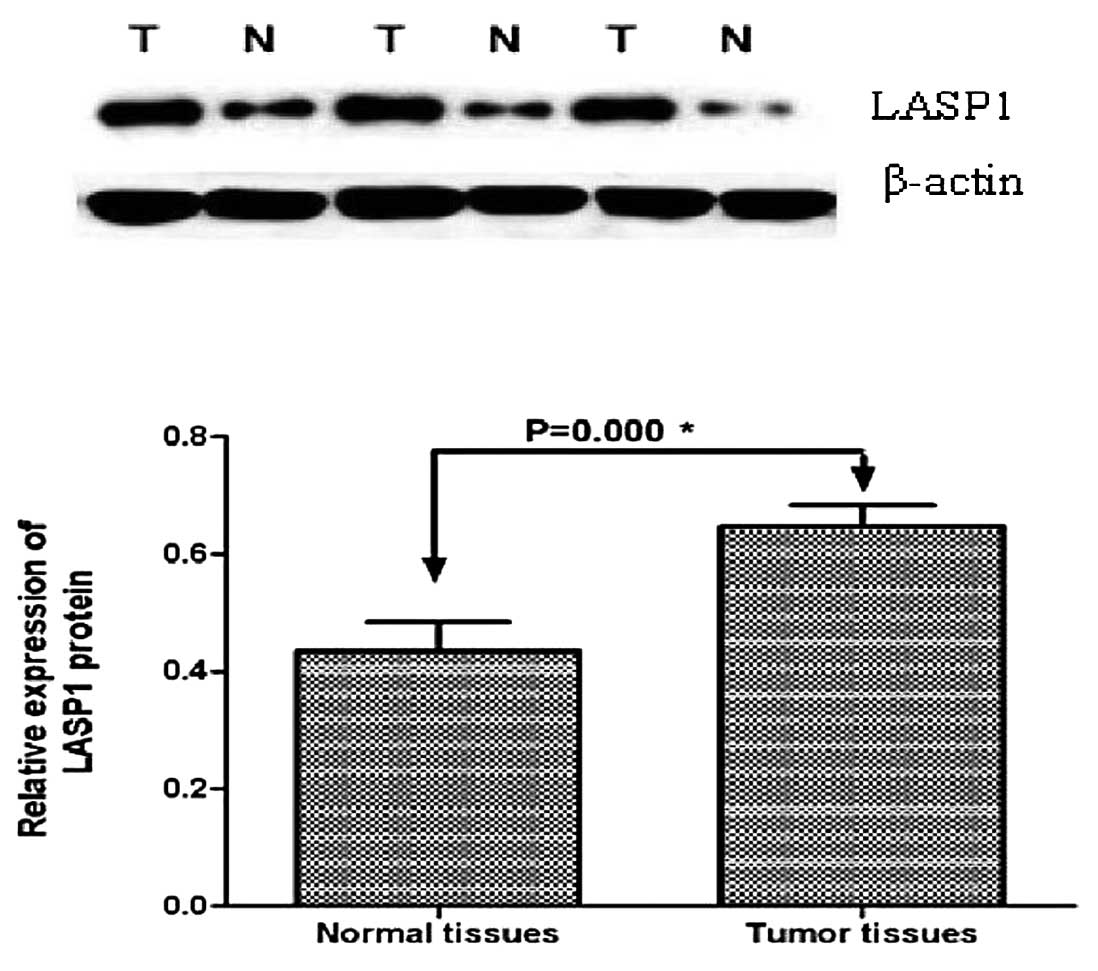
To confirm the qRT-PCR results, western blot
analysis was used to detect the LASP1 expression in another 13
pairs of primary ESCC and adjacent normal esophageal tissues. The
LASP1 protein was normalized to β-actin. Western blot analysis
revealed that LASP1 expression was upregulated in the ESCC tissues
(P=0.000) (Fig. 2) compared to that
of the adjacent normal esophageal tissues. This result was
consistent with that found in the previous qRT-PCR.
Increased expression of LASP1 as detected
by immunohistochemistry
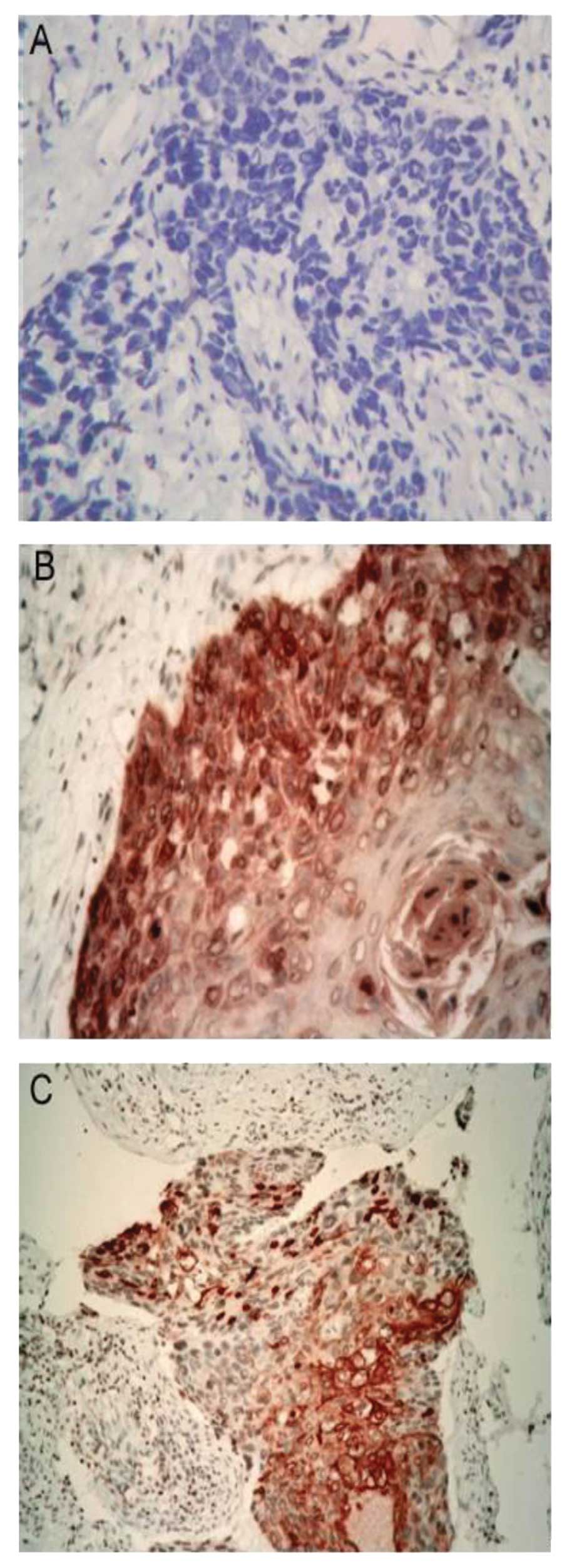
To further confirm the above-mentioned results, IHC
was performed to evaluate the expression of LASP1 in 20 pairs of
paraffin wax slices of ESCC and adjacent normal tissues. IHC showed
positive staining of LASP1 in 18 out of 20 (90%) ESCC tissues.
Strong immunoreactivity was observed in 13 cases, whereas 5 samples
showed a medium to low LASP1 expression and 2 (10%) specimens were
LASP1 negative. In contrast, all of the adjacent normal esophageal
tissues were LASP1-negative. Furthermore, we found that positive
staining of LASP1 was localized not only in the cytoplasm but also
in the nucleolus (Fig. 3).
Efficiency of the transient transfection
of siRNA-LASP1
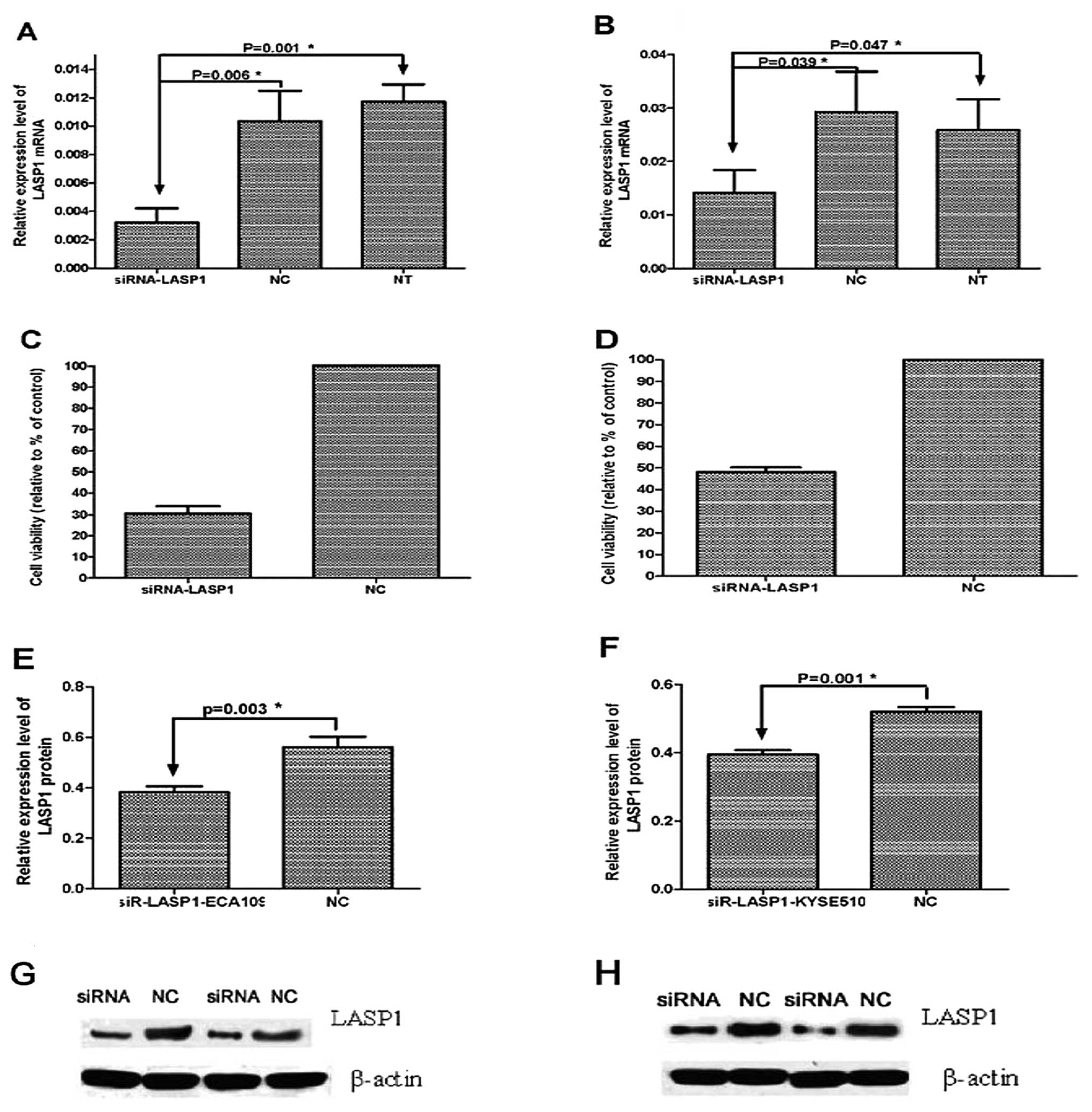
Using a knockdown gene technique, we investigated
the function of LASP1 in ECA109 and KYSE510 cells. To evaluate the
efficiency of the transient transfection of siRNA-LASP1, qRT-PCR
and western blot analysis were performed at 24, 48 and 72 h after
transfection. Results showed that the mRNA expression of LASP1 was
obviously repressed at 72 h after transfection with siRNA-LASP1,
and was reduced by 30.5±3.33 and 48±2.18% in ECA109 and KYSE510
cells compared with the negative-control siRNA-transfected and
non-transfected cells (P=0.006, P=0.001 and P=0.039, P=0.047,
respectively) (Fig. 4). The
expression of LASP1 protein was also inhibited at 72 h after
transfection with siRNA-LASP1 in the ECA109 and KYSE510 cells
compared with the negative-control siRNA-transfected cells (P=0.003
and P=0.001, respectively). These results demonstrated that the
expression of LASP1 in the ECA109 and KYSE510 cells was effectively
suppressed following transfection with specific siRNA-LASP1.
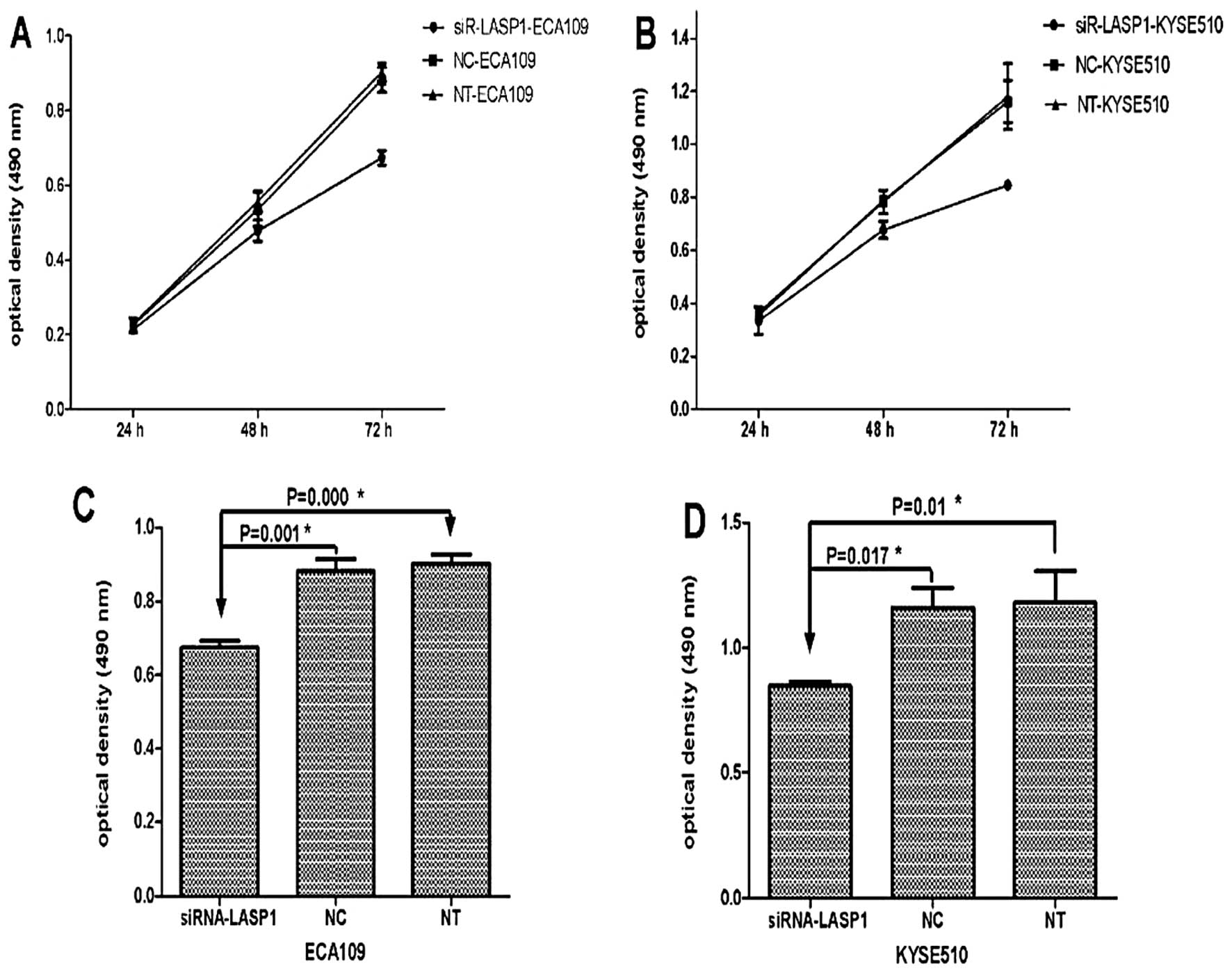
Silencing of LASP1 in ESCC cells inhibits
proliferation in vitro
To evaluate the effect of LASP1 on cell growth,
ECA109 and KYSE510 cells were transiently transfected with either
the siRNA-LASP1 or negative-control siRNA. At 24, 48 and 72 h after
transfection, MTT was performed to detect the cell numbers. The
results showed marked cell growth inhibition in the ECA109 and
KYSE510 cell lines transfected with siRNA-LASP1 compared to the
negative-control siRNA-transfected cells. The percentage of growth
suppression of the cells was 23.6% in ECA109 and 27.1% in KYSE510
cells at 72 h following transfection with siRNA-LASP1, respectively
[optical density (OD), 0.674±0.019 vs. 0.882±0.033; P=0.001 and
0.847±0.017 vs. 1.162±0.079; P=0.017] (Fig. 5). These results indicated that
inhibition of LASP1 prevents the proliferation of ECA109 and
KYSE510 cells.
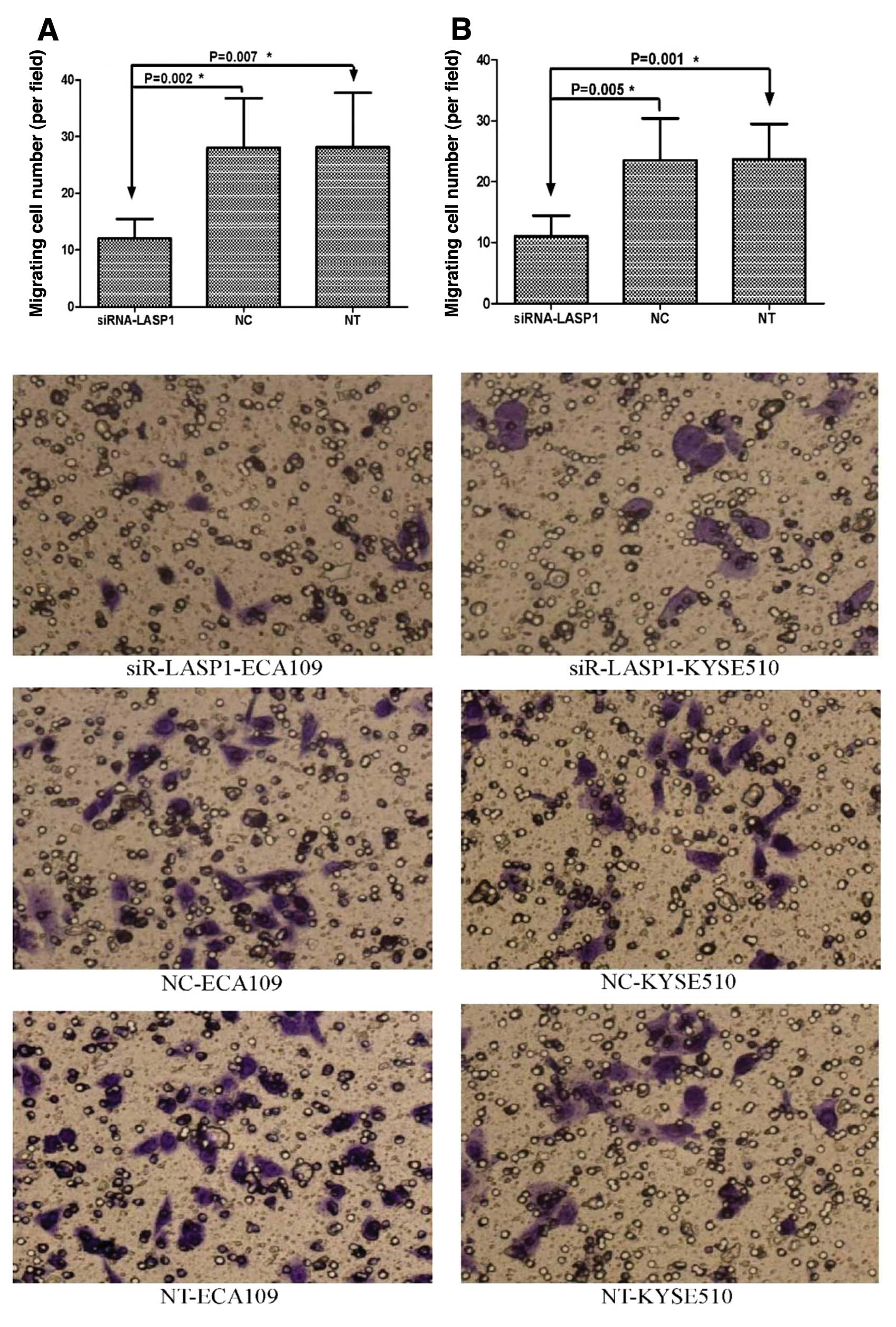
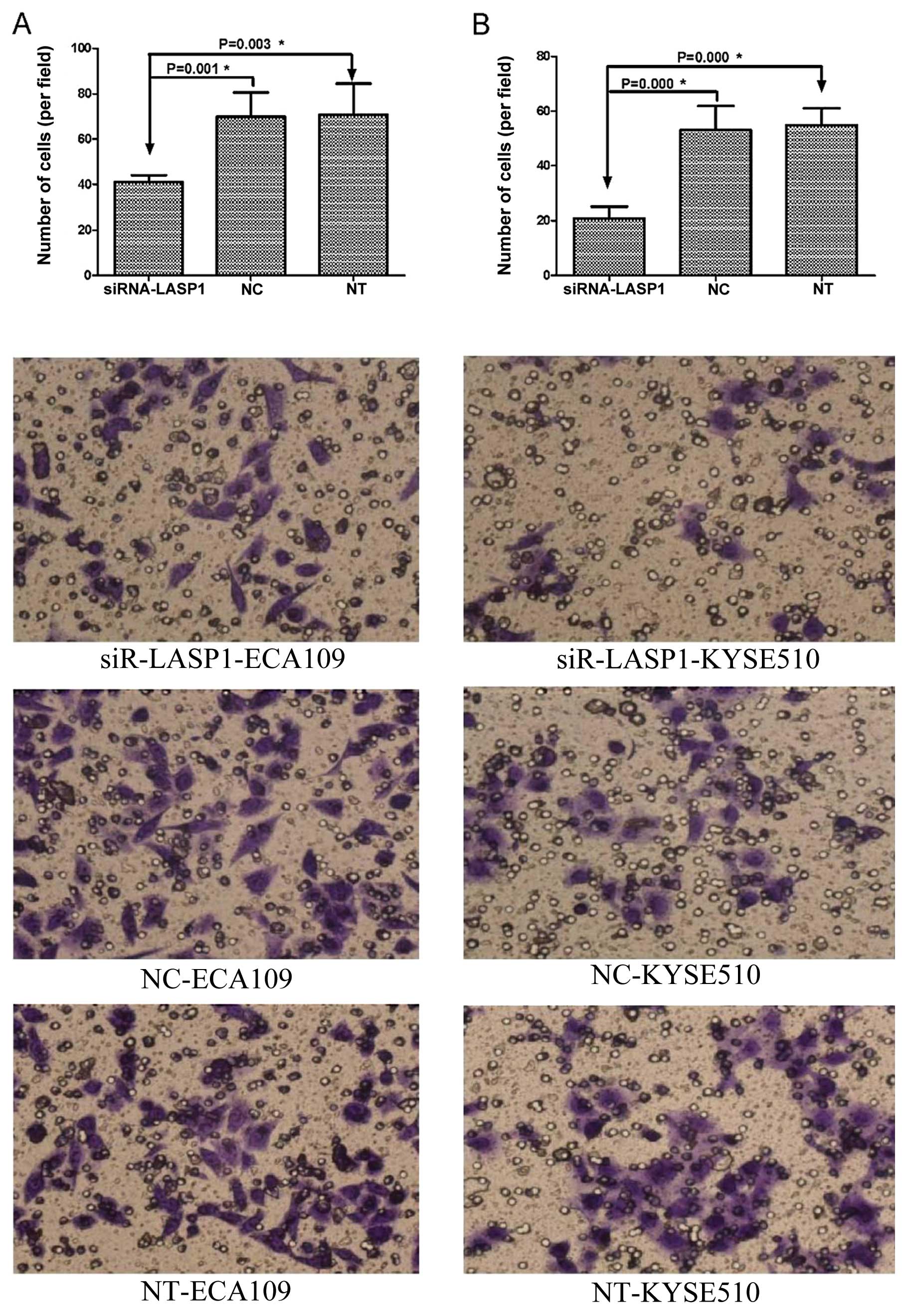
Silencing of LASP1 in ESCC cells inhibits
migration and invasion in vitro
Migration and invasion experiments were performed to
evaluate the role of LASP1 in ESCC cell motility and invasiveness.
Cell migration assay demonstrated that silencing of LASP1 by
siRNA-LASP1 in ECA109 and KYSE510 cells strongly reduced cell
migration compared to the negative-control siRNA-transfected and
non-transfected cells (12±3.46 vs. 28±8.76 and 28.17±9.56; P=0.002
and P=0.007; 11±3.41 vs. 23.5±6.89 and 23.67±5.79; P=0.005 and
P=0.001, respectively) (Fig. 6).
Cell invasion assay showed significant reduction in cell invasion
in siRNA-LASP1-transfected ECA109 cells (41±3.03 vs. 69.83±10.68
and 70.67±13.74; P=0.001, P=0.003) and KYSE510 cells compare to the
negative-control siRNA-transfected and non-transfected cells
(20.83±4.31 vs. 53±8.81 and 54.8±6.18; P=0.000 and P=0.000)
(Fig. 7). These results suggest
that the inhibition of LASP1 expression inhibits the migratory
ability and invasiveness of ECA109 and KYSE510 cell lines in
vitro.
Discussion
EC is the leading cause of cancer-related death
worldwide. ESCC is the most common subtype of EC in China. Since
ESCC patients are associated with an increase incidence of relapse
and metastasis, the prognoses of these patients are still poor
despite improvements in therapeutic techniques. Yet, the mechanisms
involved in the relapse and metastasis in ESCC to date have not
been fully elucidated.
LASP1 is an actin-binding protein. Previous studies
have revealed that the expression of LASP1 is higher in several
types of cancers, and the overexpression of LASP1 plays significant
roles in carcinogenesis and cancer progression. In this study, we
evaluated the expression level of LASP1 in 89 ESCC tissues at the
mRNA and protein levels. Results showed that LASP1 expression at
the mRNA and protein levels was obviously higher in human ESCC
tissues and ESCC cell lines than that in adjacent normal esophageal
tissues. Simultaneously, consistent with the results of studies in
other types of cancers (18), IHC
demonstrated that positive staining of LASP1 was noted in the
cytoplasm and nucleolus of the tumor cells. This observation
indicates that LASP1 is not only a cytosolic protein, but is also a
nuclear protein. This result is consistent with a previous study
(18). Recent data showed that
cytosolic overexpression and nuclear localization significantly
correlates with tumor size and nodal-positivity in many cancers,
and the postoperative relevance of LASP1 expression for prediction
of nodal-positivity has a sensitivity of approximately 85%
(15,18); Cserni found that LASP1 could be used
as a predictive marker for lymph node metastasis together with
other markers such as the superior method of sentinel lymph node
biopsy with an average sensitivity of approximately 95% (19). Consequently, we hypothesized that
overexpression of LASP1 plays an important role in the progression
of ESCC.
Tumor cell motility is a sign of invasiveness and an
essential step in metastasis (20–22).
Several studies have indicated that LASP1 is an important factor
for increasing the viability and motility of tumor cells and it
plays important roles in proliferation, migration and invasion of
several types of cancers. Inhibition of highly expressed LASP1
using siRNA in human breast and ovarian cancer cells induced a
reduction in cell proliferation and migration (13,14).
Furthermore, Zhao et al demonstrated that use of
gain-of-function analysis with gene transfection-mediated
overexpression of LASP-1 in SW480 CRC cells resulted in an
aggressive phenotype of cancer cells and promoted cancer growth and
metastasis in vitro and promoted an aggressive phenotypes of
CRC cells in vivo(15). In
this study, we investigated the function of LASP1 in ECA109 and
KYSE510 cells using a knockdown gene technique. Our study showed
that the proliferation, migration and invasion of ESCC cell lines,
ECA109 and KYSE510, folowing transfection with siRNA-LASP1 were
obviously reduced compared to the negative control cells. These
results suggest that LASP1 may be an important factor for promoting
cell viability, migratory ability and invasiveness in ESCC
progression, and it may function as an oncogene. Moreover,
alterations in LASP1 levels can have an impact on cell growth.
Therefore, further studies with a large sample size are needed to
comfirm these findings and establish the role of LASP1 in the
prognosis of ESCC.
Migration and invasion are central features of the
molecular pathology of malignant tumors and are the main causes of
death from malignant tumors. Alterations in cell shape are crucial
in the first step of migration and invasion in the early stage of
tumor progression. The changes are closely correlated with
parapodium formation by cytoskeleton rearrangements and
concentrations of actin-binding protein. Adhesion proteins have a
crucial role in tumor growth and metastasis. To data, more than 50
different adhesion proteins that regulate the rate and organization
of actin polymerization and focal adhesion turnover in protrusions
have been identified. LASP1 is an actin-binding protein, of which
the C-terminal SH3 domain has an important function that is
involved in protein-protein interactions through binding to
proline-rich sequences, specifically to lipoma preferred partner
(LPP), zyxin, palladin and vasodilator stimulated phosphoprotein
(VASP) (8,23,24).
However, the potential molecular mechanism of LASP1 in the
promotion of cell viability, migratory ability and invasiveness in
carcinogenesis is unclear. The zinc-finger containing LIM domain of
LASP1 is a morphologically and perhaps functionally independent
folding-unit offering the possibility of direct binding to DNA
(25) and it is known to be a
nuclear shuttle protein involved in cell cycle control and cell
migration (26,27). LASP1 silencing is accompanied by
reduced binding of the LASP1-binding partner zyxin which is
necessary for proper cell migration and growth possibly through
influencing zyxin localization (13,14).
Mutation analysis of LASP1 demonstrated that its SH3 domain is
necessary for pseudopodial extension and invasion (28,29).
However, in the present study, we did not investigate the mechanism
of inhibition of cell growth and motility after LASP1 silencing.
Based on the LASP1 molecular mechanism and previous data, we
presumed that inhibition of LASP1 may change the assembly of
cytoskeletal proteins, causing a reduction in cell proliferation,
migration and invasion in particular actin microfilaments and in
filopodia. The potential molecular mechanisms of LASP1 in
carcinogenesis require further research.
Our study firstly observed that LASP1 was
overexpressed in ESCC. Using a knockdown gene technique, we found
that silencing of LASP1 reduced cell proliferation, migration and
invasion in ESCC cell lines in vitro. Our study contributes
to the growing understanding of the role of LASP1 in the
pathogenesis of ESCC. Further prospective studies are necessary to
define the potential of migration and invasion of LASP1 in
carcinogenesis.
Acknowledgements
The authors would like to thank Yerong Hu for her
help. We appreciate support from the Hunan Provincial Health
Department Science Foundation (B2007041 to N.Y.), Hunan Provincial
Nature Science Foundation (2011TT2058 to N.Y.) and Natural Science
Foundation of Changsha (K0803130-31 to NY).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomasetto C, Moog-Lutz C, Régnier CH,
Schreiber V, Basset P and Rio MC: Lasp-1 (MLN 50) defines a new LIM
protein subfamily characterized by the association of LIM and SH3
domains. FEBS Lett. 373:245–249. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomasetto C, Régnier C, Moog-Lutz C,
Mattei MG, Chenard MP and Lidereau R: Identification of four novel
human genes amplified and overexpressed in breast carcinoma and
localised to the q11eq21.3 region of chromosome 17. Genomics.
28:367–376. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schreiber V, Moog-Lutz C, Régnier CH,
Chenard MP, Boeuf H and Vonesch JL: Lasp-1, a novel type of
actin-binding protein accumulating in cell membrane extensions. Mol
Med. 4:675–687. 1998.PubMed/NCBI
|
|
6
|
Chew CS, Chen X, Parente JA Jr, Tarrer S,
Okamoto C and Qin HY: Lasp-1 binds to non-muscle F-actin in vitro
and is localized within multiple sites of dynamic actin assembly in
vivo. J Cell Sci. 115:4787–4799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Butt E, Gambaryan S, Göttfert N, Galler A,
Marcus K and Meyer HE: Actin binding of human LIM and SH3 protein
is regulated by cGMP- and cAMP-dependent protein kinase
phosphorylation on serine 146. J Biol Chem. 278:15601–15607. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keicher C, Gambaryan S, Schulze E, Marcus
K, Meyer HE and Butt E: Phosphorylation of mouse LASP-1 on
threonine 156 by cAMP- and cGMP-dependent protein kinase. Biochem
Biophys Res Commun. 324:308–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa H, Terasaki AG, Suzuki H, Ohashi
K and Miyamoto S: Short-term retention of actin filament binding
proteins on lamellipodial actin bundles. FEBS Lett. 580:3223–3228.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chew CS, Parente JA Jr, Zhou C, Baranco E
and Chen X: Lasp-1 is a regulated phosphoprotein within the cAMP
signaling pathway in the gastric parietal cell. Am J Physiol.
275:C56–C67. 1998.PubMed/NCBI
|
|
11
|
Chew CS, Parente JA Jr, Chen X, Chaponnier
C and Cameron RS: The LIM and SH3 domain-containing protein,
lasp-1, may link the cAMP signaling pathway with dynamic membrane
restructuring activities in ion transporting epithelia. J Cell Sci.
113:2035–2045. 2000.PubMed/NCBI
|
|
12
|
Lin YH, Park ZY, Lin D, Brahmbhatt AA, Rio
MC and Yates JR III: Regulation of cell migration and survival by
focal adhesion targeting of Lasp-1. J Cell Biol. 165:421–432. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grunewald TG, Kammerer U, Schulze E,
Schindler D, Honig A and Zimmer M: Silencing of LASP-1 influences
zyxin localization, inhibits proliferation and reduces migration in
breast cancer cells. Exp Cell Res. 312:974–982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grunewald TG, Kammerer U, Winkler C,
Schindler D, Sickmann A and Honig A: Overexpression of LASP-1
mediates migration and proliferation of human ovarian cancer cells
and influences zyxin localization. Br J Cancer. 96:296–305. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao L, Wang H, Liu C, Liu Y, Wang X and
Wang S: Promotion of colorectal cancer growth and metastasis by the
LIM and SH3 domain protein 1. Gut. 59:1226–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiyomaru T, Enokida H, Kawakami K,
Tatarano S, Uchida Y and Kawahara K: Functional role of LASP1 in
cell viability and its regulation by microRNAs in bladder cancer.
Urol Oncol. 30:434–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Feng P, Xiao Z and Ren EC: LIM and
SH3 protein 1 (Lasp1) is a novel p53 transcriptional target
involved in hepatocellular carcinoma. J Hepatol. 50:528–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grunewald TG, Kammerer U, Kapp M, Eck M,
Dietl J and Butt E: Nuclear localization and cytosolic
overexpression of LASP-1 correlates with tumor size and
nodal-positivity of human breast carcinoma. BMC Cancer. 7:198–208.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cserni G: Evaluation of sentinel lymph
nodes in breast cancer. Histopathology. 46:697–702. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Goswami S and Sahai E: Tumor cells
caught in the act of invading: their strategy for enhanced cell
motility. Trends Cell Biol. 15:138–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ridley AJ, Schwartz MA and Burridge K:
Cell migration: integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rachlin AS and Otey CA: Identification of
palladin isoforms and characterization of an isoform-specific
interaction between Lasp-1 and palladin. J Cell Sci. 119:995–1004.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Zhuang L and Trueb B: Zyxin
interacts with the SH3 domains of the cytoskeletal proteins
LIM-nebulette and Lasp-1. J Biol Chem. 279:20401–20410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hammarstrom A, Berndt KD and Sillard R:
Solution structure of a naturally-occurring zinc-peptide complex
demonstrates that the N-terminal zinc-binding module of the Lasp-1
LIM domain is an independent folding unit. Biochemistry.
35:12723–12732. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beckerle MC: Zyxin: zinc fingers at sites
of cell adhesion. Bioessays. 19:949–957. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kadrmas JL and Beckerle MC: The LIM
domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell
Biol. 5:920–931. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spence HJ, McGarry L and Chew CS: AP-1
differentially expressed proteins Krp1 and fibronectin
cooperatively enhance RhoROCK-independent mesenchymal invasion by
altering the function, localization, and activity of
nondifferentially expressed proteins. Mol Cell Biol. 26:1480–1495.
2006. View Article : Google Scholar
|
|
29
|
Viney RL, Morrison AA and van den Heuvel
LP: A proteomic investigation of glomerular podocytes from a
Denys-Drash syndrome patient with a mutation in the Wilms tumour
suppressor gene WT1. Proteomics. 7:804–815. 2007. View Article : Google Scholar : PubMed/NCBI
|