Introduction
The cancer stem cell (CSC) theory proposes that
tumor cells are composed of a heterotypic population of cells and
the CSCs, which comprise a small percentage of the total tumor,
remain at the top of the cellular hierarchy with unlimited
proliferation potential and pluripotency (1,2).
Recent evidence indicates that breast CSCs are relatively resistant
to both radiation and chemotherapy (3,4).
Therefore, new treatment strategies that effectively target both
NCSCs and CSCs show promise as an effective treatment for breast
cancer.
The oncolytic herpes simplex virus (oHSV) vector has
been shown to be a safe and effective therapeutic approach for
various types of cancer, including breast cancer (5,6). G47Δ
is a third-generation replication-competent HSV-1 vector based on
G207 by deletion of the ICP47 gene (7). The deletion of ICP47 not only
upregulates the expression of MHC class I on the surface of
G47Δ-infected cells, but also deletes the US11 promoter which makes
the late US11 gene under the control of immediate early ICP47
promoter, thereby enhancing growth of G47Δ by precluding shutoff of
protein synthesis. This makes G47Δ more effective than G207 in
killing tumor cells. We previously reported that G47Δ effectively
targeted breast cancer both in vitro and in
vivo(5,6).
In the present study, we showed that G47Δ
effectively killed different types of breast cancer cells in
vitro. We found that G47Δ also effectively targeted CSCs in
vitro and impaired their self-renewal ability. Finally, we
demonstrated that G47Δ could induce the regression of tumor
xenografts in BALB/c mice and worked synergistically with
paclitaxel in killing both NCSCs and CSCs.
Materials and methods
Cells and viruses
Breast cancer cell lines MCF-7, SK-BR-3, MDA-MB-435,
MDA-MB-436 and MDA-MB-468 were obtained from Dr Xie Xiao-ming (Sun
Yat-sen University Cancer Center, China). G47Δ was constructed as
previously described (7) and was
obtained from Dr Samuel D. Rabkin, Molecular Neurosurgery
Laboratory, Massachusetts General Hospital, Harvard Medical School,
Boston, MA, USA). Viruses were grown and titered using Vero cells
(African green monkey kidney cells; ATCC, Manassas, VA, USA).
In vitro cytotoxicity of the parental
cell lines
Cells were infected with mock or viruses at
multiplicity of infection (MOI) of 0.1 and 0.01, then incubated in
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, USA)
supplemented with 1% heat-inactivated fetal calf serum. Cells were
stained with trypan blue (Sigma, USA) and counted on a
hemocytometer.
For combination therapy, cells were seeded into a
10-cm culture dish and infected with viruses at a MOI of 0.5, 1 nM
paclitaxel (Sigma), G47Δ + paclitaxel or a mock treatment. Cells
were incubated at 37°C for 4 days in DMEM supplemented with 10%
heat-inactivated fetal calf serum. Cells were harvested and counted
on Day 4 and stained with fluorescein isothiocyanate-labeled (FITC)
anti-CD44 and phycoerythrin-labeled (PE) anti-CD24 antibodies (both
from Beckman Coulter, Brea, CA, USA). The stained cells were
assayed by flow cytometry (Becton-Dickinson), and the percent of
CD44+CD24− cells was calculated.
Isolation and identification of CSCs
As previously described (8), cells were cultured in DMEM/F12 medium
supplemented with 10 μg/l basic fibroblast growth factor, 20 μg/l
epidermal growth factor, 5 mg/l insulin and B27 (all from
Invitrogen). Cells generally formed mammospheres within 10
days.
The mammospheres and parental cell lines were
trypsinized and stained with anti-CD44 and anti-CD24 antibodies as
described above. The Aldefluor assay was performed according to the
manufacturer’s protocol (Stemcell Technologies, Canada).
In vitro cytotoxicity of G47Δ on
CSCs
Mammospheres were dissociated, the resulting single
cells were resuspended at 1×107 cells/ml and infected at
an MOI of 0.1 or mock-infected for 90 min at 37°C. The cells were
then centrifuged to remove unadsorbed viruses and seeded in 6-well
plates at 1×105 cells per well. Cells were counted on
Days 3 and 7 using a hemocytometer.
Cells were harvested along with the associated
supernatant at 12, 36 and 60 h post-infection. After three
freeze/thaw cycles, the titers of infectious virus were tested
using a plaque assay with Vero cells.
Seven days following virus infection, the viable
cells were collected, resuspended in fresh medium, and seeded into
96-well plates at a density of 1 or 10 cells per well. Two weeks
later, the number of wells containing mammospheres (diameter >40
μm) was recorded.
In vivo treatment studies
MDA-MB-468 mammosphere cells (1×106) were
implanted into the left flank of 6-week-old female BALB/c nude mice
(Vital River Laboratory Animal Technology Co., Ltd., China). When
tumors reached a maximal diameter of ~5 mm, mice were randomized
into 4 groups of 7 mice per group. The groups consisted of a G47Δ
treatment group that was given a 50-μl intratumor (i.t.) injection
of G47Δ virus (2×107 plaque-forming units, pfu) on Days
0 and 3, a paclitaxel treatment group treated with an
intraperitoneal (i.p.) injection of 3 mg/kg paclitaxel on Days 0
and 7, a combination treatment group that was treated with G47Δ and
paclitaxel on the same dosing schedule, and a mock treatment group.
Tumor volume was calculated using the formula: width
(mm)2 × length (mm) × 0.5.
Tumor tissue was minced and digested for ~6 h at
37°C using a solution of 100 U/ml collagenase I, 150 U/ml
hyaluronidase, 10% calf serum, and 5 mg/l bovine insulin in DMEM
(all from Invitrogen). Digested tissue was strained using a 40-μm
strainer, the cell suspension was washed with PBS and the red blood
cells were lysed for 5 min (Bomeike Biotechnology, China). The
samples were analyzed for the percentage of
CD44+CD24− cells using flow cytometry. All
animal procedures were approved by the Animal Care and Use
Committee of Sun Yat-sen University.
X-gal histochemistry
X-gal staining was performed according to the
manufacturer’s protocol (Beyotime Institute of Biotechnology,
China).
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Each experiment was repeated in at least three independent
trials. A Student’s t-test was used to analyze the significance of
differences between two treatment groups. SPSS version 13.0
software was used, and P<0.05 was considered to indicate a
statistically significant difference.
Results
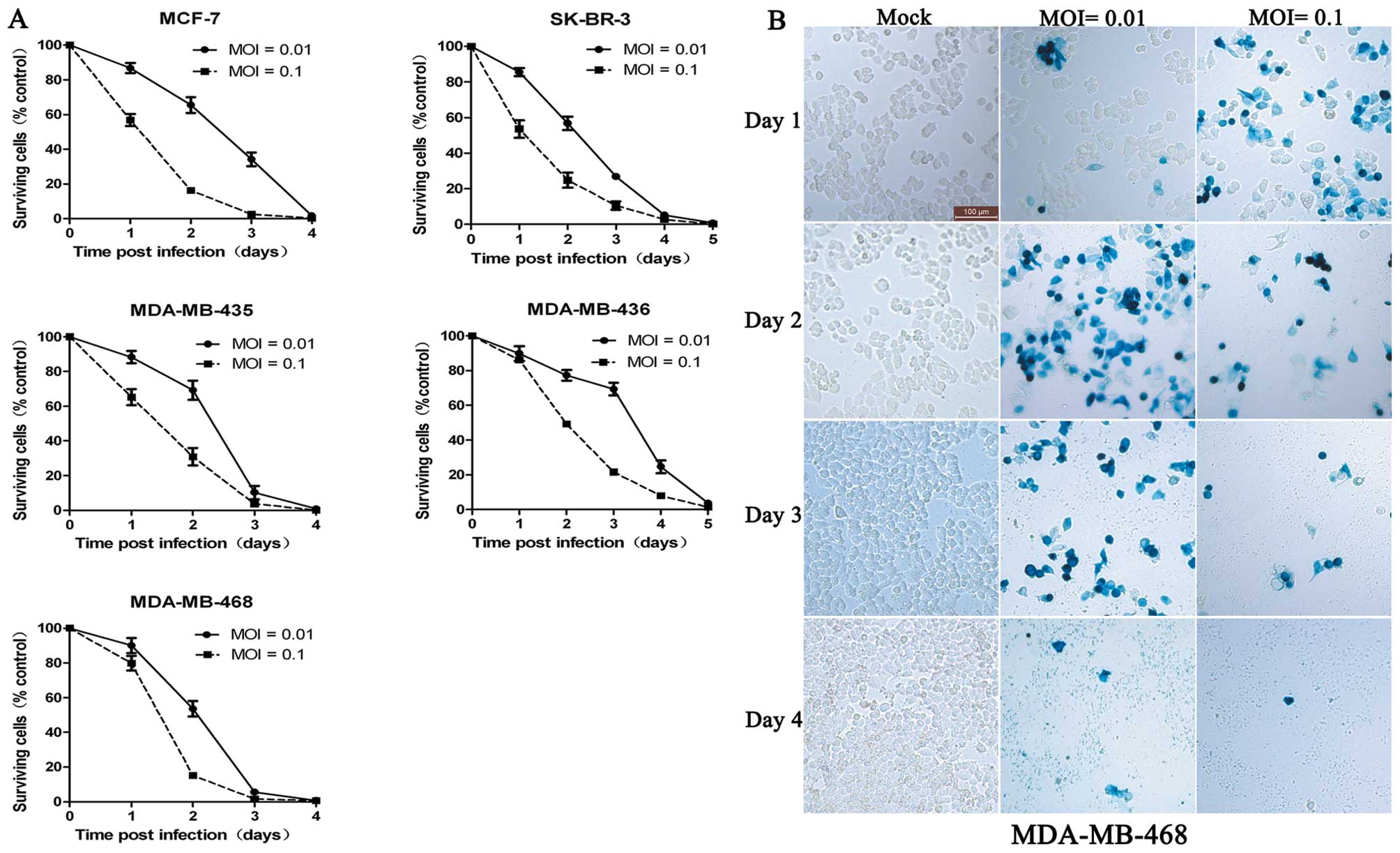
G47Δ effectively kills different types of
breast cancer cell lines in vitro
Neve et al(9)
reported that breast cancer cell lines effectively model primary
breast tumors. MCF-7, SK-BR-3 and three other cell lines represent
luminal subtype tumors, HER-2 overexpression subtype and basal-like
subtype, respectively. We showed that G47Δ effectively killed
different subtypes of tumors (Fig.
1A). MCF-7, MDA-MB-435 and MDA-MB-468 were highly sensitive to
G47Δ-mediated killing with >98% of these tumor cells dying by
Day 4 with an MOI of 0.01. By Day 3, almost all the MDA-MB-468
cells were infected by G47Δ (Fig.
1B). SK-BR-3 and MDA-MB-436 cells displayed a moderate
sensitivity to G47Δ-mediated killing with >98% of cells dying by
Day 5. These findings demonstrate that G47Δ can effectively kill
different subtypes of breast cancer cells.
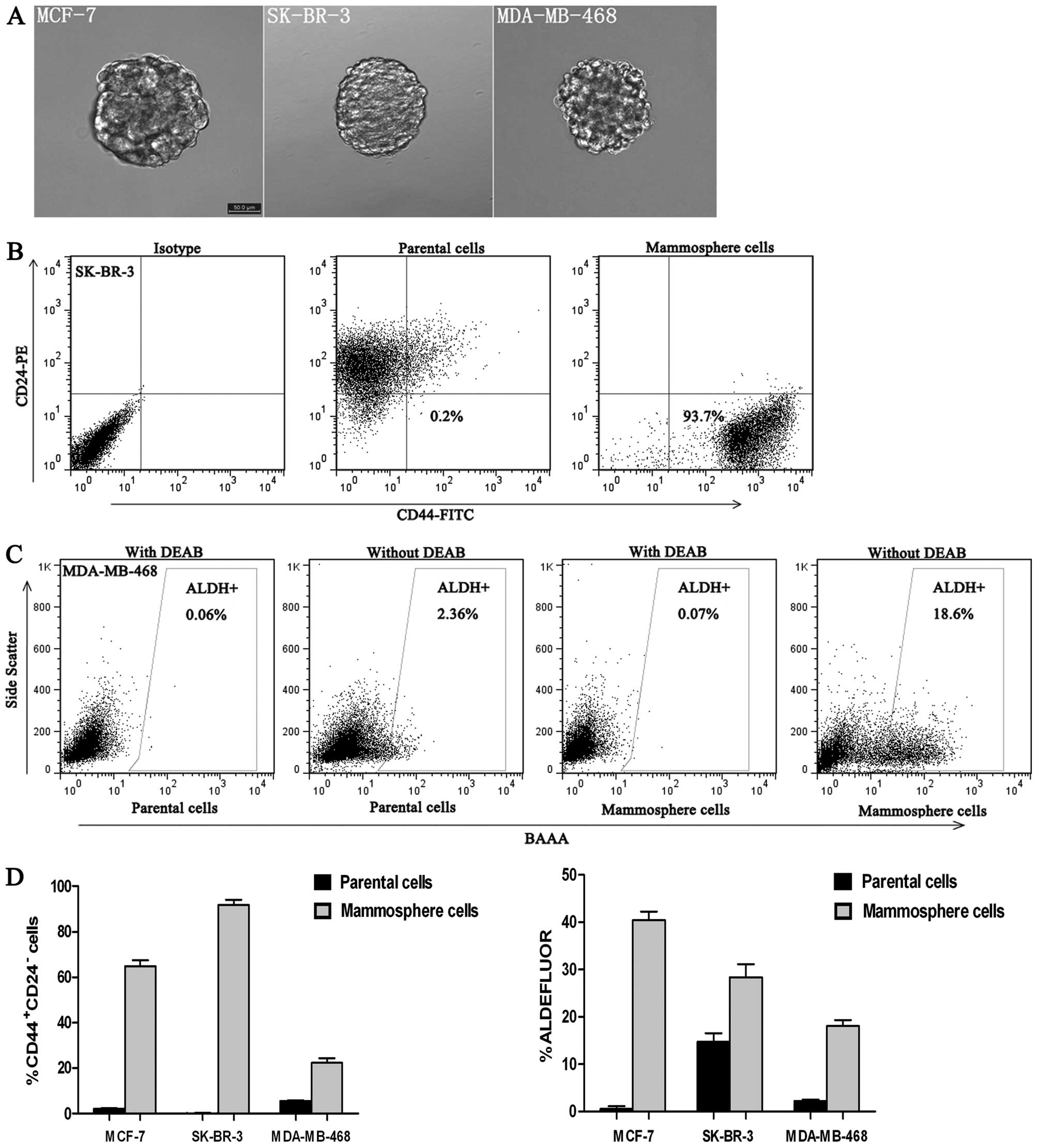
Isolation and identification of CSCs
CSCs typically form mammospheres within 10 days of
anchorage-independent culture. Our mammosphere cultures could be
passaged for >3 months. Moreover, single dissociated mammosphere
cells were able to regenerate spheres, thereby indicating their
clonogenicity (Fig. 2A and Table I). Using the cultured CSCs, we
investigated the percentage of mammosphere cells that displayed the
stem cell-like phenotype CD44+CD24− and
aldehyde dehydrogenase (ALDH. In contrast to their respective
parental cell lines, cells derived from mammosphere cells were
enriched for CD44+CD24− and ALDH+
cells. CD44+CD24− cells accounted for
0.23±0.08% of parental SK-BR-3 cells, whereas
CD44+CD24− cells accounted for 91.69±2.27% of
mammosphere cells derived from the SK-BR-3 cell line (Fig. 2B). The percentage of
ALDH+ cells in the MDA-MB-468 parental cell line was
2.31±0.27%, whereas ALDH+ cells accounted for
18.14±1.21% of mammosphere cells derived from the MDA-MB-468 cell
line (P<0.001) (Fig. 2C).
Mammosphere cells from the other breast cancer cell lines displayed
a similar enrichment for CD44+CD24− or
ALDH+ cells relative to their respective parental cell
line (Fig. 2D).
 | Table ICSCs that survive infection with G47Δ
are unable to form secondary mammospheres. |
Table I
CSCs that survive infection with G47Δ
are unable to form secondary mammospheres.
| Cell | MOI | Cell no. per
well | No. of wells with
spheres/96-wells |
|---|
| MCF-7 | 0 | 10 | 70 |
| 0 | 1 | 13 |
| 0.1 | 10 | 0 |
| SK-BR-3 | 0 | 10 | 88 |
| 0 | 1 | 21 |
| 0.1 | 10 | 0 |
| 0 | 10 | 58 |
| MDA-MB-468 | 0 | 1 | 10 |
| 0.1 | 10 | 0 |
These results show that anchorage-independent cell
culture results in the production of mammosphere cells that are
enriched with cells expressing breast CSC markers, and these cells
display undifferentiated stem-like characteristics.
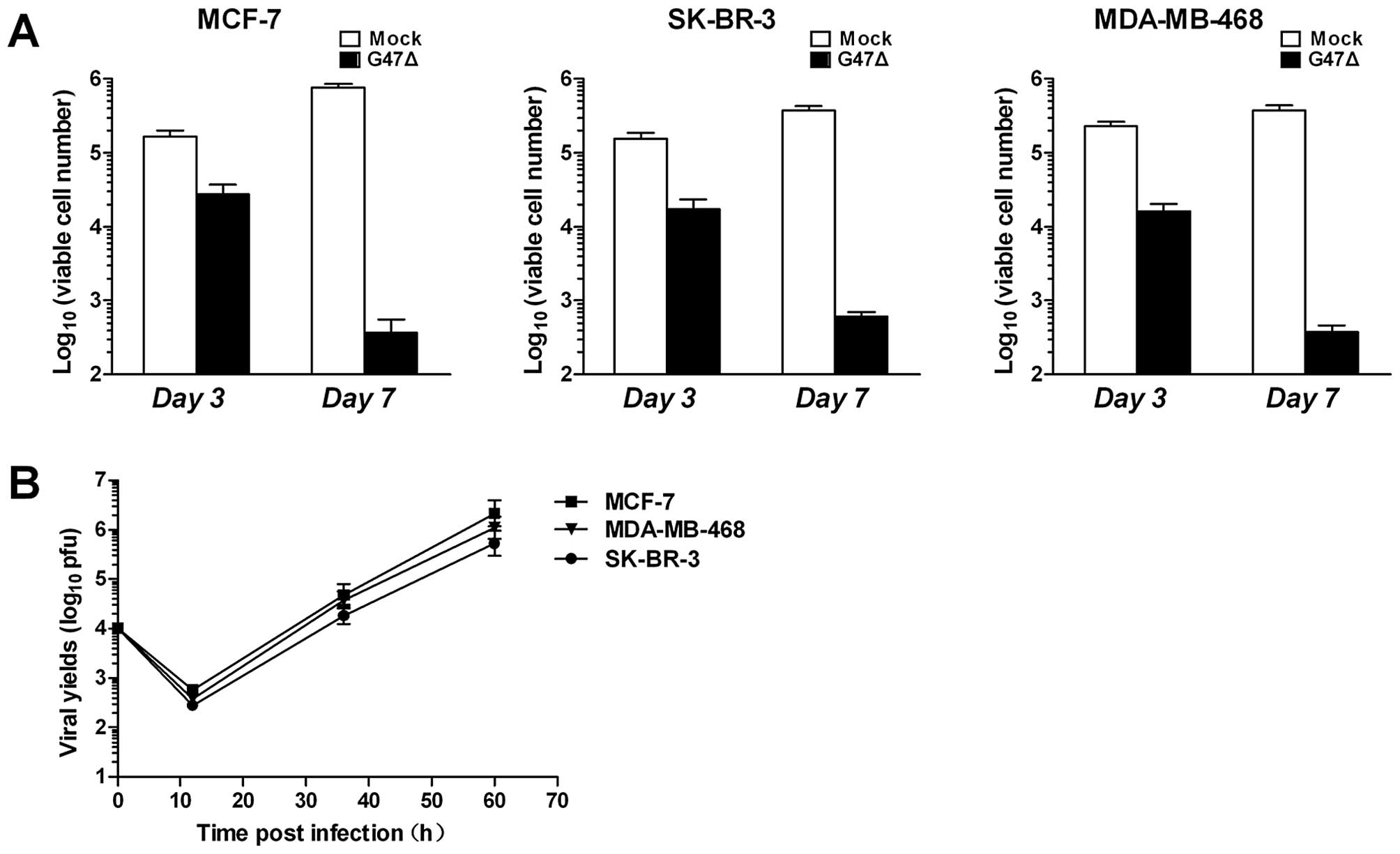
G47Δ effectively targets breast CSCs in
vitro
CSCs were cultured under anchorage-independent
conditions and infected with G47Δ at a MOI of 0.1. Our results
showed that CSCs were sensitive to G47Δ (Fig. 3A). Similar to the parental cell
lines, MCF-7, SK-BR-3 and MDA-MB-468 grown under
anchorage-independent conditions were also effectively killed by
G47Δ with >98% of CSCs killed by Day 7. To determine whether
G47Δ could replicate and spread among CSCs, we quantified viral
replication using the plaque-forming unit (PFU) assay. The CSCs and
their associated supernatants were collected at indicated time
points after infection, and virus titers were determined after
three freeze/thaw cycles by the PFU assay using Vero cells. We
found that G47Δ replicated considerably in MCF-7, SK-BR-3 and
MDA-MB-468 cells (Fig. 3B).
Collectively, these results demonstrate that G47Δ effectively
targets breast CSCs.
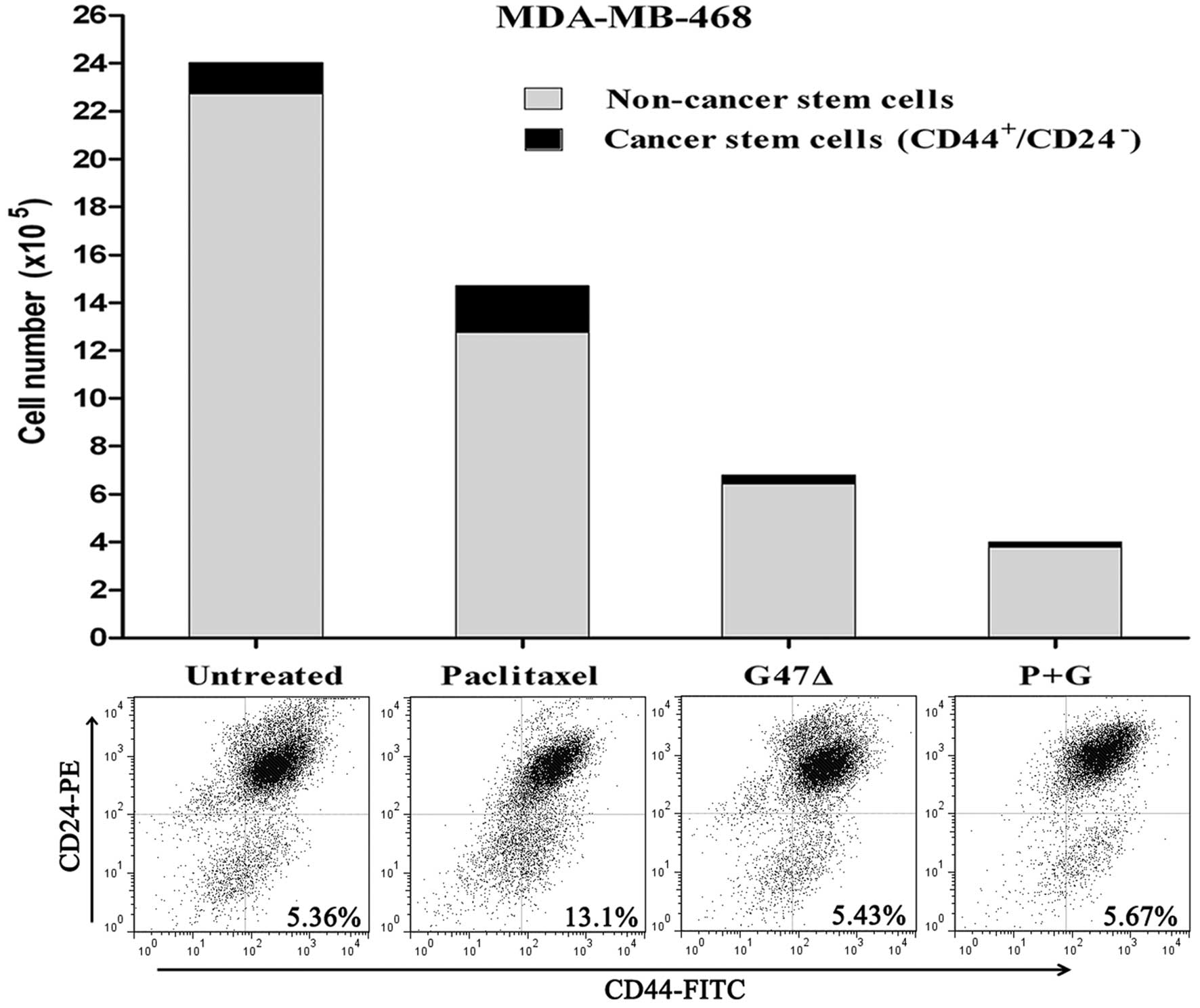
G47Δ kills breast NCSCs and CSCs equally
and synergizes with paclitaxel by killing both NCSCs and CSCs in
vitro
A previous study demonstrated that CSCs are
relatively resistant to chemotherapy (4). This study showed that treatment with
the standard breast cancer drug paclitaxel resulted in an increase
in the proportion of CD44+CD24− cells
(4). Therefore, we determined
whether NCSCs were more sensitive to G47Δ than CSCs. To this end,
we tested the proportion of CD44+CD24− cells
before and after treatment with G47Δ or paclitaxel. Similar to
previous research, our results showed that CSCs were generally
resistant to paclitaxel-mediated cell death, and, following
treatment with paclitaxel, the proportion of
CD44+CD24− cells increased (13.13±0.43%) when
compared with the control group (5.41±0.42%, P<0.001) (Fig. 4). By contrast, the proportion of
CD44+CD24− cells did not change relative to
the control cells after treatment with G47Δ (5.44±0.39%,
P>0.05). Moreover, when G47Δ was used in combination with
paclitaxel, we observed a synergistic effect resulting in the death
of both NCSCs and CSCs, the percentage of stem cells remained
unchanged (5.55±0.32%, P>0.05). Collectively, these results
demonstrate that G47Δ is equally effective in killing both breast
NCSCs and CSCs. Furthermore, G47Δ can synergize with paclitaxel to
kill both NCSCs and CSCs in vitro.
The self-renewal of CSCs is impaired
after G47Δ infection
Self-renewal is one of the defining properties of
CSCs, and the ability to form spheres in vitro is considered
an indicator of self-renewal ability. Wakimoto et
al(10) found that G47Δ
infection impairs the self-renewal ability of glioblastoma stem
cells. Therefore, we used single-cell-derived mammosphere cells to
determine whether G47Δ could impair the self-renewal ability of
breast CSCs. Although G47Δ killed almost all mammosphere cells by
Day 7, we observed a minor population of cells that remained alive
after 7 days of infection with G47Δ. Live cells were collected and
subjected to a limiting dilution assay to determine whether these
cells maintained the ability to generate secondary mammospheres.
Our results showed that none of the cells that survived following
G47Δ infection were able to generate secondary mammospheres when
seeded at up to 10 cells per well (Table I). By contrast, as few as one live
cell from the control treatment group was able to form secondary
mammospheres. Collectively, these results suggest that G47Δ may
inhibit the self-renewal of breast CSCs regardless of whether they
undergo oncolysis. However, the underlying mechanism remains to be
determined and warrants further investigation.
G47Δ-induced regression of tumor
xenografts in BALB/c mice
We chose MDA-MB-468 mammosphere cells to form tumors
in BALB/C mice, as MDA-MB-468 is a basal-like cell line that is
very aggressive and forms large tumors that are resistant to
tamoxifen or trastuzumab (11).
Mice were injected with MDA-MB-468 mammosphere cells, and after
flank tumors developed, the mice were divided into four treatment
groups. One group was treated with a mock treatment, another group
received an i.t. G47Δ injection, one group received an i.p.
paclitaxel injection, and one group received G47Δ + paclitaxel. Our
results showed that G47Δ effectively killed tumor cells in
vivo since the mice that received G47Δ alone showed a
significant reduction in their mean tumor volume (101.6±46.7
mm3) when compared with the mock treatment group
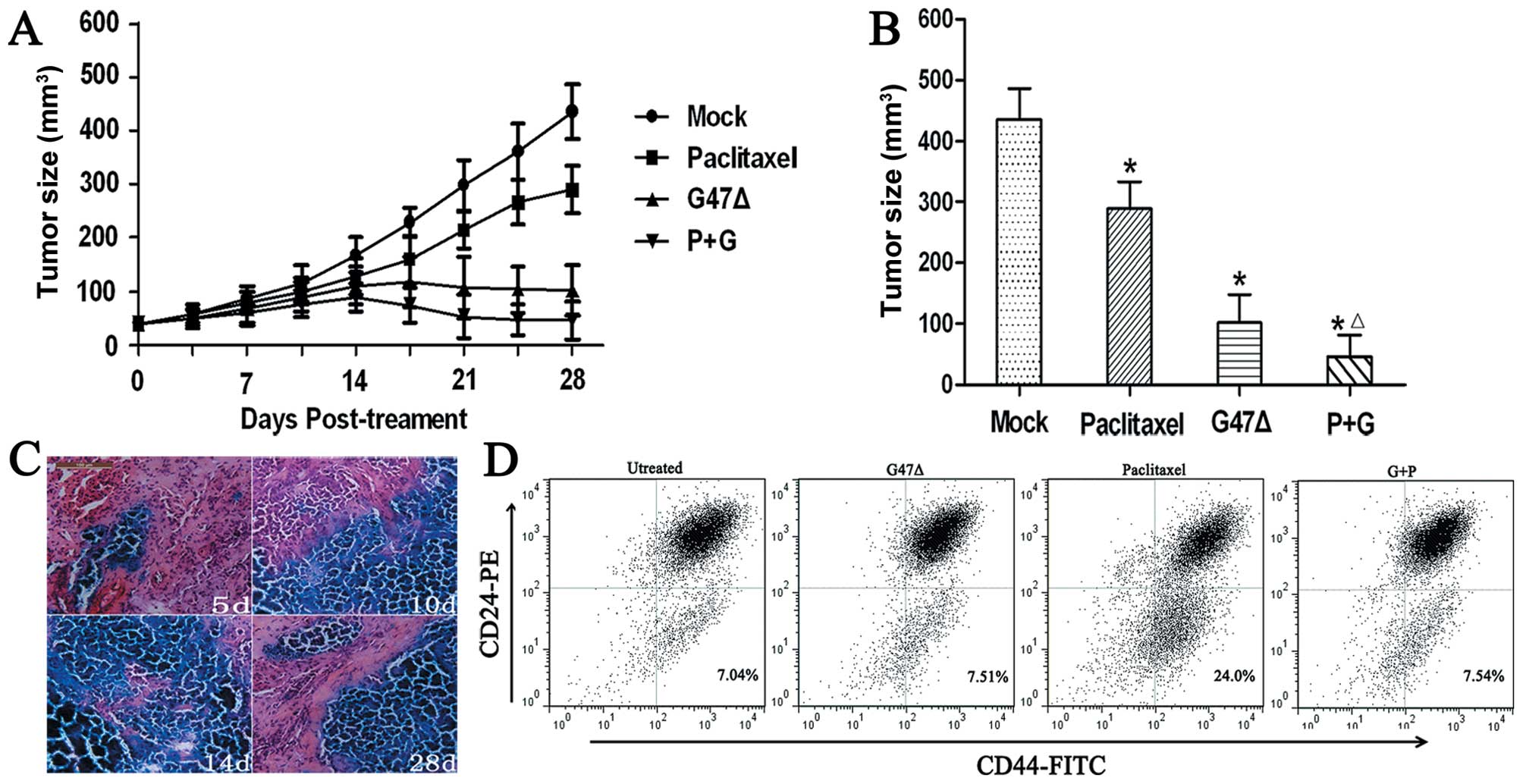
(435.3±50.25 mm3) by Day 28 (P<0.001) (Fig. 5A and B). However, we also observed
that the group of mice that received G47Δ + paclitaxel displayed a
synergistic effect in vivo and a significant reduction in
the mean tumor volume (45.9±35.4 mm3) when compared with
G47Δ alone (101.6±46.7 mm3), paclitaxel alone
(288.9±44.4 mm3), or mock treatment groups (435.3±50.25
mm3) by Day 28 (P<0.05 for all three comparisons)
(Fig. 5A and B). Furthermore, the
combined treatment showed no signs of toxicity as the body weights
of the mice were similar among the four groups (mean body weight,
22.3 g), and body weight did not significantly change over the
course of the study.
We also found that G47Δ effectively replicated and
spread among tumor cells in vivo, and the most extensive
X-gal staining was apparent 14 days post-inoculation (Fig. 5C). By Day 28, we found that G47Δ
remained capable of in vivo replication.
We found that mammosphere cells derived from
MDA-MB-468 could differentiate into different types of cancer cells
in vivo. The proportion of CD44+CD24−
cells of the control treatment group (7.23±0.45%) was similar to
the MDA-MB-468 cell line in vitro (5.35±0.42%) (Fig. 5D). G47Δ effectively killed both CSCs
and NCSCs in vivo, and the proportion of the
CD44+CD24− cells (7.48±0.37%, P>0.05,
compared with the control group) did not increase after treatment
with G47Δ, which was similar to the in vitro results.
However, the proportion of CD44+CD24− cells
(23.6±2.54%, P<0.001, compared to the control group) increased
2.27-fold after treatment with paclitaxel. Notably, we found G47Δ
and paclitaxel showed a synergistic effect by killing both NCSCs
and CSCs in vivo. The proportion of
CD44+CD24− cells in the combination treatment
group was 7.51±0.63%, which was similar to the control treatment
group (P>0.05, compared to the control group).
Collectively, these results suggest that G47Δ
effectively kills breast NCSCs and CSCs in vivo and has a
synergistic effect with paclitaxel by killing both CSCs and
NCSCs.
Discussion
Different breast cancer subtypes categorized based
on molecular characteristics have been shown to be highly
associated with patient prognosis; these characteristics also serve
as predictive tools for the efficacy of a particular therapy in a
given patient. Luminal subtype of breast tumors usually have an
excellent prognosis and do not benefit significantly from
chemotherapy (12). For patients
whose tumors highly express the HER-2 gene, trastuzumab
significantly improves the disease-free and overall survival
(13,14). However, although triple-negative
breast cancers (TNBCs) or basal-like tumors tend to be highly
sensitive to chemotherapy, they tend to have the worst prognosis
and highest recurrence rates (15–17).
Moreover, there are no approved drugs that specifically target
TNBCs. There is a substantial ongoing effort to develop
therapeutics that effectively target all subtypes of breast cancer
and TNBC in particular. In this study, we have shown that G47Δ
effectively kills all types of breast cancer cells regardless of
the ER, PR and HER-2 status. G47Δ may represent a promising
therapeutic agent for the treatment of all subtypes of breast
cancer.
Breast CSCs were isolated based on the expression of
the two cell surface markers CD44 and CD24 or expression of
aldehyde dehydrogenase 1 (ALDH1) (18,19).
Previous studies have demonstrated that
CD44+CD24− cells are highly tumorigenic and
can give rise to a large and diverse population of tumor cell types
(18). Ginestier et
al(19) showed ALDH+
cells were highly tumorigenic, and patients with a higher
percentage of ALDH+ cells had the worst clinical
outcomes. Recent studies reveal that stem/progenitor tumor cells
can grow in non-adherent conditions based on the unique properties
of stem/progenitor cells that allow them to survive and grow in a
serum-free suspension culture (8).
Thus, the tumorsphere assay allows researchers to culture breast
tumor cells with stem/progenitor cell properties for a long period
of time, which represents a suitable in vitro model for the
study of breast cancer-initiating cells. We used
anchorage-independent cell culture conditions to enrich for CSCs
and found that mammosphere cells were enriched with cells
expressing breast CSC markers, and these cells display
undifferentiated stem-like characteristics.
Despite the improvements in treatment regimens,
approximately 30% of patients with an early-stage disease
eventually develop recurrent and metastatic lesions (20). CSCs are considered the main source
of recurrence and metastasis (1,2). CSCs
are relatively resistant to chemotherapy and radiotherapy;
moreover, breast CSCs appear to be ER negative and may play an
important role in resistance to endocrine therapy (21).
oHSV viral vectors selectively replicate within
tumors and directly destroy tumor cells by virus-induced cell
lysis. This mechanism is distinctly different from routine cancer
therapies, such as chemotherapy and endocrine therapy. We
demonstrated that G47Δ equally killed CSCs and NCSCs; G47Δ also
displayed a synergistic effect with paclitaxel by killing both CSCs
and NCSCs, and G47Δ showed no obvious signs of toxicity in mice.
G47Δ might be an effective new strategy to target CSCs and to use
in conjunction with conventional chemotherapy to achieve the
greatest effect. Ahtiainen et al(22) found that breast CSCs had innate
immunity defects which rendered breast CSCs permissive to oncolytic
adenovirus. Next, we may focus on the immune response of breast
CSCs to G47Δ infection both in vitro and in vivo.
CSCs have the ability of self-renewal, and only CSCs
are believed to have the ability to proliferate indefinitely to
generate new tumors. Through asymmetric division, CSCs divide to
produce one daughter stem cell and one daughter cell that may
differentiate into a nontumorigenic cell. This theory suggests that
agents that target the self-renewal pathways in CSCs could
represent effective routes of therapy. Our results show that CSCs
that survive G47Δ infection are unable to form secondary
mammospheres, thereby indicating that the self-renewal ability of
CSCs remains impaired by G47Δ even if lysis does not occur.
Similarly, Wakimoto et al(10) reported that the inhibition of
self-renewal by G47Δ may be due to factors secreted by G47Δ or due
to G47Δ directly inhibiting the self-renewal pathways. The
mechanism whereby G47Δ inhibits the self-renewal capability of stem
cells remains unknown and warrants further investigation.
In conclusion, we have shown that G47Δ effectively
and equally targets NCSCs and CSCs derived from different breast
cancer subtypes. Furthermore, G47Δ appeared to impair the
self-renewal ability of CSCs. G47Δ also demonstrated a synergistic
effect with paclitaxel to amplify the killing of both NCSCs and
CSCs in vitro and in vivo. To our knowledge, this is
the first report on the targeted treatment of breast CSCs with
oHSV.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30672410) and Natural
Science Foundation of Guangdong Province, China (grant no.
06104599).
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
1:105–111. 2001. View
Article : Google Scholar
|
|
2
|
Charafe-Jauffret E, Monville F, Ginestier
C, Dontu G, Birnbaum D and Wicha MS: Cancer stem cells in breast:
current opinion and future challenges. Pathobiology. 75:75–84.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating
cells to radiation. J Natl Cancer Inst. 98:1777–1785. 2006.
|
|
4
|
Fillmore CM and Kuperwasser C: Human
breast cancer cell lines contain stem-like cells that self-renew,
give rise to phenotypically diverse progeny and survive
chemotherapy. Breast Cancer Res. 10:R252008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu R, Varghese S and Rabkin SD: Oncolytic
herpes simplex virus vector therapy of breast cancer in C3(1)/SV40
T-antigen transgenic mice. Cancer Res. 65:1532–1540. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Hu P, Zeng M, Rabkin SD and Liu R:
Oncolytic herpes simplex virus treatment of metastatic breast
cancer. Int J Oncol. 40:757–763. 2012.PubMed/NCBI
|
|
7
|
Todo T, Martuza RL, Rabkin SD and Johnson
PA: Oncolytic herpes simplex virus vector with enhanced MHC class I
presentation and tumor cell killing. Proc Natl Acad Sci USA.
98:6396–6401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T,
Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL,
Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB,
Johnson MD, Lippman M, Ethier S, Gazdar A and Gray JW: A collection
of breast cancer cell lines for the study of functionally distinct
cancer subtypes. Cancer Cell. 10:515–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wakimoto H, Kesari S, Farrell CJ, Curry WT
Jr, Zaupa C, Aghi M, Kuroda T, Stemmer-Rachamimov A, Shah K, Liu
TC, Jeyaretna DS, Debasitis J, Pruszak J, Martuza RL and Rabkin SD:
Human glioblastoma-derived cancer stem cells: establishment of
invasive glioma models and treatment with oncolytic herpes simplex
virus vectors. Cancer Res. 69:3472–3481. 2009. View Article : Google Scholar
|
|
11
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim E and Winer EP: Adjuvant chemotherapy
in luminal breast cancers. Breast. (Suppl 3): 128–131. 2011.
View Article : Google Scholar
|
|
13
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
Baselga J and Norton L: Use of chemotherapy plus a monoclonal
antibody against HER2 for metastatic breast cancer that
overexpresses HER2. N Engl J Med. 344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG,
Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas
EP, Lingle WL, Klein PM, Ingle JN and Wolmark N: Trastuzumab plus
adjuvant chemotherapy for operable HER2-positive breast cancer. N
Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS
and Millikan RC: Race, breast cancer subtypes, and survival in the
Carolina Breast Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banerjee S, Reis-Filho JS, Ashley S,
Steele D, Ashworth A, Lakhani SR and Smith IE: Basal-like breast
carcinomas: clinical outcome and response to chemotherapy. J Clin
Pathol. 59:729–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fulford LG, Reis-Filho JS, Ryder K, Jones
C, Gillett CE, Hanby A, Easton D and Lakhani SR: Basal-like grade
III invasive ductal carcinoma of the breast: patterns of metastasis
and long-term survival. Breast Cancer Res. 9:R42007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS and Dontu G: ALDH1
is a marker of normal and malignant human mammary stem cells and a
predictor of poor clinical outcome. Cell Stem Cell. 1:555–567.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O’Brien CS, Farnie G, Howell SJ and Clarke
RB: Breast cancer stem cells and their role in resistance to
endocrine therapy. Horm Cancer. 2:91–103. 2011.PubMed/NCBI
|
|
22
|
Ahtiainen L, Mirantes C, Jahkola T,
Escutenaire S, Diaconu I, Osterlund P, Kanerva A, Cerullo V and
Hemminki A: Defects in innate immunity render breast cancer
initiating cells permissive to oncolytic adenovirus. PLoS One.
5:e138592010. View Article : Google Scholar : PubMed/NCBI
|