Introduction
Osteosarcoma is the most common primary malignant
tumor of bone. Remarkable advances in the treatment of osteosarcoma
have occurred, including the introduction of adjuvant chemotherapy
and appropriate surgical excision (1–4).
However, there have also been advances in the field of
immunotherapy for the treatment of osteosarcoma that have received
less attention (5,6). Following cryotreatment, the necrotic
tumor lesion remains within the body, and the release of tumor
antigens by dying cells has been hypothesized to activate a
tumor-specific immune response via antigen presentation by
antigen-presenting cells (APCs) such as dendritic cells (DCs) to T
cells (7,8). A number of tumor studies combining
immunomodulation methods such as the injection of Toll-like
receptor agonists with cryotreatment have demonstrated a
synergistic effect on tumor rejection, and this was attributed to
the enhanced activation of APC function (9,10). We
developed a method using cryotreated tumor tissue and DCs to
enhance tumor-specific immunoreactions since DCs are the main APCs
initiating cell-mediated immune responses in vivo(11,12).
Monoclonal antibodies (mAbs) that block the function
of cytotoxic T lymphocyte antigen-4 (CTLA-4), a transmembrane
protein expressed by activated T cells, represent a promising novel
therapy for treating cancer through the depletion of regulatory T
cells (Tregs) (13–15). CTLA-4 inhibits the activation of
self-reactive T cells, and several years ago blockade of this
pathway was proposed to enhance tumor T-cell responses. As
expected, in preclinical studies, CTLA-4 blockade led to tumor
rejection (16–19). Clinical trials to validate the
efficacy of anti-CTLA-4 mAb (anti-CTLA-4) therapy for the treatment
of various types of cancers, including melanoma and prostate
cancer, in humans have been completed or are currently underway
(20,21).
In the present study, we investigated how
immunotherapies that target the inhibitory pathways of Tregs using
anti-CTLA-4 mAbs potentially synergize the effects of cryotreated
tumor lysate-pulsed DCs to generate systemic antitumor immunity. We
verify that, in contrast to tumor lysate-pulsed DC or CTLA-4
treatment alone, the combination therapy enhanced antitumor
immunity and slowed the growth of a secondary tumor from a large
primary tumor by using a mouse metastatic osteosarcoma model.
Materials and methods
Cell line
LM8 cells, derived from Dunn osteosarcoma, were
provided by the Riken BioResource Center (Saitama, Japan). The
cells were maintained in complete medium consisting of RPMI-1640
supplemented with 10% heat-inactivated fetal bovine serum, 100
μg/ml streptomycin and 100 U/ml penicillin. Cells were cultured at
37°C in 5% CO2.
Animals
A total of 1×106 LM8 cells (a murine
osteosarcoma cell line) was hypodermically implanted into the
subcutaneous gluteal region of 60 female C3H mice 6–8 weeks old. We
purchased the C3H mice from Sankyo Labo, Inc. (Toyama, Japan) and
housed them in a specific pathogen-free animal facility in our
laboratory.
Study design
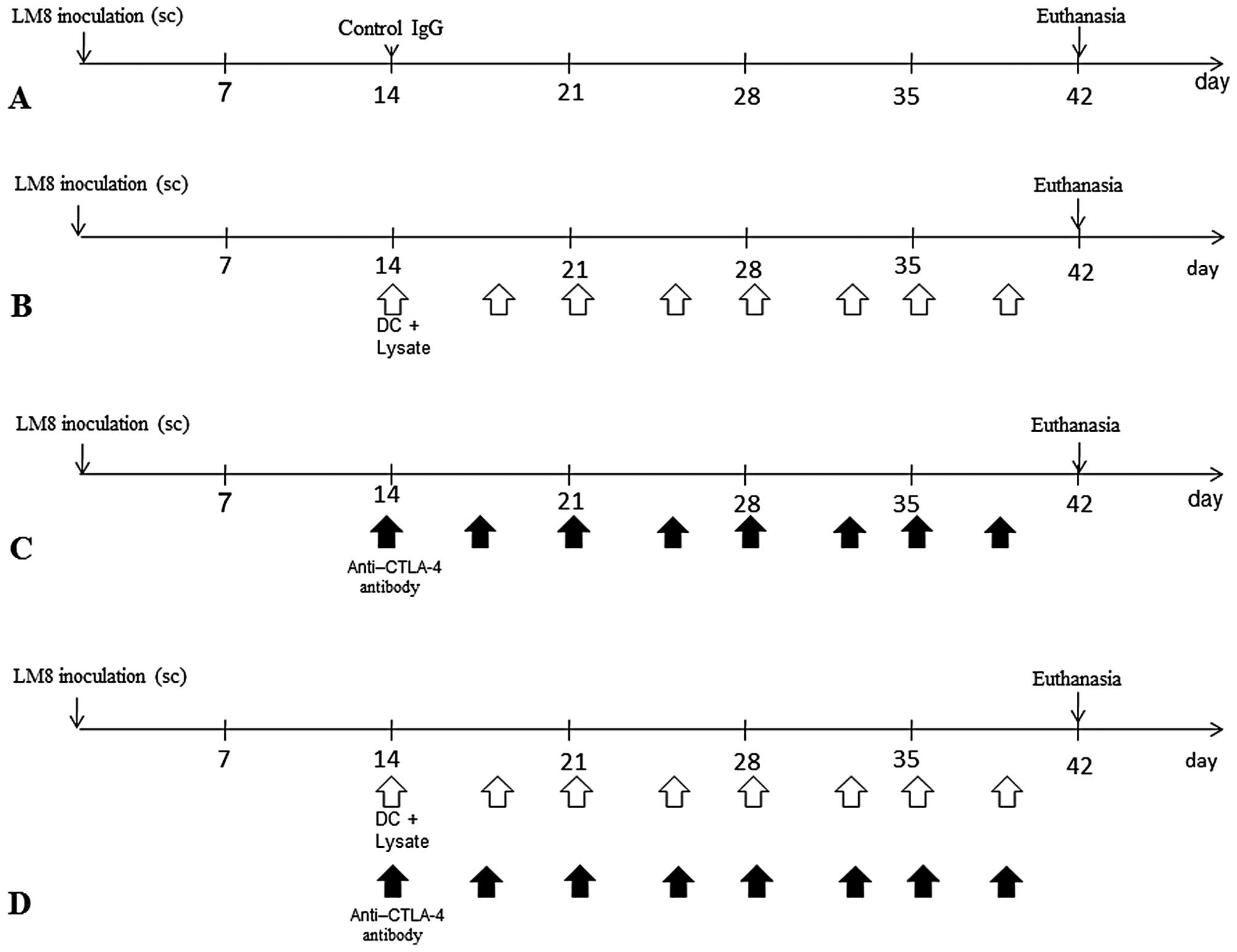
All animals developed tumors. The following 4 groups
were established (Fig. 1): i)
control immunoglobulin G (IgG) (control; n=15); ii) DCs exposed to
cryotreated tumor lysates were injected twice a week into the
subcutaneous contralateral gluteal region [DC(Ly); n=15]; iii)
intraperitoneal injection of anti-CTLA-4 Ab was performed
twice/week (anti-CTLA-4 Ab; n=15) and iv) DCs exposed to
cryotreated tumor lysates were injected twice a week into the
subcutaneous contralateral gluteal region and intraperitoneal
injection of agonist anti-CTLA-4 antibody was performed twice/week
[DC(Ly) + anti-CTLA-4 Ab; n=15]. This study was performed at the
Department of Orthopaedic Surgery, Faculty of Medicine, Oita
University, Oita, Japan. All experiments were performed under the
guidelines for animal experiments as stipulated by the Oita
University Graduate School of Medical Science.
Generation of DCs
Bone marrow-derived DCs were generated as described
by Lutz and Rössner (22) with
minor modifications. Briefly, erythrocyte-depleted mouse bone
marrow cells obtained from flushed marrow cavities
(1×106 cells/ml) were cultured in complete medium with
20 ng/ml recombinant mouse granulocyte-macrophage
colony-stimulating factor (GM-CSF) (PeproTech EC Ltd., London, UK)
in 10-cm tissue culture dishes at 37°C in an atmosphere containing
50 ml CO2/l.
Preparation of tumor lysate
Four weeks after tumor inoculation, we resected the
primary tumor lesion and soaked the entire tumor in liquid nitrogen
to kill the tumor cells. The freeze-thawed tumor lysate was added
to the DC cultures on Day 6 at a ratio of 5 DC equivalents to 1
tumor cell (i.e., 5:1). The homogenate was passed through a 0.2-μm
filter to remove bacteria and tissues and mixed with the DCs for 24
h. After 24 h of incubation, nonadherent cells including DCs were
harvested by gentle pipetting.
Antibody
The monoclonal antibody CTLA-4 (hamster IgG, mCD152
antibody; 0.2 mg/mouse) was provided by BioXcell (Lebanon, NH,
USA). The CTLA-4 blocking activity was confirmed using the mink
lung cell assay. The monoclonal control antibody IgG ( hamster IgG,
isotype control antibodies; 0.2 mg/mouse) was provided by
BioXcell.
Flow cytometry
The markers Foxp3 and CD4, which are expressed on
the surface of Tregs, were counted with a FACSCalibur™ Flow
Cytometer (Becton Dickinson, San Jose, CA, USA) and were stained
with fluorochrome-conjugated antibody (BD Pharmingen, Tokyo, Japan)
for the following markers: phycoerythrin (PE)-conjugated anti-mouse
Foxp3 staining kit (eBioscience, San Diego, CA, USA) and
fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4 (BD
Pharmingen; clone: RM4-5). Data analysis was performed with
CellQuest™ software (Becton Dickinson).
Immunofluorescence
Immunohistochemistry was used to measure the levels
of Foxp3, a marker of Tregs, and CD8, a marker of cytotoxic T
lymphocytes, within metastatic tumor lesions. Lung specimens were
fixed in 20% formalin and embedded in paraffin. In each case, we
examined all formalin-fixed, paraffin-embedded tumor tissue blocks.
Five samples/mouse were cut into 4-μm slices. Rehydrated tissue
sections were incubated with primary Abs against CD8+
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Foxp3 (Abcam,
Cambridge, MA, USA) diluted at 1:200 in Ab diluent (Dako ChemMate,
Dako, Japan) overnight at room temperature. For CD8+
staining with FITC donkey anti-rabbit IgG and Foxp3+
staining with Texas red goat anti-rat IgG (Invitrogen, Carlsbad,
CA, USA), secondary antibodies were diluted at 1:300 in Ab diluent
and added for 60 min at room temperature in the dark. Digital
images were captured using a BIOREVO microscope equipped with a
confocal microscopy system (BZ-9000; Keyence, Japan).
Tumor volume
Tumor volumes were measured using the micro-CT
apparatus (R_mCT) to obtain high-resolution CT images in small
living animals. The I-view-R (J. Morita Mfg Corp., Kyoto, Japan)
was used as the viewer, and diagnosis was made with slice images
viewed in all directions. Tumor volumes were estimated using the
formula (π × long axis × short axis × short axis)/6.
ELISA
We measured murine interferon-γ (IFN-γ) and
interleukin-10 (IL-10) release by enzyme-linked immunosorbent assay
using Quantikine® (R&D Systems, Minneapolis, MN,
USA) according to the manufacturer’s protocol using an Easy Reader
EAR340 microtest plate reader (SLT-Lab Instruments, Salzburg,
Austria).
Statistical analysis
We determined differences among the 4 groups using a
non-repeated measures analysis of variance (ANOVA) and the Scheffe
test. All analyses were conducted using SPSS® 18.0
software (SPSS Japan, Inc., Tokyo, Japan). Results were expressed
as the means ± standard deviation and P<0.01 was considered to
indicate a statistically significant difference. For survival
analysis, the differences in survival rates were analyzed by the
log-rank test.
Results
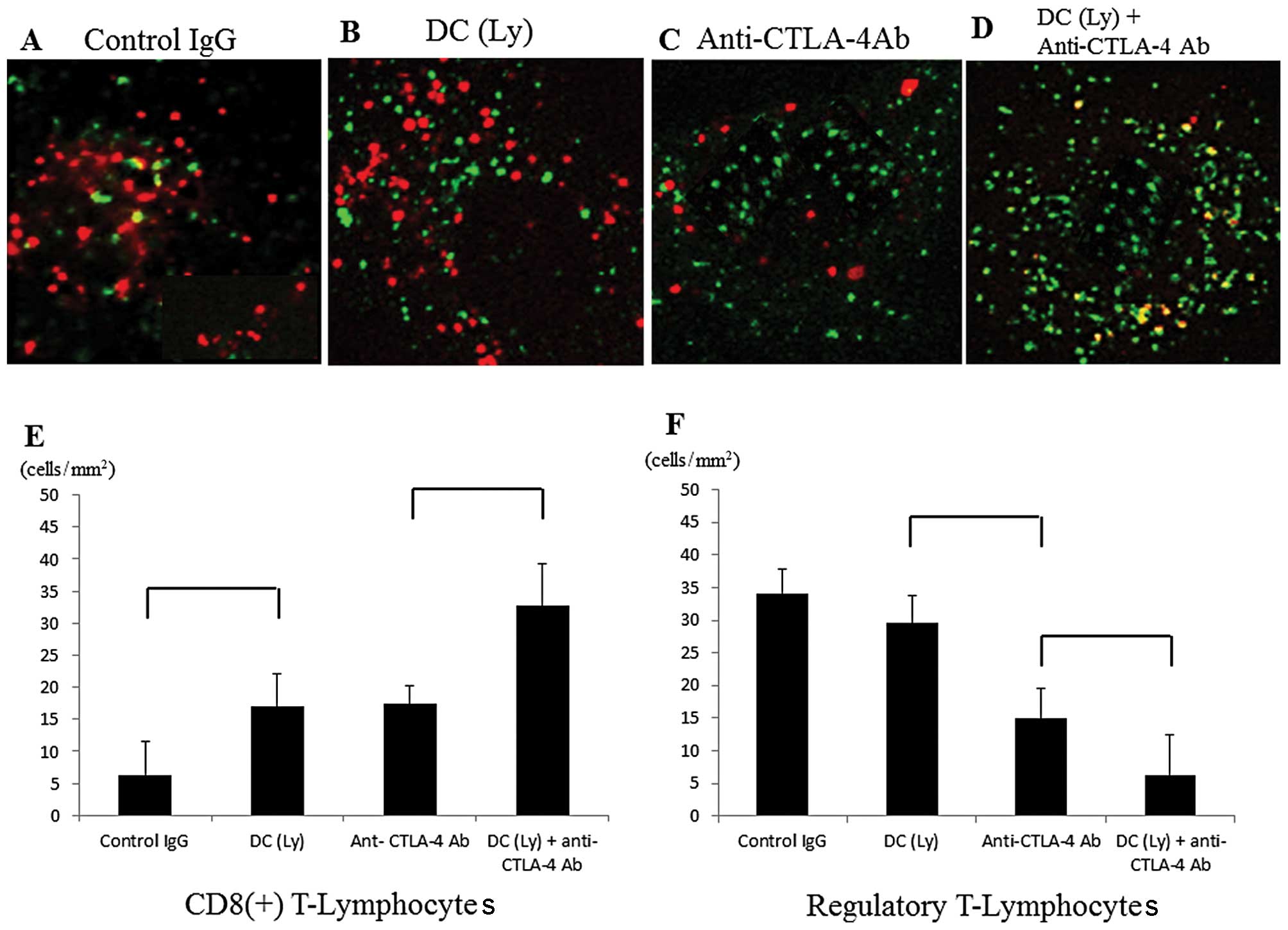
Infiltration of CD8+ T
lymphocytes and Tregs inside the tumor
The levels of Foxp3 were significantly decreased and
the numbers of CD8+ cells were significantly increased
inside the metastatic tumor lesions in the anti-CTLA-4
antibody-treated groups. Foxp3+ cells were not recruited
to the metastatic area in the anti-CTLA-4 antibody-treated groups
compared with the findings in the control IgG-treated group
(Fig. 2A–D). The number of
CD8+ T lymphocytes/unit area was higher (P<0.01) in
the mice that received tumor lysate-pulsed DCs and the anti-CTLA-4
antibody (32.79±6.39 cells/mm2) compared to those that
received tumor lysate-pulsed DCs (16.98±5.16 cells/mm2)
or anti-CTLA-4 antibody alone (17.41±2.88 cells/mm2;
Fig. 2E). The number of
Foxp3+ T lymphocytes/unit area was lower (P<0.01) in
the mice that received the anti-CTLA-4 antibody (14.94±4.59
cells/mm2) compared to those that received tumor
lysate-pulsed DCs (29.54±4.22 cells/mm2). The number of
Foxp3+ T lymphocytes/unit area was lower (P<0.01) in
the mice that received tumor lysate-pulsed DCs and the anti-CTLA4
antibody (6.29±6.12 cells/mm2) compared to those that
received the anti-CTLA-4 antibody alone (Fig. 2F).
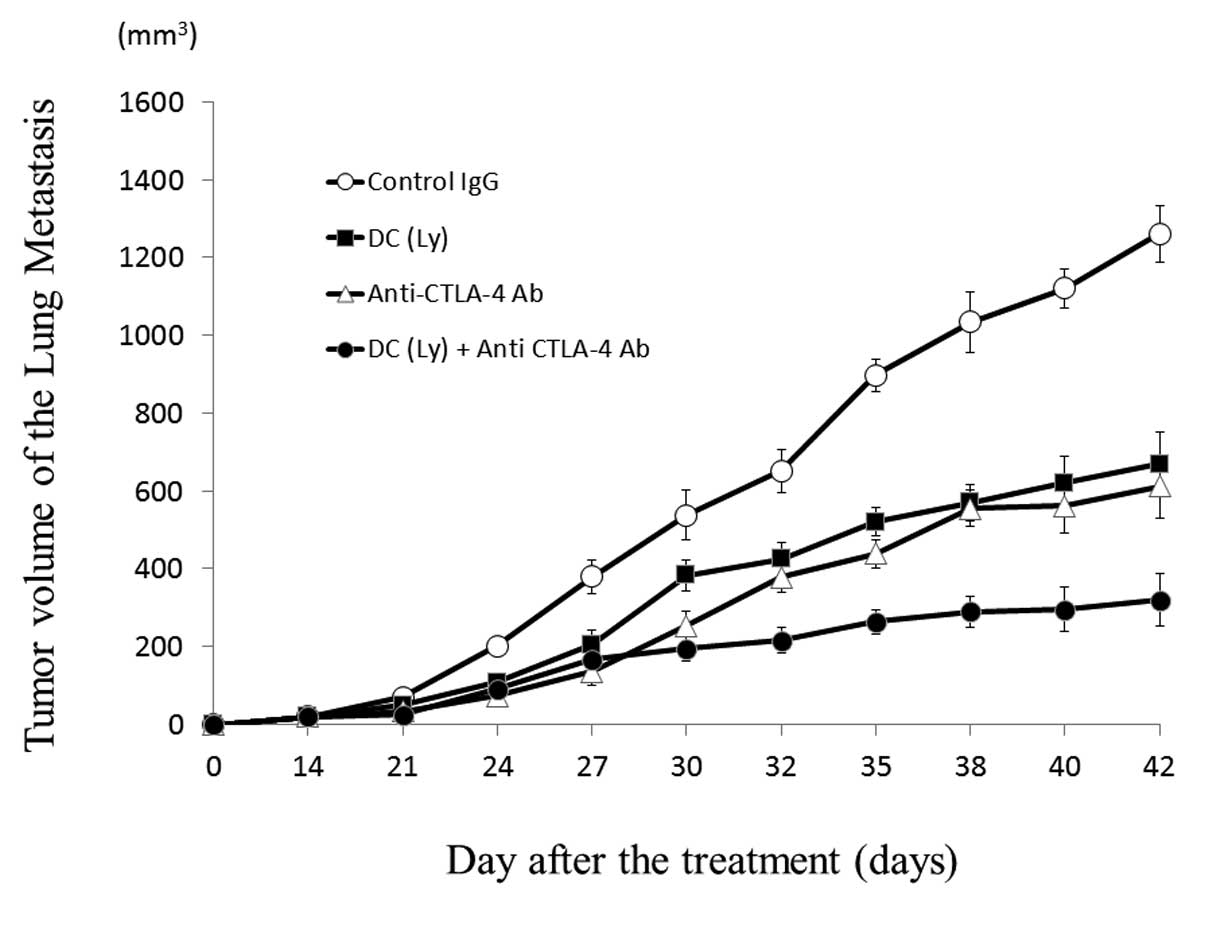
Tumor volume of the lung metastases
Forty-two days after inoculation, the volume of the
metastatic lesion (P<0.01) in the mice that received tumor
lysate-pulsed DCs and the anti-CTLA-4 antibody (319.58±49.96
mm3) was lower compared to that in those that received
tumor lysate-pulsed DCs (669.04±40.19 mm3) or the
anti-CTLA-4 antibody alone (611.54±31.97 mm3) (Fig. 3).
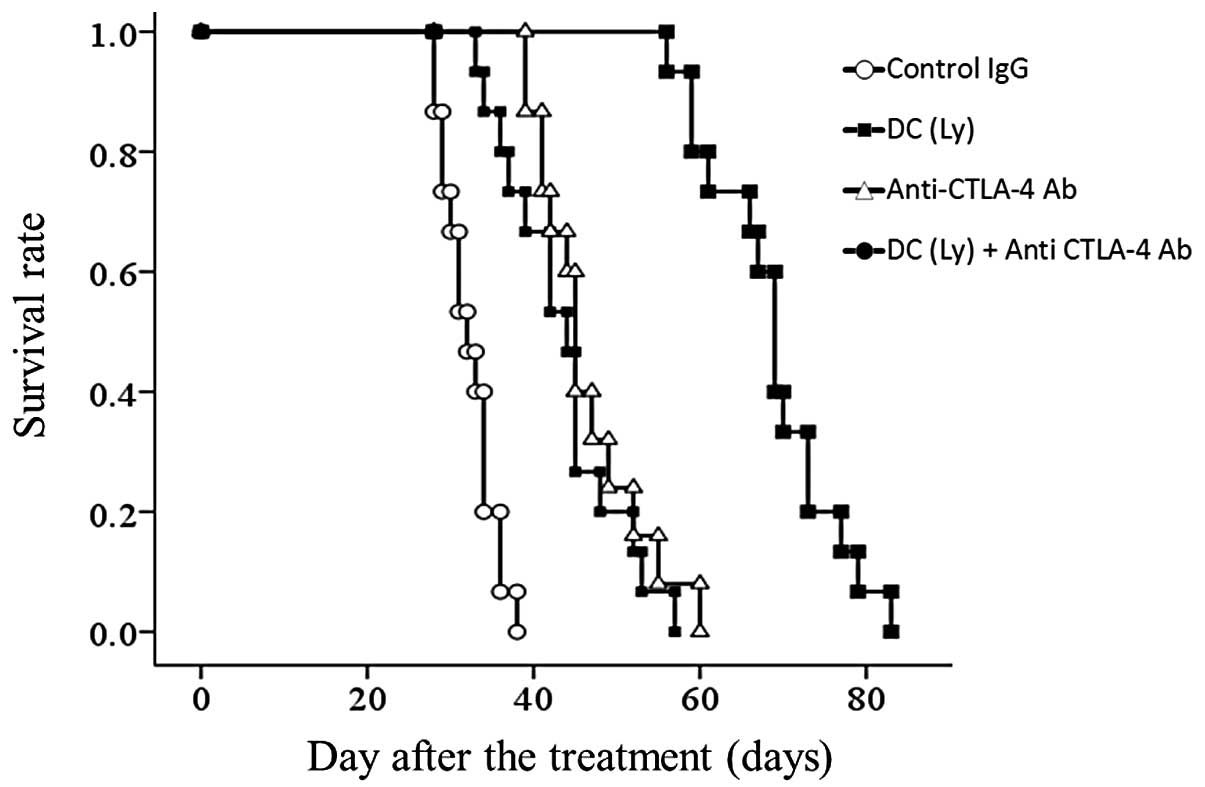
Survival rate
The median survival time was: IgG control, 32.27
days (range, 28–38); tumor lysate-pulsed DC-treated group, 43.47
days (range, 33–57); anti-CTLA4 antibody-treated group, 45.93 days
(range, 39–60) and for the tumor lysate-pulsed DCs and the
anti-CTLA-4 antibody group, 68.67 days (range, 56–83). All mice in
the control IgG group were sacrificed within 42 days following
formation of an enlarged tumor at the inoculated site. Survival was
significantly prolonged but differences in the tumor lysate-pulsed
DCs alone and the anti-CTLA4 antibody alone groups were small
compared with the control IgG group (P<0.001). There was no
significant difference between the tumor lysate-pulsed DCs alone
and anti-CTLA4 antibody alone groups. Further lifetime prolongation
was observed in the tumor lysate-pulsed DCs and anti-CTLA-4
antibody group compared with the tumor lysate-pulsed DCs alone and
anti-CTLA4 antibody alone groups (P<0.01) (Fig. 4.).
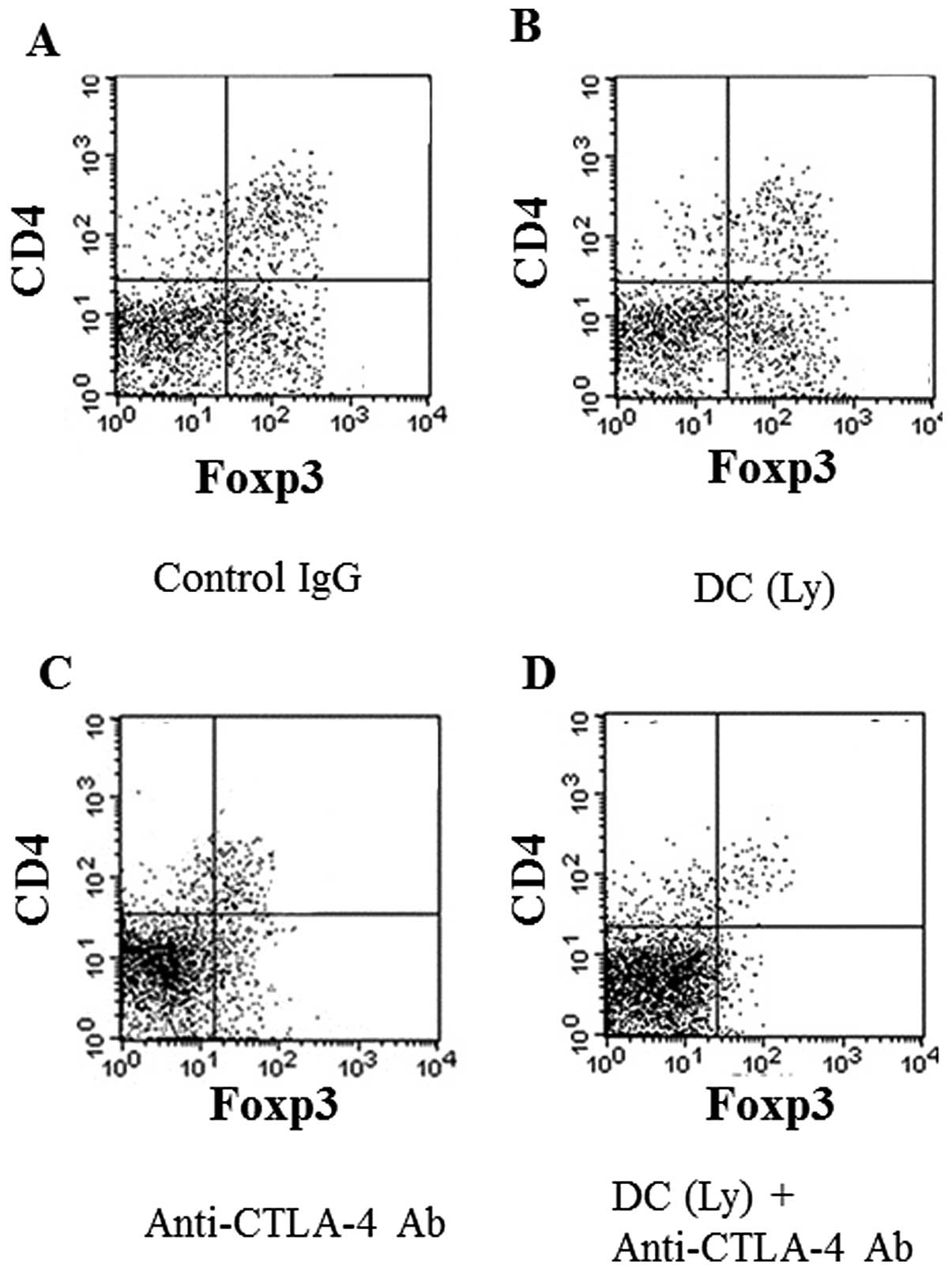
Reduction of Tregs in the spleen
The anti-CTLA-4 antibody markedly reduced the cell
population of Tregs, the 2 markers of which are CD4+ and
Foxp3+, in the spleen. The groups that received
anti-CTLA-4 antibody alone (Fig.
5C) or in combination with tumor lysate-pulsed DCs (Fig. 5D) displayed marked decreases in the
percentage of CD4+Foxp3+ cells compared with
the findings in the control IgG (Fig.
5A) or tumor lysate-pulsed DC-treated group (Fig. 5B).
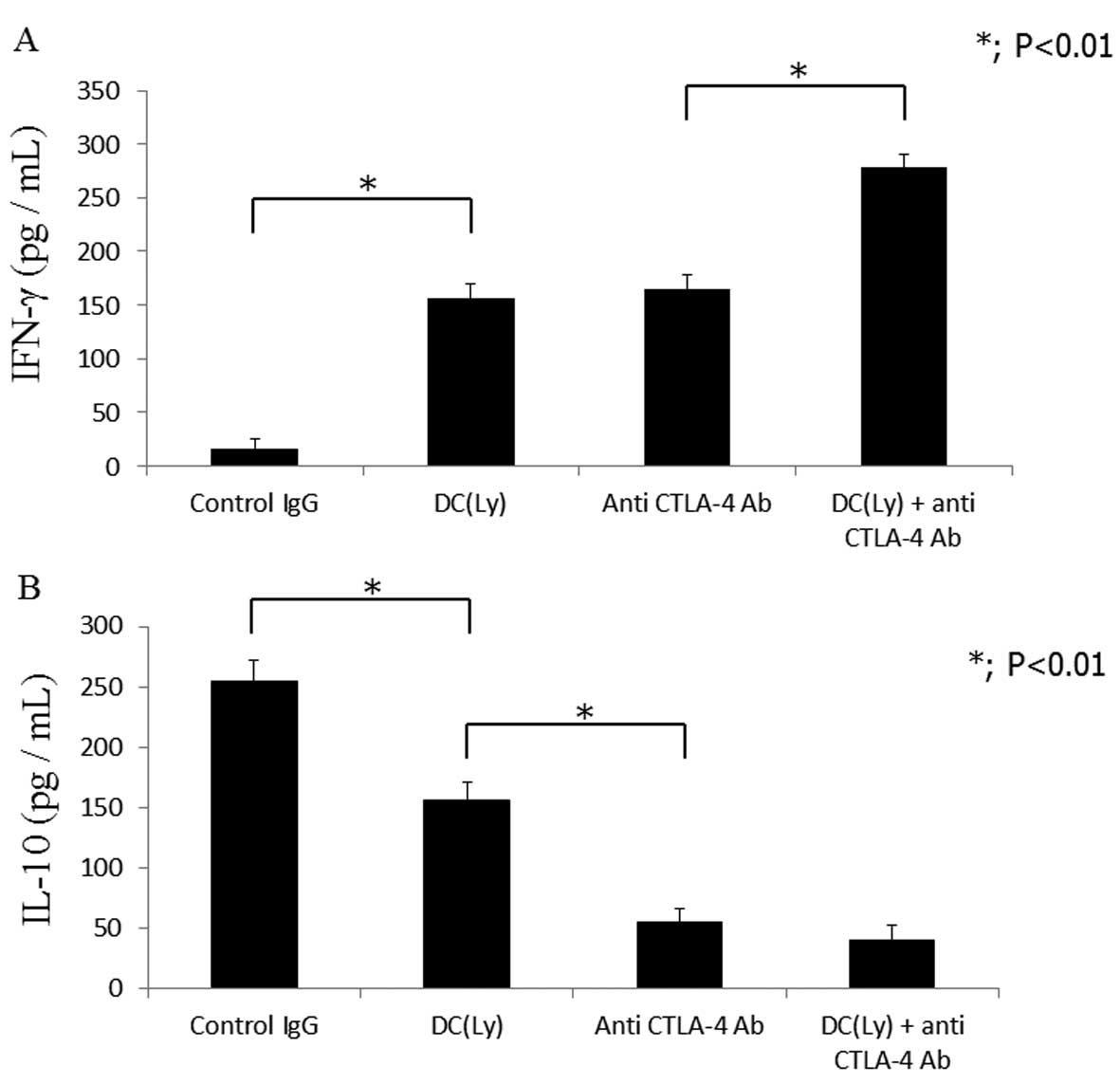
Cytokine release
Mice treated with tumor lysate-pulsed DCs and the
anti-CTLA-4 antibody displayed higher serum IFN-γ levels
(278.33±18.64 pg/ml; P<0.01) compared to those that received
tumor lysate-pulsed DCs (129.6±13.28 pg/ml) or the anti-CTLA4
antibody alone (126.98±20.37 pg/ml) (Fig. 6A). Serum IL-10 levels were lower
(P<0.01) in the mice that received the anti-CTLA-4 antibody
alone (53.24±21.29 pg/ml) compared to those that received tumor
lysate-pulsed DCs alone (145.43±16.38 pg/ml). Serum IL-10 levels
were lower (P<0.01) in the mice that received tumor
lysate-pulsed DCs and the anti-CTLA4 antibody (15.38±9.26 pg/ml)
compared to those that received the anti-CTLA-4 antibody alone
(53.24±21.29 pg/ml) (Fig. 6B).
Discussion
Most osteosarcoma patients are treated with a
combination of surgery and chemotherapy. Despite recent advances in
local therapy with curative intent, chemotherapeutic treatments for
metastatic disease often remain unsatisfactory owing to severe
adverse effects and incomplete long-term remission. Therefore, the
evaluation of novel therapeutic options is of great interest. Since
the discovery of the regression of metastases after cryotreatment,
investigators have examined immune responses in animal models in
hopes of showing a ‘cryoimmunologic’ effect (12,23–25).
Despite the lack of response to cryotreatment alone, we observed a
synergistic effect when cryotreatment was combined with DCs
(11). Since the initial discovery
that CTLA-4 stimulation drives T-cell immunity (13–15),
anti-CTLA-4 therapy has been used extensively for tumor
immunotherapy. In addition, CTLA-4 stimulation has been
demonstrated to suppress the function of Treg cells and drive
potent CD8+ T cell-mediated tumor protection (16–19).
However, the synergistic effect of anti-CTLA-4 antibody and
cryotreated tumor lysate-pulsed DCs has not been investigated in
osteosarcoma models. In the present study, we examined the
synergistic effect of cryotreated tumor lysate-pulsed DCs and
CTLA-4 blockade on preventing the development of a secondary tumor
from a large primary tumor by using a mouse metastatic osteosarcoma
model.
The anti-CTLA-4 antibody inhibited the accumulation
of Tregs and induced the infiltration of CD8+ T cells
inside the metastatic lesions. CTLA-4 signaling in
CD4+Foxp3+ Tregs is required for their
immunosuppressive capacity (13,16).
Our findings revealed that CTLA-4 stimulation led to the inhibition
of Foxp3+ T cells inside the tumor tissues. We report
that after combination therapy, the numbers of intratumoral
CD8+ T cells were significantly increased and Treg cells
were depleted by the combination treatment, supporting the ability
of the therapy to enhance the tumor-specific cell mediated immune
response.
The group treated with the combination of tumor
lysate-pulsed DCs and the anti-CTLA-4 antibody also displayed
smaller lung metastases with a prolonged lifetime. Tregs comprise
one of the major components of the immunosuppressive condition of
tumor lesions (15). Noteworthy,
the result of tumor rejection in lung metastatic lesions in the
combined therapy group correlated with the intratumor ratio of
CD8+ T cells to Tregs. This suggests that controlling
immunosuppressive factors may facilitate the activity of DCs and
cytotoxic T lymphocytes in the tumor. Identically, the Treg
depletion using anti-CTLA-4 antibody treatment combined with tumor
lysate-pulsed DCs treatment displayed a significantly improved
survival in comparison to the tumor lysate-pulsed DCs or
anti-CTLA-4 antibody monotherapy groups.
The combination therapy also resulted in the
enhancement of the number of CD8+ T cells and prevented
the proliferation of Tregs in the spleen, which is evidence of a
systemic response with the potential to eradicate disseminated
disease. Inhibition of Treg accumulation in the spleen enhances
systemic cell-mediated immunity through the activation of DCs or
cytotoxic T lymphocytes.
Tumor lysate-pulsed DCs and CTLA-4 blockade induced
the activation of cell-mediated immunity by increasing serum IFN-γ
levels and decreasing serum IL-10 levels. Tregs are among the major
elements that cause potent immunosuppression mediated by cytokines
from tumor cells and the inhibition of CTLA-4 stimulation may be
useful for enhancing the efficacy of cancer therapy or vaccines
(26–28). Our results revealed that blocking
CTLA-4 signaling using anti-CTLA-4 antibody enhanced cell-mediated
immunity.
Although the immune response to tumor lysate-pulsed
DCs or anti-CTLA-4 antibody alone may vary or may be insufficient
to suppress metastatic tumors, the combination of tumor
lysate-pulsed DCs and CTLA-4 blockade has the potential to create a
robust antitumor immune response that controls the growth of
metastases. The present study could lead to a generation of
proposals for clinical trials, in which tumor lysate-pulsed DCs
will be combined with CTLA-4 blockade to treat cancer. Ipilimumab
is a human monoclonal immunoglobulin G (IgG) antibody against
CTLA-4, an immune inhibitory molecule that the US Food and Drug
Administration has approved for the treatment of unresectable or
metastatic melanoma in 2011 (29).
The combination of ipilimumab and chemotherapy in the treatment of
melanoma (30) and lung cancer
(31) has been evaluated in
clinical trials.
Chemotherapy for human osteosarcoma is the standard
treatment and it should be performed before immunotherapy. However,
additional methods have yet to be developed for treating patients
who are resistant to the standard osteosarcoma treatment (32). Therefore, the development of novel
treatment strategies for metastatic osteosarcoma is critical. The
combination of chemotherapy, DC vaccination and anti-CTLA-4
antibody treatment may be effective in the treatment of
osteosarcoma patients with residual tumors or distant metastasis
after chemotherapy. These issues will be addressed in the future to
enable the clinical application of our therapy in treating human
osteosarcoma and to facilitate further research efforts.
Acknowledgements
We thank Hiroyuki Tsuchiya, Hideji Nishida,
Katsuhiro Hayashi, Akihiko Takeuchi and Katsuro Tomita for
participating in this study.
References
|
1
|
Ferrari S, Smeland S, Mercuri M, et al:
Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose
methotrexate, cisplatin, and doxorubicin for patients with
localized osteosarcoma of the extremity: a joint study by the
Italian and Scandinavian Sarcoma Groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kager L, Zoubek A, Dominkus M, Lang S,
Bodmer N, Jundt G, Klingebiel T, Jürgens H, Gadner H and Bielack S;
COSS Study Group. Osteosarcoma in very young children: experience
of the Cooperative Osteosarcoma Study Group. Cancer. 116:5316–5324.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis VO: What’s new in musculoskeletal
oncology. J Bone Joint Surg Am. 89:1399–1407. 2007.
|
|
4
|
Stiller CA, Bielack SS, Jundt G and
Steliarova-Foucher E: Bone tumours in European children and
adolescents, 1978–1997. Report from the Automated Childhood Cancer
Information System project. Eur J Cancer. 42:2124–2135. 2006.
|
|
5
|
Campbell CJ, Cohen J and Enneking WF:
Editorial: New therapies for osteogenic sarcoma. J Bone Joint Surg
Am. 57:143–144. 1975.PubMed/NCBI
|
|
6
|
Kawaguchi S, Wada T, Tsukahara T, Ida K,
Torigoe T, Sato N and Yamashita T: A quest for therapeutic antigens
in bone and soft tissue sarcoma. J Transl Med. 3:312005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soanes WA, Ablin RJ and Gonder MJ:
Remission of metastatic lesions following cryosurgery in prostatic
cancer: immunologic considerations. J Urol. 104:154–159.
1970.PubMed/NCBI
|
|
8
|
Sabel MS: Cryo-immunology: a review of the
literature and proposed mechanisms for stimulatory versus
suppressive immune responses. Cryobiology. 58:1–11. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawano M, Nishida H, Nakamoto Y, Tsumura H
and Tsuchiya H: Cryoimmunologic antitumor effects enhanced by
dendritic cells in osteosarcoma. Clin Orthop Relat Res.
468:1373–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishida H, Tsuchiya H and Tomita K:
Re-implantation of tumour tissue treated by cryotreatment with
liquid nitrogen induces anti-tumour activity against murine
osteosarcoma. J Bone Joint Surg Br. 90:1249–1255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
den Brok MH, Sutmuller RP, Nierkens S,
Bennink EJ, Toonen LW, Figdor CG, et al: Synergy between in situ
cryoablation and TLR9 stimulation results in a highly effective in
vivo dendritic cell vaccine. Cancer Res. 66:7285–7292.
2006.PubMed/NCBI
|
|
12
|
Redondo P, del Olmo J, López-Diaz de Cerio
A, Inoges S, Marquina M, Melero I and Bendandi M: Imiquimod
enhances the systemic immunity attained by local cryosurgery
destruction of melanoma lesions. J Invest Dermatol. 127:1673–1680.
2007.PubMed/NCBI
|
|
13
|
Wing K, Onishi Y, Prieto-Martin P, et al:
CTLA-4 control over Foxp3+ regulatory T cell function.
Science. 322:271–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang B, Workman C, Lee J, et al:
Regulatory T cells inhibit dendritic cells by lymphocyte activation
gene-3 engagement of MHC class II. J Immunol. 180:5916–5926. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Viguier M, Lemaître F, Verola O, et al:
Foxp3 expressing CD4+CD25(high) regulatory T cells are
overrepresented in human metastatic melanoma lymph nodes and
inhibit the function of infiltrating T cells. J Immunol.
173:1444–1453. 2004.PubMed/NCBI
|
|
16
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hurwitz AA, Yu TF, Leach DR and Allison
JP: CTLA-4 blockade synergizes with tumor-derived
granulocyte-macrophage colony-stimulating factor for treatment of
an experimental mammary carcinoma. Proc Natl Acad Sci USA.
95:10067–10071. 1998. View Article : Google Scholar
|
|
18
|
van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentation. J Exp Med. 190:355–366. 1999.
|
|
19
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005.PubMed/NCBI
|
|
20
|
Small EJ, Sacks N, Nemunaitis J, Urba WJ,
Dula E, Centeno AS, et al: Granulocyte macrophage
colony-stimulating factor-secreting allogeneic cellular
immunotherapy for hormone-refractory prostate cancer. Clin Cancer
Res. 13:3883–3891. 2007. View Article : Google Scholar
|
|
21
|
Hodi FS, O’Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, et al: Improved survival with ipilimumab in
patients with metastatic melanoma. N Engl J Med. 363:711–723. 2010.
View Article : Google Scholar
|
|
22
|
Lutz MB and Rössner S: Factors influencing
the generation of murine dendritic cells from bone marrow: the
special role of fetal calf serum. Immunobiology. 212:855–862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabel MS, Arora A, Su G and Chang AE:
Adoptive immunotherapy of breast cancer with lymph node cells
primed by cryoablation of the primary tumor. Cryobiology.
53:360–366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sabel MS, Nehs MA, Su G, Lowler KP,
Ferrara JLM and Chang AE: Immunologic response to cryoablation of
breast cancer. Breast Cancer Res Treat. 90:97–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Urano M, Tanaka C, Sugiyama Y, Miya K and
Saji S: Antitumor effects of residual tumor after cryoablation: the
combined effect of residual tumor and a protein-bound
polysaccharide on multiple liver metastases in a murine model.
Cryobiology. 46:238–245. 2003. View Article : Google Scholar
|
|
26
|
Yang YF, Zou JP, Mu J, Wijesuriya R, Ono
S, Walunas T, Bluestone J, Fujiwara H and Hamaoka T: Enhanced
induction of antitumor T-cell responses by cytotoxic T
lymphocyte-associated molecule-4 blockade: the effect is manifested
only at the restricted tumor-bearing stages. Cancer Res.
57:4036–4041. 1997.PubMed/NCBI
|
|
27
|
Persson J, Beyer I, Yumul R, et al:
Immuno-therapy with anti-CTLA4 antibodies in tolerized and
non-tolerized mouse tumor models. PLoS One. 6:223032011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shrikant P, Khoruts A and Mescher MF:
CTLA-4 blockade reverses CD8+ T cell tolerance to tumor
by a CD4+ T cell- and IL-2-dependent mechanism.
Immunity. 11:483–493. 1999.PubMed/NCBI
|
|
29
|
Traynor K: Ipilimumab approved for
metastatic melanoma. Am J Health Syst Pharm. 68:7682011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hersh EM, O’Day SJ, Powderly J, et al: A
phase II multicenter study of ipilimumab with or without
dacarbazine in chemotherapy-naïve patients with advanced melanoma.
Invest New Drugs. 29:489–498. 2011.
|
|
31
|
Lynch TJ, Bondarenko I, Luft A,
Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H,
Cuillerot JM and Reck M: Ipilimumab in combination with paclitaxel
and carboplatin as first-line treatment in stage IIIB/IV
non-small-cell lung cancer: results from a randomized,
double-blind, multicenter phase II study. J Clin Oncol.
30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bacci G, Briccoli A, Rocca M, Ferrari S,
Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C,
Manfrini M and Galletti S: Neoadjuvant chemotherapy for
osteosarcoma of the extremities with metastases at presentation:
recent experience at the Rizzoli Institute in 57 patients treated
with cisplatin, doxorubicin, and a high dose of methotrexate and
ifosfamide. Ann Oncol. 14:1126–1134. 2003. View Article : Google Scholar
|