Introduction
Pancreatic cancer is one of the common malignant
tumors in Western countries with the highest incidence rate, and is
also one of the cancers with the highest mortality rate (1). The characteristics of pancreatic
cancer include high-grade malignancy, specific biological behavior,
non-specific symptoms and tendency for early metastasis,
insensitivity to chemotherapy and radiotherapy and a low rate of
surgical resection (2). Compared
with other cancers, the survival rate is so low that the incidence
rate is basically equal to the mortality rate (3). Thus, molecular targeted therapy may be
the most effective treatment for pancreatic cancer.
The proto-oncogene Pim family exhibits
serine/threonine kinase activity. In humans, the Pim family
includes 3 members: Pim-1, Pim-2 and Pim-3 (4). Crystal structures of Pim family
kinases have revealed that the ATP-binding sites of Pim family
kinases share high structural homology (5–7). The
overexpression of Pim-1 and Pim-2 has been found in a variety of
human hematopoietic malignancies (8–11),
such as leukemia and lymphoma and some solid tumors, such as
prostate cancer (12–14). Pim-3 has mainly been found in solid
tumors, particularly endoderm-derived organs such as the liver,
stomach, pancreas and colon (15–17)
and Pim kinases have been demonstrated to inhibit apoptosis by
phosphorylating the pro-apoptotic molecule, Bad at
Ser112. Thus, Pim-3 may be an effective target for the
treatment of cancer of endoderm-derived organs, particularly the
pancreas (18–21). Several Pim kinase inhibitors have
been reported (22,23), but only a few of them are effective
against all Pim family kinases (24–27).
This prompted us to develop a specific inhibitor for each Pim
kinase.
In this study, we investigated whether T-18, a
stemonamide synthetic intermediate, may be used as a Pim-3 kinase
inhibitor. T-18 reduced the levels of phosphorylated Bad at
Ser112, resulting in the decreased growth of pancreatic
carcinoma cells in vitro and the induction of apoptosis.
Furthermore, T-18 also inhibited the growth of human pancreatic
cancer cells in nude mice even when the forming tumor was observed.
These results indicate that T-18 may be a lead compound that can be
modified to exert a more potent inhibitory effect on Pim-3 kinase,
and may thus be effectively used in the treatment of cancers with
an aberrant Pim-3 overexpression.
Materials and methods
Cell culture and antibodies
The human pancreatic cancer cell lines, PCI35
(28), PCI55 (28), PCI66 (28), PANC-1 (29) and MIA PaCa-2 (30), and the human colon cancer cell line,
SW480 (31), were cultured in
RPMI-1640 medium, while another human pancreatic cancer cell line,
L3.6pl (32), and the human colon
cancer cell line, SW48 (31), were
maintained in minimum essential medium. The human colon cancer cell
lines, HCT29 (33) and HCT116
(34), were cultured in McCoy’s 5A
medium and the human hepatocarcinoma cell lines, HepG2, Hep3B
(35) and HuH7 (36), were maintained in Dulbecco’s
modified Eagle’s medium. All media were supplemented with 10% FBS,
50 U/ml of penicillin and 50 U/ml of streptomycin. The following
antibodies were used: mouse anti-human Bad (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), rabbit anti-phosphorylated
(p-) Ser112-Bad (Cell Signaling Technology, Beverly, MA,
USA) and anti-β-actin antibodies (Sigma-Aldrich). The rabbit
anti-human Pim-3 antibodies were prepared as previously described
(16).
Preparation of stemonamide synthetic
intermediates
The stemonamide synthetic intermediates were
synthesized as previously described (37). T-10 and T-18 were dissolved in DMSO
(Sigma-Aldrich) at the concentration of 100 mM as stocking solution
and the solutions were stable within 1 week. T-18 was dissolved in
DMSO at the concentration of 100 mg/ml and the solution was diluted
with olive oil to reduce the final DMSO concentration to 4% v/v for
the animal experiment.
In vitro Pim kinase assay and Akt
kinase assay
Based on the ability of Pim kinases to phosphorylate
Bad at Ser112, in vitro kinase assay for Pim-1,
Pim-2 and Pim-3 was established and conducted as previously
described (38,39). The IC50 values were
calculated using logistic regression.
Akt kinase activities were determined based on their
capacity to phosphorylate Bad at Ser136 as described
previously (38,39). Either recombinant Akt-1 (10 ng)
(Cell Signaling Technology) or Akt-2 (10 ng) (Assay Designs, Ann
Arbor, MI, USA) was used as the source of the enzymes. Akt-1/2
kinase inhibitor (Sigma-Aldrich) was used as the positive control
inhibitor.
Cell viability assay
Cells were inoculated into 96-multiwell plate at
3,000 cells/100 μl per well and incubated at 37°C for 18 h. The
chemicals were then added to each well at the indicated
concentrations and the cells were incubated for the indicated
periods of time. After removing the supernatants, the medium
containing CCK-8 (Dojin Chemicals, Kumamoto, Japan) was added to
each well followed by a 2-h incubation. Subsequently, the optical
density was measured at 450 nm. The number of viable cells at day 0
was regarded as the control to determine the ratios of viable cell
numbers. Logistic regression was used to calculate the
IC50 values.
Cell apoptosis assay
The cell apoptosis assay was operated according to
the instruction manual of human Annexin V-FITC apoptosis kit
(Bender MedSystems GmbH, Vienna, Austria). The cells were harvested
after exposure to the chemicals and 20,000 stained cells were
acquired for the analysis on the FACSCalibur system (BD
Biosciences).
Western blot analysis
The L3.6pl or MIA PaCa-2 cells (2×106
cells) were seeded in 100-mm culture plates. After an 18-h
incubation, the chemicals were added to the plate at the final
concentration of 10 μM. Subsequently, 60 μl CellLytic™ Cell Lysis
Reagent (Sigma-Aldrich) with Complete Proteinase Inhibitor Cocktail
(Roche Diagnostics AG, Rotkreutz, Switzerland) was added followed
by ultrasound sonication after treatment with the chemicals.
Following centrifugation, the supernatants were collected for
western blot analysis using the antibodies mentioned above.
Animal experiments
L3.6pl cells were suspended in HBSS at the
concentration of 5×106 cells/ml and 100 μl cell
suspensions were inoculated subcutaneously into the back of BALB/c
nu/nu mice (SLC, Shizuoka, Japan). Twelve days after tumor
injection, the chemical (20 mg/kg) or olive oil vehicle was
injected intraperitoneally once a day for 5 days as one course. The
course was repeated 3 times with an interval of 2 days. Tumor sizes
were measured every 3–4 days with a caliper and tumor volumes were
determined using the following the formula: tumor volume
(mm3) = (the longest diameter) (mm) × (the shortest
diameter)2 (mm)/2. On day 32 after the tumor cell
injection, blood was taken simultaneously at tumor removal. Serum
levels of alanine aminotransferase (ALT) were measured using the
Fuji Drichem 5500V analyzer (Fujifilm Medical Systems, Tokyo,
Japan).
Statistical analysis
Data are expressed as the means ± SD unless
otherwise indicated. One-way ANOVA and the Tukey-Kramer test were
employed to analyze the differences between groups and a value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of T-18 on Pim kinase
activity
We previously reported that some synthetic
intermediates of stemonamide (T-2) can inhibit the activity of
Pim-3 kinase in vitro(38).
However, the IC50 of T-2 was at a micromolar
concentration, which prompted us to find additional chemicals which
can exert a more profound inhibitory effect at low doses. T-18 was
synthesized based on T-2. We found that T-18, one of the
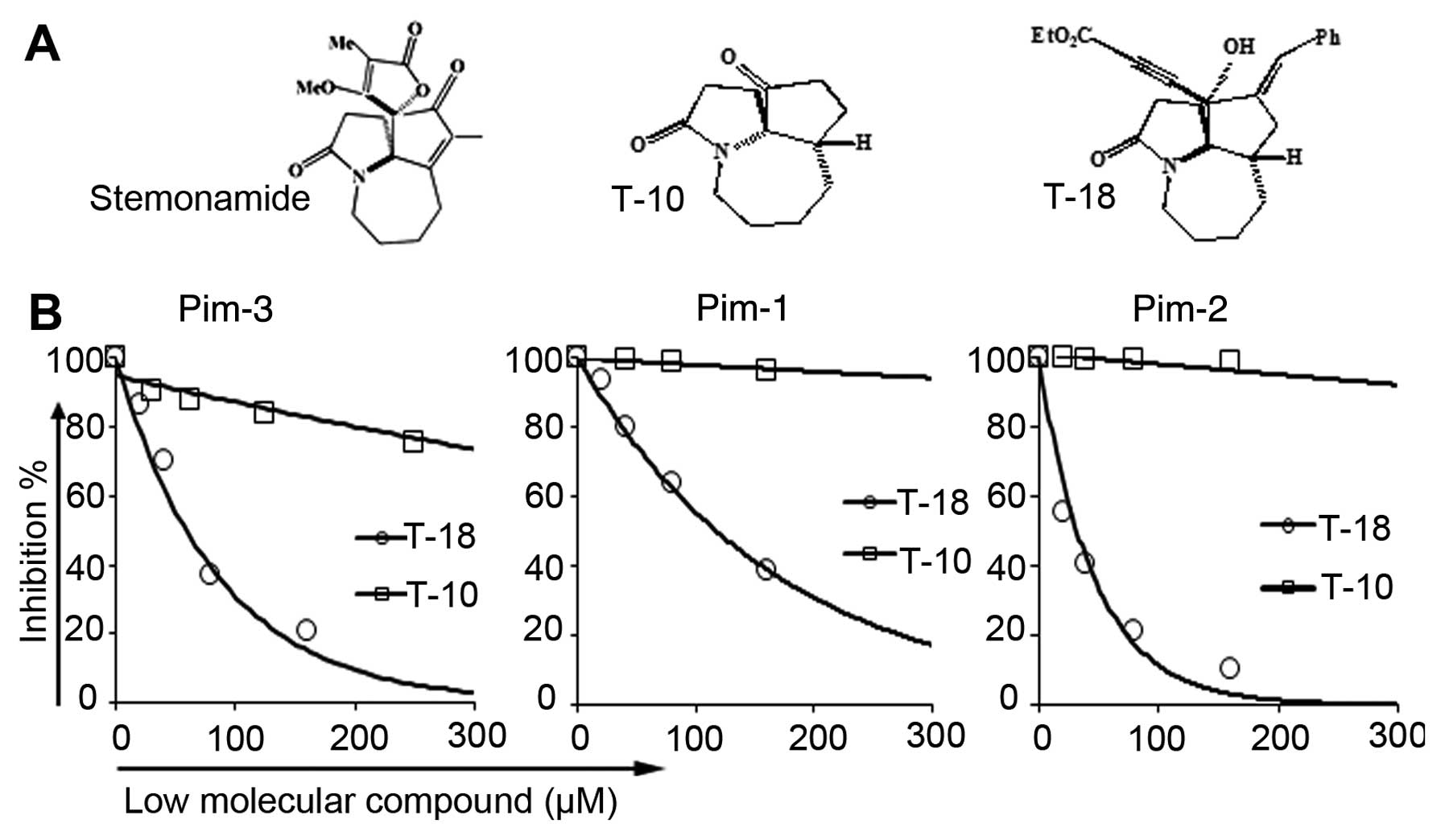
stemonamide synthetic intermediates (Fig. 1A), inhibited Pim kinase activity
(Fig. 1B). Compared with T-10
(38), T-18 inhibited Pim kinase
activity to a lower extent. Moreover, T-18 inhibited Pim-1 and
Pim-2 kinase activity to a similar extent as Pim-3 (Table I). We then examined the effects of
T-18 on the human Akt/protein kinase B (PKB) protein kinase,
another serine/threonine kinase which can phosphorylate a similar
set of proteins, such as Bad (40,41).
Akt-1/2, a specific inhibitor of Akt-1 and Akt-2, was used in our
study as the positive control for a comparison against T-18
(Table I). Akt-1/2 efficiently
inhibited Akt-1 and Akt-2 activity, while T-18 did not exert any
effect on the Akt protein kinase. Thus, T-18 can potently and
selectively inhibit Pim-3 kinase activity, but not that of Akt.
 | Table IInhibitory activity of T-18 on Pim
and Akt kinases. |
Table I
Inhibitory activity of T-18 on Pim
and Akt kinases.
| IC50
(μmol/l) |
|---|
|
|
|---|
| Chemical name | Pim-3 | Pim-1 | Pim-2 | Akt-1 | Akt-2 |
|---|
| T-10 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 |
| T-18 | 57 | 118 | 27 | >2,000 | >2,000 |
| Akt-1/2 | ND | ND | ND | 31.6 | 72.5 |
Effects of T-18 on cell proliferation
in vitro
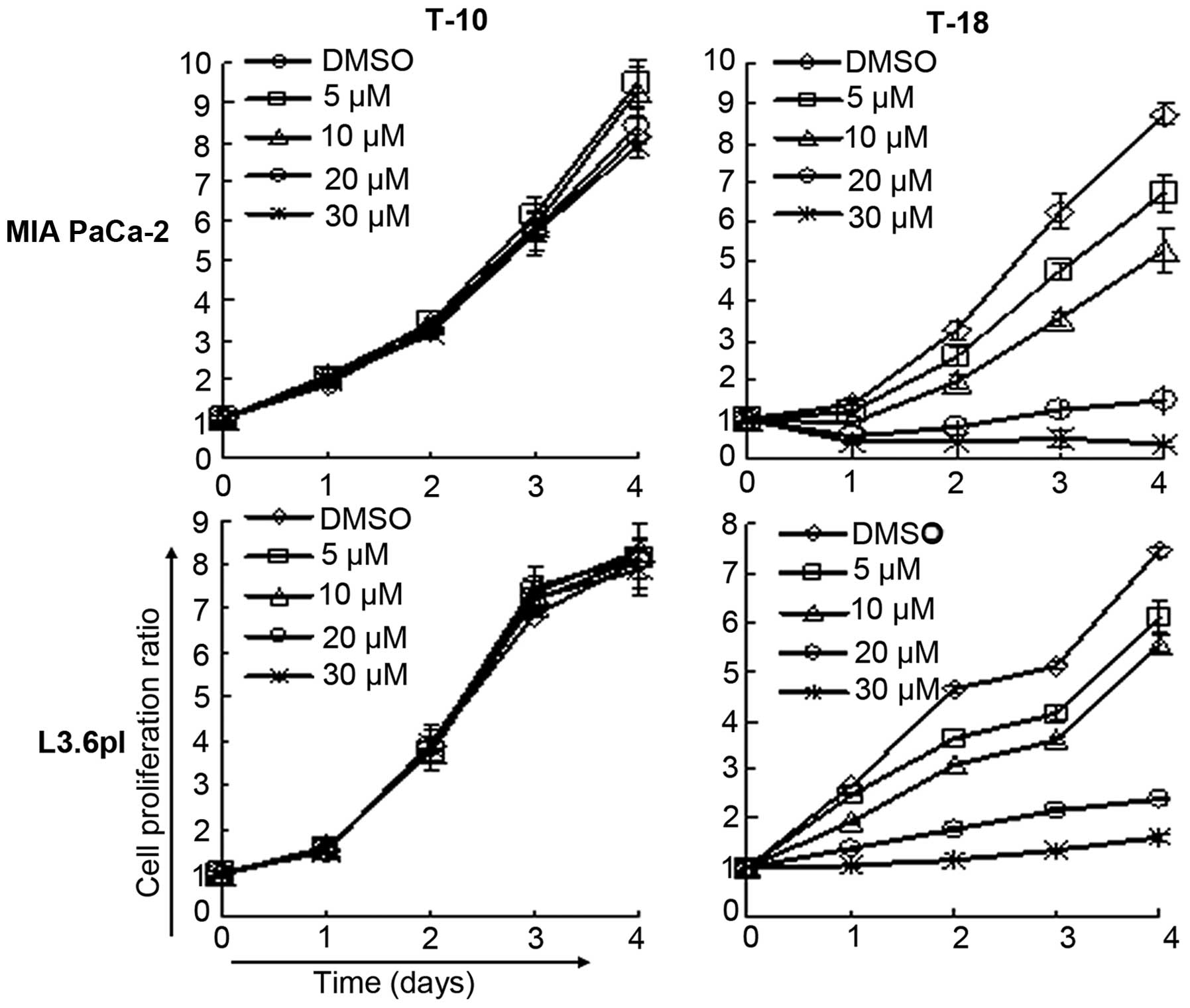
Two human pancreatic cancer cell lines, MIA PaCa-2
and L3.6pl, were recruited to examine the effects of T-18 on cell
proliferation in vitro (Fig.
2). The results showed that T-18 effectively inhibited cell
proliferation in a dose-dependent manner. However, T-10 had little
effect on the cell proliferation of these cancer cell lines, and
had little influence on Pim-3 kinase activity. T-18 was added to
the medium of the other human pancreatic cancer cell lines (PCI66,
PCI35, PCI55 and PANC-1), hepatocellular carcinoma cell lines
(HuH7, HepG2 and Hep3B) and colon cancer cell lines (SW480, SW48,
HT29 and HCT116) to determine the effect of T-18 on the
proliferation of these cells (Table
II). Almost all the cancer cell lines were sensitive to T-18.
These observations suggest that T-18 inhibits Pim-3 kinase activity
and has an effect on the proliferation of these cells.
 | Table IIEffects of T-18 on the proliferation
of human cancer cells in vitro. |
Table II
Effects of T-18 on the proliferation
of human cancer cells in vitro.
| Cell type | Cell line name | Proliferation
IC50 (μmol/l) T-18 |
|---|
| Pancreas | L3.6pl | 3.46 |
| MIA PaCa-2 | 2.09 |
| PCI66 | 2.24 |
| PCI35 | 2.25 |
| PCI55 | 3.31 |
| PANC-1 | 2.96 |
| Liver | HuH7 | 5.25 |
| HepG2 | 21.4 |
| Hep3B | - |
| Colon | SW480 | 1.78 |
| SW48 | 2.75 |
| HT29 | 4.32 |
| HCT116 | 1.79 |
Effects of T-18 on cell apoptosis
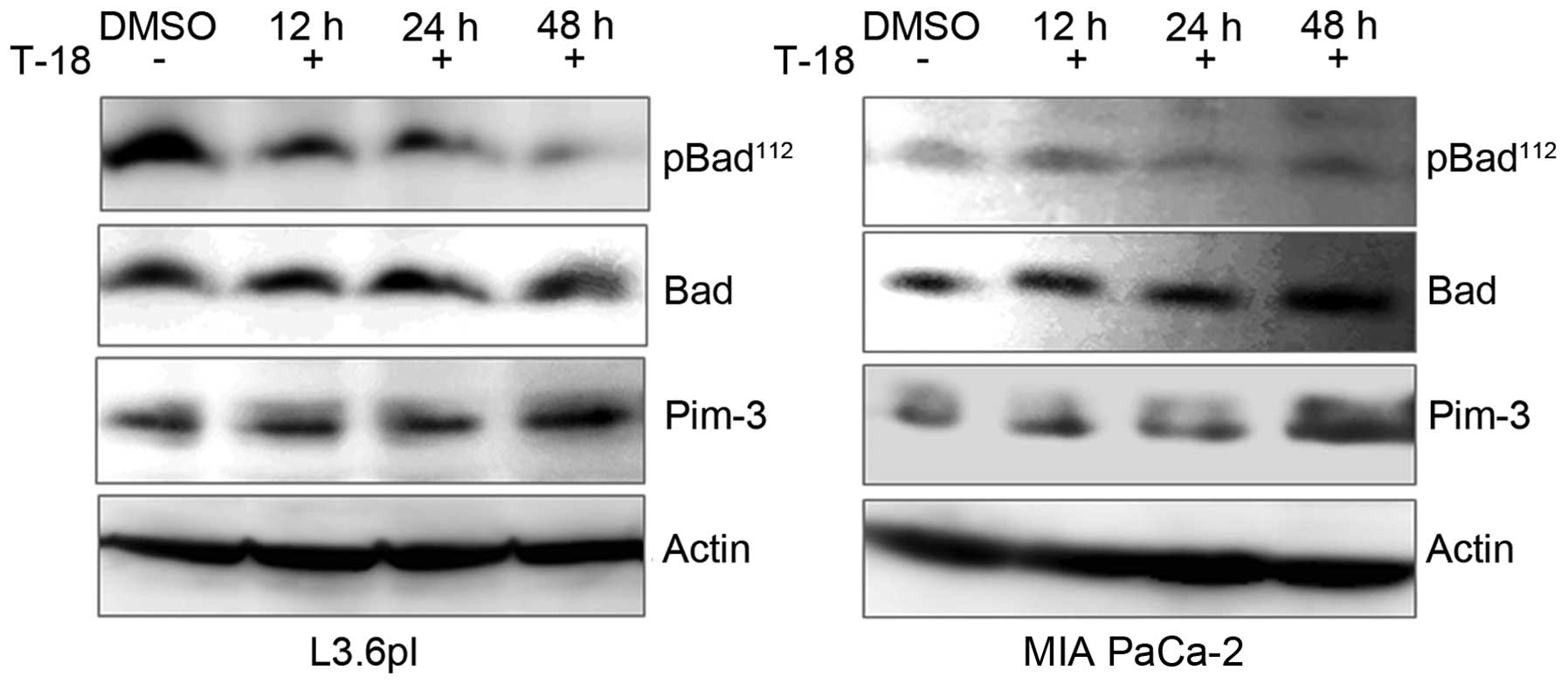
In our previous studies, we demonstrated that a
Pim-3 shRNA-mediated reduction in Pim-3 protein expression
decreased the levels of phospho-Ser112-Bad and increased
the number of apoptotic cells in human pancreatic and colon cancer
cell lines (16,17). In this study, we investigated
whether T-18 can affect the phosphorylation state of Bad in the
human pancreatic cancer cell lines, MIA PaCa-2 and L3.6pl.
Consistent with our previous results, the levels of
phospho-Ser112-Bad decreased in the cell lines following
treatment with T-18. However, the levels of Pim-3 kinase protein or
total Bad protein were not altered with the treatment time
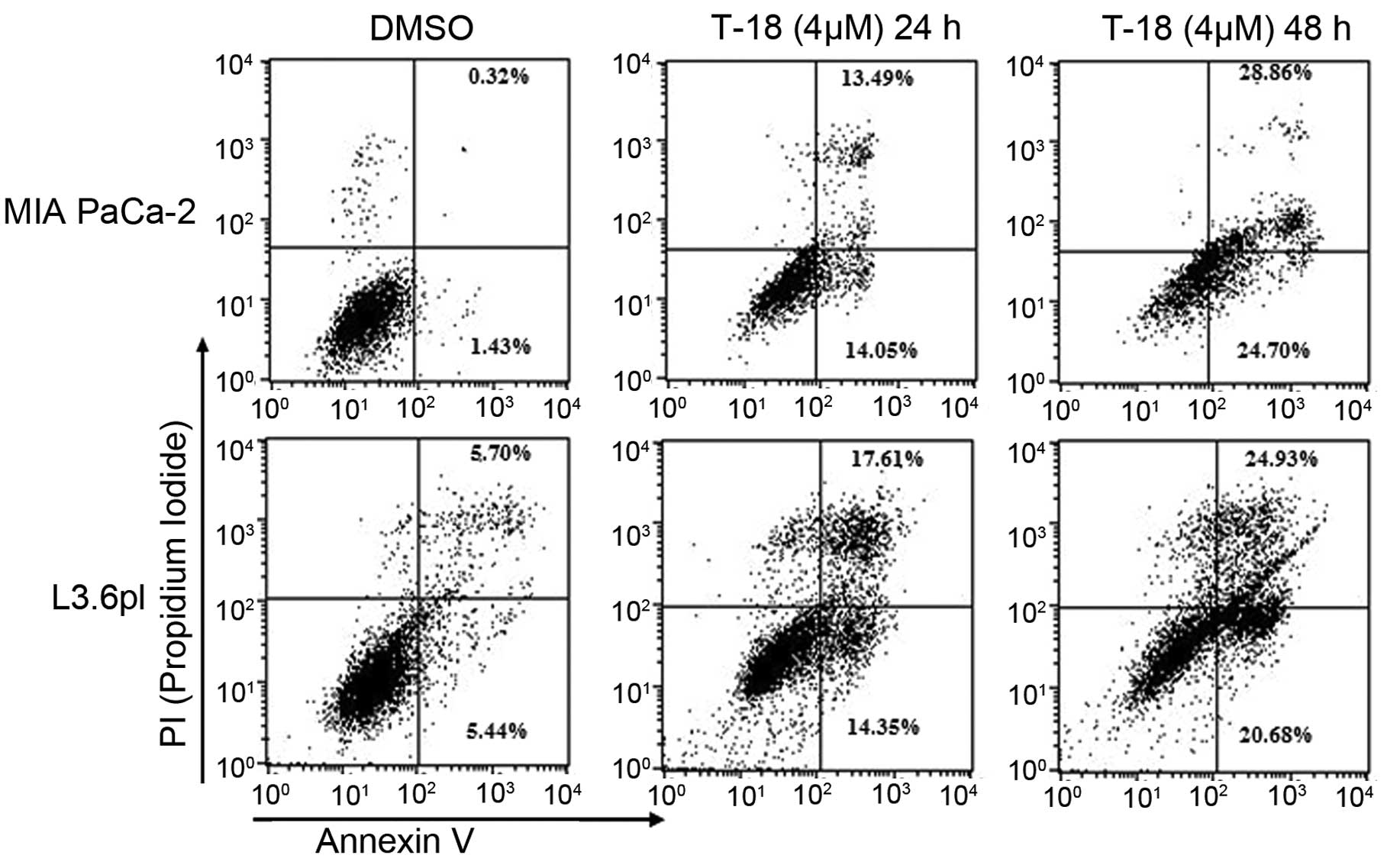
(Fig. 3). Moreover, early apoptotic
and late apoptotic cells were enhanced in both cell lines (Fig. 4) following treatment with T-18 for
24 and 48 h. These observations indicate that T-18 prevents the
phosphorylation of Bad, eventually leading to apoptosis in human
pancreatic cancer cells.
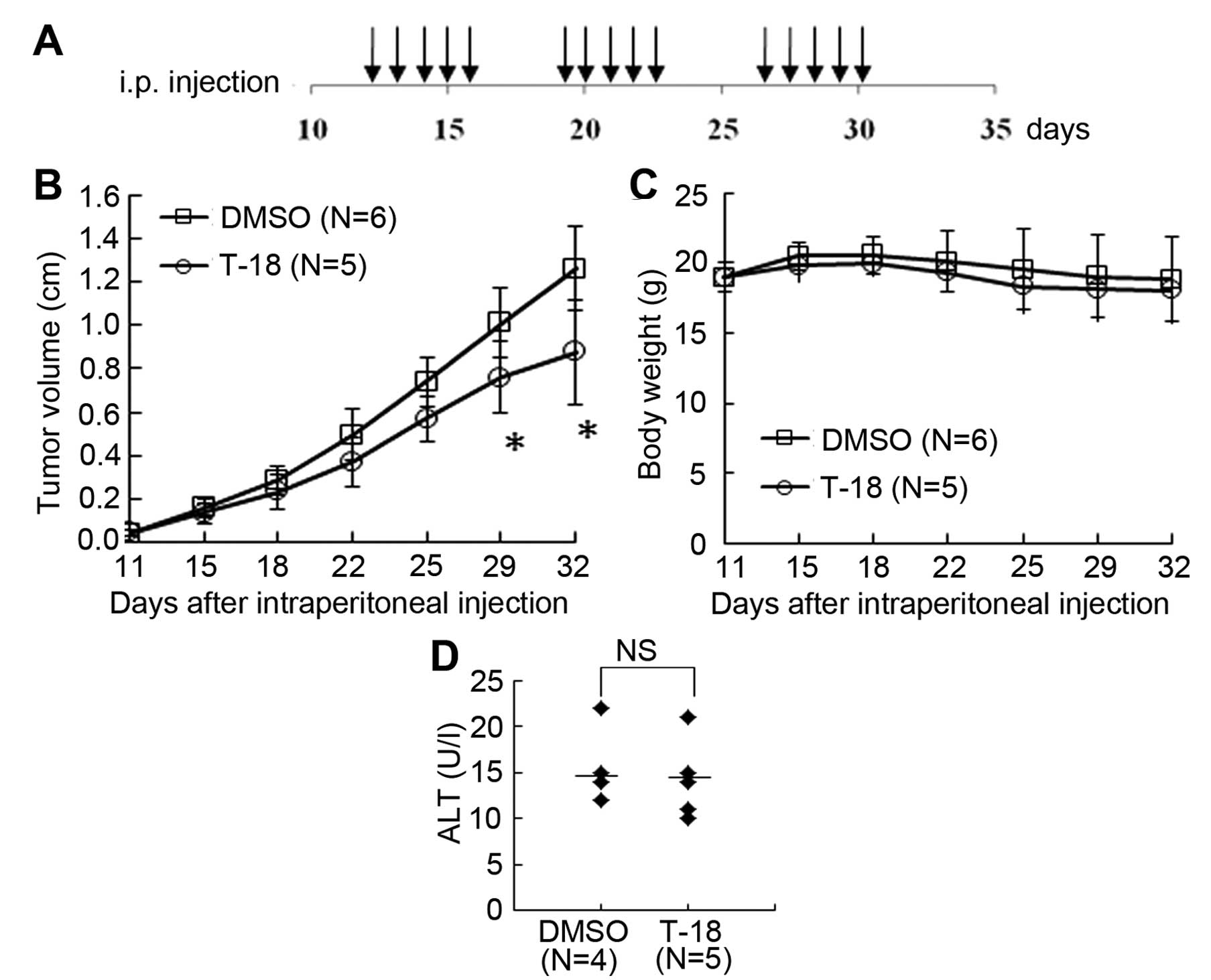
Effects of T-18 on tumor growth in
vivo
Finally, we investigated the effects of T-18 on
tumor growth in vivo. We subcutaneously injected L3.6pl
pancreatic cancer cells into nude mice as described above and T-18
treatment commenced on day 12 after the tumor injection, when the
tumor was palpable (Fig. 5A). The
tumors of the mice in the vehicle-treated group grew more rapidly
than those of the mice in the T-18-treated group (Fig. 5B). During the course of the
experiment, all mice tolerated T-18 well, as evidenced by the fact
that no body weight loss was observed (Fig. 5C) and the changes in serum ALT
levels (Fig. 5D). These results
suggest that T-18 suppresses tumor growth in vivo without
any significant side-effects.
Discussion
Pancreatic cancer is a malignant tumor of the
pancreas, and poses a threat to human health. The majority of
patients are diagnosed at an advanced stage, thus surgical
treatment is not valid and the only treatment options are
radiotherapy or chemotherapy, with the survival time being less
than a year (42,43). Thus, molecular targeted therapy is
considered to be a very promising treatment. These targeted
molecules are not expressed in normal tissues but are aberrantly
expressed in tumor tissues and play a key role in regulating tumor
cell proliferation. The Pim-3 kinase is aberrantly expressed in
premalignant and malignant lesions of endoderm-derived organs, such
as the liver, pancreas, colon and stomach (16,17,44,45)
but not in normal tissues. The Pim-3 kinase inactivates the Bad
protein by phosphorylating Bad at Ser112 to promote cell
proliferation (25), providing the
characteristics of a targeted molecule. Thus, blocking Pim-3 kinase
activity may be a new strategy for the treatment of pancreatic
cancer.
The crystal structure of Pim-1 and Pim-2 reveals
that there is a special hinge region that can connect the 2 lobes
of the protein kinase domain, which presents a unique way for ATP
to bind with Pim kinases instead of other protein kinases (5,46,47).
Thus, it may be possible to develop an inhibitor that selectively
targets Pim kinases, not other seine/threonine kinases (48). Existing small molecule inhibitors
against Pim kinases that have been reported include flavonol
quercetargetin (7),
imidazole[1,2-b]pyridazines (26,49),
bezylindene-thiazolidine-2,4-dione (50–52),
3,5-disubstituted indole derivatives (53), pyrazolo[3,4-g]quinoxaline
derivatives (54) and N-10
substituted pyrrolo[2,3-a]carbazole derivatives (55–57).
These molecules have been certified to inhibit the proliferation of
human cancer cells in vitro and/or in vivo. However,
most of the compounds are multi-Pim kinase inhibitors and Pim-3 has
received the least attention among the Pim family kinases. Thus, a
novel and safe Pim-3 targeting inhibitor is required. We have
previously demonstrated that other stemonamide synthetic
intermediates (T-2, T-5) can inhibit Pim kinases activity and
suppress cancer cell proliferation in vitro, as well as
tumor growth in vivo(38).
However, the IC50 of T-2 was at a micromolar
concentration. In this study, we investigated another low molecular
compound (T-18) that shares homoplastic structure with T-2 and may
exert a more effective anticancer effect.
To recognize a Pim-3 inhibitor, a series of low
molecule chemicals were screened and a stemonamide synthetic
intermediate, T-18, was observed, which was similar to T-2 which
was investigated in our previous study (38). In the current study, T-18 inhibited
the activity of Pim-3, Pim-1 and Pim-2 but not that of Akt-1 or
Akt-2 kinase, showing the specific inhibitory effect on Pim family
kinases and not on Akt kinases. Moreover, T-18 suppressed the
proliferation of human pancreatic carcinoma cells in vitro,
as well as the in vivo tumor growth of human pancreatic
cancer cells injected into nude mice, without any severe adverse
effects. Furthermore, T-18 prevented Bad phosphorylation at
Ser112, while increasing the number of apoptotic
pancreatic cancer cells; however, the levels of Pim-3 protein and
total Bad protein were not altered. Since Pim-1 and Pim-2 protein
can phosphorylate Bad at Ser112, which were not found in
human pancreatic cancer cells (unpublished data), Pim-3 kinase was
regarded as the target of T-18. T-18 inhibited Pim-3 activity,
inactivating Bad and finally inducing apoptosis.
As we have previously demonstrated, T-2 (38), similar to T-18, can inhibit Pim-3
activity and reduce the growth of pancreatic cancer cells in
vitro and in vivo. T-2 also inhibited in vivo
tumor growth effectively. However, as shown in this study, T-18
inhibited the proliferation of human cancer cells in vitro
with a lower IC50 value. Perhaps T-18 is an ideal
compound that can be modified to exert a more potent inhibitory
effect without any severe side-effects.
Another serine/threonine kinase, namely Akt, can
phosphorylate similar sets of substrates, such as Bad, as Pim
kinases, thus initiating the proliferation of cancer cells
(41). Akt is aberrantly activated
in various types of tumors and Akt inhibitors have been extensively
investigated (40). Although the
Akt inhibitor, GSK690693, has exhibited potent antitumor activity
in pre-clinical animal experiments (41), the genetic disruption of each Akt
kinase gene results in severe phenotypic changes, such as neonatal
mortality, severe growth retardation and reduced brain size
(58–60) and Akt-2 inhibition induces severe
hyperglycemia (41). This is a
serious impediment for the clinical use of Akt inhibitors in
anticancer treatment. The Pim-3 kinase is aberrantly expressed in
malignant lesions but not in endoderm-derived normal tissues, such
as the liver, pancreas, colon and stomach (16,17,44,45)
and promotes tumor development. Moreover, Pim-3 gene deficiency
does not induce apparent phenotypic changes, suggesting that Pim-3
may be physiologically dispensable (61). Pim kinases are not localized
downstream of the insulin receptor signaling pathway but the Akt
kinase is. The inhibition of Pim kinases has few effects on normal
metabolism. Thus, Pim kinases may be more effective targets
compared to Akt in the molecular targeted therapy of various types
of cancer, particularly pancreatic cancer, exerting potent effects
and few adverse effects. In this study, we showed that T-18
inhibited Pim kinases activity but failed to suppress Akt-1 and
Akt-2 activity, suggesting that T-18 may be a safe drug for use in
molecular targeted therapy. However, it may be difficult to
discovery a specific inhibitor against either Pim-1 or Pim-3 kinase
due to their extraordinarily similar peptide substrate identity
(46). It would be of interest to
determine whether a specific inhibitor against anyone of the Pim
family kinases will prove to be advantageous over existing Pim
kinase inhibitors.
Acknowledgements
The authors gratefully acknowledge grant support
from The National Science Foundation of China (NSFC) (30973476),
the Shanghai Pujiang Program (KW201028464), Fudan University ‘985
Project’ Phase III Cancer Research Projects II (985III-YFX0102),
‘Start Up’ Project of the Shanghai Cancer Center (YJRC0901), and
Shanghai Committee of Science and Technology (12DZ2260100). The
authors thank Professor Hiroyuki Ishibashi of School of
Pharmaceutical Sciences, Kanazawa University, for providing T-18
and T-10.
References
|
1
|
Aune D, Vieira AR, Chan DS, et al: Height
and pancreatic cancer risk: a systematic review and meta-analysis
of cohort studies. Cancer Causes Control. 23:1213–1222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
3
|
Hochster HS, Haller DG, de Gramont A, et
al: Consensus report of the international society of
gastrointestinal oncology on therapeutic progress in advanced
pancreatic cancer. Cancer. 107:676–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikkers H, Allen J, Knipscheer P, et al:
High-throughput retroviral tagging to identify components of
specific signaling pathways in cancer. Nat Genet. 32:153–159. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian KC, Wang L, Hickey ER, et al:
Structural basis of constitutive activity and a unique nucleotide
binding mode of human Pim-1 kinase. J Biol Chem. 280:6130–6137.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar A, Mandiyan V, Suzuki Y, et al:
Crystal structures of proto-oncogene kinase Pim1: a target of
aberrant somatic hypermutations in diffuse large cell lymphoma. J
Mol Biol. 348:183–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holder S, Zemskova M, Zhang C, et al:
Characterization of a potent and selective small-molecule inhibitor
of the PIM1 kinase. Mol Cancer Ther. 6:163–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alvarado Y, Giles FJ and Swords RT: The
PIM kinases in hematological cancers. Expert Rev Hematol. 5:81–96.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim KT, Baird K, Ahn JY, et al: Pim-1 is
up-regulated by constitutively activated FLT3 and plays a role in
FLT3-mediated cell survival. Blood. 105:1759–1767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pasqualucci L, Neumeister P, Goossens T,
et al: Hypermutation of multiple proto-oncogenes in B-cell diffuse
large-cell lymphomas. Nature. 412:341–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaidano G, Pasqualucci L, Capello D, et
al: Aberrant somatic hypermutation in multiple subtypes of
AIDS-associated non-Hodgkin lymphoma. Blood. 102:1833–1841. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dhanasekaran SM, Barrette TR, Ghosh D, et
al: Delineation of prognostic biomarkers in prostate cancer.
Nature. 412:822–826. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valdman A, Fang X, Pang ST, Ekman P and
Egevad L: Pim-1 expression in prostatic intraepithelial neoplasia
and human prostate cancer. Prostate. 60:367–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Zhang T, Tang H, et al:
Overexpression of PIM-1 is a potential biomarker in prostate
carcinoma. J Surg Oncol. 92:326–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu LM, Zhang JX, Wang XP, Guo HX, Deng H
and Luo J: Pim-3 protects against hepatic failure in
D-galactosamine (D-GalN)-sensitized rats. Eur J Clin Invest.
40:127–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YY, Popivanova BK, Nagai Y, Ishikura H,
Fujii C and Mukaida N: Pim-3, a proto-oncogene with
serine/threonine kinase activity, is aberrantly expressed in human
pancreatic cancer and phosphorylates Bad to block Bad-mediated
apoptosis in human pancreatic cancer cell lines. Cancer Res.
66:6741–6747. 2006. View Article : Google Scholar
|
|
17
|
Popivanova BK, Li YY, Zheng H, et al:
Proto-oncogene, Pim-3 with serine/threonine kinase activity, is
aberrantly expressed in human colon cancer cells and can prevent
Bad-mediated apoptosis. Cancer Sci. 98:321–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lilly M, Sandholm J, Cooper JJ, Koskinen
PJ and Kraft A: The PIM-1 serine kinase prolongs survival and
inhibits apoptosis-related mitochondrial dysfunction in part
through a bcl-2-dependent pathway. Oncogene. 18:4022–4031. 1999.
View Article : Google Scholar
|
|
19
|
Fox CJ, Hammerman PS, Cinalli RM, Master
SR, Chodosh LA and Thompson CB: The serine/threonine kinase Pim-2
is a transcriptionally regulated apoptotic inhibitor. Genes Dev.
17:1841–1854. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan B, Zemskova M, Holder S, et al: The
PIM-2 kinase phosphorylates BAD on serine 112 and reverses
BAD-induced cell death. J Biol Chem. 278:45358–45367. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macdonald A, Campbell DG, Toth R,
McLauchlan H, Hastie CJ and Arthur JS: Pim kinases phosphorylate
multiple sites on Bad and promote 14-3-3 binding and dissociation
from Bcl-XL. BMC Cell Biol. 7:12006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brault L, Gasser C, Bracher F, Huber K,
Knapp S and Schwaller J: PIM serine/threonine kinases in the
pathogenesis and therapy of hematologic malignancies and solid
cancers. Haematologica. 95:1004–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anizon F, Shtil AA, Danilenko VN and
Moreau P: Fighting tumor cell survival: advances in the design and
evaluation of Pim inhibitors. Curr Med Chem. 17:4114–4133. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akue-Gedu R, Rossignol E, Azzaro S, et al:
Synthesis, kinase inhibitory potencies, and in vitro
antiproliferative evaluation of new Pim kinase inhibitors. J Med
Chem. 52:6369–6381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao ZF, Hasvold LA, Leverson JD, et al:
Discovery of 3H-benzo[4,5]thieno[3,2-d]pyrimidin-4-ones as potent,
highly selective, and orally bioavailable inhibitors of the human
protooncogene proviral insertion site in moloney murine leukemia
virus (PIM) kinases. J Med Chem. 52:6621–6636. 2009.
|
|
26
|
Chen LS, Redkar S, Bearss D, Wierda WG and
Gandhi V: Pim kinase inhibitor, SGI-1776, induces apoptosis in
chronic lymphocytic leukemia cells. Blood. 114:4150–4157. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mumenthaler SM, Ng PY, Hodge A, et al:
Pharmacologic inhibition of Pim kinases alters prostate cancer cell
growth and resensitizes chemoresistant cells to taxanes. Mol Cancer
Ther. 8:2882–2893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yano T, Ishikura H, Kato H, Ogawa Y, Kondo
S and Yoshiki T: Vaccination effect of interleukin-6-producing
pancreatic cancer cells in nude mice: a model of tumor prevention
and treatment in immune-compromised patients. Jpn J Cancer Res.
92:83–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lieber M, Mazzetta J, Nelson-Rees W,
Kaplan M and Todaro G: Establishment of a continuous tumor-cell
line (panc-1) from a human carcinoma of the exocrine pancreas. Int
J Cancer. 15:741–747. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yunis AA, Arimura GK and Russin DJ: Human
pancreatic carcinoma (MIA PaCa-2) in continuous culture:
sensitivity to asparaginase. Int J Cancer. 19:128–135. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leibovitz A, Stinson JC, McCombs WB III,
McCoy CE, Mazur KC and Mabry ND: Classification of human colorectal
adenocarcinoma cell lines. Cancer Res. 36:4562–4569.
1976.PubMed/NCBI
|
|
32
|
Bruns CJ, Harbison MT, Kuniyasu H, Eue I
and Fidler IJ: In vivo selection and characterization of
metastatic variants from human pancreatic adenocarcinoma by using
orthotopic implantation in nude mice. Neoplasia. 1:50–62. 1999.
View Article : Google Scholar
|
|
33
|
Marshall CJ, Franks LM and Carbonell AW:
Markers of neoplastic transformation in epithelial cell lines
derived from human carcinomas. J Natl Cancer Inst. 58:1743–1751.
1977.PubMed/NCBI
|
|
34
|
Brattain MG, Brattain DE, Fine WD, et al:
Initiation and characterization of cultures of human colonic
carcinoma with different biological characteristics utilizing
feeder layers of confluent fibroblasts. Oncodev Biol Med.
2:355–366. 1981.
|
|
35
|
Knowles BB, Howe CC and Aden DP: Human
hepatocellular carcinoma cell lines secrete the major plasma
proteins and hepatitis B surface antigen. Science. 209:497–499.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakabayashi H, Taketa K, Miyano K, Yamane
T and Sato J: Growth of human hepatoma cells lines with
differentiated functions in chemically defined medium. Cancer Res.
42:3858–3863. 1982.PubMed/NCBI
|
|
37
|
Taniguchi T, Tanabe G, Muraoka O and
Ishibashi H: Total synthesis of (+/-)-stemonamide and
(+/-)-isostemonamide using a radical cascade. Org Lett. 10:197–199.
2008.
|
|
38
|
Li YY, Wang YY, Taniguchi T, et al:
Identification of stemonamide synthetic intermediates as a novel
potent anticancer drug with an apoptosis-inducing ability. Int J
Cancer. 127:474–484. 2010.PubMed/NCBI
|
|
39
|
Wang YY, Taniguchi T, Baba T, Li YY,
Ishibashi H and Mukaida N: Identification of a phenanthrene
derivative as a potent anticancer drug with Pim kinase inhibitory
activity. Cancer Sci. 103:107–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amaravadi R and Thompson CB: The survival
kinases Akt and Pim as potential pharmacological targets. J Clin
Invest. 115:2618–2624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rhodes N, Heerding DA, Duckett DR, et al:
Characterization of an Akt kinase inhibitor with potent
pharmacodynamic and antitumor activity. Cancer Res. 68:2366–2374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
André T, Balosso J, Louvet C, et al:
Adenocarcinoma of the pancreas. General characteristics. Presse
Med. 27:533–536. 1998.(In French).
|
|
43
|
Jian J, Hu ZF and Huang Y: Effect of
ginsenoside Rg3 on Pim-3 and Bad proteins in human pancreatic
cancer cell line PANC-1. Ai Zheng. 28:461–465. 2009.(In
Chinese).
|
|
44
|
Fujii C, Nakamoto Y, Lu P, et al: Aberrant
expression of serine/threonine kinase Pim-3 in hepatocellular
carcinoma development and its role in the proliferation of human
hepatoma cell lines. Int J Cancer. 114:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng HC, Tsuneyama K, Takahashi H, et al:
Aberrant Pim-3 expression is involved in gastric
adenoma-adenocarcinoma sequence and cancer progression. J Cancer
Res Clin Oncol. 134:481–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bullock AN, Debreczeni J, Amos AL, Knapp S
and Turk BE: Structure and substrate specificity of the Pim-1
kinase. J Biol Chem. 280:41675–41682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bullock AN, Russo S, Amos A, et al:
Crystal structure of the PIM2 kinase in complex with an
organoruthenium inhibitor. PLoS One. 4:e71122009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Swords R, Kelly K, Carew J, et al: The Pim
kinases: new targets for drug development. Curr Drug Targets.
12:2059–2066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pogacic V, Bullock AN, Fedorov O, et al:
Structural analysis identifies imidazo[1,2-b]pyridazines as PIM
kinase inhibitors with in vitro antileukemic activity.
Cancer Res. 67:6916–6924. 2007.PubMed/NCBI
|
|
50
|
Dakin LA, Block MH, Chen H, et al:
Discovery of novel benzylidene-1,3-thiazolidine-2,4-diones as
potent and selective inhibitors of the PIM-1, PIM-2, and PIM-3
protein kinases. Bioorg Med Chem Lett. 22:4599–4604. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xia Z, Knaak C, Ma J, et al: Synthesis and
evaluation of novel inhibitors of Pim-1 and Pim-2 protein kinases.
J Med Chem. 52:74–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin YW, Beharry ZM, Hill EG, et al: A
small molecule inhibitor of Pim protein kinases blocks the growth
of precursor T-cell lymphoblastic leukemia/lymphoma. Blood.
115:824–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nishiguchi GA, Atallah G, Bellamacina C,
et al: Discovery of novel 3,5-disubstituted indole derivatives as
potent inhibitors of Pim-1, Pim-2, and Pim-3 protein kinases.
Bioorg Med Chem Lett. 21:6366–6369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gavara L, Saugues E, Alves G, Debiton E,
Anizon F and Moreau P: Synthesis and biological activities of
pyrazolo[3,4-g]quinoxaline derivatives. Eur J Med Chem.
45:5520–5526. 2010.
|
|
55
|
Akue-Gedu R, Letribot B, Saugues E,
Debiton E, Anizon F and Moreau P: Kinase inhibitory potencies and
in vitro antiproliferative activities of N-10 substituted
pyrrolo[2,3-a]carbazole derivatives. Bioorg Med Chem Lett.
22:3807–3809. 2012.PubMed/NCBI
|
|
56
|
Akue-Gedu R, Nauton L, Thery V, et al:
Synthesis, Pim kinase inhibitory potencies and in vitro
antiproliferative activities of diversely substituted
pyrrolo[2,3-a]carbazoles. Bioorg Med Chem. 18:6865–6873.
2010.PubMed/NCBI
|
|
57
|
Letribot B, Akue-Gedu R, Santio NM, et al:
Use of copper(I) catalyzed azide alkyne cycloaddition (CuAAC) for
the preparation of conjugated pyrrolo[2,3-a]carbazole Pim kinase
inhibitors. Eur J Med Chem. 50:304–310. 2012.PubMed/NCBI
|
|
58
|
Chen WS, Xu PZ, Gottlob K, et al: Growth
retardation and increased apoptosis in mice with homozygous
disruption of the Akt1 gene. Genes Dev. 15:2203–2208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Easton RM, Cho H, Roovers K, et al: Role
for Akt3/protein kinase Bgamma in attainment of normal brain size.
Mol Cell Biol. 25:1869–1878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Woulfe D, Jiang H, Morgans A, Monks R,
Birnbaum M and Brass LF: Defects in secretion, aggregation, and
thrombus formation in platelets from mice lacking Akt2. J Clin
Invest. 113:441–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mukaida N, Wang YY and Li YY: Roles of
Pim-3, a novel survival kinase, in tumorigenesis. Cancer Sci.
102:1437–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|