Introduction
Lung cancer, and particularly non-small cell lung
cancer (NSCLC), is the leading cause of cancer-related mortality
worldwide. In China, approximately 500,000 patients were diagnosed
with lung cancer and 430,000 deaths were reported in 2005. In 2025,
the number of deaths is expected to be more than one million
(1–4). After diagnosis, less than 15% of
patients survive beyond 5 years. Therefore, identification of
molecular pathways involved in lung tumorigenesis and metastasis
may lead to new targeted therapies. Among the known pathways are
CXCR4 and EGFR, where both proteins are expressed in NSCLC and may
predict prognosis (1,5).
Chemokines are small secreted chemotactic (8–10 kDa)
proteins which play an important role in the regulation of
leukocyte trafficking and extravasations toward the sites of tissue
inflammation (6). Many recent
reports have shown that these chemokines are involved in every
aspect of cancer biology (7). Among
chemokines and their receptors is the CXCL12/CXCR4 system. CXCR4 is
a seven-transmembrane trimeric G-protein-coupled receptor (GPCR);
CXCL12, also called stromal cell-derived factor 1α (SDF1–1α), is a
powerful chemoattractant cytokine that directs locomotion of
hematopoietic and non-hematopoitic cells (8,9).
Interaction between the chemokine receptor CXCR4 and its cognate
ligand CXCL12 has been implicated in tumorigenicity, cell
proliferation, angiogenesis and metastasis in 23 types of cancers,
such as lung cancer, breast cancer, melanoma, glioblastoma,
pancreatic cancer, colorectal carcinoma, cholangiocarcinoma basal
cell carcinoma cells and prostate cancer, and it has been proposed
as a prognostic marker for these malignancies (10–13).
All NSCLC cell lines have been shown to express
CXCR4 on the cell surface (14).
Kijima et al reported that the expression of the CXCR4
receptor in NSCLC cells regulates their homing to organs that
express higher levels of CXCL12 such as lung, liver, bone marrow
and lymph node. Similarly, Phillips et al reported that the
CXCR4/CXCL12 biological axis can regulate the overall metastatic
behavior of NSCLC, and they found that in vivo
neutralization of CXCL12 in an SCID mouse system of human NSCLC by
anti-CXCL12 antibodies resulted in a marked attenuation of NSCLC
metastasis to adrenal gland, lymph node, liver, lung and bone
marrow (15,16).
Moreover, NSCLC patients with clinical metastasis
have been shown to express a high level of CXCR4 mRNA in their
tissue specimen compared with patients without metastasis,
suggesting that the activation of the CXCR4/CXCL12 pathway
increases the ability of metastasis-associated behavior such as
cell invasion and migration (14,17,18).
Oonakanava et al found that the CXCL12/CXCR4 axis is
involved in the metastasis of NSCLC cells into the pleural space
(14), while Tang et al
reported that the CXCL12/CXCR4 pathway mediates bone-specific
metastasis of NSCLC (8). Wagner
et al reported that cytomembranous expression of CXCR4 is an
independent risk factor associated with poor outcome in
adenocarcinoma (ADC) of the lung (19).
Epidermal growth factor receptor (EGFR) is a
membrane bound receptor, which is frequently mutated or functions
anomalously in cancer. These receptors homodimerize or
heterodimerize, after the binding of their cognate ligands,
resulting in activation of their tyrosine kinase domain,
subsequently initiating a cascade of signals that affects cell
cycle progression, angiogenesis, apoptosis and metastasis leading
to the development and progression of cancer (20,21).
NSCLC tissues have been shown to overexpress EGFR at all tumor
stages compared with uninvolved lung tissues. The EGFR gene
copy number and EGFR mRNA transcript level are closely associated
with the expression of EGFR in NSCLC tumors (5,22). The
frequency of EGFR overexpression has been reported to range from 34
to 84%, and contradictory results have been reported regarding the
correlation between EGFR overexpression and clinicopathological
factors or patient survival. Either shorter or longer survival
associated with EGFR expression has been reported by several
studies (23). Despite the
implication of EGFR signaling in the progression of NSCLC, a
marginal survival benefit and limited response rates have been
demonstrated with the use of anti-EGFR targeted therapies (24–26).
Interaction has been found between CXCR4 and EGFR in
cancer cells. In ovarian cancer, EGFR has been shown to enhance the
expression of CXCR4 in ovarian cancer cell lines through the
activation of Src kinase that enhances tumor growth
(27). Another study showed that
EGFR activates not only CXCR4 but also MMP9, leading to the
increased metastatic potential of tumors (28). Similarly, concerning breast cancer,
the activation of both EGFR and ErbB2 has been shown to increase
CXCR4 expression in breast cancer cells (29). Furthermore, it was found that
patients with tumors co-expressing CXCR4 and EGFR had a high
incidence of inflammatory breast cancer-related death and a lower
overall survival rate (30).
Regarding colon cancer, co-expression of both EGFR and CXCR4 has
been shown to be positively correlated with lymph node metastasis
and distant metastasis, when compared with high expression of each
molecule alone (31). However dual
expression of EGFR and CXCR4 and its relationship with prognosis
has not been previously investigated in NSCLC.
The mechanism of EGFR-induced CXCR4 activation
appears to be complex, yet a close relationship has been suggested.
Therefore, we carried out the present study using NSCLC tissue
samples and cell lines to evaluate the potential correlation
between CXCR4 and EGFR, and to investigate the relationship between
their expression and clinicopathological parameters and to evaluate
the prognostic impact of individual and combined expression of
CXCR4 and EGFR in NSCLC.
Materials and methods
Patients
We retrospectively selected 125 patients with a
histological diagnosis of NSCLC at Zhong Nan Hospital who were
followed-up on a regular basis during a qualified follow-up program
lasting from 2003 to 2011. Tumors were staged according to the
American Joint Committee on Cancer (AJCC) pathologic
tumor-node-metastasis (TNM) classification. The study was conducted
in conformity to the Helsinki Declaration and was approved by the
Ethics Committee of Zhongnan Hospital, Wuhan, China.
Patient characteristics and recorded clinical
features including age, gender, type of surgery, eventual
concomitant treatment and evidence of recurrence were obtained from
office files and hospital records. Follow-up was carried out on an
outpatient basis at 3-month intervals for the first 2 years and
thereafter, for 6-month intervals. The follow-up evaluation
consisted of a physical examination and blood examination including
the detection of pertinent tumor markers and contrast-enhanced
computed tomography (CT) scan of the chest. Further evaluation
including CT of the abdomen, bone scintigraphy and MRI of the brain
were performed when any signs or symptoms of recurrence were
observed. Recurrences were detected by imaging techniques and were
confirmed histologically when necessary. Neither chemotherapy nor
radiotherapy were administered prior to surgery, and none of the
patients received EGFR-targeted anticancer therapy during the
follow-up period.
Tissue preparation and
immunohistochemical analysis
Immunohistochemical staining by a standard
streptavidin-peroxidase technique was used to examine CXCR4 and
EGFR expression. Briefly, 5-μm paraffin sections were made,
deparaffinized in xylene and dipped in a graded series of ethanol.
In order to block endogenous peroxidase activity, the slides were
incubated in 3% H2O2 for 10 min, for antigen
retrieval; deparaffinized sections were boiled in citric acid, pH
6.0, in a pressure cooker for 3 min. The slides were dipped and
cleansed in Tris buffer and incubated with anti-CXCR4 antibodies
(Abcam, ab2074) or with anti-EGFR antibodies (Abcam, ab2430)
overnight at 4°C. The bound antibody was detected with a
biotinylated secondary antibody (Maixin-Bio). The enzyme complex
was visualized with 3,3′-diaminobenzidine tetrachloride. Negative
control experiments included omitting the primary antibody and
replacing it with normal serum. The slides were assessed by two
pathologists in a blinded manner.
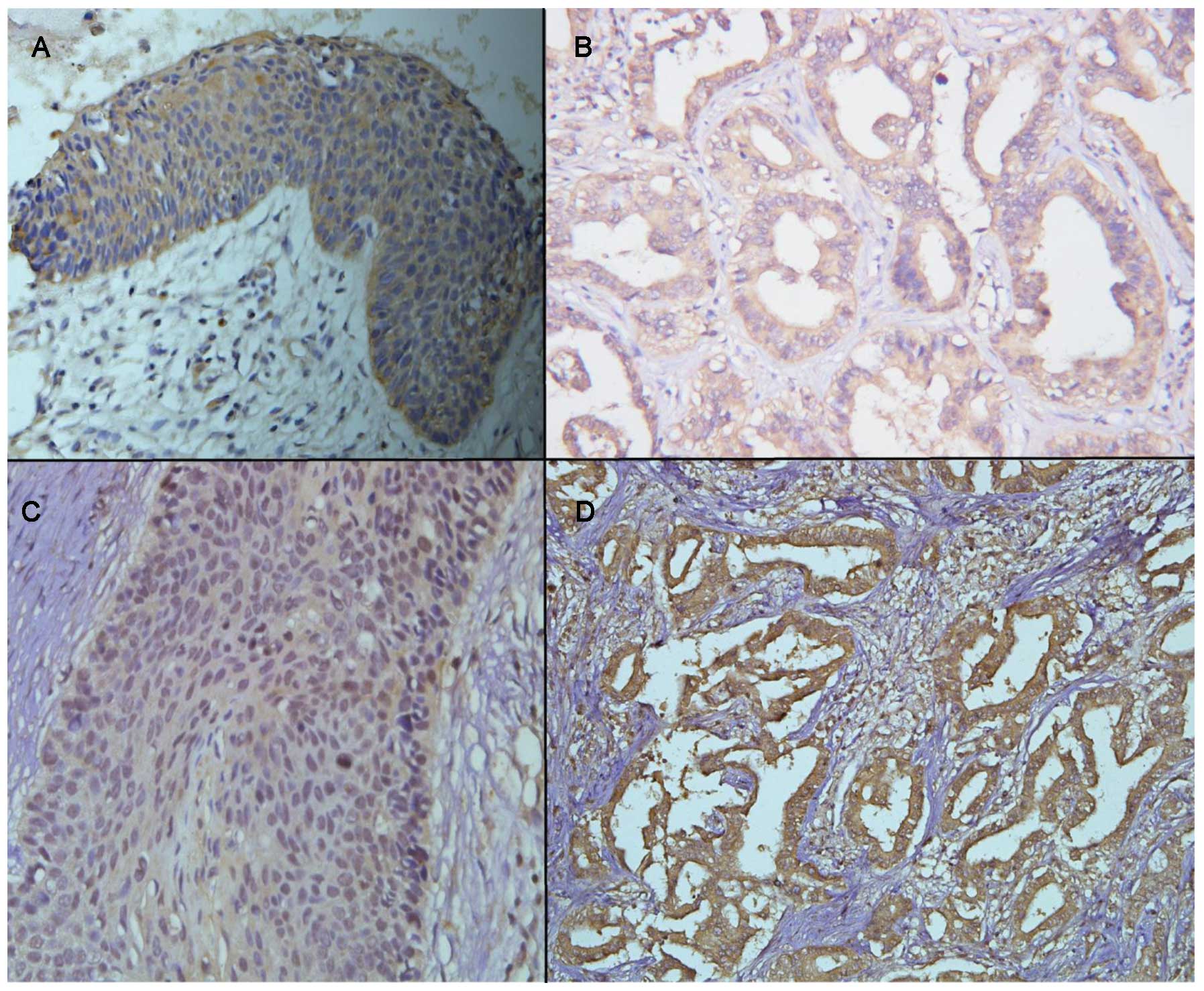
Specific immunoreactivity was observed in the nuclei
and cytoplasm of the tumor cells. We used a system that was based
on staining intensity and the percentage of stained cells, as
previously described for EGFR (33)
and for CXCR4 (32). As shown in
Fig. 1, IHC was regarded as
positive only when evident distinct cell membrane staining was
noted. An average of 1,500 cells per section was evaluated using a
semi-quantitative grading system based on four scores (0, no
staining; 1+, staining in 1–10% of the scrutinized cells; 2+,
staining in 11–25% of the scrutinized cells; 3+, staining in
>25% of the scrutinized cells). In order to avoid inclusion of
scattered positivity of the same intensity found in the normal
bronchial tissue, a cutoff value of 10% positive cells was
used.
Cell line
The human NSCLC cell line A549 was obtained from the
Shanghai Institute of Health Sciences (Shanghai, China), The A549
cell line was cultured in RPMI-1640 media together with 1 mM
L-glutamine, 25 mM HEPES buffer, 100 ng/ml streptomycin, 100 U/ml
penicillin and 10% FCS (RPMI complete media).
Antibodies and reagents
The anti-CXCR4 and anti-EGFR antibodies were
purchased from Abcam (Hong Kong). Recombinant human EGF was
obtained from Peprotech (USA). LY294002 (PI-3K inhibitor) was from
Beyotime Institute of Biotechnology (Jiangsu, China) and AG1478
from Sigma (St. Louis, MO, USA).
Real-time PCR
Total RNA was isolated from A549 cells using TRIzol
reagent (Invitrogen) according to the manufacturer's instructions.
Cells were lysed in TRIzol reagent and then mixed with chloroform,
to separate the RNA, DNA and protein. The lysates were then
centrifuged. Total RNA was precipitated with isopropanol, and then
the RNA pellet was washed with 75% ethanol and subsequently
dissolved in water. RNA (1.5 μg) was reverse transcribed into cDNA
using a Revert Aid cDNA synthesis kit, following the manufacturer's
instructions. Finally, the cDNA was evaluated for changes in CXCR4
expression by real-time PCR using the Takara SYBR First Strand
RT-PCR kit and MX3000P (Stratagene, La Jolla, CA, USA).
Western blotting
Using SDS-PAGE, immunoblotting was performed using
40 μg of total protein. The proteins were transferred to PVDF
membranes by electrophoresis at 100 V for 1 h at room temperature
and subsequently blocked in non-fat milk for 30 min. Following
this, the membranes were incubated overnight at 4°C with rabbit
anti-human CXCR4 (ab2074; 1:1500; Abcam, Cambridge, CA, USA) and
the blots were washed in TTBS before incubation with the secondary
antibodies (goat anti-rabbit IgG-HRP) at a dilution of 1:3000 for
40 min. After washing in TTBS, detection was carried out using
enzyme linked chemiluminescence (ECL) detection reagents (Beyotime
Institute of Biotechnology). To demonstrate equal loading of each
lane, the membranes were then reprobed with a β-actin antibody
(1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Statistical analysis
The statistical analysis and data management were
conducted using SPSS for Windows (version 18; SPSS). The
χ2 test was used to assess significant
clinicopathological differences between patients with positive and
negative EGFR and CXCR4 expression.
The duration of overall survival was calculated from
the date of first diagnosis of the disease to the date of death or
the last follow-up. Kaplan-Meier test was performed for survival
analysis, and Cox proportional hazards models were used to evaluate
the relationship between the tumor expression of CXCR4 and EGFR and
the prognosis of NSCLC patients. Significant prognostic variables
such as patient age, tumor stage and type of treatment were
included in these models. Cox regression plots were constructed for
CXCR4+ vs. CXCR4− and EGFR+ vs.
EGFR−. The following additional comparisons were made:
CXCR4+/EGFR+ vs.
CXCR4−/EGFR−,
CXCR4+/EGFR+ vs. others,
CXCR4+/EGFR− vs. others,
CXCR4−/EGFR+ vs. others,
CXCR4−/EGFR− vs. others.
Results
The median age of the patients was 59 years (range
37–80), and the majority of the patients were male (70%) (M:F=
6:1). Of the 125 examined biopsies, distribution of the
histological types were 64 (51.2%) ADCs and 61 (48.8%) squamous
cell carcinomas (SCCs). After diagnosis, 16 (12.8%) patients were
treated with surgery alone, 27 patients (21.6%) were treated with
surgery followed by radiochemotherapy, 48 patients (38.4%) were
treated with surgery followed by chemotherapy, 27 patients (21.6%)
were treated with chemoradiotherapy and 7 patients (5.6%) were
treated with chemotherapy.
EGFR and CXCR4 protein expression in
NSCLC samples
The positive frequency of EGFR and CXCR4 expression
and co-expression of both receptors are shown in Table I. Using immunohistochemical
techniques, EGFR expression was detected as a membranous and
cytoplasmic staining pattern. Forty-nine (39.2%) cases showed high
expression and 76 (60.8%) cases showed low expression. CXCR4
expression was detected as a cytoplasmic and nuclear staining
pattern. Out of the 125 cases, 62 (49.6%) showed high cytoplasmic
expression while 63 (50.4%) showed low cytoplasmic expression. High
nuclear expression was found in 19 cases (15.2%).
 | Table ICorrelation of EGFR and CXCR4
expression with clinicopathological features of the NSCLC
cases. |
Table I
Correlation of EGFR and CXCR4
expression with clinicopathological features of the NSCLC
cases.
| | EGFR | CXCR4 | EGFR/CXCR4 |
|---|
| |
|
|
|
|---|
| Variables | N | + (%) | − (%) | P-value | + (%) | − (%) | P-value | Both + (%) | Others | P−value |
|---|
| Total no. | 125 | 39.2 | 60.8 | | 49.6 | 50.4 | | 26.4 | 73.6 | |
| Gender |
| Male | 87 | 30.4 | 39.2 | | 33.6 | 36.0 | | 20.8 | 48.8 | |
| Female | 38 | 8.8 | 21.6 | 0.163 | 16.0 | 14.4 | 0.70 | 5.6 | 24.8 | 0.196 |
| Histologic
subtype |
| ADC | 64 | 16.0 | 35.2 | | 27.2 | 24 | | 14.4 | 36.8 | |
| SCC | 61 | 23.2 | 25.6 | 0.098 | 22.4 | 26.4 | 0.419 | 12.0 | 36.8 | 0.654 |
| Metastasis |
| Presence | 31 | 12.8 | 12.0 | | 20.8 | 4.0 | | 12.0 | 12.8 | |
| Absence | 94 | 26.4 | 48.8 | 0.137 | 28.8 | 46.4 | 0.001 | 14.4 | 60.8 | 0.002 |
| Tumor stage |
| I | 16 | 3.2 | 9.6 | | 2.4 | 10.4 | | 1.6 | 11.2 | |
| II | 23 | 4.8 | 13.6 | | 5.6 | 12.8 | | 1.6 | 16.8 | |
| III | 55 | 16.8 | 27.2 | | 20.8 | 23.2 | | 11.2 | 32.8 | |
| IV | 31 | 14.4 | 10.4 | 0.053 | 20.8 | 4.0 | 0.001 | 12.0 | 12.8 | 0.005 |
Dual EGFR/CXCR4 protein expression for the 125 cases
of NSCLC included: CXCR4+/EGFR+ in 33 (26.4%)
cases, CXCR4−/EGFR+ in 16 (12.8%) cases,
CXCR4+/EGFR− in 29 (23.2%) cases and
CXCR4−/EGFR− in 47 (37.6%) cases.
Clinicopathological characteristics
Neither EGFR tumor expression nor CXCR4 tumor
expression were associated with the gender of the patients or
histological subtype. Overexpression of the CXCR4 protein was
observed more frequently in patients with clinical metastasis than
in patients without metastasis (P=0.001), and CXCR4 was noted more
frequently in advanced stage than in early stage NSCLC (P=0.001).
EGFR overexpression was also found to be more frequent in advanced
stage than in a localized stage, although this difference was not
statistically significant (P=0.053). Thirty-three patients (26.4%)
showed concomitant overexpression of the EGFR and CXCR4 receptors.
This group (CXCR4+/EGFR+) was also
significantly associated with metastatic and advanced stage disease
(P=0.002, P=0.005, respectively).
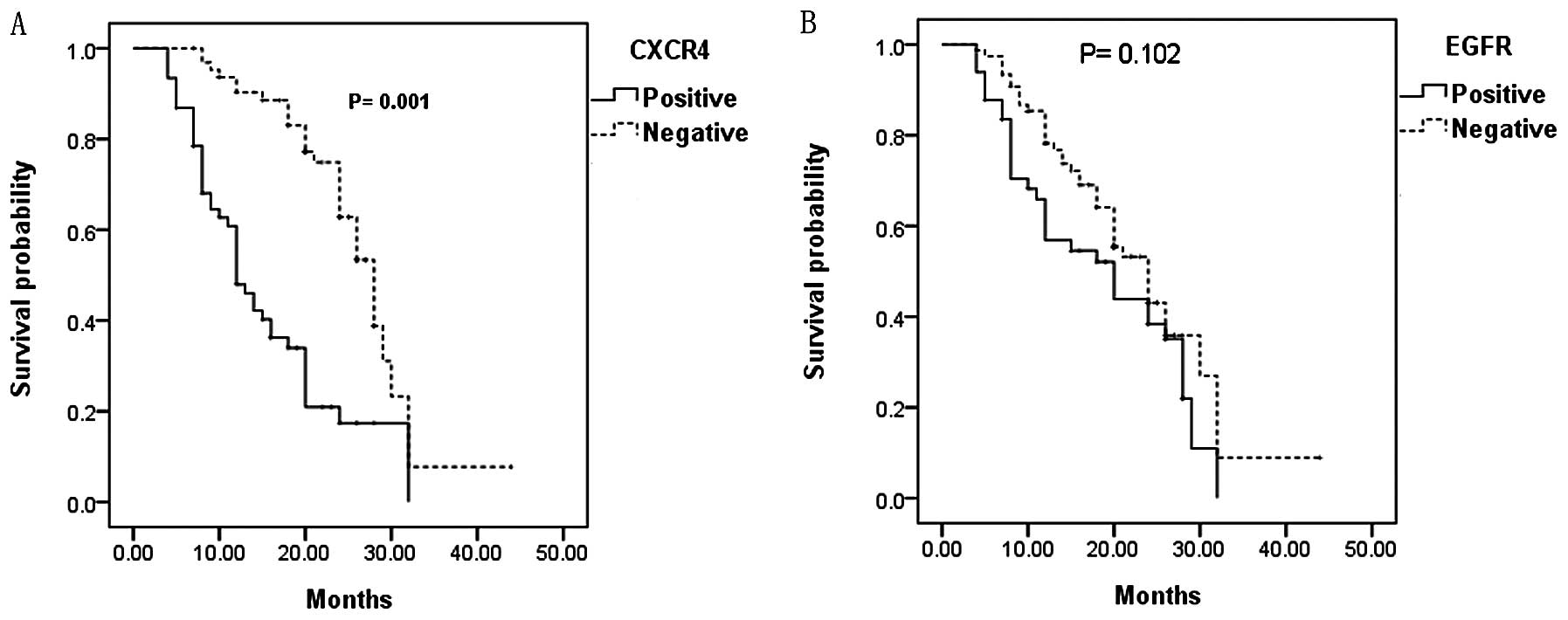
Survival analysis
The median survival time for cases with
CXCR4+ was 12.8 months and 21.1 months for patients with
CXCR4−, while the median survival time for cases with
nuclear staining of CXCR4 was 24 months and 16.4 months for
patients with no nuclear staining of CXCR4. The median survival
time for patients with EGFR+ was 15.8 months and 16.2
months for patients with EGFR−. Patients with
CXCR4+ tumors had statistically significant shorter
overall survival when compared with patients with CXCR4−
tumors (P=0.001) (Fig. 2).
Moreover, patients with CXCR4+ tumors had a
statistically significant higher cumulative incidence of
cancer-related death than patients with CXCR4− tumors
(HR=2.172), whereas there was no significant difference in overall
survival between patients with positive or negative immunostaining
for EGFR (Fig. 2).
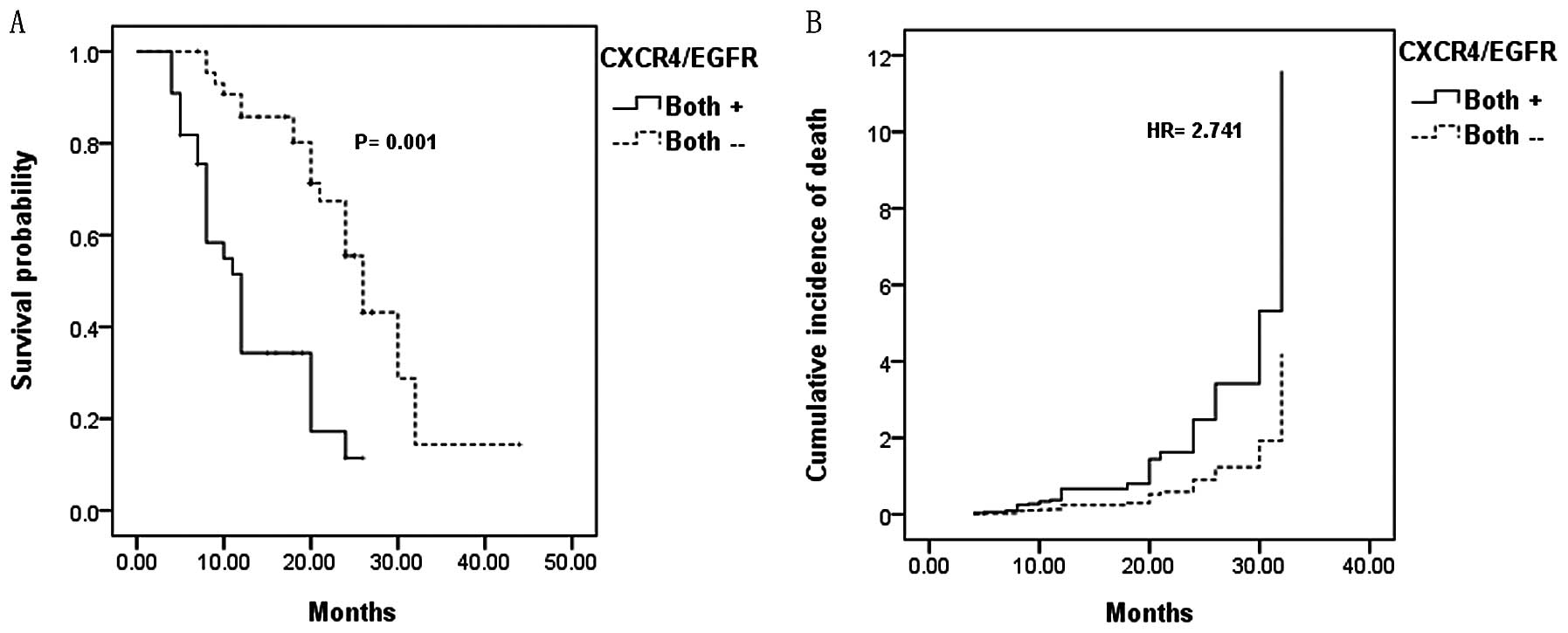
The different association patterns for CXCR4 and
EGFR protein expression were also compared. Patients were divided
into 4 groups: group I with positive CXCR4 and positive EGFR
(CXCR4+/EGFR+), group II with positive CXCR4
and negative EGFR (CXCR4+/EGFR−), group III
with negative CXCR4 and positive EGFR
(CXCR4−/EGFR+) and group IV with negative
CXCR4 and negative EGFR (CXCR4−/EGFR−).
The median survival times among these groups were:
group I (CXCR4+/EGFR+), 11.6 months; group II
(CXCR4+/EGFR−), 14.86 months; group III
(CXCR4−/EGFR+), 19.8 months and group IV
(CXCR4−/EGFR−), 24.8 months. The estimated
risk of death for these groups adjusted for patient age, tumor
stage and type of treatments that patients received is shown in
Table II.
 | Table IIEstimated risk of death associated
with EGFR and CXCR4 positivity in NSCLC. |
Table II
Estimated risk of death associated
with EGFR and CXCR4 positivity in NSCLC.
| Strata | Hazard ratio | Confidence interval
(95%) | P-value |
|---|
| CXCR4 | 2.172 | 1.229–3.839 | 0.008 |
| EGFR | 1.334 | 0.819–2.664 | 0.248 |
|
CXCR4+/EGFR+ vs.
others | 2.415 | 1.399–4.170 | 0.01 |
|
CXCR4+/EGFR− vs.
others | 1.197 | 0.680–2.107 | 0.533 |
|
CXCR4−/EGFR+ vs.
others | 0.953 | 0.295–1.718 | 0.199 |
|
CXCR4−/EGFR− vs.
others | 0.688 | 0.394–1.200 | 0.188 |
|
CXCR4+/EGFR+ vs.
CXCR4−/EGFR− | 2.741 | 1.330–5.741 | 0.006 |
The incidence of death was not different in patients
with EGFR+ when compared with EGFR− (HR=1.33,
P=0.248), but the addition of CXCR4 expression resulted in a
statistically significant increase in the incidence of death in
group I patients (CXCR4+/EGFR+) when compared
with the other groups (HR=2.415, P=0.01). Furthermore, when we
compared group I patients (CXCR4+/EGFR+) with
group IV patients (CXCR4−/EGFR−), the
incidence of cancer-related death was much higher (HR=2.741,
P=0.006) (Fig. 3).
When we compared group II
(CXCR4+/EGFR−) or III
(CXCR4−/EGFR+) with the other groups, no
significant difference was found concerning the risk of death
(HR=1.197, P=0.533 and HR=0.953, P=0.199, respectively). The risk
of death was reduced in group IV patients
(CXCR4−/EGFR−) when compared with the other
groups, yet this reduction was not statistically significant
(HR=0.688, P=0.188).
Expression of CXCR4 and EGFR in a human
NSCLC cell line
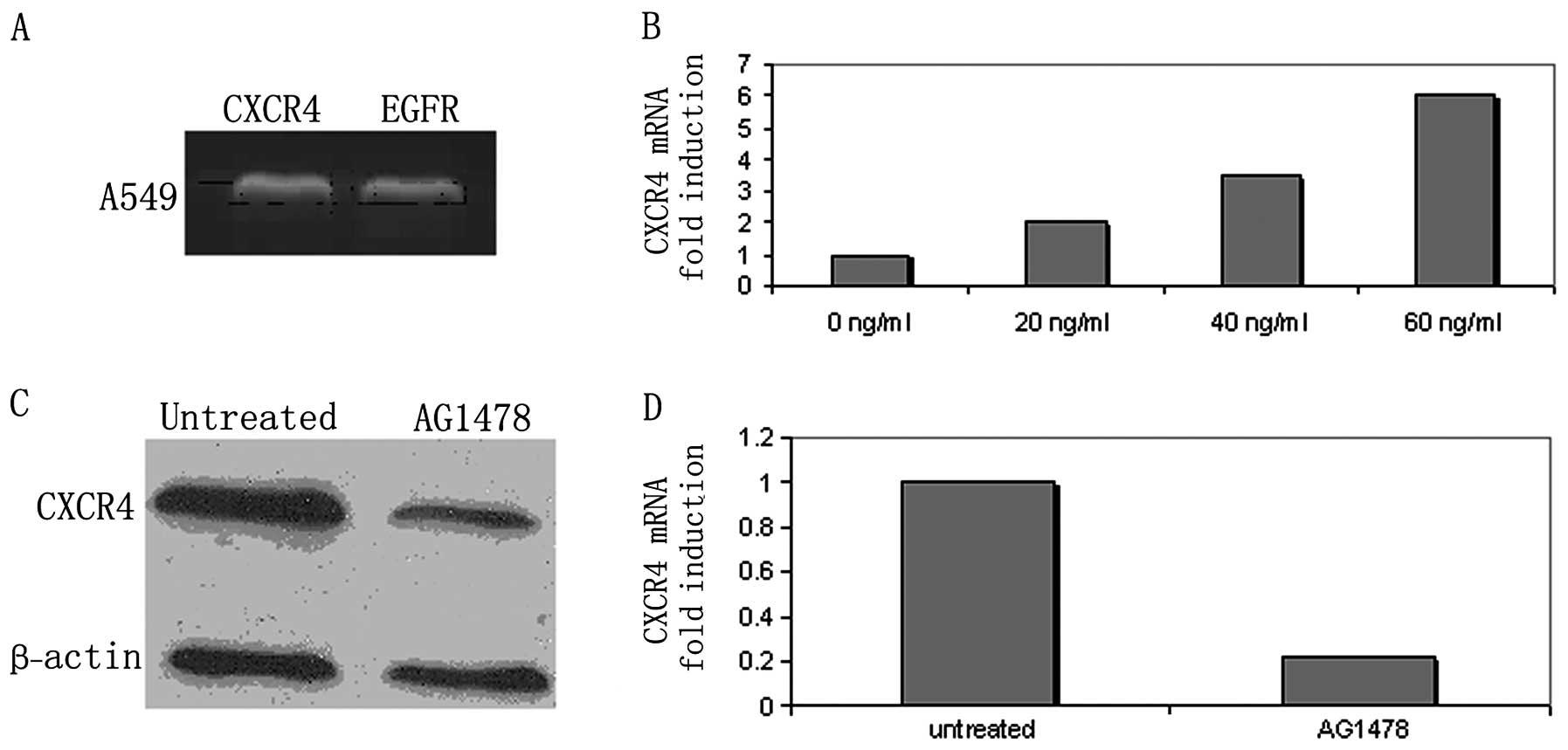
Total RNA was isolated from the A549 cell line, and
reverse transcriptase PCR was performed to evaluate the expression
of CXCR4 and EGFR. CXCR4 and EGFR mRNAs were highly expressed in
the A549 cell line (Fig. 4A).
Expression of CXCR4 in A549 cells after
stimulation by EGF
A549 cells (cultured in RPMI starved media for 24 h)
were stimulated with different concentrations of EGF for an
additional 24 h, and CXCR4 expression levels were then evaluated by
real-time PCR. When compared to the control, the expression of
CXCR4 mRNA was found to be concentration-dependent (Fig. 4B). To confirm that CXCR4
upregulation was secondary to EGF, A549 cells were treated with
AG1478 (a specific inhibitor of EGFR phosphorylation) (10 μM) for
24 h, and CXCR4 expression was re-evaluated by real-time PCR and
western blotting (Fig. 4C and D).
Our results demonstrate that CXCR4 mRNA and protein levels were
reduced after treatment with AG1478.
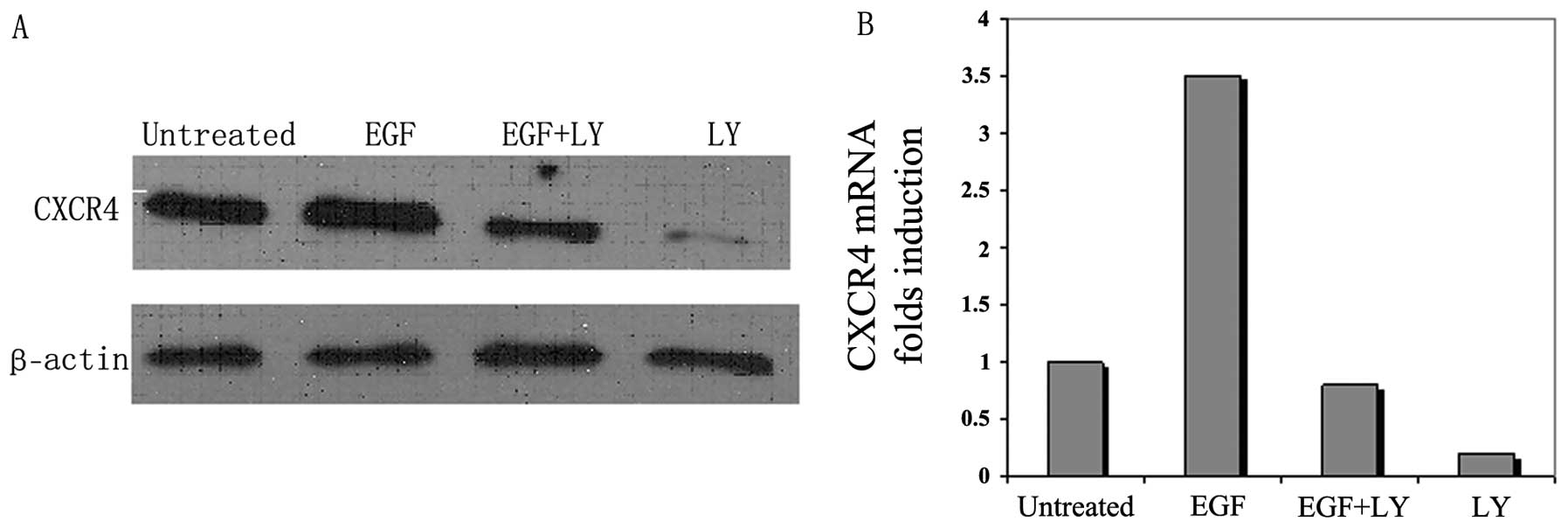
Role of the PI-3 kinase pathway in
EGF-induced upregulation of CXCR4 expression in A549 cells
EGF was previously shown to enhance the expression
of CXCR4 through the PI-3 kinase pathway, therefore we hypothesized
that EGF induces upregulation of CXCR4 through the PI-3 kinase
pathway in NSCLC. Hence, to prove this hypothesis, serum-starved
A549 cells were treated with EGF in the presence or absence of
LY294002 (a PI-3 kinase pathway inhibitor). Cells were cultured in
serum-starved media for 24 h, pretreated or not for 2 h with
LY294002, then stimulated with EGF (40 ng/ml) for 24 h. CXCR4
expression was assessed by real-time PCR and western blotting
(Fig. 5). Our results showed that
the EGF-enhanced upregulation of CXCR4 was blocked by inhibition of
the PI-3 kinase pathway.
Discussion
Research has demonstrated that cross-talk between
EGFR and CXCR4 is important in the proliferation and metastasis of
ovarian and breast cancers (7,27,28).
Therefore, in this study, we investigated the correlation between
EGFR and CXCR4 in a human NSCLC cell line and tumor specimens, and
also analyzed the correlation between co-expression of both
molecules (EGFR and CXCR4), as compared with the high expression of
a single molecule alone using immunohistochemical staining.
Previously, several studies have shown an
association between the expression of the EGFR protein and patient
survival in NSCLC (34). However,
other studies have either demonstrated no significant association
(35,36), or an inverse relationship between
the expression of EGFR protein and survival (5). A meta-analysis reported by Meert et
al showed that an average of 51% of NSCLC cases exhibited
overexpression of the EGFR protein in 14 studies based on
immunohistochemical analysis (37).
Twelve of the 16 studies (approximately three-fourths) did not find
EGFR expression to be a prognostic indicator. Similarly, Nicholson
et al(38) carried out
another review and included studies that were published from 1985
to 2000. They demonstrated that 20% of the studies found an
association between increased EGFR expression and a lower
recurrence-free survival in NSCLC, whereas, only 10% of the studies
showed an association between the overexpression of the EGFR
protein and overall survival.
In the present study, patients with EGFR+
had a higher estimated risk of death than those patients with
EGFR− (HR=1.33), but this trend did not reach
statistical significance (P=0.248). These findings remain
controversial as many studies did not adjust for significant
prognostic factors such as tumor stage, patient age at diagnosis
and type of treatment. In contrast, our study did address the
relationship between EGFR expression and clinical variables.
Furthermore, many studies suggest that the improvement in survival
among patients with high expression of EGFR is associated with
enhanced responsiveness to anti-EGFR-targeting therapies (5) In contrast, all of the patients that
were included in our study had not received any type of target
therapy.
Previous studies have attempted to determine the
clinical significance of CXCR4 expression in NSCLC. Spano et
al, studying only stage I lesions, found a significant
association between nuclear CXCR4 staining and prolonged survival
of patients with NSCLC (32). Su
et al showed a significant correlation between high
cytoplasmic CXCR4 staining intensity and metastasis (18). Recently, Wagner et al
demonstrated an independent association between CXCR4 expression
and survival depending on its subcellular location; nuclear
staining was correlated with good prognosis, whereas cytomembranous
staining was correlated with poor prognosis (19). Our results are consistent with
previous studies. We found that patients with cytoplasmic
overexpression of CXCR4 had a significant association with
metastasis and had a significantly shorter overall survival and a
high incidence of cancer-related death. However, we found nuclear
staining in only 15.2% of patients with early-stage disease, and
these patients had a comparatively long overall survival. This is
quite reasonable since only 31% of the patients included in our
study had stage I and II disease which is consistent with previous
studies that demonstrated that strong nuclear CXCR4 staining is
associated with a good prognosis in patients with early-stage NSCLC
(32).
Furthermore, our study analyzed the
clinicopathological and prognostic significance of co-expression of
CXCR4 and EGFR in NSCLC. A positive correlation was found between
co-expression of the two molecules and distant metastasis and
advanced stage disease (P=0.005). Moreover, patients with
co-expression of EGFR and CXCR4 significantly had shorter OS (11.6
months) when compared to the other groups:
CXCR4+/EGFR−,
CXCR4−/EGFR+ and
CXCR4−/EGFR−. Additionally, patients with
CXCR4+/EGFR+ had a higher risk of
cancer-related death (HR=2.415), whereas the risk of death for
patients with only EGFR+ or CXCR4+ was lower
(HR=1.33 and 2.172, respectively). These results suggest that
concomitant overexpression of EGFR and CXCR4 is associated with
poorer prognosis as compared with that of high expression of a
single molecule.
To our knowledge, this is the first study to
investigate the dual CXCR4/EGFR tumor status and determine its
prognostic impact in NSCLC. These results suggest a possible
important relationship between CXCR4 and EGFR intracellular
pathways that may stimulate different cell proliferation- and
metastasis-related pathways in NSCLC. Based on EGFR and CXCR4
expression in our study, patients with
CXCR4−/EGFR− had the most favorable prognosis
followed by CXCR4−/EGFR+ and
CXCR4+/EGFR−. Patients with
CXCR4+/EGFR+ were associated with a worse
prognosis.
Noteworthy, similar results were found in previous
studies regarding other tumor types. High expression of EGFR and
CXCR4 was found to be associated with increased risk of death and
recurrence in inflammatory breast cancer (30). Another study demonstrated that
combined high expression of cytoplasmic CXCR4 and VEGFR was
significantly associated with lymphatic and distant metastasis in
colorectal cancer (31). This
suggests the existence of a shared mechanism of CXCR4 and EGFR
interaction in common epithelial malignancies. Similarly, CXCR4 was
shown to be frequently expressed with EGFR in synovial sarcoma
(39).
An interaction between EGFR and CXCR4 has been noted
in many types of cancer. Regarding NSCLC, Phillips et al
emphasized that activation of EGFR by EGF increased CXCR4
expression in normoxia and hypoxia in NSCLC (40) In the present study, our results are
consistent with this finding. We demonstrated that EGF upregulated
CXCR4 expression through the PI-3 kinase pathway. Furthermore, we
showed that CXCR4 mRNA upregulation increased in a
concentration-dependent manner. In addition, CXCR4 expression was
reduced when A549 cells were treated with an EGFR phosphorylation
inhibitor (AG1478). These results further confirm the relationship
between EGFR and CXCR4 and explain the reason as to why higher
tumor grades and a shorter overall survival rate are noted in
patients with co-expression of CXCR4 and EGFR.
The mechanisms of EGF-induced CXCR4 upregulation are
not yet well understood, and may be explained by the fact that
mechanisms involved in CXCR4 ubiquitination and sorting may share
some common features with EGFR endocytosis and degradation
processes, although they are structurally unrelated membrane
receptors. Internalization of CXCR4 through early endosomes, and
then sorting into late endosomes and lysosomes is one mechanism of
CXCR4 degradation. Therefore, we suggest that EGFR may inhibit the
process of CXCR4 ubiquitination and subsequently abrogate sorting
and prevent its degradation (41).
Taken together, regulation of CXCR4 expression can be accomplished
through different mechanisms. When and where each mechanism should
be used to regulate CXCR4 expression warrants further
exploration.
In conclusion, according to our data, NSCLC tumor
cells with concomitant expression of both CXCR4 and EGFR may
represent a subpopulation that is able to achieve a more aggressive
clinical progression. Furthermore, CXCR4 and EGFR could be used as
new biomarkers for indicating poor prognosis in NSCLC. However, due
to the limited percentage of patients with co-expression of both
CXCR4 and EGFR and the small sample size of our study, these
results must be confirmed in large prospective studies with
cautious interpretation. It will also be worthwhile to evaluate the
efficacy of different treatments targeting both CXCR4 and EGFR in
comparison with tyrosine kinase inhibitor treatment in patients
with concomitant overexpression of CXCR4 and EGFR.
Acknowledgements
This study was supported from the Hubei Provincial
Natural Science Foundation of China (no. 2008CDA065).
References
|
1
|
Chen G, Wang Z, Liu XY and Liu FY:
High-level CXCR4 expression correlates with brain-specific
metastasis of non-small cell lung cancer. World J Surg. 35:56–61.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong J, Dai J, Shu Y, et al: Polymorphisms
in EGFR and VEGF contribute to non-small-cell lung cancer survival
in a Chinese population. Carcinogenesis. 31:1080–1086. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Li L, Chen Y and Parkin DM:
Mortality time trends and the incidence and mortality estimation
and projection for lung cancer in China. Zhongguo Fei Ai Za Zhi.
8:274–278. 2005.(In Chinese).
|
|
4
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
5
|
Van Dyke AL, Cote ML, Prysak GM, et al:
COX-2/EGFR expression and survival among women with adenocarcinoma
of lung. Carcinogenesis. 29:1781–1787. 2008.PubMed/NCBI
|
|
6
|
Sung B, Jhurani S, Ahn KS, et al:
Zerumbone down-regulates chemokine receptor CXCR4 expression
leading to inhibition of CXCL12-induced invasion of breast and
pancreatic tumor cells. Cancer Res. 68:8938–8944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahimi M, George J and Tang C: EGFR
variant-mediated invasion by enhanced CXCR4 expression through
transcriptional and post-translational mechanisms. Int J Cancer.
126:1850–1860. 2010.PubMed/NCBI
|
|
8
|
Tang CH, Tan TW, Fu WM and Yang RS:
Involvement of matrix metalloproteinase-9 in stromal cell-derived
factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis.
29:35–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stromal-derived factor (SDF-1/CXCL2) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–C995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imai H, Sunaga N, Shimizu Y, et al:
Clinicopathological and therapeutic significance of CXCL12
expression in lung cancer. Int J Immunopathol Pharmacol.
23:153–164. 2010.PubMed/NCBI
|
|
11
|
Andre F, Xia W, Conforti R, et al: CXCR4
expression in early breast cancer and risk of distant recurrence.
Oncologist. 14:1182–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chinni RS, Yamamoto H, Dong Z, et al:
CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer
cells and promotes growth of metastatic deposit in bone. Mol Cancer
Res. 6:446–457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong D and Korz W: Translating an
antagonist of chemokine receptor CXCR4: from bench to bedside. Clin
Cancer Res. 14:7975–7980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oonakahara K, Matsuyama W, Higashimoto I,
et al: Stromal-derived factor-1alpha/CXCL12-CXCR 4 axis is involved
in the dissemination of NSCLC cells into pleural space. Am J Respir
Cell Mol Biol. 30:671–677. 2004. View Article : Google Scholar
|
|
15
|
Kijima T, Maulik G, Ma PC, et al:
Regulation of cellular proliferation, cytoskeletal function, and
signal transduction through CXCR4 and c-Kit in small cell lung
cancer cells. Cancer Res. 62:6304–6311. 2002.PubMed/NCBI
|
|
16
|
Phillips RJ, Burdick MD, Lutz M, et al:
The stromal derived factor-1/CXCL12-CXC chemokine receptor 4
biological axis in non-small cell lung cancer metastases. Am J
Respir Crit Care Med. 167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martínez-García E, Irigoyen M,
González-Moreno O, et al: Repetitive nicotine exposure leads to a
more malignant and metastasis-prone phenotype of SCLC: a molecular
insight into the importance of quitting smoking during treatment.
Toxicol Sci. 116:467–476. 2010.
|
|
18
|
Su L, Zhong J, Xu H, et al: Differential
expression of CXCR4 is associated with the metastatic potential of
human non-small cell lung cancer cells. Clin Cancer Res.
11:8273–8280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wagner PL, Hyjek E, Vazquez MF, et al:
CXCL12 and CXCR4 in adenocarcinoma of the lung: association with
metastasis and survival. J Thorac Cardiovasc Surg. 137:615–621.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cappuzzo F, Hirsch FR, Rossi E, et al:
Epidermal growth factor receptor gene and protein and gefitinib
sensitivity in non-small-cell lung cancer. J Natl Cancer Inst.
97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morita S, Okamoto I, Kobayashi K, et al:
Combined survival analysis of prospective clinical trials of
gefitinib for non-small cell lung cancer with EGFR mutation.
Clin Cancer Res. 15:4493–4498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Z, Zhang J, Zeng X, et al:
Relationship between EGFR expression, copy number and mutation in
lung adenocarcinomas. BMC Cancer. 10:3762010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki S, Dobashi Y, Yamane T, et al:
Protein overexpression and gene amplification of epidermal growth
factor receptor in nonsmall cell lung carcinomas. An
immunohistochemical and fluorescence in situ hybridization study.
Cancer. 103:1265–1273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer OM, Hart S, Gschwind A and Ullrich
A: EGFR signal transactivation in cancer cells. Biochem Soc Trans.
31:1203–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaman SN, Resek ME and Robbins SM: Dual
acylation and lipid raft association of Src-family protein tyrosine
kinases are required for SDF-1/CXCL12-mediated chemotaxis in the
Jurkat human T cell lymphoma cell line. J Leukoc Biol.
84:1082–1091. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peipp M, Schneider-Merck T, Dechant M, et
al: Tumor cell killing mechanisms of epidermal growth factor
receptor (EGFR) antibodies are not affected by lung
cancer-associated EGFR kinase mutations. J Immunol. 180:4338–4345.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porcile C, Bajetto A, Barbieri F, et al:
Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates
ovarian cancer cell growth through the EGF receptor
transactivation. Exp Cell Res. 308:241–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo Z, Cai S, Fang R, et al: The
synergistic effects of CXCR4 and EGFR on promoting EGF-mediated
metastasis in ovarian cancer cells. Colloids Surf B Biointerfaces.
60:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cabioglu N, Summy J, Miller C, et al:
CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu
in breast cancer cells by a novel pathway involving Src kinase
activation. Cancer Res. 65:6493–6497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cabioglu N, Gong Y, Islam R, et al:
Expression of growth factor and chemokine receptors: new insight in
the biology of inflammatory breast cancer. Ann Oncol. 18:1021–1029.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu YG, Jin M, Xu H, et al:
Clinicopathologic significance of HIF-1α, CXCR4, and VEGF
expression in colon cancer. Clin Dev Immunol. 7:5375312010.
|
|
32
|
Spano JP, Andre F, Morat L, et al:
Chemokine receptor CXCR4 and early-stage non-small cell lung
cancer: pattern of expression and correlation with outcome. Ann
Oncol. 15:613–617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ludovini V, Bellezza G, Pistola L, et al:
High coexpression of both insulin-like growth factor receptor-1
(IGFR-1) and epidermal growth factor receptor (EGFR) is associated
with shorter disease-free survival in resected non-small-cell lung
cancer patients. Ann Oncol. 20:842–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niemiec J, Kolodziejski L and Dyczek S:
EGFR LI and Ki-67 LI are independent prognostic parameters
influencing survivals of surgically treated squamous cell lung
cancer patients. Neoplasma. 52:231–237. 2005.PubMed/NCBI
|
|
35
|
Brabender J, Danenberg KD, Metzger R, et
al: Epidermal growth factor receptor and HER2-neu mRNA expression
in non-small cell lung cancer is correlated with survival. Clin
Cancer Res. 7:1850–1855. 2001.PubMed/NCBI
|
|
36
|
Kanematsu T, Yano S, Uehara H, et al:
Phosphorylation, but not overexpression, of epidermal growth factor
receptor is associated with poor prognosis of non-small cell lung
cancer patients. Oncol Res. 13:289–298. 2003.PubMed/NCBI
|
|
37
|
Meert AP, Martin B, Delmotte P, et al: The
role of EGF-R expression on patient survival in lung cancer: a
systematic review with meta-analysis. Eur Respir J. 20:975–981.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar
|
|
39
|
Grisanti S, Rossi L, Ardighieri C, et al:
CXCR4, EGFR and HER2 expression in patients with high-risk synovial
sarcoma (SS): a clinicopathologic study. J Clin Oncol.
24:95802006.
|
|
40
|
Phillips RJ, Mestas J, Gharaee-Kermani M,
et al: Epidermal growth factor and hypoxia-induced expression of
CXC chemokine receptor 4 on non-small cell lung cancer cells is
regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian
target of rapamycin signaling pathway and activation of hypoxia
inducible factor-1alpha. J Biol Chem. 280:22473–22481. 2005.
|
|
41
|
Li YM, Pan Y, Wei Y, et al: Upregulation
of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer
Cell. 6:459–469. 2004. View Article : Google Scholar : PubMed/NCBI
|